全世界超过8×108 hm2的土地受到土壤盐渍化的影响,并且这一问题仍在持续恶化[1]。土壤盐渍化严重影响种子萌发、作物生长和生产力,是限制全球农业生产的一个主要因素。
土壤环境中高浓度的可溶性盐会降低植物根系表面的水势,影响根部对水分的吸收,降低植物水分利用效率,进而对植物产生渗透胁迫、离子胁迫及氧化胁迫等[2]。NaCl是自然界中最主要的盐,也是引起盐胁迫的主要成分。高盐环境下,植物胞质中大量积累的Na+会打破细胞的Na+/K+平衡,降低植物细胞维持内外离子动态平衡的能力进而阻碍对其他离子(如K+)的吸收,最终影响植物的初级代谢和次级代谢[3]。为适应高盐环境,植物通过对Na+外排或区隔化以降低细胞内的Na+浓度,进而建立新的离子稳态。
探究盐胁迫下植物维持Na+动态平衡的分子机制将有助于解析植物耐盐性分子机理。近年来,利用分子遗传学、基因组学等技术手段,在植物维持Na+动态平衡的分子调节机制方面取得了诸多重要进展[4],但仍有许多关键问题尚不清楚。本文主要综述了盐胁迫下植物Na+吸收与转运的动态平衡机制的研究进展,以期为培育及开发耐盐作物新品种并合理利用改良后盐碱地资源奠定理论基础。
1 植物对盐胁迫信号的感知土壤中高浓度的Na+和Cl+会引发植物产生离子胁迫、高渗胁迫以及氧化损伤等次级胁迫[5],植物体内的叶绿素酶被激活,加速了叶绿素分解,造成植物光合速率下降,植物不能获取足够的物质和能量使生长受到抑制[6],因而植物会通过表型、代谢、生理性状和基因表达等诸多方面的调节以应对盐胁迫。Essah等[7]研究发现经NaCl溶液处理拟南芥2 min后,根组织内便积累了大量Na+,而Bose等[8]发现经NaCl溶液处理拟南芥10 min后,其根部积累的Na+便开始外排。
胁迫条件下,感知盐信号是启动细胞离子稳态重建的先决条件。生物体可以通过感受器或受体以感受胞质中钠离子水平的增加。钙调磷酸酶途径[9]在酵母(Saccharomyces cerevisiae)对Na+胁迫信号的感受中发挥着重要作用。在该途径中,高浓度Na+会引起胞质中Ca2+与钙调蛋白和钙调磷酸酶B亚基(B subunit of calcineurin,CnB)的结合,Ca2+-钙调蛋白和Ca2+-CnB进而激活钙调磷酸酶(Calcineurin)的催化亚基CnA。活化的钙调磷酸酶去磷酸化锌指转录因子CRZ1,并转位到细胞核中,进而激活ENA1和其他目的基因的表达[4]。ENA1编码Na+-ATPase,将过多的Na+泵出细胞,维持细胞内的离子动态平衡。据推测,植物细胞可能是通过感受器或受体来感知胞质中钠离子的水平。尽管类钙调磷酸酶(CBL)已经被广泛地用来指代植物EF钙结合蛋白家族,但是,植物基因组并不编码任何钙调磷酸酶蛋白[10]。人们至今也未从植物中鉴定出Na+的感受器或受体。因此,目前植物细胞如何感受Na+的机制仍不清楚。
2 Na+调节机制高盐环境下,为降低细胞内Na+的大量积累,植物通过限制Na+摄取、增加Na+外排和对细胞内Na+的区隔等策略以降低细胞质中Na+浓度,维持相对较高的K+/Na+比,进而保证植物的正常生命活动[11]。在这些过程中起关键作用的是质膜Na+/H+逆转运蛋白(Plasma membrane Na+/H+ antiporter)、高亲和性K+转运蛋白(High-affinity K+ channel transporter 1,HKT1)和液泡膜Na+(K+)/H+逆转运蛋白(Na+/H+ exchanger,NHX)。SOS1主要在植物质膜上起调控作用,以质膜上质子H+梯度为驱动力,促使H+顺化学势进入胞内,Na+逆化学势排出胞外,以减轻Na+的毒害,是植物响应盐胁迫的重要机制[12]。植物的根和地上部均存在大量的钾转运载体和通道蛋白,它们大多定位在质膜和液泡膜,从而保证植物从土壤中吸收K+,并利于在不同组织间的分配,使植物在细胞及整体水平上均能维持高的K+/Na+比率。其中,植物阳离子载体——HKT家族,在Na+、K+均衡过程中发挥重要作用。NHX主要在液泡膜上起作用,以液泡膜上H+-ATPase和H+-PPiase两种质子泵产生的跨膜质子梯度为驱动力,将细胞质中的Na+区隔在液泡,从而降低盐胁迫的损伤[13-14]。
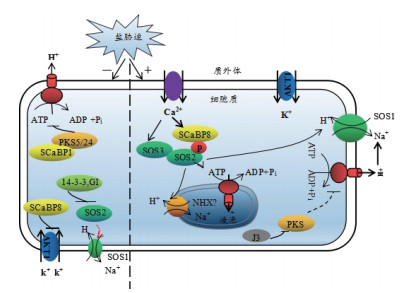
2.1 Na+的吸收与转运盐超敏感(Salt overly sensitive,SOS)途径特异性调控离子动态平衡,是目前研究最清楚的植物应答盐胁迫信号转导途径之一[5]。植物受到高浓度Na+胁迫时,植物运用SOS途径的钙依赖蛋白激酶途径介导盐胁迫信号,通过外排Na+来增强对盐胁迫的耐受性。SOS途径中的3个核心成员为SOS1、SOS2和SOS3,分别为质膜Na+/H+逆转运蛋白,丝氨酸/苏氨酸蛋白激酶(Serine/threonine protein kinase)和Ca2+感知蛋白。植物受到盐胁迫时,钙结合蛋白SOS3和SCaBP8/CBL10(SOS3-LIKE CALCIUM BINDING PROTEIN8/CALCINEURIN B-LIKE PROTEIN10)首先感知Ca2+的增加[16-19]。随后SOS3/SCaBP8和SOS2结合将SOS2激酶磷酸化[18, 20-21],磷酸化的SOS2激酶可激活质膜上的SOS1蛋白,将细胞质中多余的Na+排出细胞外,进而维持细胞质中Na+的动态平衡[20, 22-23]。正常条件下(无盐胁迫),14-3-3和GI(GIGANTEA)作为负调节蛋白与SOS2结合抑制SOS途径的激酶活性,以确保SOS2处于非激活状态[24]。此外,SOS2与磷酸酶2C类的蛋白磷酸酶ABI2相互作用,也使SOS2处于非激活状态[25]。盐胁迫下,14-3-3蛋白和GI蛋白通过26S蛋白酶体途径降解[26-27],PKS5活性受到抑制进而激活质膜H+-ATP酶的活性,使SOS途径激活[28-29]。
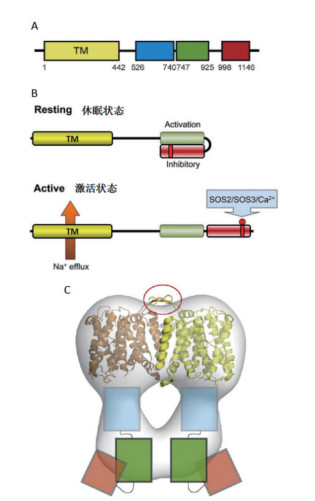
SOS1(图 1)蛋白是SOS途径中将Na+从胞质运输到胞外的关键蛋白。拟南芥AtSOS1蛋白的N端是高度疏水区,含有12个跨膜区,与细菌及一些真核生物的Na+/H+逆转运蛋白同源性较高。C端是亲水性区域,约含有700个氨基酸,SOS1也成为目前已知的最长的Na+/H+逆转运蛋白,SOS1蛋白N端的跨膜区和C端位于细胞质中的长链尾巴共同构成了一个同源二聚体结构。SOS1蛋白的跨膜结构域与胞质结构域相互作用来抑制其转运活性(图 2)[30-31]。无盐胁迫时,AtSOS1的C末端自抑制域(Autoinhibitory domains)与相邻的激活域相互结合,AtSOS1蛋白保持休眠状态[32]。在盐胁迫下,AtSOS1蛋白C末端自抑制域的1 138位丝氨酸磷酸化,Na+/H+逆转运蛋白的自抑制域被解除,转为活化状态,4个结构域的单体产物形成二聚体,能把细胞内过多的Na+排出细胞外,从而减少Na+对细胞的危害,使植物表现出较高的耐盐性[30, 32]。
|
| 14-3-3: 14-3-3蛋白; GI: (GIGANTEA)GIGANTEA蛋白; SCaBP1/8: (calcium-binding protein1/8)钙结合蛋白1/8; NHX: (Na+/H+ exchanger)钠离子/氢质子交换蛋白; PKS5/24: (SOS2-like protein5/24 serine/threonine protein kinases)丝氨酸、苏氨酸蛋白激酶5/24; AKT1: (Arabidopsis K+ transporter1)拟南芥钾离子转运蛋白; J3: (DnaJ homolog 3)热休克蛋白3; SOS1/2/3: (salt overly sensitive1/2/3)SOS1/2/3蛋白 图 1 植物细胞中SOS途径示意图[15] |
拟南芥AtSOS1在根尖表皮和木质部薄壁细胞膜中表达,AtSOS1蛋白通过将Na+泵出根细胞的方式以减少共质体途径运输至维管束的Na+含量[33]。将番茄SOS1完全沉默,植物则表现出根部和叶片中高Na+含量的盐敏感表型,而特异性沉默茎部维管束中SISOS1,则表现低Na+含量的耐盐表型,表明SISOS1蛋白主要在番茄根细胞将Na+排出[34]。SOS1蛋白通过调节甜土植物和盐生植物木质部Na+的装载以缓解植物受高盐环境影响[35]。例如,在莲子根细胞中通过增强SOS1的表达将Na+由木质部转运至地上部分以缓解盐分胁迫[36]。
SOS1蛋白在木质部薄壁细胞膜上表达具有调控木质部汁液Na+含量的功能。在中度盐胁迫(如25 mmol/L NaCl)条件下,拟南芥AtSOS1蛋白具有装载Na+至木质部向上运输的功能,在重度盐胁迫(如100 mmol/L NaCl)条件下,在根部及地上部维管束薄壁细胞膜上表达的SOS1蛋白限制Na+装载入木质部,避免地上部Na+含量升高[34]。小花碱茅Pt SOS1蛋白也具有控制木质部汁液Na+含量的功能[37]。
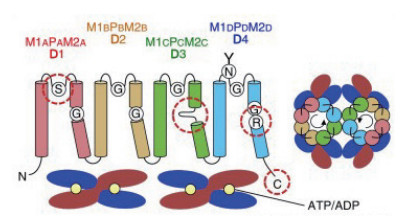
HKT1(High-affinity K+ channel transporter)蛋白是另一个重要的离子转运蛋白。其功能包括介导植株跨细胞膜的Na+、K+转运或K+-Na+共转运; 参与植物韧皮部Na+的外排和木质部Na+的卸载,阻止过度的Na+转运到幼叶,同时积累更多的K+离子,保持植物体内的Na+和K+稳态平衡,从而赋予植物盐耐受性[38-39]。基于对拟南芥AtHKT1蛋白结构分析,该蛋白含有8个跨膜结构域,中间是4个孔状区域[40],组成4个高度保守的重复跨膜结构域跨膜-环-跨膜(Membrane -pore-membrane motif,MPM)结构,每个MPM结构包括2个跨膜螺旋M1、M2及中间1个P环,其N末端135-142残基区域、C末端377-384残基区域均位于细胞质一侧,而55-62残基区域暴露在膜外侧(图 3)。
HKT1蛋白因其结构的差异,功能也有不同,可将HKT1蛋白分为2个亚类:第一类HKT1蛋白在第一个环状区域的位置有一个丝氨酸残基,另外3个环状区域的位置是甘氨酸残基,构成Ser-Gly-Gly-Gly(SGGG)类型[41],该类型是Na+特异性载体,主要作用是高度选择性转运Na+,对调节植物中Na+动态平衡具有重要作用[42]。第二类HKT1蛋白在第一个孔状区域的位置由甘氨酸残基替换丝氨酸残基,另外3个孔状区域的位置仍是甘氨酸残基,构成Gly-Gly-Gly-Gly(GGGG)类型[41],这类HKT1蛋白不仅是Na+- K+共转运载体。例如,水稻亚家族2成员OsHKT2; 1与OsHKT2; 2的氨基酸序列同源性比对高达91%,OsHKT2; 1是Na+转运蛋白,Os HKT2; 2则是K+/Na+协同转运蛋白[43],这是因为在功能区域OsHKT2; 1是Ser残基,而OsHKT2; 2是Gly残基[44]。
植物维管束鞘组织内分布的HKT转运蛋白对Na+具有高度的选择性,可将已进入木质部的Na+卸载在维管束薄壁细胞中,减少木质部汁液中Na+含量,从而降低钠离子的向上运输,提高植物耐盐性[45]。AtHKT1; 1在植物的根部和叶的木质部薄壁细胞中表达将Na+从根的木质部细胞卸载,控制Na+从根部向地上部分的转运[46]。在盐胁迫下,大麦HKT1; 5通过卸载根部和叶鞘木质部Na+来减少Na+向地上部叶片运输,提高大麦的耐盐性[47]。Hamamoto等[40]、Garriga等[41]和Zhang等[42]证明小麦、拟南芥、玉米等植物中的HKT1类离子转运蛋白能够将木质部维管束中的Na+转运到其他部位来增加植物耐盐性。植物通过排钠机制,即控制木质部汁液中钠离子向上运输,维持地上部较高K+/Na+比值来缓解盐胁迫对植物的危害。此外,植物将吸收的Na+通过木质部运输至地上叶片等组织中,也有证据表明一部分Na+分泌到韧皮部中再运回根中,最后分泌到环境中[48]。盐胁迫下,拟南芥tus-1突变体中At HKT1; 1在茎部起主要调节作用,通过回流茎部木质部汁液减少Na+向花器官运输,即通过降低花器官Na+含量来提高植株的耐盐性[49]。
2.2 Na+的区隔化植物将Na+区隔化入特定组织细胞的液泡或囊泡内,可以降低离子毒害,该过程主要由液泡膜上的Na+/H+逆转运体(Na+/H+ exchanger,NHX)的跨膜转运实现。地上部叶片表皮细胞或维管束细胞内有液泡,植物利用液泡膜上H+-ATPase和焦磷酸酶产生的质子梯度,驱动Na+/H+逆向转运蛋白,将Na+泵到液泡内贮藏,减轻Na+对胞质酶的伤害,以确保胞质生理功能[50]。植物(Na+、K+/H+)逆转运体(Antiporter NHX)利用质子泵产生的H+电化学梯度进行离子交换,根据其亚细胞分布分为质膜NHX(Plasma membrane NHX)、液泡NHX(Vacuolar NHX)和内膜NHX(Endosomal NHX)。液泡膜Na+/H+逆向转运蛋白广泛存在于植物界中,从藻类到开花植物中都被证实有该蛋白的存在[38-39]。在植物的整个生活史中,该蛋白扮演着非常重要的角色。在盐胁迫下,液泡膜Na+/H+逆向转运蛋白参与细胞质Na+浓度和pH的调节,将Na+和K+区隔化入液泡,在植物盐胁迫响应中起到关键作用[51-53]。
在高盐环境下,Na+进入细胞后,液泡膜上的NHX类Na+/H+逆向转运蛋白开始工作。Na+/H+逆向转运蛋白是一种跨膜蛋白(图 4)。AtNHX1蛋白N-端位于细胞质,C-端位于液泡内,含有9个跨膜结构域(Transmembrane domains TM),3个疏水结构域(TM3,TM5,TM6),将细胞质中多余的Na+区域化到液泡中,降低胞质Na+浓度,缓解植物对盐胁迫压力[55]。该过程所需的能量主要由液泡膜的H+-异位酶、H+-ATP酶(H+-ATPase)和H+-焦磷酸酶(H+-PPiase)产生的质子驱动力提供。H+-ATP酶和H+-焦磷酸酶产生的质子梯度,将H+运输到细胞质,产生H+电化学梯度驱动液泡膜上的Na+/H+逆向转运蛋白活性使Na+跨膜运输,实现Na+区域化以此提高植物耐盐性[56]。
研究表明,过表达NHX逆向转运蛋白可以提高植物耐盐性。200 mmol/L NaCl胁迫下,番茄和油菜AtNHX1蛋白过表达植株,仍能正常生长和结实[14]。过表达GmNHX1的百脉根在盐胁迫条件下,再生能力、光合作用及存活时间明显高于对照组,耐盐性提高[57]。He等[58]将AtNHX1转入棉花,在温室200 mmol/L NaCl胁迫下转基因植株产量提高,棉纤维含量增多,光合作用增强,氮的同化速率增高。也有研究表明,盐胁迫下,植物可以将体内积累的Na+运输到液泡内,避免Na+对植物的伤害[59]。盐胁迫下根系伸长区中的Na+被区隔化到液泡中,缓解盐胁迫对植物影响[60]。研究发现nhx5和nhx6双敲除植物对盐胁迫更敏感[61]。NHX蛋白在植物响应盐胁迫中的主要作用是将Na+区域化进入液泡中,使体内多余的Na+向根、茎基部、叶鞘等薄壁细胞发达的组织中运输,以降低胞内有毒离子的浓度,实现植物体内离子平衡稳态。
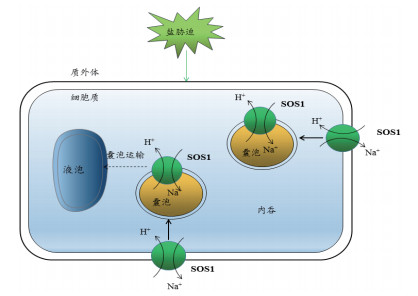
Na+区隔化还可能是通过吞饮途径完成的。细胞内膜动力学推动了人们对该途径的认识。盐胁迫下,植物可通过促进内吞作用及减少液泡膜融合,增加囊泡上Na+/H+逆转运蛋白的数量,从而增强囊泡对Na+的摄取,降低胞浆中Na+的浓度,这也许是植物适应盐胁迫的一种机制(图 5)。盐胁迫下,在拟南芥的根尖细胞和悬浮培养细胞的液泡内可以观察到囊泡的相互运动和检测到Na+的积累[62]。在拟南芥中,过表达AtRab7促进根、叶片细胞及原生质体的内吞作用,拟南芥耐盐性增强[63]。
3 结论与展望高盐胁迫下,植物由于受到生理干旱的影响首先会导致渗透胁迫; 其次,在细胞质中可能过度积累Na+离子,使胞质Na+/K+比显著升高,导致其细胞遭受离子毒害[5, 64]; 随后,植物体内的活性氧物质大量积累导致植物遭受严重的氧化胁迫[11]。植物进化出离子转运机制、渗透调节机制和活性氧清除机制来适应盐胁迫,这三种机制相互作用、协同调节使植物能够在盐渍化的土壤环境下生存[65]。
关于植物离子转运机制的研究较为广泛,而且取得了一定的成果,但是仍有许多内容需要进行深入研究。如鉴定盐胁迫下植物Na+的感受器或受体,阐明离子转运机制涉及的不同途径中蛋白质的调节机制,如SOS1和HKT1的拮抗作用是如何被协调的。另外,多重的离子转运代表如何协同作用来维持植物体内相对稳定的Na+-K+环境,以及离子转运蛋白是否通过以及如何参与植物多个代谢调控机制来赋予植物盐耐受性的,这些问题都有待深入研究。
| [1] |
Munns R, Tester M. Mechanisms of salinity tolerance[J]. Annual Review of Plant Biology, 2008, 59: 651-681. DOI:10.1146/annurev.arplant.59.032607.092911 |
| [2] |
李建锐.谷子SiASR4基因参与植物响应干旱和盐胁迫的功能研究[D].北京: 中国农业大学, 2018.
|
| [3] |
赵盼盼.棉花GhACR1沉默株系的构建及其功能初步研究[D].新乡: 河南师范大学, 2018. http://cdmd.cnki.com.cn/Article/CDMD-10476-1018241173.htm
|
| [4] |
朱健康, 倪建平. 植物非生物胁迫信号转导及应答[J]. 中国稻米, 2016, 22(6): 52-60. DOI:10.3969/j.issn.1006-8082.2016.06.012 |
| [5] |
Zhu JK. Salt and drought stress signal transduction in plants[J]. Annu Rev Plant Biol, 2002, 53: 247-273. DOI:10.1146/annurev.arplant.53.091401.143329 |
| [6] |
Rao GG, Ramaiah JK, Rao GR. Salinity induced changes in the activities of aspartate & alanine amino transferases & glutamate dehydrogenase in peanut(Arachis hypogaea L.)leaves[J]. Indian Journal of Experimental Biology, 1981, 19(8): 771. |
| [7] |
Essah PA, Davenport R, Tester M. Sodium influx and accumulation in Arabidopsis[J]. Plant Physiology, 2003, 133: 307-318. DOI:10.1104/pp.103.022178 |
| [8] |
Bose J, Rodrigomoreno A, Lai D, et al. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa[J]. Annals of Botany, 2015, 115: 481-494. DOI:10.1093/aob/mcu219 |
| [9] |
Thewes S. Calcineurin-Crz1 signaling in lower eukaryotes[J]. Eukaryot Cell, 2014, 13: 694-705. DOI:10.1128/EC.00038-14 |
| [10] |
Yu Q, An L, Li W. The CBL-CIPK network mediates different signaling pathways in plants[J]. Plant Cell Rep, 2014, 33: 203-214. DOI:10.1007/s00299-013-1507-1 |
| [11] |
Zhu JK. Plant salt tolerance[J]. TRENDS in Plant Science, 2001, 6(2): 66-71. DOI:10.1016/S1360-1385(00)01838-0 |
| [12] |
Yue Y, Zhang M, Zhang J, et al. SOS1 gene overexpression increa-sed salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ratio[J]. Journal of Plant Physiology, 2012, 169(3): 255-261. DOI:10.1016/j.jplph.2011.10.007 |
| [13] |
Gaxiola RA, Li J, Undurraga S, et al. Drought and salt tolerant plants result from overexpression of the AVP1 H+-pump[J]. Proceedings of the National Academy of Sciences of the USA, 2001, 98(20): 11444-11449. DOI:10.1073/pnas.191389398 |
| [14] |
Zhang GH, Su Q, An LJ, et al. Characterization and expression of a vacuolar Na+/H+ antiporter gene from the monocot halophyte Aeluropus littoralis[J]. Plant Physiology and Biochemistry, 2008, 46(2): 117-126. |
| [15] |
Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses[J]. New Phytologist, 2018, 217(2): 523-539. DOI:10.1111/nph.14920 |
| [16] |
Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance[J]. Science, 1998, 280(5371): 1943-1945. DOI:10.1126/science.280.5371.1943 |
| [17] |
Ishitani M, Liu J, Halfter U, et al. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding[J]. The Plant Cell, 2000, 12(9): 1667. DOI:10.1105/tpc.12.9.1667 |
| [18] |
Quan R, Lin H, Mendoza I, et al. SCABP8/CBL10, a putative calc-ium sensor, interacts with the protein kinase SOS2 to protect Ara-bidopsis shoots from salt stress[J]. The Plant Cell, 2007, 19(4): 1415-1431. DOI:10.1105/tpc.106.042291 |
| [19] |
Zhu JK. Abiotic Stress signaling and responses in plants[J]. China Rice, 2016, 167(2): 313. |
| [20] |
Lin H, Guo Y. Phosphorylation of SOS3 like calcium binding protein8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis[J]. The Plant Cell, 2009, 21(5): 1607-1619. DOI:10.1105/tpc.109.066217 |
| [21] |
Halfter U, Ishitani M, Zhu JK. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3[J]. Proceedings of the National Academy of Sciences of the USA, 2000, 97(7): 3735. DOI:10.1073/pnas.97.7.3735 |
| [22] |
Shi HZ, Ishitani M, Cheolsoo K, et al. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter[J]. Proceedings of the National Academy of Sciences of the USA, 2000, 97(12): 6896. DOI:10.1073/pnas.120170197 |
| [23] |
Qiu QS, Guo Y, Dietrich MA, et al. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3[J]. Proceedings of the National Academy of Sciences of the USA, 2002, 99(12): 8436. DOI:10.1073/pnas.122224699 |
| [24] |
Zhou H, Lin H, Chen S, et al. Inhibition of the Arabidopsis salt overly sensitive pathway by 14-3-3 proteins[J]. The Plant Cell, 2014, 26: 1166-1182. DOI:10.1105/tpc.113.117069 |
| [25] |
Ohta M, Guo Y, Halfter U, et al. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2[J]. Proceedings of the National Academy of Sciences of the USA, 2003, 100: 11771-11776. DOI:10.1073/pnas.2034853100 |
| [26] |
Kim WY, Ali Z, Park HJ, et al. Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis[J]. Nature Communications, 2013, 4: 273-275. |
| [27] |
Tan T, Cai J, Zhan E, et al. Stability and localization of 14-3-3 proteins are involved in salt tolerance in Arabidopsis[J]. Plant Molecular Biology, 2016, 92: 391-400. DOI:10.1007/s11103-016-0520-5 |
| [28] |
Yang Y, Qin Y, Xie C, et al. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase[J]. The Plant Cell, 2010, 22: 1313-1332. DOI:10.1105/tpc.109.069609 |
| [29] |
Fuglsang AT, Guo Y, Cuin TA, et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein[J]. The Plant Cell, 2007, 19(5): 1617-1634. DOI:10.1105/tpc.105.035626 |
| [30] |
Quintero FJ, Martinez-Atienza J, Villalta I, et al. Activation of the plasma membrane Na+/H+ antiporter salt-overly-sensitive 1(SOS1)by phosphorylation of an auto-inhibitory C-terminal domain[J]. Proceedings of the National Academy of Sciences of the USA, 2011, 108(6): 2611-2616. DOI:10.1073/pnas.1018921108 |
| [31] |
Núñez-Ramírez R, Sánchez-Barrena MJ, Villalta I, et al. Structural insights on the plant salt-overly-sensitive 1(SOS1)Na+/H+ antiporter[J]. Journal of molecular biology, 2012, 424(5): 283-294. DOI:10.1016/j.jmb.2012.09.015 |
| [32] |
Feki K, Quintero FJ, Pardo JM, et al. Regulation of durum wheat Na+/H+ exchanger TdSOS1 by phosphorylation[J]. Plant molecular biology, 2011, 76(6): 545-556. DOI:10.1007/s11103-011-9787-8 |
| [33] |
Shi H, Quintero FJ, Pardo JM, et al. The putative plasma membrane NA/H antiporter SOS1 controls long-distance NA+ transport in plants[J]. The Plant Cell, 2002, 14(2): 465-477. DOI:10.1105/tpc.010371 |
| [34] |
Olías R, Eljakaoui Z, Li J, et al. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs[J]. Plant, Cell & Environment, 2009, 32(7): 904-916. |
| [35] |
Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops[J]. Ann Bot, 2013, 112(7): 1209-1221. DOI:10.1093/aob/mct205 |
| [36] |
Katschnig D, Bliek T, Rozema J, et al. Constitutive high-level SOS1, expression and absence of HKT1; 1, expression in the salt-accumulating halophyte Salicornia dolichostachya[J]. Plant Science, 2015, 234: 144-154. DOI:10.1016/j.plantsci.2015.02.011 |
| [37] |
Zhang WD, Wang P, Bao Z, et al. SOS1, HKT1; 5, and NHX1 synergisti-cally modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora[J]. Frontiers in Plant Science, 2017, 8: 576. |
| [38] |
Benito B, Haro R, et al. The twins K+ and Na+ in plants[J]. Journal of Plant Physiology, 2014, 171(9): 723-731. DOI:10.1016/j.jplph.2013.10.014 |
| [39] |
Huang Y, Guan C, Liu Y, et al. Enhanced growth performance and salinity tolerance in transgenic switchgrass via overexpressing Vacuolar Na+(K+)/H+ antiporter gene(Pv NHX1)[J]. Frontiers in Plant Science, 2017, 8: 458. |
| [40] |
Hamamoto S, Horie T, Hauser F, et al. HKT transporters mediate salt stress resistance in plants: from structure and function to the field[J]. Current opinion in biotechnology, 2015, 32: 113-120. DOI:10.1016/j.copbio.2014.11.025 |
| [41] |
Garriga M, Raddatz N, Véry AA, et al. Cloning and functional characterization of HKT1 and AKT1 genes of fragaria spp. -relationship to plant response to salt stress[J]. Journal of Plant Physiology, 2016, 210: 9-17. |
| [42] |
Zhang M, Cao Y, Wang Z, et al. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize[J]. New Phytologist, 2018, 217(3): 1161-1176. DOI:10.1111/nph.14882 |
| [43] |
Horie T, Yoshida K, Nakayama H, et al. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa[J]. The Plant Journal, 2010, 27(2): 129-138. |
| [44] |
Pascal M, Hosoo Y, Goshima S, et al. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants[J]. Proceedings of the National Academy of Sciences of the USA, 2002, 99(9): 6428-6433. DOI:10.1073/pnas.082123799 |
| [45] |
Pascal M, Brendan E, Rama V, et al. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1[J]. Febs Letters, 2002, 531(2): 157-161. DOI:10.1016/S0014-5793(02)03488-9 |
| [46] |
Rus A, Yokoi S, Sharkhuu A, et al. At HKT1 is a salt tolerance determinant that controls Na+ entry into plant roots[J]. Proceedings of the National Academy of Sciences of the USA, 2001, 98: 14150-14155. DOI:10.1073/pnas.241501798 |
| [47] |
Hazzouri KM, Basel K, et al. Mapping of HKT1; 5 gene in barley using GWAS approach and its implication in salt tolerance mechanism[J]. Frontiers in Plant Science, 2018, 9: 156. DOI:10.3389/fpls.2018.00156 |
| [48] |
Berthomieu P. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance[J]. The EMBO Journal, 2003, 22(9): 2004-2014. DOI:10.1093/emboj/cdg207 |
| [49] |
An D, Chen JG, Gao YQ, et al. AtHKT1 drives adaptation of Arabidopsis thaliana to salinity by reducing floral sodium content[J]. Plos Genetics, 2017, 13(10): e1007086. DOI:10.1371/journal.pgen.1007086 |
| [50] |
Blumwald E. Sodium transport and salt tolerance in plants[J]. Current Opinion in Cell Biology, 2000, 12(4): 431-434. DOI:10.1016/S0955-0674(00)00112-5 |
| [51] |
Chanroj S, Wang GY, Venema K, et al. Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants[J]. Frontiers in Plant Science, 2012, 3: 25. |
| [52] |
Apse MP, Aharon GS, Snedden WA, et al. Salt tolerance conferred by over expression of a vacuolar Na CMC antiport in Arabidopsis[J]. Science, 1999, 285(12): 1256-1258. |
| [53] |
Fukuda A, Nakamura A, Tanaka Y. Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa[J]. BBA-Gene Structure and Expression, 1999, 1446: 149-155. DOI:10.1016/S0167-4781(99)00065-2 |
| [54] |
Yamaguchi T, Apse M P, Shi H, et al. Topological analysis of a plant vacuolar Na+/H+ antiporter reveals a luminal C terminus that regulates antiporter cation selectivity[J]. Proceedings of the National Academy of Sciences of the USA, 2003, 100(21): 12510-12515. DOI:10.1073/pnas.2034966100 |
| [55] |
Bassil E, Blumwald E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development[J]. The Plant Cell, 2011, 23(1): 224-239. DOI:10.1105/tpc.110.079426 |
| [56] |
Blumwald E, Aharon GS, Apse MP. Sodium transport in plant cells[J]. Biochim Biophys Acta, 2000, 1465(1): 140-151. |
| [57] |
Zhang HX, Blumwald E. Transgenic salt tolerant tomato plants accumulate salt in fruit[J]. Nature Biotechnology, 2001, 19(8): 765-768. DOI:10.1038/90824 |
| [58] |
Xue ZY, Zhi DY, Xue GP. Enhanced salt tolerance of transgenic wheat(Tritivum aestivum L.)expressing a vacuolar Na+/ H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+[J]. Plant Science, 2004, 167(4): 859-899. |
| [59] |
He CX, Yan JQ, Shen GX. Expression of an Arabidopsis vacuolar sodium/proton antiporter gene in cotton improves photosynthetic performance under salt conditions and increases fiber yield in the fields[J]. Plant Cell Physiology, 2005, 46(11): 1848-1854. DOI:10.1093/pcp/pci201 |
| [60] |
Julkowska MM, et al. Tuning plant signaling and growth to survive salt[J]. Trends in Plant Science, 2015, 20(9): 586-594. DOI:10.1016/j.tplants.2015.06.008 |
| [61] |
Hernández A, Jiang X, Cubero B, et al. Mutants of the Arabidopsis thaliana cation/H+ antiporter AtNHX1 conferring increased salt tolerance in yeast: the endosome/prevacuolar compartment is a target for salt toxicity[J]. J Biol Chem, 2009, 284(21): 14276-14285. DOI:10.1074/jbc.M806203200 |
| [62] |
Hamaji K, Nagira M, Yoshida K, et al. Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis[J]. Plant & Cell Physiology, 2009, 50: 2023-2033. |
| [63] |
Mazel A, Leshem Y, Tiwari BS, et al. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRAB7(AtRABG3E)[J]. Plant Physiology, 2004, 134: 118-128. DOI:10.1104/pp.103.025379 |
| [64] |
Blumwald E, Aharon GS, Apse MP. Sodium transport in plant cells[J]. Biochimicaet Biophysica Acta, 2000, 1465: 140-151. DOI:10.1016/S0005-2736(00)00135-8 |
| [65] |
Blumwald E, Engineering. Solt tolerance in plants[J]. Biotech-nology and Genetic Engineering Reviews, 2003, 20(1): 261-276. DOI:10.1080/02648725.2003.10648046 |