在整个生命周期中,植物经常暴露于不同的生物和非生物胁迫条件下,为了对抗这些环境对植物造成的不良影响,植物进化出非常复杂而多样的防御机制[1-2]。这些防御反应包括,细胞壁加厚、抗毒素合成、病程相关基因表达等[3]。van Loon等[4]发现至少17个病程相关家族蛋白(Pathogenesis-related protein,PR)能被卵菌、真菌、细菌、线虫、病毒和类病毒侵染,以及昆虫啃咬所诱导表达。植物类甜蛋白(Thaumatin-like protein,TLP)属于第5家族,主要包括类甜蛋白、渗透蛋白(Osmotin)和Zeamatin。植物类甜蛋白因与热带植物西非竹竽(Thaumatococcus danielli Benth)果实中分离到的甜蛋白(thaumatin)氨基酸序列有很高的同源性而得名,两种蛋白的同源性很高,但其功能完全不一样,甜蛋白具有甜味无抗真菌活性,而TLP蛋白无甜味具有抗真菌活性[4]。目前,陆续从多种植物、动物及微生物中发现了TLP蛋白[5-6]。TLP基因能被多种胁迫诱导表达[7-11],体内或体外试验表明TLP蛋白具有抗真菌活性[12],TLP转基因植物对真菌性病害的耐受性和抗性明显增强[13-21]。然而,TLP蛋白抗真菌的分子机制及其具体生物学功能还不是很清楚。因此,对植物TLP家族的研究受到越来越的关注。本文主要从TLP蛋白结构功能及应用方面综述了最新的研究成果,以期为今后的研究提供借鉴。
1 TLP蛋白结构及其进化特征根据蛋白分子量的大小,TLP蛋白主要分为两种类型:L(Large)型TLP蛋白分子量介于21到26 kD,包含16个保守的半胱氨酸残基;S(Small)型TLP蛋白分子量大约16 kD,只有10个半胱氨酸残基[13],虽然缺少了部分氨基酸序列,但它们仍能被病原菌诱导,并显示出抗真菌活性[22]。Petre等[23]根据非典型区域将TLPs分为small-TLPs、TLP激酶、small-TLP激酶,这有助于预测与病原感知和病原信号有关的受体激酶。大多数TLP蛋白均具有索玛甜家族标签G-X-[GF]-X-C-X-T-[GA]-D-C-X-(1,2)-G-X-(2,3)-C[24]和5个保守的REDDD(1个精氨酸,1个谷氨酸和3个天门冬氨酸)残基,后者参与蛋白维持适当的拓扑结构和酸裂周围的表面静电势,对TLP蛋白抗真菌活性必不可少[16]。
典型的TLP蛋白由16个半胱氨酸残基对形成8个二硫键,不仅稳定了分子结构,也保证蛋白的正确折叠,能够抵抗热变性、酸、碱和蛋白酶降解作用[25-26]。目前,多个TLP蛋白三维结构已被解析,显示具有保守的酸性裂缝的3D构象。Ban-TLP(1Z3Q)由3个功能域组成:domain Ⅰ为N端核心功能域,由2个反向平行的β片层(分别包含5个和6个β折叠)组成(图 1-A);domain Ⅱ由半胱氨酸二硫键形成的3个较短的α螺旋构成;domain Ⅲ由2个β折叠和1个大环构成(图 1-B)[27]。番茄TLP-NP24-Ⅰ在其结构域Ⅰ和Ⅱ之间有一个明显的“V”字形裂缝,TLP-NP24-Ⅰ一级结构中构成这个裂缝的侧链具有4个保守的氨基酸残基Glu84、Asp97、Asp102和Asp185,在三维结构中它们均从结构域Ⅱ插入到该裂缝中[28]。NP24-Ⅰ和其它具有抗真菌活性的TLP蛋白类似,其“V”字形裂缝呈酸性(带负电)[27-28]。一些TLP蛋白含有N端信号肽,用于引导成熟的蛋白到分泌路径。逆渗透蛋白(osmotin)和植物OLPs还具有C端前导肽,用于引导蛋白到达液泡[29]。4个拟南芥和7个水稻PR-5蛋白具有疏水结构,能结合到膜上。其他TLP蛋白具有跨膜片段和激酶结构域,可能具有感知胞外信号和信号转导功能[30]。这些发现说明一些防御相关蛋白具有对病原和生长发育信号传导的作用[4]。疏水性TLP蛋白和抗菌肽的“V”字形裂缝的边缘都具有2个疏水氨基酸残基Phe91和Phe96,而在甜蛋白的裂缝中这两个氨基酸残基均被被疏水性差的Tyr所取代。
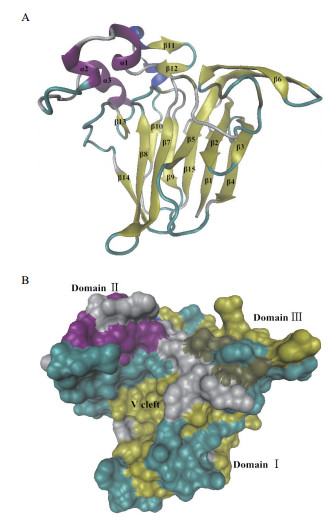
|
| 图 1 小果野蕉1Z3Q三维结构 |
有多项研究对TLP家族的进化关系进行了分析[6, 13, 23, 31]。蛋白结构同源建模分析表明,动物和植物TLP蛋白高度相似,二者仅在表面暴露的环结构存在差异[6]。通过对昆虫、线虫、水稻和拟南芥TLP家族蛋白序列进化分析发现,动物TLP蛋白单独分在一支,可能是以单一祖先序列的形式,且来自于植物;而水稻和拟南芥TLP蛋白分布于多个支系,并存在染色体内和染色体间的复制[6]。单子叶和双子叶植物进化上发生分离后,TLP蛋白基因在10个进化枝上发生了不对称的增加[6]。植物、动物和真菌等真核生物TLP蛋白序列进化分析显示,陆生植物进化过程中TLP基因含量和多样性显著增加了[23]。对从18个植物基因组序列获取的TLP家族基因比较发现,从莱茵衣藻(Chlamydomonas reinhardtii)到毛果杨(Populus trichocarpa)TLP基因发生了多样性的进化[23]。Liu等[13]将获得的118个TLP蛋白序列分为9组,植物TLP蛋白主要包括5个组(Ⅳ、Ⅵ、Ⅶ、Ⅷ和Ⅸ),他们认为TLP基因来自于大约10亿年前的植物、动物和真菌的共同祖先。在有些植物中,同一染色体上甚至同一位点存在TLP基因簇,说明串联重复是TLP超家族不对称扩张的重要机制。
2 TLP蛋白生物学功能 2.1 抗真菌活性近年来,TLP蛋白的抗真菌活性得到深入研究。超过20个来自动物、真菌和植物的TLP蛋白具有抗真菌活性[13]。Osmotin通过改变真菌细胞膜的透性而具有抗真菌活性[5]。植物TLP蛋白主要通过裂解真菌孢子,抑制孢子萌发,降低幼嫩菌丝活力等方式对致病和非致病真菌产生抑制作用[5, 32-33]。Zeamatin通过极化活性而穿透微生物的质膜[34]。TLP蛋白的电子极化的酸性裂隙直接作用于真菌细胞膜,是其抑菌活性必需的[33, 35],TLP蛋白转基因植物能够延缓多种真菌病害发展过程,增强植物对病原真菌的抗性[36-37]。TLP蛋白参与多种转基因植物对不同病原真菌的抗性。野生花生中的AdTLP重组蛋白比报道的其他TLP蛋白具有更强的抗真菌活性[17]。体外实验表明,龙葵TLP重组蛋白对多种植物病原真菌具有抗性[38-39]。TLP蛋白的疏水部位有利于其对真菌细胞膜的作用,番茄的各种TLP蛋白中,疏水性TLP蛋白的抗真菌活性高于非疏水性TLP蛋白,具有抗真菌活性的TLP蛋白其domainⅠ和domain Ⅱ之间存在负电荷的表面裂缝[40]。但到目前为止,TLP蛋白的抗真菌机制仍不很清楚。
2.2 葡聚糖酶活性TLP蛋白具有葡聚糖结合或葡聚糖酶活性[41-43],TLP蛋白的葡聚糖酶活性与其抗真菌活性有关,葡聚糖酶活性使TLP蛋白能结合并降解真菌细胞壁的主要组分β-1,3-葡聚糖[44],为TLP蛋白进一步破坏真菌细胞膜奠定了基础[45]。TLP8能结合到谷物提取物中不溶性的(1,3,1,4)-β-D葡聚糖上,有助于去除啤酒发酵中此类不需要的物质,这对啤酒发酵过程的理解和酿造工艺的改进具有重要意义[46]。模型预测发现,葡聚糖的多糖链与蛋白酸性裂在性状上能很好的互补,并且Glu84与两个Asp(Asp102、Asp97)分别位于酸性裂的两侧,因Glu84与Asp97距离太近不能进行催化反应,而Glu84与Asp102的距离又太大,也许是作为质子供体和亲核剂成为内切葡聚糖酶活性所必需的催化中心[28]。一般认为,功能域Ⅰ和Ⅱ之间酸性氨基酸是TLP蛋白具有β-1,3-葡聚糖酶活性所必需的[27]。也有研究表明,虽然TLP家族一些蛋白具有β-1,3-葡聚糖酶活性,但活性偏低[47]。但也有些TLP蛋白并不具有β-1,3-葡聚糖酶活性[24]。虽然TLP蛋白抗真菌能力可能与葡聚糖酶活性有关,但也有研究表明有些具有低的葡聚糖酶活性的TLP蛋白,不具有抗真菌能力。香蕉TLP蛋白具有可检测的低活性的β-1,3-葡聚糖酶活性,并不具有抗真菌活性[48]。
2.3 过敏原活性多种植物果实和花粉TLP蛋白具有过敏原活性[49]。植物来源的过敏原能引起人类Ⅰ型过敏反应,通过饮食或者上呼吸道吸入诱发。研究发现,TLP蛋白在多种水果和作物中具有过敏原活性,如猕猴桃、苹果、樱桃、橄榄、香蕉、番茄和小麦及柏树的花粉等[49]。在苹果中发现的32 kD的TLP蛋白Mal d 2具有过敏原活性[50]。在桃中发现3个TLP蛋白亚型Pru p 2.0101、Pru p 2.0201和Pru p 2.0301具有过敏原活性[51]。人心果酸性TLP基因无内含子和非糖基化,其蛋白在临床上能引起口腔过敏综合征,被鉴定为新的过敏原[52]。NP24属于食品过敏类蛋白[53],NP24-Ⅰ致敏表位(152-186)位于功能域Ⅱ的螺旋中,其含有7个Ig-E结合基序(图 2),均位于三维结构的表面[28]。这些TLP蛋白3-D结构中的功能域Ⅱ表现出非常保守的构象,预测这些氨基酸序列为主要的IgE结合表位[27]。鉴于果实和花粉中TLP致敏原的结构高度相似,可以通过蛋白结构预测设计有针对性的治疗剂,对过敏原特异性免疫治疗进行指导。
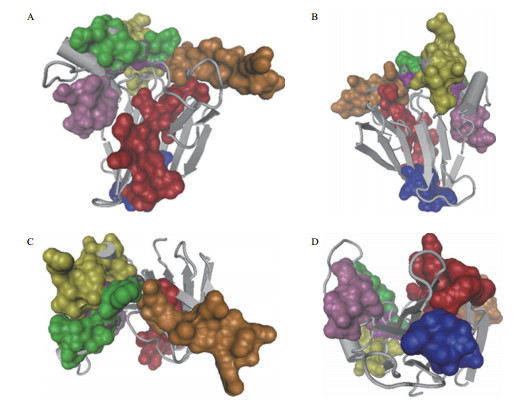
|
| 图 2 NP24-Ⅰ Ig-E结合表位分布 A、B、C和D分别为从正面、反面、顶部和底部角度观察 |
细胞程序性死亡是生物体发育过程中普遍存在的,是一个由基因决定的细胞主动的有序的死亡方式。植物与病原的非亲和互作过程中会伴随着过敏反应(Hypersensitive response,HR)发生,包括宿主细胞程序性死亡(Bogrammed cell death,PCD),亲和互作中也会发生PCD,其具体机制还不是很清楚[54]。植物渗透蛋白诱导了酵母菌MAPK信号通路,并通过刺激细胞壁成分的改变而增加了细胞毒性,导致渗透蛋白能够通过细胞膜并引起细胞死亡[55]。烟草TLP蛋白通过RAS2/cAMP通路介导的胞内活性氧(Reactive oxygen species,ROS)积累诱导了酵母细胞凋亡[56],说明植物TLP蛋白具有抗菌蛋白活性。渗透蛋白诱导了目标微生物胞内积累抗菌肽,抗菌肽通过结合或破坏核酸而起作用[57]。宿主因子诱导病原PCD是宿主-病原互作的重要因素[56]。
2.5 其他活性研究发现TLP蛋白还具有酶抑制剂活性,在植物的防御中发挥作用[26]。TLP蛋白也提高了植物对多种非生物胁迫的耐受性[32]。研究发现,植物TLP蛋白在质外体中积累,提高了植物对冷环境的适应性[58]。TLP1在桃冷害敏感品系Hermoza的细胞壁中后期积累,而在抗寒品系Oded中早期积累,TLP蛋白在核果冷害发生过程中参与了水果细胞壁结构改变,有助于水果对寒冷的抵抗[59]。TLP蛋白作为防冻剂,在控制冷冻酸奶冰晶体的形成中起作用[60]。巴西橡胶树渗透蛋白通过提高水势,降低植物对渗透胁迫的耐受性,参与了乳汁细胞的渗透调节[61],渗透蛋白过表达植株通过程序性细胞死亡、细胞骨架构建以及钙离子信号等,增强了对冷胁迫的防御能力[62]。除了冷胁迫,TLP蛋白过表达植株显著提高了对其他多种非生物胁迫的抗性[17, 63]。
TLP蛋白除了参与植物的胁迫反应之外,也参与了生长发育的多项进程。一些TLP蛋白在植物的组织器官或特定的发育阶段高度表达,有些植物(如樱桃、苹果、香蕉)果实成熟时很多TLP蛋白高表达[64-66]。光照延长时,PpTLP转录增加,而幼叶中其表达很低,这可能有利于植物保护营养器官和生殖器官[16]。
3 TLP家族基因表达特征TLP基因一般在植物特定组织、器官中低水平组成型表达,而且表达活性不同。多种高等植物遭受病原侵染时,TLP蛋白上调表达[13, 67]。杨树中TLP在细胞和组织中的定位较复杂,TLP1主要在维管系统如中脉、叶柄和茎中表达,而另一个31 kD的TLP主要在幼叶和茎的淀粉粒中表达[68]。多项研究表明TLP蛋白定位于胞外空间,能够增强植物对多种胁迫的耐受[14, 17]。定位研究显示,病原诱导型TLP蛋白分泌到质外体中起作用[7]。
TLP基因的表达能被多种信号所调控,如水杨酸、茉莉酸甲酯、脱落酸、受伤、紫外、渗透胁迫以及细菌、真菌、病毒等生物体的侵袭[69]。受到外界逆境胁迫后,TLP基因的表达迅速上调。TLP蛋白与植物的抗逆反应有关,当植物受到病原物(真菌、细菌和病毒等),化学因子(乙烯、水杨酸、氨基酸衍生物和重金属离子等),物理因子(紫外线、热处理和机械损伤等)以及特定的生理因素(衰老、开花和质壁分离等)诱导时,TLP基因在植物体特定部位迅速表达并积累。Ahmed等[70]从大白菜中鉴定了20个TLP蛋白,其中多个基因能被病原菌和非生物胁迫所诱导,说明TLP蛋白参与了大白菜对胁迫的响应。通过DNA渐渗将来自于野黄瓜的CsTLP蛋白基因构建了IL5211S系,从该系中分离到CsTLP基因,该基因响应了Pseudoperonospora cubensis和其他非生物胁迫[71]。
基因上游多样的顺式调控元件是基因特异性表达所必不可少的。TLP基因表达受启动子区特定顺式元件的调控。Rtlp1启动子区的W-box能显著提前其对真菌病原的响应,在真菌侵入细胞前,Rtlp1已被诱导[72]。SA处理1 h后和MeJA处理2 h后Rtlp1启动子活性显著增强[72]。烟草渗透蛋白启动子区的GCC-boxes对其乙烯响应所必需[73]。启动子中的沉默子会抑制生姜(Zingiber officinale)防御反应中ZoPR5蛋白的表达,启动子中的GT-1 box和TGTCA可以激活植物防御反应中杧果姜CaPR5蛋白(Curcuma amada)的表达[74]。
4 TLP家簇基因的利用传统农业一般通过使用杀菌剂控制植物真菌病害,但农药的广泛应用可能导致严重的环境污染和食品安全问题。基因工程通过利用多种抗病基因为现代农业提供了一条可持续发展的道路。体外研究表明TLP蛋白有很强的抗真菌活性,希望能通过过表达或转基因来提高植物对真菌的抗性。多种转基因作物明显提高了植物对疾病的抗性以及对干旱和盐胁迫的耐受性。AdTLP转基因稻烟草中,显著增强了其对病原真菌Rhizoctonia solani的抗性,同时增强了植物对盐和氧胁迫的耐受性[17]。将水稻的TLP基因转入大麦(Hordeum vulgare)并过量表达,转基因植株的抗真菌能力提高[75]。将海岛棉具有次级细胞壁发育功能的类索玛甜蛋白基因(GbTLP1)转入烟草中,显著增强了烟草对多种胁迫的抗性[63]。将水稻的TLP基因转入香蕉植株中,增强了其对尖孢镰刀菌的抗性,控制了香蕉镰刀枯萎病的发生[15]。多数研究确实提高了植物对真菌的抗性,但却不能对病原完全免疫,说明单个基因过量表达产生的蛋白还不足以有效保护植物完全不受病原菌的侵染。
除了PR基因本身,植物PR基因启动子的可诱导性在植物抗病基因工程中也有极高的应用价值。植物抗病基因工程中经常将抗菌蛋白或对病原菌有毒性物质的基因置于超强启动子控制下来提高抗病能力。但是,高度组成性表达的抗菌化合物持续在整个生育期的所有的组织中合成,对植物的生长又极为不利。而若用PR蛋白基因的启动子控制,则转基因只在受到病原菌侵染或其他胁迫因子处理时才表达,具有重要的利用价值。
5 结语虽然研究者对植物TLP家族已开展了大量研究,但其抗真菌的核心机理还不是很清楚,这将大大限制该家族基因在植物遗传育种中的应用。植物TLP家族成员众多,在植物不同的生命进程中发挥作用的基因数量和种类还不是很清楚,这些成员在植物生长发育和对抗胁迫中的具体对应关系有待深入研究。对该家族单个基因和多个基因在植物生命进程中具体作用的深入研究,将是今后植物病理学和遗传育种研究中的重要内容。
| [1] |
Solano R, Gimenez-Ibanez S. Nuclear jasmonate and salicylate signaling and crosstalk in defense against pathogens[J]. Frontiers in Plant Science, 2013, 4: 72. |
| [2] |
Kazan K, Lyons R. Intervention of phytohormone pathways by pathogen effectors[J]. The Plant Cell, 2014, 26(6): 2285-2309. DOI:10.1105/tpc.114.125419 |
| [3] |
Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens[J]. Trends Plant Sci, 2012, 17(2): 73-90. DOI:10.1016/j.tplants.2011.11.002 |
| [4] |
van Loon LC, Rep M, Pieterse CM. Significance of inducible defenserelated proteins in infected plants[J]. Annu Rev Phytopathol, 2006, 44: 135-162. DOI:10.1146/annurev.phyto.44.070505.143425 |
| [5] |
Abad LR, D'Urzo MP, Liu D, et al. Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization[J]. Plant Science, 1996, 118(1): 11-23. DOI:10.1016/0168-9452(96)04420-2 |
| [6] |
Shatters RG Jr, Boykin LM, Lapointe SL, et al. Phylogenetic and structural relationships of the PR5 gene family reveal an ancient multigene family conserved in plants and select animal taxa[J]. Journal of Molecular Evolution, 2006, 63(1): 12-29. DOI:10.1007/s00239-005-0053-z |
| [7] |
Wang X, Tang C, Deng L, et al. Characterization of a pathogenesisrelated thaumatin-like protein gene TaPR5 from wheat induced by stripe rust fungus[J]. Physiologia Plantarum, 2010, 139(1): 27-38. DOI:10.1111/ppl.2010.139.issue-1 |
| [8] |
Kim BG, Fukumoto T, Tatano S, et al. Molecular cloning and characterization of a thaumatin-like protein-encoding cDNA from rough lemon[J]. Physiological and Molecular Plant Pathology, 2009, 74(1): 3-10. DOI:10.1016/j.pmpp.2009.07.001 |
| [9] |
Kim DH, Noh MY, Park KB, et al. Expression profiles of two thaumatin-like protein(TmTLP)genes in responses to various micro-organisms from Tenebrio molitor[J]. Entomological Research, 2017, 47(1): 35-40. DOI:10.1111/enr.2017.47.issue-1 |
| [10] |
Beatrice C, Linthorst JMH, Cinzia F, et al. Enhancement of PR1 and PR5 gene expressions by chitosan treatment in kiwifruit plants inoculated with Pseudomonas syringae pv. actinidiae[J]. European Journal of Plant Pathology, 2017, 148(1): 163-179. DOI:10.1007/s10658-016-1080-x |
| [11] |
Rout E, Nanda S, Joshi RK. Molecular characterization and heterologous expression of a pathogen induced PR5 gene from garlic(Allium sativum L.)conferring enhanced resistance to necrotrophic fungi[J]. European Journal of Plant Pathology, 2016, 144(2): 345-360. DOI:10.1007/s10658-015-0772-y |
| [12] |
Guo J, Zhao X, Wang H, et al. Expression of the LePR5 gene from cherry tomato fruit induced by Cryptococcus laurentii and the analysis of LePR5 protein antifungal activity[J]. Postharvest Biology and Technology, 2016, 111: 337-344. DOI:10.1016/j.postharvbio.2015.09.002 |
| [13] |
Liu JJ, Sturrock R, Ekramoddoullah AKM. The superfamily of thaumatin-like proteins:its origin, evolution, and expression towards biological function[J]. Plant Cell Reports, 2010, 29(5): 419-436. DOI:10.1007/s00299-010-0826-8 |
| [14] |
Wang Q, Li F, Zhang X, et al. Purification and Characterization of a CkTLP Protein from Cynanchum komarovii Seeds that Confers Antifungal Activity[J]. PLoS One, 2011, 6(2): e16930. DOI:10.1371/journal.pone.0016930 |
| [15] |
Mahdavi F, Sariah M, Maziah M. Expression of rice thaumatin-like protein gene in transgenic banana plants enhances resistance to fusarium wilt[J]. App Biochem Biotechnol, 2012, 166(4): 1008-1019. DOI:10.1007/s12010-011-9489-3 |
| [16] |
Liu D, He X, Li W, et al. Molecular cloning of a thaumatin-like protein gene from Pyrus pyrifolia and overexpression of this gene in tobacco increased resistance to pathogenic fungi[J]. Plant Cell, Tissue and Organ Culture(PCTOC), 2012, 111(1): 29-39. DOI:10.1007/s11240-012-0167-0 |
| [17] |
Singh NK, Kumar KRR, Kumar D, et al. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut[J]. PLoS One, 2013, 8(12): e83963. |
| [18] |
Odeny PO. Enhancement of resistance against Colletotrichum gloeosporioides in cassava over-expressing rice thaumatin-like protein(PR-5)gene[D]. Kenya: Kenyatta University, 2015.
|
| [19] |
He R, Wu J, Zhang Y, et al. Overexpression of a thaumatin-like protein gene from Vitis amurensis improves downy mildew resistance in Vitis vinifera grapevine[J]. Protoplasma, 2017, 254(4): 1579-1589. DOI:10.1007/s00709-016-1047-y |
| [20] |
Misra RC, Sandeep MK, Kumar S, et al. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis[J]. Scientific Reports, 2016, 6: 25340. DOI:10.1038/srep25340 |
| [21] |
Xue X, Cao ZX, Zhang XT, et al. Overexpression of OsOSM1 enhances resistance to rice sheath blight[J]. Plant Disease, 2016, 100(8): 1634-1642. DOI:10.1094/PDIS-11-15-1372-RE |
| [22] |
Reimmann C, Dudler R. cDNA cloning and sequence analysis of a pathogen-induced thaumatin-like protein from rice(Oryza sativa)[J]. Plant Physiol, 1993, 101(3): 1113-1114. DOI:10.1104/pp.101.3.1113 |
| [23] |
Petre B, Major I, Rouhier N, et al. Genome-wide analysis of eukaryote thaumatin-like proteins(TLPs)with an emphasis on poplar[J]. Plant Biology, 2011, 11(1): 33. |
| [24] |
Jami SK, Anuradha TS, Guruprasad L, et al. Molecular, biochemical and structural characterization of os-motin-like protein from black nightshade(Solanum nigrum)[J]. Journal of Plant Physiology, 2007, 164(3): 238-252. DOI:10.1016/j.jplph.2006.01.006 |
| [25] |
Smole U, Bublin M, Radauer C, et al. Mal d 2, the thaumatin-like allergen from apple, is highly resistant to gastrointestinal digestion and thermal processing[J]. International Archives of Allergy and Immunology, 2008, 147(4): 289-298. DOI:10.1159/000144036 |
| [26] |
Fierens E, Rombouts S, Gebruers K, et al. TLXI, a novel type of xylanase inhibitor from wheat(Triticum aestivum)belonging to the thaumatin family[J]. Biochemical Journal, 2007, 403(3): 583-591. DOI:10.1042/BJ20061291 |
| [27] |
Leone P, Menu-Bouaouiche L, Peumans WJ, et al. Resolution of the structure of the allergenic and antifungal banana fruit thaumatin-like protein at 1. 7-Å[J]. Biochimie, 2006, 88(1): 45-52. DOI:10.1016/j.biochi.2005.07.001 |
| [28] |
Ghosh R, Chakrabarti C. Crystal structure analysis of NP24-Ⅰ:a thaumatin-like protein[J]. Planta, 2008, 228(5): 883-890. DOI:10.1007/s00425-008-0790-5 |
| [29] |
Anžlovar S, Dermastia M. The comparative analysis of osmotins and osmotin-like PR-5 proteins[J]. Plant Biology, 2003, 5(2): 116-124. DOI:10.1055/s-2003-40723 |
| [30] |
Wang X, Zafian P, Choudhary M, et al. The PR5K receptor protein kinase from Arabidopsis thaliana is structurally related to a family of plant defense proteins[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(6): 2598-2602. DOI:10.1073/pnas.93.6.2598 |
| [31] |
Sakamoto Y, Watanabe H, Nagai M, et al. Lentinula edodes tlg1 encodes a thaumatin-like protein that is involved in lentinan degradation and fruiting body senescence[J]. Plant Physiol, 2006, 141(2): 793-801. DOI:10.1104/pp.106.076679 |
| [32] |
Rajam MV, Chandola N, Saiprasad Goud P, et al. Thaumatin gene confers resistance to fungal pathogen as well as tolerance to abiotic stresses in transgenic tobacco plants[J]. Biologia Plantarum, 2007, 51(1): 135-141. DOI:10.1007/s10535-007-0026-8 |
| [33] |
Ramos MV, de Oliveira RSB, Pereira HM, et al. Crystal structure of an antifungal osmotin-like protein from Calotropis procera and its effects on Fusarium solani spores, as revealed by atomic force microscopy:insights into the mechanism of action[J]. Phytochemistry, 2015, 119: 5-18. DOI:10.1016/j.phytochem.2015.09.012 |
| [34] |
Roberts WK, Selitrennikoff CP. Zeamatin, an antifungal protein from maize with membrane permeabilizing activity[J]. Microbiology, 1990, 136(9): 1771-1778. |
| [35] |
Batalia MA, Monzingo AF, Ernst S, et al. The crystal structure of the antifungal protein zeamatin, a member of the thaumatin-like, PR-5 protein family[J]. Nature Structural & Molecular Biology, 1996, 3(1): 19-22. |
| [36] |
Yan X, Qiao H, Zhang X, et al. Analysis of the grape(Vitis vinifera L.)thaumatin-like protein(TLP)gene family and demonstration that TLP29 contributes to disease resistance[J]. Scientific Reports, 2017, 7(1): 4269. DOI:10.1038/s41598-017-04105-w |
| [37] |
Chowdhury S, Basu A, Kundu S. Overexpression of a new osmotin-like protein gene(SindOLP)confers tolerance against biotic and abiotic stresses in sesame[J]. Frontiers in Plant Science, 2017, 8. |
| [38] |
Campos MA, Silva MS, Magalhães CP, et al. Expression in Escherichia coli, purification, refolding and antifungal activity of an osmotin from Solanum nigrum[J]. Microbial Cell Factories, 2008, 7(1): 7. DOI:10.1186/1475-2859-7-7 |
| [39] |
Liu C, Cheng F, Sun Y, et al. Structure-function relationship of a novel PR-5 protein with antimicrobial activity from soy hulls[J]. J Agric Food Chem, 2016, 64(4): 948-959. DOI:10.1021/acs.jafc.5b04771 |
| [40] |
Koiwa H, Kato H, Nakatsu T, et al. Crystal structure of tobacco PR-5d protein at 1. 8 Å resolution reveals a conserved acidic cleft structure in antifungal thaumatin-like proteins[J]. J Mol Biol, 1999, 286: 1137-1145. DOI:10.1006/jmbi.1998.2540 |
| [41] |
Osmond RIW, Hrmova M, Fontaine F, et al. Binding interactions between barley thaumatin-like proteins and(1, 3)-β-D-glucans[J]. European Journal of Biochemistry, 2001, 268(15): 4190-4199. DOI:10.1046/j.1432-1327.2001.02331.x |
| [42] |
Van Damme EJ, Charels D, Menu-Bouaouiche L, et al. Menu-Bouaouiche L, et al. Biochemical, molecular and structural analysis of multiple thaumatin-like proteins from the elderberry tree(Sambucus nigra L.)[J]. Planta, 2002, 214(6): 853-862. DOI:10.1007/s00425-001-0713-1 |
| [43] |
Menu-Bouaouiche L, Vriet C, Peumans WJ, et al. A molecular basis for the endo-β 1, 3-glucanase activity of the thaumatin-like proteins from edible fruits[J]. Biochimie, 2003, 85(1): 123-131. |
| [44] |
Theis T, Stahl U. Antifungal proteins:targets, mechanisms and prospective applications[J]. Cellular and Molecular Life Sciences, 2004, 61(4): 437-455. DOI:10.1007/s00018-003-3231-4 |
| [45] |
Grenier J, Potvin C, Trudel J, et al. Some thaumatin-like proteins hydrolyse polymeric β-1, 3-glucans[J]. The Plant Journal, 1999, 19(4): 473-480. DOI:10.1046/j.1365-313X.1999.00551.x |
| [46] |
Singh S, Tripathi RK, Lemaux PG, et al. Redox-dependent interaction between thaumatin-like protein and β-glucan influences malting quality of barley[J]. Proc Nat Acad Sci, 2017, 114(29): 7725-7730. DOI:10.1073/pnas.1701824114 |
| [47] |
姜晓玲, 黄秋娴, 李虹, 赵嘉平. 植物类甜蛋白基因家族研究进展[J]. 浙江农林大学学报, 2012(2): 279-287. |
| [48] |
Barre A, Peumans WJ, Menu-Bouaouiche L, et al. Purification and structural analysis of an abundant thaumatin-like protein from ripe banana fruit[J]. Planta, 2000, 211(6): 791-799. DOI:10.1007/s004250000354 |
| [49] |
Breiteneder H. Thaumatin-like proteins-a new family of pollen and fruit allergens[J]. Allergy, 2004, 59(5): 479-481. DOI:10.1046/j.1398-9995.2003.00421.x |
| [50] |
Krebitz M, Wagner B, Ferreira F, et al. Plant-based heterologous expression of Mal d 2, a thaumatin-like protein and allergen of apple(Malus domestica), and its characterization as an antifungal protein[J]. J Mol Biol, 2003, 329(4): 721-730. DOI:10.1016/S0022-2836(03)00403-0 |
| [51] |
Palacín A, Tordesillas L, Gamboa P, et al. Characterization of peach thaumatin-like proteins and their identification as major peach allergens[J]. Clini Exp Allergy, 2010, 40(9): 1422-1430. DOI:10.1111/cea.2010.40.issue-9 |
| [52] |
Kumar HGA, Hegde VL, Shetty SM, et al. Characterization and gene cloning of an acidic thaumatin-like protein(TLP 1), an allergen from sapodilla fruit(Manilkara zapota)[J]. Allergology International, 2013, 62(4): 447-462. DOI:10.2332/allergolint.12-OA-0522 |
| [53] |
Schein CH, Ivanciuc O, Braun W. Bioinformatics approaches to classifying allergens and predicting cross-reactivity[J]. Immunology and Allergy Clinics of North America, 2007, 27(1): 1-27. DOI:10.1016/j.iac.2006.11.005 |
| [54] |
Dangl J. Innate immunity:plants just say NO to pathogens[J]. Nature, 1998, 394(6693): 525-527. DOI:10.1038/28958 |
| [55] |
Yun DJ, Ibeas JI, Lee H, et al. Osmotin, a plant antifungal protein subverts signal transduction to enhance fungal cell susceptibility[J]. Molecular Cell, 1998, 1: 807-17. DOI:10.1016/S1097-2765(00)80080-5 |
| [56] |
Narasimhan ML, Damsz B, Coca MA, et al. A plant defense response effector induces microbial apoptosis[J]. Molecular Cell, 2001, 8(4): 921-930. DOI:10.1016/S1097-2765(01)00365-3 |
| [57] |
Park CB, Kim HS, Kim SC. Mechanism of action of the antimicrobial peptide buforin Ⅱ:buforin Ⅱ kills microorganisms by penetrating the cell membrane and inhibiting cellular functions[J]. Biochemical and Biophysical Research Communications, 1998, 244(1): 253-257. DOI:10.1006/bbrc.1998.8159 |
| [58] |
Hon WC, Griffith M, Mlynarz A, et al. Antifreeze proteins in winter rye are similar to pathogenesis-related proteins[J]. Plant Physiol, 1995, 109(3): 879-889. DOI:10.1104/pp.109.3.879 |
| [59] |
Dagar A, Friedman H, Lurie S. Thaumatin-like proteins and their possible role in protection against chilling injury in peach fruit[J]. Postharvest Biology and Technology, 2010, 57(2): 77-85. DOI:10.1016/j.postharvbio.2010.03.009 |
| [60] |
Yasmin N, Saleem M, Naz M, et al. Molecular characterization, structural modeling, and evaluation of antimicrobial activity of basrai thaumatin-like protein against fungal infection[J]. Biomed Research International, 2017, 504651. |
| [61] |
Tong Z, Sun Y, Wang D, et al. Identification and functional characterization of HbOsmotin from Hevea brasiliensis[J]. Plant Physiology and Biochemistry, 2016, 109: 171-180. DOI:10.1016/j.plaphy.2016.09.017 |
| [62] |
D'Angeli S, Altamura MM. Osmotin induces cold protection in olive trees by affecting programmed cell death and cytoskeleton organization[J]. Planta, 2007, 225(5): 1147-1163. DOI:10.1007/s00425-006-0426-6 |
| [63] |
Munis MF, Tu L, Deng F, et al. A thaumatin-like protein gene involved in cotton fiber secondary cell wall development enhances resistance against Verticillium dahliae and other stresses in transgenic tobacco[J]. Biochemical and Biophysical Research Communications, 2010, 393(1): 38-44. DOI:10.1016/j.bbrc.2010.01.069 |
| [64] |
Fils-Lycaon BR, Wiersma PA, Eastwell KC, et al. A cherry protein and its gene, abundantly expressed in ripening fruit, have been identified as thaumatin-like[J]. Plant Physiol, 1996, 111(1): 269-273. DOI:10.1104/pp.111.1.269 |
| [65] |
Kim SH, Lee JR, Kim SR. Molecular characterization of a fruit-preferential thaumatin-like gene from apple(Malus domestica cv. Fuji)[J]. Journal of Plant Biology, 2003, 46(1): 52-58. DOI:10.1007/BF03030302 |
| [66] |
Ho VS, Wong JH, Ng TB. A thaumatin-like antifungal protein from the emperor banana[J]. Peptides, 2007, 28(4): 760-766. DOI:10.1016/j.peptides.2007.01.005 |
| [67] |
Mukherjee AK, Carp MJ, Zuchman R, et al. Proteomics of the response of Arabidopsis thaliana to infection with Alternaria brassicicola[J]. Journal of Proteomics, 2010, 73: 709-720. DOI:10.1016/j.jprot.2009.10.005 |
| [68] |
Dafoe NJ, Gowen BE, Constabel CP. Thaumatin-like proteins are differentially expressed and localized in phloem tissues of hybrid poplar[J]. BMC Plant Biology, 2010, 10(1): 191. DOI:10.1186/1471-2229-10-191 |
| [69] |
Velazhahan R, Datta SK, Muthukrishnan S. The PR-5 family: thaumatin-like proteins[M]//Datta SK, Muthukrishnan S(eds). Pathogenesis related Proteins in Plants. Boca Raton: CRC Press, 1999: 107-129.
|
| [70] |
Ahmeda NU, Parka JI, Junga HJ, et al. Molecular characterization of thaumatin family genes related to stresses in Brassica rapa[J]. Scientia Horticulturae, 2013, 152: 26-34. DOI:10.1016/j.scienta.2013.01.007 |
| [71] |
Wan H, Chen J. Enhanced expression of a thaumatin-like gene, involved in 'Pseudoperonospora cubensis' and abiotic stresses, induced by DNA introgression from a wild relative, Cucumis hystrix[J]. Plant Omics, 2013, 6(2): 135. |
| [72] |
Hiroyuki K, Terauchi R. Regulation of expression of rice thaumatin-like protein:inducibility by elicitor requires promoter W-box elements[J]. Plant Cell Reports, 2008, 27(9): 1521-1528. DOI:10.1007/s00299-008-0536-7 |
| [73] |
Raghothama KG, Maggio A, Narasimhan ML, et al. Tissue-specific activation of the osmotin gene by ABA, C2H4 and NaCl involves the same promoter region[J]. Plant Mol Biol, 1997, 34(3): 393-402. DOI:10.1023/A:1005812217945 |
| [74] |
Prasath D, El-Sharkawy I, Sherif S, et al. Cloning and characterization of PR5 gene from Curcuma amada and Zingiber officinale in response to Ralstonia solanacearum infection[J]. Plant Cell Reports, 2011, 30(10): 1799-809. DOI:10.1007/s00299-011-1087-x |
| [75] |
Tobias DJ, Manoharan M, Pritsch C, et al. Co-bombardment, integration and expression of rice chitinase and thaumatin-like protein genes in barley(Hordeum vulgare cv. Conlon)[J]. Plant Cell Reports, 2007, 26(5): 631-639. DOI:10.1007/s00299-006-0263-x |




