2. 杂粮种质资源发掘与遗传改良山西省重点实验室,太谷 030801;
3. 农业部黄土高原基因资源与种质创制重点实验室,太原 030031
2. Shanxi Key Laboratory of Genetic Resources and Genetic Improvement of Minor Crops, Taigu 030801;
3. Key Laboratory of Crop Gene Resources and Germplasm Enhancement on the Loess Plateau, Ministry of Agriculture, Taiyuan 030031
萜类物质是一种广泛存在于生物体的次生代谢产物。至今已有5万多种萜类物质被分离鉴定,包括单萜(如柠檬烯)、倍半萜(如橙花叔醇)、二萜(如叶绿素)、三萜(如植物甾醇)、四萜(如类胡萝卜素)和多萜(如质体醌)[1]。在高等植物中,萜类物质参与多种生理代谢活动,如光合作用、呼吸作用和细胞周期控制等。除满足自身需求,植物产生的萜类物质还可用于工业、医药等领域,如萜类花香物质可以制成香水;VA和VE是保障人体健康的营养物质;紫杉醇可以用于治疗癌症等[2]。
萜类物质的前体物质是3-异戊烯基焦磷酸(isopentenyl diphosphate,IPP)及其异构体二甲基丙烯焦磷酸(dimethylallyl diphosphate,DMAPP),由2-C-甲基-D-赤藻糖醇-4-磷酸(2-C-methyl-D-erythritol 4-phosphate,MEP)和甲羟戊酸(mevalon-ate,MVA)两条途径合成[3]。1-脱氧木酮糖-5-磷酸(1-deoxy-D-xylulose 5-phosphate,DXP)合成酶(1-deoxy-D-xylulose 5-phosphate synthase,DXS)是MEP途径中的第一个酶,催化丙酮酸和3-磷酸甘油醛生成DXP[4](图 1)。许多研究表明,DXS是MEP代谢通路的关键酶[4-5],在拟南芥中更是被鉴定为主要的限速酶[6]。虽然金蓉等[7]在2007年对DXS基因的酶学和分子生物学特征进行过综述,但在近10年内,对DXS的作用机理和功能研究取得了很多新的进展。本文综述了近10年内在DXS基因克隆、DXS酶学特性、基因表达、调控和遗传转化方面的最新研究成果,以期为植物育种和DXS调控提供理论参考。

|
| 图 1 植物萜类物质的合成途径 MVA途径:甲羟戊酸途径;MEP途径:2-C-甲基-D-赤藻糖醇-4-磷酸途径;DMAPP:3,3-二甲基丙烯基焦磷酸;IPP:异戊烯基焦磷酸;IPI:异戊烯基焦磷酸异构酶;FPP:法尼基焦磷酸;Squalene:鲨烯;DXS:5-磷酸脱氧木酮糖合酶;DXP:5-磷酸脱氧木酮糖;GPP:香叶基焦磷酸;GGPPS:香叶基香叶基焦磷酸合酶;GGPP:香叶基香叶基焦磷酸 |
数量性状基因座(Quantitative trait loci,QTL)的定位可以帮助人们找到感兴趣的数量性状的遗传标记,明确其在染色体上的位置,以便于了解某一基因调控特定性状的遗传机制,最终为分子标记辅助育种服务。然而,DXS在植物中的QTL定位研究并不深入。使用在线软件,Sharma等[8]将鼠耳芥的4个DXS基因定位在3、4、5染色体上,水稻5个DXS基因定位在4、5、6、7染色体上。在葡萄上对DXS的QTL定位研究较为详细,依赖于两个F1作图群体和候选基因法,Battilana等[9]将控制芳樟醇、橙花醇和香叶醇合成的DXS基因定位于连锁群LG5上,解释了DXS基因调控麝香味葡萄香味产生的遗传机制。
2 DXS酶活性研究及结构特征DXS酶活性一直是科学家的研究热点,并由此派生出许多研究方法。由放射性化学法[10]到分光光度法[11-12]再到荧光高效液相色谱法(High performance liquid chromatography,HPLC)[13-14],研究方法的改进,降低了检测技术要求,提高了检测灵敏度,但HPLC法未能测定DXS酶稳态动力学常数。Brammer等[15]建立的柱前衍生法可对DXS酶活性的稳态动力学常数作测定。胡玥等[16]建立了2,4-二硝基苯肼柱前衍生HPLC法,利用含羰基化合物与2,4-二硝基苯肼(2,4-dinitrophenylhydrazine,DNPH)在酸性条件下反应得到的产物腙对紫外-可见光有吸收的特性,改进了Brammer的方法,使检测方法更易执行。
通过酶活性研究,DXS被定位于植物质体的类囊体中[5, 17-18]。DXS是一个依赖焦磷酸硫胺素(Thiamine pyrophosphate,TPP)的酶。不同生物的DXS均存在与TPP结合的结构域保守区[19],与植物转酮酶和丙酮酸脱氢酶E1亚基高度同源[12]。TPP结合位点大致位于第140-260氨基酸处,共6个氨基酸参与,其反应机制为:硫胺二磷酸TPP与DXS酶活性中心结合,形成一个介于TPP和磷酸丙酮盐的共价中间体,该物质催化丙酮酸脱羧,并使3-磷酸甘油醛(GAP)结合到丙酮酸剩余碳部位形成DXP[20]。MEP代谢通路终产物IPP或DMAPP通过与TPP竞争DXS结合位点抑制其活性[21]。将杨树DXS(PtDXS)第147位丙氨酸突变为甘氨酸,可以减弱IPP抑制作用从而提高DXS酶活性[22]。从动力学角度讲,TPP是DXS酶起活性作用的关键性物质,相当于反应的底物而非共反应因子,其作用是不可或缺的[22]。
除TPP结合位点外,所有DXS的N端均有一段质体转运肽,是氨基酸序列的前30-60个左右的氨基酸残基[7]。这段序列通常保守性较差,如冬凌草IrDXS蛋白中这段信号肽包括64个氨基酸残基[23],但在有玫瑰香味的天竺葵中,这段信号肽的长度为55个氨基酸残基[19]。不过,DXS质体转运肽均富含羟基化的丝氨酸和苏氨酸,缺乏天冬氨酸和谷氨酸等酸性氨基酸。属于TPP超家族的DXS酶还有另外两个保守结构域,一个是嘧啶(Pyrimidine,PYR)结合结构域(TPP_PYR_DXS_like),一个是C端的转酮酶结构域(Transketolase-C)[19]。
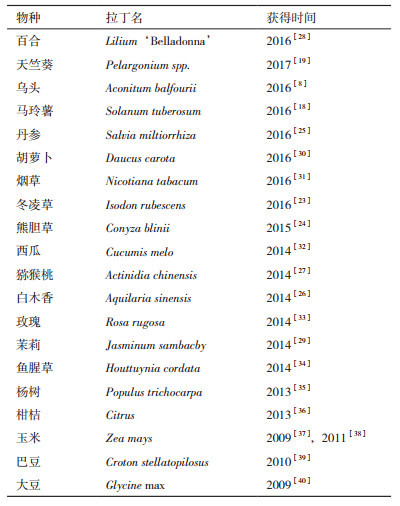
3 DXS基因的类型及特征自首次从拟南芥和薄荷[6]中分离到DXS基因后,截至2017年8月4日,在NCBI登录的DXS基因序列已达1 556个,涉及到178种植物。许多物种通过全基因组鸟枪测序法获得了DXS的基因组DNA全长,包括常见的作物如欧洲油菜(Brassica napus)、大豆(Glycine max)、水稻(Oryza sativa)、葡萄(Vitis vinifera)及拟南芥(Arabidopsis thaliana)等。表 1列举的是近10年来部分物种获得DXS基因的情况及NCBI登录号,包括药用植物如熊胆草(Conyza blinii)[24]、丹参(Salvia miltiorrhiza)[25]和白木香(Aquilaria sinensis)[26],也包括许多果实或花朵能散发芳香气味的植物,如猕猴桃(Actinidia chinensis)[27]、百合(Lilium ‘Belladonna’)[28]和茉莉(Jasminum sambacby)[29]。以前的研究表明,DXS酶多由1-3个基因编码[7],但基于RNA-seq测序技术的最新研究表明,胡萝卜(Daucus carota)[30]有5个DXS的同源基因,烟草(Nicotiana tabacum)[31]有6个DXS基因。
DXS基因通常被分为3类(图 2),Ⅰ型为管家基因,其所编码的酶催化前体物质形成叶绿素、类胡萝卜素等与光合有关的萜类物质,如拟南芥中的AtDXS1(CLA1);Ⅱ型基因编码植物特异性的次生代谢产物,如玉米的DXS2和苜蓿的MtDXS2可能跟植物的防御功能有关;Ⅲ型基因通常存在于植物基因组中,但其所编码酶的功能尚不清晰[5, 8],也有研究认为Ⅲ型基因所编码的蛋白已失活[32]。在4个胡萝卜的DXS基因中,DCAR_030576属于DXS1型,DCAR_009911、DCAR_014178属于DXS2型,DCAR_022887属于DXS3型[30]。

|
| 图 2 不同植物DXS蛋白的聚类分析 |
DXS1基因编码区长2 100 bp左右,编码700个左右氨基酸链,相对分子量70 kD左右[18, 41]。如马铃薯DXS基因cDNA全长2 421 bp,编码区长2 160 bp,编码719个氨基酸,分子量为77.8 kD[18]。玫瑰DXS基因全长2 091 bp,包含1 494 bp的开放阅读框,编码498个氨基酸[33]。天竺葵(Pelargonium spp.)GrDXS基因cDNA全长2 505 bp,包含长2 157 bp的开放阅读框,编码792个氨基酸,分子量为77.5 kD[19]。我们利用NCBI数据库分析3种类型DXS氨基酸的长度发现,Ⅰ型植物DXS长度略大于Ⅱ型(如银杏DXS2)和Ⅲ型(如拟南芥DXS3)。
4 DXS基因的表达及调控DXS基因表达水平与花、果实发育程度、昼夜节律及品种有关,且具有组织差异性。DXS基因在玫瑰花瓣中表达量从花蕾到盛开期持续增加,在盛开期达到顶峰,然后随着花朵的衰败表达量降低,与花朵发育程度密切相关[33]。茉莉花瓣中DXS基因一天的表达量变化受生物钟的调控,具有昼夜节律[29]。番茄DXS(SlDXS2)基因在花被片中高表达[42],DXS基因在铁皮石斛原球茎中高表达[43]。对不同品种百合花被片DXS基因表达水平的研究发现:浓香型百合‘Conca d’Or’基因表达量比淡香型百合‘Santander’高,具有品种差异性[28]。果实发育程度不同,DXS基因表达水平也不同。随着果实成熟,猕猴桃果实中DXS(AcDXS1)基因表达量增加[27]。在以叶片为萜类合成主要器官的植物体中(如玫瑰香味天竺葵),DXS的表达在叶片发育的早期最高[19]。
同一物种不同拷贝的DXS基因的表达模式可能相同,也可能不同。白木香的3个DXS基因的表达模式十分相似,均在茎中高表达,其次为叶片,在根中的表达量最低[26]。丹参SmDXS1在叶、茎、根中均有表达,在叶中表达量最高,茎中次之,根中的表达量十分微弱,然而丹参SmDXS2主要在根中表达,但表达量不高[25]。
很多信号可以在转录和蛋白水平调节DXS,如激素、光、昼夜节律、发育程度和蔗糖浓度[38, 44]。4种外源植物激素处理铁皮石斛后发现,生长素(ABA)、水杨酸(SA)、茉莉酸(JA)和油菜素内脂(BR)均可以提高原球茎中DXS基因的转录水平,但达到最高值所需时间不同[43]。在白木香中的研究发现,AsDXS1受物理、化学和H2O2的调控,但AsDXS2和AsDXS3只受物理和化学调控,不受H2O2调控,同时,3个基因均受茉莉酸甲酯(MJ)调控,但受调控的时间不同[26]。DXS的表达受光的调节,光下天竺葵GrDXS基因的表达量是暗处表达量的150倍[19]。
DXS的活性还受内源产物和底物的调节。如3-异戊烯基焦磷酸(IPP)和二甲基丙烯焦磷酸(DMAPP)的反馈调节[5, 35],即IPP和DMAPP会竞争性地结合DXS酶的活性中心,使TPP脱离,从而抑制DXS酶的活性。卡尔文循环的中间产物GAP也可以加速DXS酶对丙酮酸的脱羧作用,从而形成底物对DXS的前馈调节[20, 45]。
5 DXS基因的功能DXS基因指导合成萜类次生代谢物质,是下游产物的关键调控位点。作为萜烯类生物合成途径第一个关键酶基因,DXS的过表达或抑制可以引起下游代谢产物含量发生改变。对植物DXS基因功能研究最多的是其对器官着色的影响。导入外源DXS基因的胡萝卜和拟南芥的类胡萝卜素和叶绿素以及其他内源激素(如ABA)含量增加[18, 30]。但是,也有研究发现,拟南芥中转入马铃薯StDXS1基因后,ABA和赤霉素(GA)的含量下降,但并不能引起JA和SA水平的改变,同时可以促进拟南芥叶片叶绿素和类胡萝卜素的积累[18]。在番茄的研究中还证明,DXS基因的表达水平与果实类胡萝卜素含量正相关,将SIDXS2基因沉默后,会引起β-水芹烯含量的降低[42]。
DXS基因的另一个功能与植物抗病性有关。早在2000年Walter等[46]通过研究表明,小麦、玉米、水稻和大麦的根部被菌根真菌侵染后会引起DXS基因表达水平发生变化。菌根真菌侵染蒺藜苜蓿(Medicago truncatula)的根后会诱发MtDXS2在根部高表达[40],使用RNAi技术把MtDXS2沉默掉以后,菌根MtDXS2的表达水平降低,丛枝菌根诱导的脱辅基类胡萝卜素也随之下降[47]。马铃薯晚疫病的发生伴随着DXS基因表达水平的降低,而DXS基因表达水平的降低又会导致与抗病性相关的异戊二烯类物质含量的降低[18]。
利用土壤农杆菌介导的转基因技术将拟南芥DXS基因导入宽叶薰衣草基因组后发现转基因植株叶片和花朵中精油(主要成分为单萜)产量比对照显著提高[48]。瞬时过表达猕猴桃果实DXS(AcDXS1)基因导致转基因烟草叶片中单萜类花香物质显著增加[27],这为改良观赏植物花香提供了新的思路。
前人的研究还表明,一个物种内的多拷贝DXS基因的功能并不等同。Zhou等[25]认为,在丹参两个拷贝的DXS基因中,SmDXS2是主要的限速酶基因,因为在丹参中过表达SmDXS1和SmDXS2,可以显著促进转基因株系根中丹参酮的积累,但只有下调SmDXS2的表达才能导致丹参酮含量的显著降低。同样,沉默掉MtDXS2基因后,蒺藜苜蓿根部丛枝菌根的减少,主要是由MtDXS2表达水平的降低引起的,与MtDXS1无关[47]。可见DXS2基因在调控次生代谢物中起着独特的作用。
6 DXS与其他基因的协同作用研究表明,某一个基因往往不是单独起作用,而与其代谢通路中上下游相关基因共同表达产生某一特定功能。将马铃薯DXS基因转入拟南芥中,DXS基因的上调表达会引起其下游GGPPS基因显著上调表达,从而促进了类胡萝卜素含量的增加;同时,也会引起PSY基因(Phytoene synthase)表达水平的上调,从而促进转基因植株中叶黄素(Lutein)和β-胡萝卜素的增加[18]。将拟南芥DXS基因转入胡萝卜中,也发现类似的结果,即DXS基因表达水平的增加可以显著增强PSY基因的表达[30]。
同一代谢通路的两个基因对代谢产物的合成所起的作用并不总是等同,如将拟南芥DXS和DXR基因转入胡萝卜中,DXS是叶绿素和胡萝卜素合成的主要限制因子,DXR的作用微乎其微[30],在薰衣草的研究中也有类似结果[48]。不过,不同物种中DXS与特定基因的共同表达,比单个基因更能有效提升代谢产物的含量。将DXS与GGPPS同时转入丹参中,转基因丹参的毛根所合成的丹参酮含量显著高于两个基因单独转入和非转基因植株中丹参酮的含量[49]。将猕猴桃DXS和单萜合成酶基因(Monoterpene synthases,TPS)同时转入烟草中,可以将萜类物质的含量提高100倍,比单独转其中一个基因的效率高很多[27]。显然,研究DXS与相关基因的协同作用,可以有效提高基因工程的效率。
7 结语随着对DXS基因在MEP萜类物质代谢途径中重要性认识的深入,其DNA序列已在越来越多的植物材料中被克隆。最早认为,DXS酶只由1-3个基因编码,高通量测序技术的进步,使得某些物种中(如水稻和烟草)发现有5-6个基因共同参与DXS酶的编码。随着基因克隆的深入,可能会在某一物种发现更多的DXS同源基因。不过,总体上说,DXS基因被分为3类,研究最多的是作为管家基因的Ⅰ型DXS,少数物种上验证了Ⅱ型DXS在次生代谢产生中的特异作用,今后,应有更多的研究用于阐明Ⅱ型和Ⅲ型基因的功能,使对DXS基因的认识更全面。
在对DXS基因的研究中,人们越来越重视DXS基因与其它基因的互作,这些均为通过生物合成法生产有益萜类物质奠定了坚实的基础。不过,现有的研究表明DXS基因在不同物种不同器官中的表达具有特异性,调控其表达的条件不尽相同,有必要根据物种的特性有针对性地开展相关研究。此外,萜类是一个庞大的家族,其合成是一个复杂的过程,底物和产物的前馈调控和反馈调节错综复杂,还有许多调控机制尚未探明,其调控特定性状的遗传机制亟待深入研究。采用转录组学和代谢组学相结合的方法进行完整的基因网络研究将有利于阐明萜类物质合成调控机理和遗传机制,加速分子设计育种的步伐。
| [1] |
孙丽超, 李淑英, 王凤忠, 等. 萜类化合物的合成生物学研究进展[J]. 生物技术通报, 2017, 33(1): 64-75. |
| [2] |
Muhlemann JK, Klempien A, Dudareva N. Floral volatiles:from biosynthesis to function[J]. Plant Cell & Environ, 2014, 37(8): 1936-1949. |
| [3] |
Dudareva N, Klempien A, Muhlemann JK, et al. Biosynthesis, function and metabolic engineering of plant volatile organic compounds[J]. New Phytol, 2013, 198(1): 16-32. DOI:10.1111/nph.12145 |
| [4] |
Battistini MR, Shoji C, Handa S, et al. Mechanistic binding insights for 1-deoxy-D-Xylulose-5-Phosphate synthase, the enzyme catalyzing the first reaction of isoprenoid biosynthesis in the malaria-causing protists, Plasmodium falciparum and Plasmodium vivax[J]. Protein Expres Purif, 2016, 120: 16-27. DOI:10.1016/j.pep.2015.12.003 |
| [5] |
Rodriguez-Concepcion M, Boronat A. Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis[J]. Curr Opin Plant Biol, 2015, 25: 17-22. DOI:10.1016/j.pbi.2015.04.001 |
| [6] |
Wright LP, Rohwer JM, Ghirardo A, et al. Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis[J]. Plant Physiol, 2014, 4: 1488-1504. |
| [7] |
金蓉, 朱长青, 徐昌杰. 1-脱氧木酮糖-5-磷酸合成酶(DXS)及其编码基因[J]. 细胞生物学杂志, 2007(29): 706-712. |
| [8] |
Sharma E, Pandey S, Gaur AK. Identification and expression analysis of DXS1 gene isolated from Aconitum balfourii Stapf[J]. Acta Physiol Plant, 2016, 38(10): 1-9. |
| [9] |
Battilana J, Costantini L, Emanuelli F, et al. The 1-deoxy-D-xylulose 5-phosphate synthase gene co-localizes with a major QTL affecting monoterpene content in grapevine[J]. Theor Appl Genet, 2009, 118(4): 653-669. DOI:10.1007/s00122-008-0927-8 |
| [10] |
Chahed K, Oudin A, Guivarc H N, et al. 1-deoxy-D-xylulose 5-phosphate synthase from periwinkle. cDNA identification and induced gene expression in terpenoid indole alkaloid-producing cells[J]. Plant Physiol Biochem, 2000, 38(7-8): 559-566. DOI:10.1016/S0981-9428(00)00781-6 |
| [11] |
Altincicek B, Hintz M, Sanderbrand S, et al. Tools for discovery of inhibitors of the 1-deoxy-D -xylulose 5-phosphate(DXP)synthase and DXP reductoisomerase:an approach with enzymes from the pathogenic bacterium Pseudomonas aeruginosa[J]. Fems Microbiol Lett, 2000, 190(2): 329-333. |
| [12] |
Xiang S, Usunow G, Lange G, et al. 1-deoxy-D-Xylulose 5-phosphate synthase(DXS), a crucial enzyme for isoprenoids biosynthesis[J]. J Biol Chem, 2007, 282(4): 2676. DOI:10.1074/jbc.M610235200 |
| [13] |
Querol J, Besumbes O, Lois LM, et al. A fluorometric assay for the determination of 1-deoxy-D-xylulose 5-phosphate synthase activity[J]. Anal Biochem, 2001, 296(1): 101-105. DOI:10.1006/abio.2001.5234 |
| [14] |
Han YS, Sabbioni C, Van dHR, et al. High-performance liquid chromatography assay for 1-deoxy-D-xylulose 5-phosphate synthase activity using fluorescence detection[J]. J Chromatogra A, 2003, 986(2): 291-296. DOI:10.1016/S0021-9673(02)02016-2 |
| [15] |
Brammer LA, Meyers CF. Revealing substrate promiscuity of 1-deoxy-D-xylulose 5-phosphate synthase[J]. Org Lett, 2009, 11(20): 4748-4751. DOI:10.1021/ol901961q |
| [16] |
胡玥, 王雪娇, 李恒, 等. 2, 4-二硝基苯肼柱前衍生高效液相色谱法测定1-脱氧-D-木酮糖-5-磷酸合酶稳态动力学参数[J]. 分析化学, 2012, 40(12): 1859-1864. |
| [17] |
Fung PK, Krushkal J, Weathers PJ. Computational analysis of the evolution of 1-deoxy-d -xylulose-5-phosphate reductoisomerase, an important enzyme in plant terpene biosynthesis[J]. Chem Biodivers, 2010, 7(5): 1098-1110. DOI:10.1002/cbdv.v7:5 |
| [18] |
Henriquez MA, Soliman A, Li G, et al. Molecular cloning, functional characterization and expression of potato(Solanum tuberosum)1-deoxy-D-xylulose 5-phosphate synthase 1(StDXS1)in response to Phytophthora infestans[J]. Plant Sci, 2016, 243: 71-83. DOI:10.1016/j.plantsci.2015.12.001 |
| [19] |
Jadaun JS, Sangwan NS, Narnoliya LK, et al. Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera:active involvement of plastid isoprenogenic pathway in their biosynthesis[J]. Physiol Plant, 2017, 159(4): 381-400. DOI:10.1111/ppl.2017.159.issue-4 |
| [20] |
Patel H, Nemeria NS, Brammer LA, et al. Observation of thiaminbound intermediates and microscopic rate constants for their interconversion on 1-deoxy-D-xylulose 5-phosphate synthase:600-fold rate acceleration of pyruvate decarboxylation by D-glyceraldehyde-3-phosphate[J]. J Am Chem Soc, 2012, 134(44): 18374-18379. DOI:10.1021/ja307315u |
| [21] |
Banerjee A, Wu Y, Banerjee R, et al. Feedback inhibition of deoxy-D-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway[J]. J Biol Chem, 2013, 288(23): 16926-16936. DOI:10.1074/jbc.M113.464636 |
| [22] |
Banerjee A, Preiser AL, Sharkey TD. Engineering of recombinant poplar deoxy-D-xylulose-5-phosphate synthase(PtDXS)by sitedirected mutagenesis improves its activity[J]. PLoS One, 2016, 11(8): e0161534. DOI:10.1371/journal.pone.0161534 |
| [23] |
朱畇昊, 苏秀红, 董诚明, 等. 冬凌草1-脱氧-D-木酮糖-5-磷酸合成酶基因克隆与表达分析[J]. 广西植物, 2016, 36(12): 1476-1482. DOI:10.11931/guihaia.gxzw201511015 |
| [24] |
Sykora R, Legut D. Cloning and expression analysis of 1-deoxy-D-xylulose-5-phosphate synthase gene from the medicinal plant Conyza blinii H. Lév[J]. Turk J Biol, 2015, 38(5): 664-670. |
| [25] |
Zhou W, Huang F, Li S, et al. Molecular cloning and characterization of two 1-deoxy-D-xylulose-5-phosphate synthase genes involved in tanshinone biosynthesis in Salvia miltiorrhiza[J]. Mol Breeding, 2016, 36(9): 124. DOI:10.1007/s11032-016-0550-3 |
| [26] |
Xu Y, Liu J, Liang L, er al. Molecular cloning and characterization of three cDNAs encoding 1-deoxy-D-xylulose-5-phosphate synthase in Aquilaria sinensis(Lour.)Gilg[J]. Plant Physiol Biochem, 2014, 82: 133-141. DOI:10.1016/j.plaphy.2014.05.013 |
| [27] |
Nieuwenhuizen NJ, Chen X, Wang MY, et al. Natural variation in monoterpene synthesis in kiwifruit:transcriptional regulation of terpene synthases by NAC and ETHYLENE-INSENSITIVE3-like transcription factors[J]. Plant Physiol, 2015, 167(4): 1243-1258. DOI:10.1104/pp.114.254367 |
| [28] |
Johnson TS, Schwieterman ML, Kim JY, et al. Lilium floral fragrance:A biochemical and genetic resource for aroma and flavor[J]. Phytochemistry, 2016, 122: 103-112. DOI:10.1016/j.phytochem.2015.11.010 |
| [29] |
孙君, 陈桂信, 叶乃兴, 等. 茉莉花香气相关基因JsDXS及其启动子的克隆与表达分析[J]. 园艺学报, 2014, 41(6): 1236-1244. |
| [30] |
Simpson K, Quiroz LF, Rodriguez-Concepcion M, et al. Differential contribution of the first two enzymes of the MEP pathway to the supply of metabolic precursors for carotenoid and chlorophyll biosynthesis in carrot(Daucus carota)[J]. Front Plant Sci, 2016, 7: e6373. |
| [31] |
Yan N, Zhang H, Zhang Z, et al. Organ-and growing stage-specific expression of solanesol biosynthesis genes in Nicotiana tabacum reveals their association with solanesol content[J]. Molecules, 2016, 21(11): 1536. DOI:10.3390/molecules21111536 |
| [32] |
Saladie M, Wright LP, Garcia-Mas J, et al. The 2-C-methylerythritol 4-phosphate pathway in melon is regulated by specialized isoforms for the first and last steps[J]. J Exp Bot, 2014, 17: 5077-5092. |
| [33] |
Feng L, Chen C, Li T, et al. Flowery odor formation revealed by differential expression of monoterpene biosynthetic genes and monoterpene accumulation in rose(Rosa rugosa Thunb.)[J]. Plant Physiol Biochem, 2014, 75: 80-88. DOI:10.1016/j.plaphy.2013.12.006 |
| [34] |
魏麟, 伍贤进, 李胜华, 等. 鱼腥草1-脱氧-D-木酮糖-5-磷酸合成酶1基因克隆与表达分析[J]. 中草药, 2014, 45(11): 1607-1612. |
| [35] |
Banerjee A, Wu Y, Banerjee R, et al. Feedback inhibition of deoxy-D-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway[J]. J Biol Chem, 2013, 288(23): 16926-16936. DOI:10.1074/jbc.M113.464636 |
| [36] |
Peng G, Wang C, Song S, et al. The role of 1-deoxy-d-xylulose-5-phosphate synthase and phytoene synthase gene family in citrus carotenoid accumulation[J]. Plant Physiol Biochem, 2013, 71: 67-76. DOI:10.1016/j.plaphy.2013.06.031 |
| [37] |
Vallabhaneni R, Wurtzel ET. Timing and biosynthetic potential for carotenoid accumulation in genetically diverse germplasm of maize[J]. Plant Physiol, 2009, 150(2): 562-572. DOI:10.1104/pp.109.137042 |
| [38] |
Cordoba E, Porta H, Arroyo A, et al. Functional characterization of the three genes encoding 1-deoxy-D-xylulose 5-phosphate synthase in maize[J]. J Exp Bot, 2011, 62(6): 2023. DOI:10.1093/jxb/erq393 |
| [39] |
Sitthithaworn W, Wungsintaweekul J, Sirisuntipong T, et al. Cloning and expression of 1-deoxy-d-xylulose 5-phosphate synthase cDNA from Croton stellatopilosus and expression of 2C-methyl-d-erythritol 4-phosphate synthase and geranylgeranyl diphosphate synthase, key enzymes of plaunotol biosynthesis[J]. J Plant Physiol, 2010, 167(4): 292-300. DOI:10.1016/j.jplph.2009.09.001 |
| [40] |
Zhang M, Li K, Zhang C, et al. Identification and characterization of class 1 DXS gene encoding 1-deoxy-D-xylulose-5-phosphate synthase, the first committed enzyme of the MEP pathway from soybean[J]. Mol Biol Rep, 2009, 36(5): 879. DOI:10.1007/s11033-008-9258-8 |
| [41] |
Walter MH, Hans J, Strack D. Two distantly related genes encoding 1-deoxy-D-xylulose 5-phosphate synthases:differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots[J]. Plant J, 2002, 31(3): 243-254. DOI:10.1046/j.1365-313X.2002.01352.x |
| [42] |
Paetzold H, Garms S, Bartram S, et al. The isogene 1-deoxy-D-xylulose 5-phosphate synthase 2 controls isoprenoid profiles, precursor pathway allocation, and density of tomato trichomes[J]. Mol Plant, 2010, 3(5): 904-916. DOI:10.1093/mp/ssq032 |
| [43] |
Fan H, Wu Q, Wang X, et al. Molecular cloning and expression of 1-deoxy-D-xylulose-5-phosphate synthase and 1-deoxy-D -xylulose-5-phosphate reductoisomerase in Dendrobium officinale[J]. Plant Cell Tiss Org, 2016, 125(2): 381-385. DOI:10.1007/s11240-016-0945-1 |
| [44] |
Iorizzo M, Ellison S, Senalik D, et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution[J]. Nat Genet, 2016, 6: 657-666. |
| [45] |
Banerjee A, Sharkey TD. Methylerythritol 4-phosphate(MEP)pathway metabolic regulation[J]. Nat Prod Rep, 2014, 31(8): 1043-1055. DOI:10.1039/C3NP70124G |
| [46] |
Walter MH, Fester T, Strack D. Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the 'yellow pigment' and other apocarotenoids[J]. Plant J, 2000, 21(6): 571-578. DOI:10.1046/j.1365-313x.2000.00708.x |
| [47] |
Floss DS, Hause B, Lange PR, et al. Knock-down of the MEP pathway isogene 1-deoxy-D-xylulose 5-phosphate synthase 2 inhibits formation of arbuscular mycorrhiza-induced apocarotenoids, and abolishes normal expression of mycorrhiza-specific plant marker genes[J]. Plant J Cell Mol Biol, 2008, 56(1): 86-100. DOI:10.1111/tpj.2008.56.issue-1 |
| [48] |
Muñoz-Bertomeu J, Arrillaga I, Ros R, et al. Up-regulation of 1-deoxy-D-xylulose-5-phosphate synthase enhances production of essential oils in transgenic spike lavender[J]. Plant Physiol, 2006, 142(3): 890-900. DOI:10.1104/pp.106.086355 |
| [49] |
Shi M, Luo X, Ju G, et al. Enhanced diterpene tanshinone accumulation and bioactivity of transgenic Salvia miltiorrhiza hairy roots by pathway engineering[J]. J Agr Food Chem, 2016, 64(12): 2523-2530. DOI:10.1021/acs.jafc.5b04697 |