植物病毒是农林生产最严重的威胁,目前尚无有效的治理措施。由于病毒无法突破植物的细胞壁障碍,大多数植物病毒的传播需要介体来完成,刺吸式口器昆虫因其特殊的口器类型和特异性的取食方式而成为植物病毒最重要的介体类群。刺吸式昆虫中的飞虱、蚜虫、粉虱和叶蝉等是植物病毒介体中最重要的类群,它们传播病毒的方式各不相同,如飞虱一般以持久方式传播病毒;蚜虫多以非持久性或半持久性方式进行传播;粉虱中的烟粉虱是双生病毒的主要传播媒介,以持久性方式进行传播。病毒传播方式是影响病毒与媒介昆虫相互作用的重要因素,紧密调控着病原体的媒介传播[1, 2]。植物-病毒-介体昆虫的互作关系是复杂而特异的,其间的协同进化关系是生态学、进化生物学等理论学科的重要研究内容,同时也是病毒防控措施制定的理论基础。病毒因其繁殖迅速、变异快而成为三者互作及进化的推动者和主动因素,其结果是有利于病毒的传播[2, 3]。病毒对介体的影响分为直接和间接两个方面,直接影响即是病毒病毒在媒介昆虫体内滞留,可直接影响昆虫的存活率、产卵量、发育历期、取食行为及寄主选择行为等生物学特性[4, 5];而间接影响是指病毒的侵染可触发寄主植物的应激反应,进而在一定程度上影响介体昆虫,以此改变介体昆虫的生态适应性。
病毒诱导的植物对刺吸式媒介昆虫的间接互作涉及生理、行为、进化层面的拮抗或协同关系。通过对这些关系的系统性研究,可为研究病毒-寄主植物-介体昆虫三者互作奠定基础,将有利于人们研究新的应对媒介传播的植物病毒病的防控机制,并为害虫防治和抗虫植物培育提供新的思路。本文主要综述了病毒侵染诱导植物的次生代谢、信号通路、初生代谢及物理结构等的变化对介体昆虫所造成的间接影响,以期为防治植物病毒性病害提供依据。
1 病毒诱导植物次生代谢的变化病毒通过侵染寄主植物使其产生一系列次生代谢产物,主要包括植物挥发性物质(volatile organic compound,VOC)、抗氧化物酶、病程相关蛋白(pathogenesis-related protein,PR)等。一些次生代谢产物可与游离氨基酸发生反应,以此改变植物的营养价值。这些变化可能会对媒介昆虫产生有利、不利或无显著影响[1, 6, 7],这种差异与物种特异性、寄主抗性等相关[8]。
1.1 植物挥发性物质大量研究发现,植物感染病毒后,其挥发性化学物质发生了很大变化,对介体昆虫的寄主选择行为造成影响。与病害相关的植物挥发物大致分为绿叶性气味、芳香族化合物、萜类化合物,也包含一些含氮及含硫的化合物等[9]。Mauck等[1, 2]发现蚜虫可能会因嗅觉感知而优先选择病毒侵染过的植株;Luan等[10]采用气相色谱-串联质谱(GC-MS)对感染番茄黄化曲叶病毒(tomato yellow leaf curl virus,TYLCV)的烟草进行分析发现,植株的萜类化合物质合成受到抑制;通过基因沉默技术发现,这种抑制现象增加了植株对烟粉虱的吸引力。据此认为,这些挥发性物质可能会对介体产生一定影响。这也与Howe等[11]在另一个研究中发现萜类物质可作为驱虫剂或毒素进而影响昆虫的习性的结论相一致。
不同的生物或非生物因子可诱导同种植物释放不同的挥发物,因此可通过监测VOC的变化研制开发监控植物健康状况的探测系统,以便能在植物病虫害初期做出预报。Cheung等[12]利用TwisterTM吸附系统,检测到月桂烯、蒈烯、罗勒烯、布藜烯等柑橘衰退病(citrus tristeza virus,CTV)的生物标志物发生显著变化,据此发现VOC的潜在应用价值,即用于一些植物病害的监测和评估分析。
1.2 抗氧化物酶病毒侵染寄主植物后能够在不同程度上诱导一些抗氧化物酶活性产生变化,且这种变化具有物种差异性。赵荣乐[13]发现黄瓜花叶病毒(cucumber mosaic virus,CMV)感染甜瓜后,植株中苯丙氨酸解氨酶(phenylalanine ammonia-lyase,PAL)活性显著增高;TYLCV侵染番茄后,过氧化物酶(peroxidase,POD)活性显著提高[14];Nair[15]发现斯里兰卡木薯花叶病毒(srilankan cassava mosaic virus,SLCMV)侵染植物后,POD、PAL和多酚氧化酶(polyphenol oxidase,PPO)酶活性均显著增加。而PPO为“营养限制酶”,可降低植物营养价值;POD可产生调节与防御有关的信号传导途径的氧化物质,这些氧化物质可增强木质化和交联作用加固细胞壁来限制昆虫取食[16],也可直接减弱其消化能力[17]。这些抗氧化物酶活性改变会对媒介昆虫生物学特性产生一定影响,进而影响病毒的传播。
1.3 病程相关蛋白病程相关蛋白参与病毒侵染诱导的植物防御反应,主要包括几丁质酶、葡聚糖酶、类甜蛋白以及一些功能未知的蛋白,这些蛋白在植物防御过程中起着重要作用[18, 19]。很多研究表明植物受病毒侵染后,能诱导几丁质酶、β-1,3-半乳糖苷酶等多种抗性酶的高水平表达[20-22];而几丁质酶能通过水解几丁质和抑制α淀粉酶活性来干扰媒介昆虫的消化[23]。
2 病毒诱导植物的信号途径变化植物进化出不同的机制来应对多种生物及非生物因素的胁迫,水杨酸(salicylic acid,SA)、茉莉酸(jasmine acid,JA)和乙烯(ethylene,ET)是目前已知的参与高等植物防御反应的重要植物激素。JA和ET信号转导途径在应对植食性害虫和腐生营养型病原微生物中发挥重要作用,而SA途径主要参与植物对活体营养型病原物微生物的防御反应。其中,SA途径的激活可诱导系统获得性抗性(systemic acquired resistance,SAR)、病程相关蛋白等抗性基因的表达,JA信号途径可诱导多种防御基因和防御相关蛋白(如多酚氧化酶、蛋白酶抑制剂等)的表达。这些激素信号途径之间的交叉互作可对昆虫产生相应的影响。
2.1 水杨酸信号途径病毒侵染可诱导SA信号途径上的相关基因表达发生变化。Shi等[24]发现TYLCV侵染显著增加番茄叶片内SA水平;Mauck等[25]也证明CMV侵染改变了寄主的SA代谢途径;Abe[20]通过表达谱测序等技术证明番茄斑萎病毒(tomato spotted wilt virus,TSWV)侵染诱导了SA相关基因表达,且西花蓟马(Frankliniella occidentalis)会优先选择感病的拟南芥,并且其存活力、增殖力也有所提高,实验表明植物体内SA信号的积累能增强昆虫的适应性。这些结果与Zarate等[26]提出的SA信号途径可抑制约束昆虫发育的防御反应的结论相符合。
2.2 茉莉酸信号途径对植株施用外源JA能降低昆虫的行为表现、存活率和繁殖力[20, 27, 28],拟南芥JA突变体coi1-1更加吸引西花蓟马取食[28],表明由JA调控的防御反应可有效调节媒介昆虫生活力。中国番茄黄化曲叶病毒(tomato yellow leaf curl China virus,TYLCCNV)的致病因子DNAβ上βC1[29]和CMV上的2b蛋白[30]可抑制JA代谢途径,从而有利于MEAM1烟粉虱和蚜虫的存活力和生殖力,促成了通过寄主植物介导的媒介昆虫-病毒之间的互惠关系。
2.3 乙烯信号途径寄主植物受病毒侵染后可改变其乙烯代谢途径[25, 31, 32]。最近研究表明,拟南芥ein2和etr1乙烯突变体可以提高植株对烟草花叶病毒(tobacco mosaic virus,TMV)和花椰菜花叶病毒(cauliflower mosaic virus,CaMV)的抗性[31, 33]。Casteel等[34]发现芜菁花叶病毒(turnip mosaic virus,TuMV)侵染可以诱导寄主植物的ET代谢途径来增加其敏感性,这种调节可以增加蚜虫的适应性。ET经常和JA协同作用,介导由植食性害虫或腐生营养性病原微生物引起的防御反应。在拟南芥JA和ET组成型激活突变体cev1上,桃蚜和B型烟粉虱的种群增长速率明显降低[26]。以上结果表明病毒可以通过诱导寄主防御信号的开启进而调节媒介昆虫的繁殖力、存活率等(表 1)。
病毒感染改变了寄主植物的性状,如初生代谢、光合作用的强弱、生长状况以及叶片颜色、筛管成分中胼胝质含量等,这些性状变化会使媒介昆虫在病株上刺探取食时表现出一些异常。
3.1 初生代谢病毒侵染能够改变植物组织中的碳水化合物、氨基酸、水等营养物质及植物光合作用的强弱,这些变化既可导致信号转导通路的激活,也可改变营养物质的运输。Su等[14]利用高效液相色谱(high performance liquid chromatography,HPLC)测定感染TYLCV及对照植株的营养组成发现,感病植株叶肉、表皮细胞中自由氨基酸浓度显著下降了55%,而碳氮化合物比例却有所增加;Mauck等[25]发现CMV侵染后的植株叶片、韧皮部组织中碳氮化合物比例显著降低。他们认为碳氮化合物比例的增加可以促进昆虫的同化吸收能力。因此,烟粉虱更偏向于在感染持久性传播的TYLCV植株上取食,而蚜虫在以非持久性传播的CMV侵染的植株上短暂取食后转移到健康植株上,从而加速了病毒的传播,这与Mauck等[1, 2]的病毒传播方式调控病原传播的研究结果一致。
3.2 植物生长结构病原可诱导植物生长、形态学性状等发生变化,如叶片黄化、卷曲、褶皱以及腺毛密度、胼胝质沉积、叶片厚度等,这些特征的变化可能会影响媒介昆虫在植株上的取食和移动[35, 36]。Casteel等[34]发现TuMV抑制了烟草中胼胝质沉积;胼胝质沉积是蚜虫取食引起的一种寄主防卫反应,可提高蚜虫的繁殖力。多数病毒都能引起寄主植物产生黄化或浅绿症状[37],而蚜虫、粉虱偏向于选择黄色或浅绿的植物组织[38, 39],介体对黄色(或黄化植物)表现一定的趋性,可能是三者长期进化过程中形成的。
3.3 其他病毒-植物的应激反应还可以对媒介昆虫的刺探产生一定影响。刺吸电位图谱(electrical penetra-tion graph,EPG)可以将昆虫的探索和取食行为可视化[40, 41]。何应琴等[42]通过EPG监测发现褐色橘蚜(Toxoptera citricida)在感病植株上C波的总持续时间显著少于健康植株,而E波增多,有利于褐色橘蚜取食及CTV的传播;Liu等[43]发现B型和Q型烟粉虱在取食感染TYLCV的番茄时口针刺探次数更频繁,取食和分泌唾液的时间更长;感染马铃薯Y病毒(potato virus Y,PVY)的植物能够显著增加桃蚜的韧皮部取食时间[44]。这些迹象表明,昆虫口针在感病植株叶片组织内移动的束缚力减弱,病毒的感染减弱了植物对刺吸式昆虫取食的抵抗性。这种由寄主植物介导的媒介昆虫与病原物之间的互作可能是决定植物病毒病流行和昆虫种群增长的主要因子之一。
病毒感染寄主植物可削弱或破坏植物的防御机制,并改变植物的营养组成或形态学性状进而对介体产生一定影响。同时这些变化可能也会对自然界中的其他病原微生物,植食性昆虫及介体天敌等产生影响[45]。此外,介体昆虫体内的内共生菌[46]、一些非生物因素(如温度、湿度、肥料和土壤类型等)等[47]在植物-病毒-介体互作中可能发挥重要作用。介体昆虫体内的内共生菌,如立氏立克次体(Rickettsia rickettsii),Rickettsia通过介体转移到植株体内并对植株防御反应进行调节[48, 49];Douglas等[46]发现烟粉虱体内的共生体组分发生变化可能会影响介体、植物病毒、寄主植物之间的相互关系。因此,开展综合互作的相关研究可为病虫害综合治理提供不可多得的借鉴。
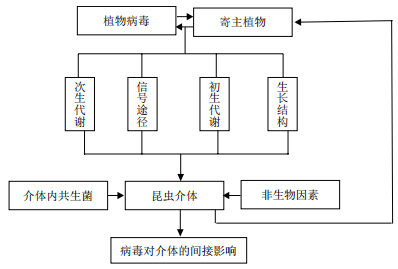
|
| 图 1 综合互作 |
利用多种技术进一步揭示植物-病毒-介体互作的复杂关系是十分必要的。植物介导的病毒与刺吸式媒介昆虫相互作用的复杂性,使得确定植物的结构特点、代谢产物及信号途径是如何影响媒介昆虫的生物学特性变得困难。因此要明确病毒侵染对媒介昆虫与寄主植物的相互影响,通过基因组、转录组、蛋白组及代谢组多组学联合分析并与EPG、种群生物学等经典研究技术相结合显得尤为重要。
植物病毒的快速繁殖及害虫的大爆发对农业生产、食品安全带来了严重性威胁。植物病毒的有效防控需要通过控制介体来实现[50]。化学防治虽能在一定程度上减少植物病毒的传播,但不同杀虫剂对媒介的防治效果差异很大;而介体昆虫一般均是繁殖力强、生活史短的r-对策者,容易产生抗药性;而且杀虫剂会对环境造成污染、农药残留等,故害虫的生态调控研究成为生态学研究的热点之一。在害虫综合治理策略中,植物抗性是最经济、最环保、最有效的措施[51]。因此,开展病毒-植物互作影响媒介昆虫生物学特性的机制研究,不仅可为其有效防控提供新的思路与途径,也可为病虫害综合治理和持续控制的问题提供一定的借鉴。
| [1] | Mauck KE, De Moraes CM, Mescher MC. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proceedings of the National Academy of Sciences, 2010, 107 (8): 3600–3605. DOI:10.1073/pnas.0907191107 |
| [2] | Mauck K, Bosque-Perez NA, Eigenbrode SD, et al. Transmission mechanisms shape pathogen effects on host-vector interactions: evidence from plant viruses. Functional Ecology, 2012, 26 (5): 1162–1175. DOI:10.1111/j.1365-2435.2012.02026.x |
| [3] | Blanc S, Michalakis Y. Manipulation of hosts and vectors by plant viruses and impact of the environment. Current Opinion in Insect Science, 2016, 16 : 36–43. DOI:10.1016/j.cois.2016.05.007 |
| [4] | Schliephake E, Habekuss A, Scholz M, et al. Barley yellow dwarf virus transmission and feeding behaviour of rhopalosiphum padi on hordeum bulbosum clones. Entomologia Experimentalis Et Applicata, 2013, 146 (3): 347–356. DOI:10.1111/eea.2013.146.issue-3 |
| [5] | Li S, Wang SJ, Wang X, et al. Rice stripe virus affects the viability of its vector offspring by changing developmental gene expression in embryos. Scientific Reports, 2015, 5 (5): 417–446. |
| [6] | Bosque-Pérez NA, Eigenbrode SD. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Research, 2011, 159 (2): 201–205. DOI:10.1016/j.virusres.2011.04.020 |
| [7] | Shapiro L, De Moraes CM, Stephenson AG, et al. Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecology Letters, 2012, 15 (12): 1430–1438. DOI:10.1111/ele.12001 |
| [8] | Nogia VK, Singh V, Meghwal RR. Effect of cotton leaf curl virus infected plants on the biology of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae): Vector-virus mutualism. Phytoparasitica, 2014, 42 (5): 619–625. DOI:10.1007/s12600-014-0402-9 |
| [9] | 刘守安, 王梦馨, 韩宝瑜. 植物挥发性物质在茶树病害监测和防御中的作用研究现状. 中国茶叶, 2010, 32(1): 12–14. |
| [10] | Luan JB, Yao DM, Zhang T, et al. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecology Letters, 2013, 16 (3): 390–398. DOI:10.1111/ele.2013.16.issue-3 |
| [11] | Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology, 2008, 59 (59): 41–66. |
| [12] | Cheung WHK, Pasamontes A, Peirano DJ, et al. Volatile organic compound (VOC) profiling of citrus tristeza virus infection in sweet orange citrus varietals using thermal desorption gas chromatography time of flight mass spectrometry (TD-GC/TOF-MS). Metabolomics, 2015, 11 (6): 1514–1525. DOI:10.1007/s11306-015-0807-6 |
| [13] | 赵荣乐. 黄瓜花叶病毒感染引起甜瓜植株苯丙氨酸解氨酶和叶绿素的变化. 吉首大学学报:自然科学版, 2006, 27(3): 78–81. |
| [14] | Su Q, Preisser EL, Zhou XM, et al. Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. Journal of Economic Entomology, 2015, 108 (1): 11–19. DOI:10.1093/jee/tou012 |
| [15] | Nair AB, Umamaheswaran K. Enzymatic Responses to Srilankan cassava mosaic virus infection in cassava plants after grafting. International Journal of Applied and Pure Science and Agriculture, 2016, 2 (3): 165–170. |
| [16] | Ralph J, Bunzel M, Marita JM, et al. Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochemistry Reviews, 2003, 3 (1): 79–96. |
| [17] | Duffey SS, Stout MJ. Antinutritive and toxic components of plant defense against insects. Archives of Insect Biochemistry & Physiology, 1996, 32 (1): 3–37. |
| [18] | Singh A, Subudhi E. Expression of a chitinase family protein at4g01700 from Arabidopsis thaliana. Austin Journal of Computational Biology & Bioinformatics, 2014, 1 (4): 23–30. |
| [19] | Eboigbe L, Tzima AK, Paplomatas EJ, et al. The role of the β-1, 6-endoglucanase gene vegB in physiology and virulence of Verticillium dahliae. Phytopathologia Mediterranea, 2014, 53 (1): 94–107. |
| [20] | Abe H, Tomitaka Y, Shimoda T, et al. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant & Cell Physiology, 2012, 53 (1): 204–212. |
| [21] | El-Morsi Adel, Abdelkhalek A, E-Shehaby O, et al. Pathogenesis-related genes as tools for discovering the response of onion defence system against Iris yellow spot virus infection. Botany-botanique, 2015, 93 (11): 735–744. DOI:10.1139/cjb-2015-0017 |
| [22] | Röhring C. Induction of Pathogenesis-related proteins of group 1 by systemic virus infections of Nicotiana tabacum L.. Beiträge Zur Tabakforschung International Contributions to Tobacco Research, 2015, 18 (2): 63–70. |
| [23] | Ary MB, Richardson M, Shewry PR. Purification and characteriza-tion of an insect α-amylase inhibitor/endochitinase from seeds of job's tears (Coix lachryma-jobi). Biochimica Et Biophysica Acta, 1989, 999 (3): 260–266. DOI:10.1016/0167-4838(89)90007-1 |
| [24] | Shi XB, Pan HP, Xie W, et al. Plant virus differentially alters the plant's defense response to its closely related vectors. PLoS One, 2013, 8 (12): e83520–e83520. DOI:10.1371/journal.pone.0083520 |
| [25] | Mauck KE, De Moraes CM, Mescher MC. Biochemical and physiological mechanisms underlying effects of cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell and Environment, 2014, 37 (6): 1427–1439. DOI:10.1111/pce.2014.37.issue-6 |
| [26] | Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiology, 2007, 143 (2): 866–875. |
| [27] | Rodriguez-Enfedaque A, Delmas E, Guillaume A, et al. zVAD-fmk upregulates caspase-9 cleavage and activity in etoposide-induced cell death of mouse embryonic fibroblasts. Biochimica Et Biophysica Acta-Molecular Cell Research, 2012, 1823 (8): 1343–1352. DOI:10.1016/j.bbamcr.2012.05.013 |
| [28] | Abe H, Narusaka M, Shimoda T, et al. Analyses of plant resistance to thrips attack using Arabidopsis and chinese cabbage. Plant Biology (Rockville), 2009, 2009 (Suppl.): 318–318. |
| [29] | Zhang T, Luan JB, Qi JF, et al. Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Molecular Ecology, 2012, 21 (5): 1294–1304. DOI:10.1111/j.1365-294X.2012.05457.x |
| [30] | Lewsey MG, Murphy AM, Maclean D, et al. Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Molecular Plant-Microbe Interactions, 2010, 23 (7): 835–845. DOI:10.1094/MPMI-23-7-0835 |
| [31] | Chen L, Zhang L, Li D, et al. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110 (21): 1963–1971. DOI:10.1073/pnas.1221347110 |
| [32] | Mandadi KK, Pyle JD, Scholthof KB. Comparative analysis of antiviral responses in Brachypodium distachyon and Setaria viridis reveals conserved and unique outcomes among C3 and C4 plant defenses. Molecular Plant-Microbe Interactions, 2014, 27 (11): 1277–1290. DOI:10.1094/MPMI-05-14-0152-R |
| [33] | Love AJ, Laval VGC, Laird J, et al. Components of arabidopsis defense-and ethylene-signaling pathways regulate susceptibility to cauliflower mosaic virus by restricting long-distance movement. Molecular Plant-Microbe Interactions, 2007, 20 (6): 659–670. DOI:10.1094/MPMI-20-6-0659 |
| [34] | Casteel CL, De AM, Bak A, et al. Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiology, 2015, 169 (1): 209–218. DOI:10.1104/pp.15.00332 |
| [35] | Moreno-Delafuente A, Garzo E, Moreno A, et al. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS One, 2013, 8 (4): e61543–e61543. DOI:10.1371/journal.pone.0061543 |
| [36] | Sade D, Shriki Oz, Cuadros-Inostroza Alvar, et al. Comparative metabolomics and transcriptomics of plant response to Tomato yellow leaf curl virus infection in resistant and susceptible tomato cultivars. Metabolomics, 2015, 11 (1): 81–97. DOI:10.1007/s11306-014-0670-x |
| [37] | Li Y, Cui H, Cui X, et al. The altered photosynthetic machinery during compatible virus infection. Current Opinion in Virology, 2016, 17 (4): 19–24. |
| [38] | Hodge S, Powell Glen. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance?. Environmental Entomology, 2015, 37 (6): 1573–1581. |
| [39] | Isaacs R, Willis MA, Byrne DN. Modulation of whitefly take-off and flight orientation by wind speed and visual cues. Physiological Entomology, 2002, 24 (4): 311–318. |
| [40] | Fred Tjallingii W, Garzo E, Fereres A. New structure in cell puncture activities by aphid stylets: a dual-mode EPG study. Entomologia Experimentalis Et Applicata, 2010, 135 (2): 193–207. DOI:10.1111/eea.2010.135.issue-2 |
| [41] | Mclean DL, Kinsey MG. A technique for electronically recording aphid feeding and salivation. Nature, 1964, 202 (4939): 1358–1359. DOI:10.1038/2021358a0 |
| [42] | 何应琴, 陈文龙, 鲁卓越, 等. 褐色橘蚜在健康与CTV植株上的EPG比较. 山地农业生物学报, 2014, 33(2): 36–39. |
| [43] | Liu BM, Preisser EL, Chu D, et al. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and Tomato Yellow Leaf curl Virus. Journal of Virology, 2013, 87 (9): 4929–4937. DOI:10.1128/JVI.03571-12 |
| [44] | Boquel S, Delayen C, Couty, et al. Modulation of aphid vector activity by potato virus Y on in vitro potato plants. Plant Disease, 2011, 96 (1): 82–86. |
| [45] | Inbar M, Dan G. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annual Review of Entomology, 2008, 53 (1): 431–448. DOI:10.1146/annurev.ento.53.032107.122456 |
| [46] | Douglas AE. The microbial dimension in insect nutritional ecology. Functional Ecology, 2009, 23 (1): 38–47. DOI:10.1111/fec.2009.23.issue-1 |
| [47] | Rodelo-Urrego M, Pagán I, González-Jara P, et al. Landscape heterogeneity shapes host-parasite interactions and results in apparent plant-virus codivergence. Molecular Ecology, 2013, 22 (8): 2325–2340. DOI:10.1111/mec.12232 |
| [48] | Casteel CL, Hansen AK, Walling LL, et al. Manipulation of plant defense responses by the tomato psyllid (Bactericerca cockerelli) and its associated endosymbiont Candidatus liberibacter psyllaurous. PLoS One, 2012, 7 (4): 1–10. |
| [49] | Frago E, Dicke M, Godfray CJH. Insect symbionts as hidden players in insect-plant interactions. Trends in Ecology & Evolution, 2012, 27 (12): 705–711. |
| [50] | Hohn T. Plant virus transmission from the insect point of view. Proceedings of the National Academy of Sciences, 2007, 104 (46): 17905–17906. DOI:10.1073/pnas.0709178104 |
| [51] | 闫凤鸣. 烟粉虱的有效治理有赖于基础研究. 中国农业科学, 2016, 49(13): 2511–251. DOI:10.3864/j.issn.0578-1752.2016.13.006 |






