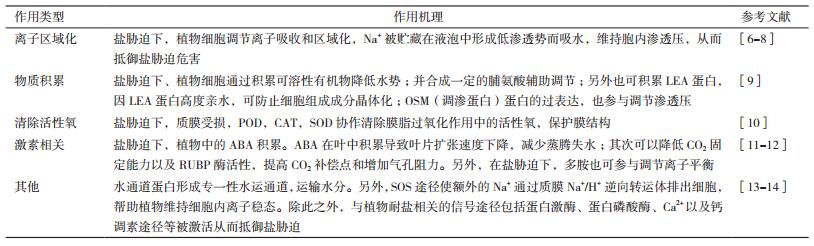
据不完全统计,全世界盐碱地面积约10亿hm2,中国盐碱地总面积约1亿hm2[1]。盐胁迫已成为植物(作物)生长发育的一个重要限制因子,可通过影响植物生长发育的各类进程对植物造成危害,严重时会导致植物死亡[2]。盐胁迫条件下,植物种子细胞内水势降低,种子生理性吸水困难,导致种子萌发率降低[3]。盐胁迫下,类囊体膜结构会发生变化,碳同化减少,同时由于植株生理性吸水困难,矿物质积累受阻,导致叶绿素合成降低,光合作用效率下降[4]。此外,盐胁迫下,植物体内的活性氧代谢紊乱,导致蛋白质聚集失活,影响蛋白质合成;同时,植物为了抵御盐胁迫会合成各类有机物质造成耗能增加,最终导致植物生长缓慢[5]。盐胁迫给农林业生产带来了极大危害,严重限制了盐碱地区的生态改善和经济发展,因此,研究人员在阐明植物耐盐机制、提高植物耐盐性等方面开展了大量研究工作。研究发现,植物自身会启动相应的耐盐机制来抵御盐胁迫。通常情况下植物的耐盐机制包括:渗透物质积累、离子区域化、清除活性氧等(表 1)。
1993年,Lee和Sunkar等[15-16]从线虫体内发现了第一个MicroRNA(miRNA)基因lin-4,随后,越来越多的miRNA在水稻、拟南芥等植物中被发现。miRNA是一类长度为19-25 nt的非编码RNA,通过对靶基因(mRNA)的降解或抑制其翻译,在转录后水平对基因的表达进行调控,在植物生长发育和环境胁迫响应等方面发挥着重要作用[17-19]。miRNA的发现为研究人员理解植物耐盐机理提供了新角度。目前,miRNA已经成为了分子生物学研究的热点领域。本文将在植物响应盐胁迫过程中发挥作用的miRNA进行整理综述,以期为阐明植物耐盐机制和耐盐植物分子育种提供参考。
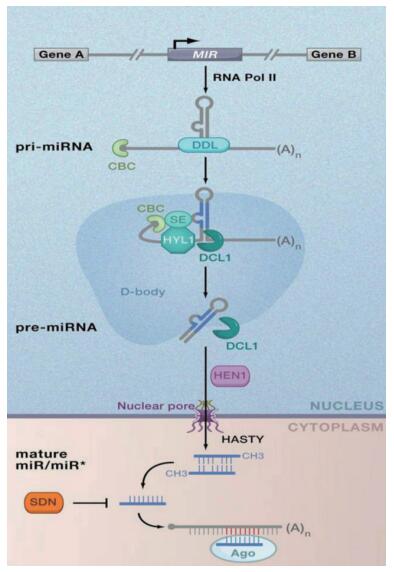
1 植物miRNA的合成、作用机制及功能 1.1 植物miRNA的合成植物miRNA的合成一般包括转录、加工、沉默复合体的装配。植物内源miRNA基因(MIR)通常位于基因间隔区,少数可能存在于内含子中。转录过程是由RNA聚合酶Ⅱ(Pol-Ⅱ)作用的。Pol-Ⅱ作用于MIR产生具有茎环结构(或称发卡结构)的初级转录产物Pri-miRNA[20]。Pri-miRNA需进行加工为成熟的RNA后才能发挥作用,Pri-miRNA的加工一般是由一种叫作DCL1的酶所进行的,DCL1是RNaseⅢ家族中Dicer的同源物[21-23],通常作用在位于细胞核内Pri-miRNA发卡结构中靠近或者远离环端的位点,产生大小约为70-300 bp的miRNA前体(Pre-miRNA)。随后进行两次切割,该过程还需要一些辅助蛋白的参与,通常包括RNA结合蛋白(HYL1)、C2H2锌指结合蛋白SE[24]。两次切割后会产生杂合双链miRNA\miRNA*(与miRNA配对的链),miRNA*互补双链在甲基转移酶HEN1的作用下使3' 末端甲基化[25-26]。随后,miRNA\miRNA*在转运蛋白exprotin-5(通常需要HASTY的辅助)的作用下被运送至细胞质中[27]。细胞质中具有RNA诱导的沉默复合体(RISC),双链分子与RISC中的AGO蛋白结合,在RNA解旋酶的作用下,去除miRNA*链,另一条也就成为了成熟的单链miRNA分子[28](图 1)。成熟的miRNA与其靶mRNA特异性互补结合,通过降解或抑制靶mRNA翻译来调节基因的表达[29]。
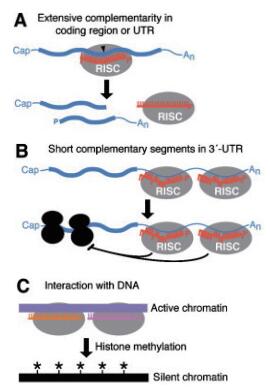
1.2 植物miRNA的作用机制植物miRNA与靶mRNA的作用机制一般有两种[30],采取何种作用机制主要取决于miRNA与其靶mRNA的序列互补程度[31]。若miRNA与其靶mRNA的序列互补程度较高,则采取切割模式,降解掉靶mRNA(图 2-A);若miRNA与其靶mRNA的序列互补程度较低,则采取抑制其靶mRNA翻译的模式(图 2-B)[32]。在切割的过程中,与miRNA配对的靶mRNA上第10-11位的核苷酸上的开放阅读框(ORF)通常作为切割位点[33]。而在抑制翻译的过程中,miRNA的靶mRNA上的3' 末端的UTR区作为主要作用位点,通过降解新合成的多肽来抑制翻译[34]。剪切和抑制过程大多是协同进行。除此之外,miRNA也可能靶向靶DNA,在转录水平上沉默基因的表达(图 2-C)[30, 35]。详细过程可参考图 2。
1.3 植物miRNA的功能植物miRNA可参与调控植物生长发育的多个过程。若参与miRNA合成过程中的DCL1基因功能丢失,将会导致植物发育异常,这些异常涉及早期胚胎发育的停滞、叶形状的改变、花期的延迟、雌性不育等[36-37]。miRNA可以控制顶端分生组织的发育,若miR164异常表达,则会导致根、茎的发育异常[38]。除此之外,miRNA还可以通过参与信号转导的调控过程来调节植物的发育,如miR164通过调控生长因素响应因子(ARF)(包括ARF2、ARF3、ARF4、ARF10、ARF16和ARF17),调控植物的发育[39]。
此外,miRNA在植物应对外界胁迫中发挥着重要的作用[40]。常见的胁迫包括以病虫害为主的生物胁迫和以温度、水分、氧化等为主的非生物胁迫。研究发现,在植物抵御生物胁迫过程中,miRNA能够通过调控靶基因等来抵御病菌的入侵。例如,植物可通过诱导产生对生长素受体基因(TIR1、AFB2和AFB3)进行负调控的miRNA,抑制生长素信号转导过程,限制假单胞杆菌(Pseudomonas syringae)生长,从而提高植物对假单胞菌的抗性[41];植物也可通过低表达miR398b,降低对其靶基因CSD1和Nod19的抑制,对病害感染做出响应[42]。在植物抵御非生物胁迫过程中,研究发现拟南芥在氧化胁迫下miR398的表达量降低,导致CSD1和CSD2表达量上调,从而减少氧化胁迫的危害[43];拟南芥在低氮条件下会使miR167a的表达量上升,抑制ARF8的表达[44];在缺磷条件下,拟南芥中的miR399表达量增加,具有在茎叶中积累磷功能的靶基因pho2和UBC24的表达受到抑制,拟南芥通过加速磷转运子基因的表达以及通过自身根系结构的改变来增加对磷的吸收[45-46];通过研究拟南芥中的miR395发现,在低硫胁迫下其表达量增加,APS1的转录水平下降,正常硫水平条件下,miR395的表达量下调,APS1的转录水平上升。可见,miR395参与了低硫胁迫的响应过程[47];干旱是植物生长发育过程中的常见逆境因子,水稻在干旱条件下诱导miR169的表达,抑制miR168、miR528、miR167的表达,其靶基因MAPK、POD、PLD表达量提高,启动ABA(脱落酸)诱导的气孔运动和氧化防御机制来抵御干旱胁迫[48]。低温是限制植物生长和分布的一种常见非生物因素,研究证实低温胁迫下,拟南芥或水稻中miR393、miR397、miR172、miR171、miR169、miR408发生差异表达[49-50]。盐胁迫是植物生长发育的重要限制因子[2],越来越多的研究表明miRNA在植物抵御盐胁迫过程中发挥着重要作用[12, 27],响应盐胁迫的miRNA及其作用机制将在下文详细阐述。
2 miRNA在植物抵御盐胁迫中的作用机理目前已发现许多响应盐胁迫的植物miRNA。盐胁迫会启动植物miRNA所参与的调控过程,不同种类的植物miRNA分别作用其靶基因,通过调控植物的种子萌发、改变植株根系生长、调控生长周期、器官形成、离子区域化、活性氧清除等过程来抵御盐胁迫的危害(图 3)。
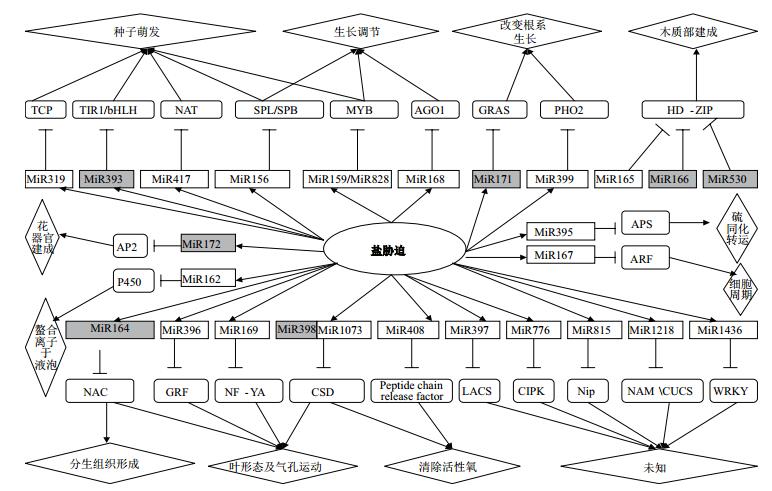
|
| 图 3 miRNA在植物抵御盐胁迫过程中发挥的作用 盐胁迫下表达量下调的植物microRNA标为灰色,上调的为无色 |
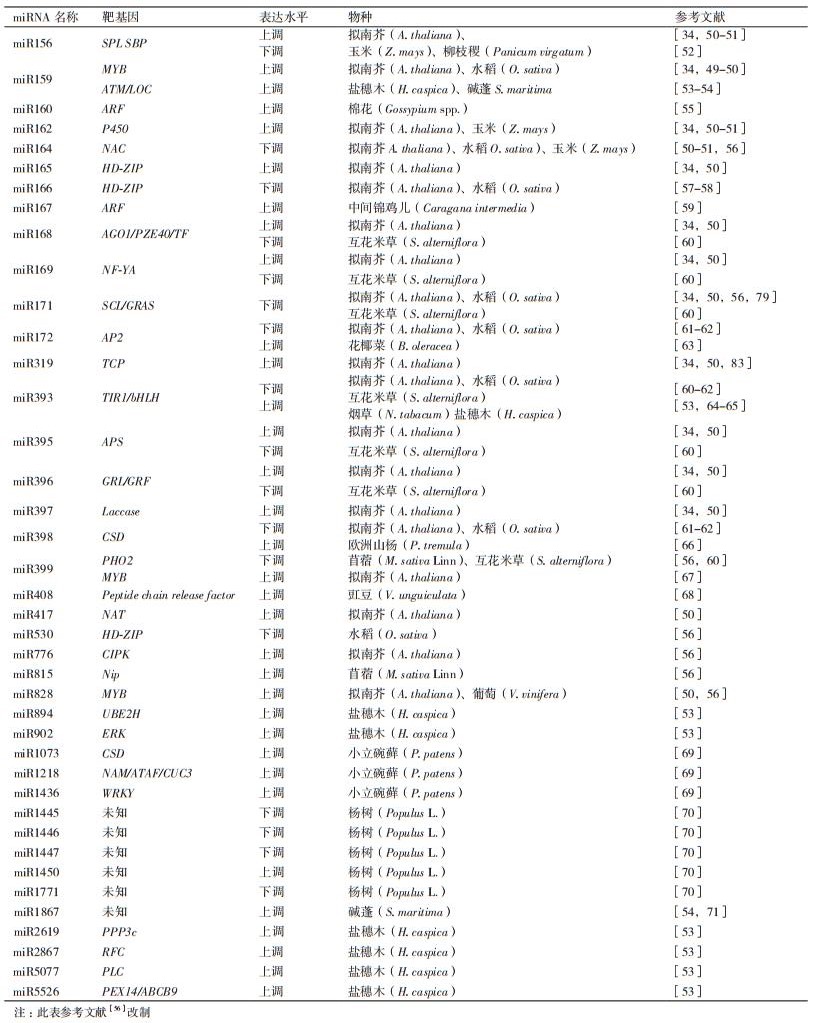
在盐胁迫条件下,miRNA发生响应(表 1)。Liu[50]和田鑫等[72]研究发现,在盐胁迫条件下,拟南芥中的miR156、miR159、miR319、miR393、miR417、miR828等表达量呈现出上升的趋势。此外,过表达miR156的苜蓿种子萌发率较高[73]。Ding[51]和Xue等[74]发现,盐胁迫下玉米中miR159的表达量上调,miR159通过与其靶基因MYB的共同作用,影响ABA信号调节过程,进而提高种子萌发率。miR393过表达的水稻和拟南芥植株耐盐碱能力增强,miR393的表达受CK(细胞分裂素)调节,miR393通过其靶基因TIR1参与耐盐相关基因P5CDH和SRO5的表达来调控种子萌发过程中的激素含量,从而提高盐碱条件下种子萌发能力[75]。Jung等[76]在转基因拟南芥中发现,盐胁迫条件下,miR417过表达的拟南芥种子萌发率较低,并且萌发以后的幼苗生长受到抑制。除此之外,Yang等[53]在研究盐生植物盐穗木响应盐胁迫的过程中发现,miR159、miR894、miR393、miR167、miR5077、miR2619、miR902、miR2867、miR5526均发生响应,其中miR5526上调的同时,启动其靶基因PEX14参与的过氧化物酶体调控过程,参与种子萌发脂肪酸的β-氧化,进入三羧酸循环,调控盐胁迫下盐穗木的种子萌发过程。
2.2 响应盐胁迫的植物miRNA与形态构建Guo等[77]和Raman等[78]发现盐胁迫条件下,miR164与NAC共同作用形成更多的侧根以维持盐胁迫下植株正常的水分和营养需求;Ma等[79]发现,在盐胁迫下,拟南芥中的miR171作用于靶基因GRAS,通过改变植株根系情况来抵御盐胁迫;盐胁迫条件下,miR399发生响应,PHO2是miR399的靶基因,植株在PHO2的作用下延长主根,通过延长主根、形成较多的侧根或分生组织以维持植株正常的水分需求[80]。miR165和miR166主要参与调控分生组织的形成[81],在盐胁迫下,拟南芥中的miR165、miR166、miR530的表达量呈上升趋势,其转录因子HD-ZIP介导的细胞信号转导途径具有调控植物木质部的形态建成的作用[82]。在拟南芥中,miR396通过其靶基因GRF来调控拟南芥叶片的宽窄,进而提高其抗盐碱和抗干旱能力[83]。研究发现,干旱和盐胁迫可以诱导水稻中miR169的表达,NF-YA转录因子为miR169的靶基因,过表达miR169使得拟南芥抵御干旱和高盐胁迫的能力增强;在玉米中,miR169c的靶基因可以控制气孔开闭,从而减少水分流失,以抵抗干旱和盐胁迫[84-85]。Jung等[86]发现,在盐胁迫下,拟南芥中的miR172表达量上升,植株耐盐能力增强,miR172通过调节AP2类转录因子控制植物开花时间,影响花器官发育和花形态建成。
2.3 响应盐胁迫的植物miRNA与活性氧清除在水稻、拟南芥和苜蓿中发现,miR398作用于CSD基因[56, 66]。盐胁迫条件下,miR398通过靶向于细胞质的CSD1和质体的CSD2,清除活性氧,从而保护细胞膜结构以抵抗盐胁迫[43]。除此之外,Higashi等[69]在小立碗藓中发现miR1073同样作用于Cu-Zn-CSD,清除盐胁迫条件下小立碗藓中活性氧的累积,保护细胞膜结构,提高植株耐盐能力。研究人员在豇豆中发现了与盐胁迫相关的miR408,目前确定其靶基因为peptide chain release factor,推测与活性氧的清除有关,但作用机制仍待进一步研究[68]。
2.4 其他除了以上几种调控类型外,植物在抵御盐胁迫过程中发生响应的miRNA还有其他的调控过程。miR167作用于ARF,参与信号调节过程,以应对盐胁迫[59],miR395与植物耐盐有关,目前仅确定其靶基因为APS1,关于miR395的研究主要集中在植物在硫盐胁迫下的调控过程,发现植株受低硫胁迫时,miR395的表达量上调,导致APS1的转录水平下降,若正常硫水平条件下,miR395表达量较低,通过调控APS1的转录水平来抵御硫盐胁迫[47]。miR162通过螯合金属离子于液泡中抵御硫盐胁迫,但在盐胁迫条件下,miR162也发生响应,其作用机制仍待进一步研究[56]。另外,在盐生植物盐穗木响应盐胁迫过程中,miR159与其靶基因ATM参与盐胁迫下的细胞凋亡调控;miR894与其靶基因UBE2H参与胁迫下蛋白质水解,miR2867在靶基因RFC的作用下参与胁迫下的DNA修复过程,miR5077、miR2619在其靶基因PLC、PPP3C的作用下分别参与Ca2+ 信号通路和MAPK的信号通路调节过程[53]。除此之外,miR776、miR815、miR1218、miR1436、miR1445、miR1446、miR1447、miR1450、miR1771等这些新发现响应盐胁迫的植物miRNA的作用机制仍待进一步研究[66, 70]。
3 展望与盐胁迫下种子萌发相关的miRNA可通过影响种子渗透势、调节激素含量、诱导各类信号过程等来提高种子萌发率。种子是植物生活史中抗性最强的阶段,但种子萌发是植物生长发育过程中最为脆弱的阶段[6]。因此,种子萌发过程中的miRNA作用机制应深入研究,同时植物形态发育、器官形成等过程也应被重视。
目前关于植物耐盐miRNA的研究大多集中在拟南芥和水稻等模式植物上,但在盐胁迫条件下,同种miRNA会因物种不同其表达量有差异,发挥的作用也有所不同,如Jia等[66]发现,在盐胁迫条件下,欧洲山杨中miR398的含量呈上升下降再上升的变化趋势,而拟南芥中miR398的含量呈持续下降趋势。开展盐胁迫条件下更多植物物种miRNA表达谱研究和综合分析,探索抵御盐胁迫过程中植物miRNA的保守性和物种特异性,对于全面了解miRNA在植物抵御盐胁迫过程中的作用机制,筛选植物耐盐相关miRNA以及开展植物耐盐分子育种尤为重要。
在盐生植物盐穗木、碱蓬等植物中新发现的响应盐胁迫的miRNA的作用方式更为多样,因此有必要进行深入研究,以便于筛选鉴定新的耐盐关键基因,揭示盐生植物的耐盐分子机制,为耐盐碱植物育种提供新的基因资源,奠定更为完善的理论基础。
新发现的与耐盐相关的miR1445、miR1446、miR1447、miR1450和miR1771[70]等是如何发挥耐盐机制的,这些miRNA都参与哪些耐盐性调节过程,作用于哪些基因,受到哪些因素影响等,这些都是有待于继续进行系统深入研究的问题。只有全面深入的了解miRNA在植株抵御盐胁迫过程中发挥的作用,才有可能更加高效的利用植物miRNA提高植物耐盐性,从而降低盐胁迫对农林业生产造成的危害。
| [1] |
王遵亲, 祝寿泉, 俞仁培, 等. 中国盐渍土[M]. 北京: 科学出版社, 1993.
|
| [2] |
Zhang JL, Shi H. Physiological and molecular mechanisms of plant salt tolerance[J]. Photosynth Res, 2013, 115(1): 1-22. DOI:10.1007/s11120-013-9813-6 |
| [3] |
Zhu JK. Abiotic stress signaling and response in plants[J]. Cell, 2016, 167(2): 313-324. DOI:10.1016/j.cell.2016.08.029 |
| [4] |
张娟, 姜闯道, 平吉成. 盐胁迫对植物光合作用影响的研究进展[J]. 农业科学研究, 2008, 29(3): 74-80. |
| [5] |
韩志平, 张海霞, 周凤. 盐胁迫对植物的影响及植物对盐胁迫的适应性[J]. 山西大同大学学报:自然科学版, 2015, 31(3): 59-64. |
| [6] |
Moez H, Chantal E, Mariama N, et al. Insights on plant salt tolerance mechanisms and their potential use for breeding[J]. Front Plant Sci, 2016, 29(7): 1787-1789. |
| [7] |
Niramaya S, Muchate Ganesh C, Nikalje Nilima S, et al. Plant salt stress:adaptive responses, tolerance mechanism and bioengineering for salt tolerance[J]. Botanical Review, 2016, 82(4): 371-406. DOI:10.1007/s12229-016-9173-y |
| [8] |
Dietz KJ, Tavakoli N, Kluge C, et al. Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level[J]. Exp Bot, 2001, 52(363): 1969-1980. DOI:10.1093/jexbot/52.363.1969 |
| [9] |
张楠楠, 徐香玲. 植物抗盐机理的研究[J]. 哈尔滨师范大学:自然科学学报, 2005, 21: 65-68. |
| [10] |
Ulrich D, Aaron B, Julian I. Plant salt-tolerance mechanisms[J]. Science Direct, 2014, 19(6): 371-379. |
| [11] |
Jia HS, Lu CM. Effects of abscisic acid on photoinhibition in maize plants[J]. Plant Sci, 2003, 165(6): 1403-1410. DOI:10.1016/j.plantsci.2003.08.004 |
| [12] |
代邹, 孙永健, 徐徽, 等. 多胺对盐胁迫下植物的作用[J]. 农业科学与技术, 2014, 3: 344-351. |
| [13] |
Zhu JK. Genetic analysis of plant salt tolerance using Arabidopsi[J]. Plant Physion, 2000, 124: 941-948. DOI:10.1104/pp.124.3.941 |
| [14] |
景昕, 张旸, 李玉花. 植物耐盐研究进展[J]. 生物技术通讯, 2010, 21(2): 290-294. |
| [15] |
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14[J]. Cell, 1993, 75(5): 843-854. DOI:10.1016/0092-8674(93)90529-Y |
| [16] |
Sunkar R, Girke T, Zhu JK. Cloning and characterization of microRNAs from rice[J]. Plant Cell, 2005, 17(5): 1397-1411. DOI:10.1105/tpc.105.031682 |
| [17] |
Ambros V. microRNAs:tiny regulators with great potential[J]. Cell, 2001, 107: 823-826. DOI:10.1016/S0092-8674(01)00616-X |
| [18] |
Dolata J, Bajczyk M, Bielewicz D, et al. Salt stress reveals a new role for ARGONAUTE1 in miRNA biogenesis at the transcriptional and posttranscriptional levels[J]. Plant Physiol, 2016, 172(1): 297-312. DOI:10.1104/pp.16.00830 |
| [19] |
Zhang B, Pan X, Cobb GP, et al. Plant microRNA:a small regul-atory molecule with big impact[J]. Dev Biol, 2006, 289: 3-16. DOI:10.1016/j.ydbio.2005.10.036 |
| [20] |
Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase Ⅱ[J]. The EMBO Journal, 2004, 23(20): 4051-4060. DOI:10.1038/sj.emboj.7600385 |
| [21] |
Wu G. Plant MicroRNAs and development[J]. J Genet Genomics, 2013, 40: 217-230. DOI:10.1016/j.jgg.2013.04.002 |
| [22] |
Millar AA, Waterhouse PM. Plant and animal microRNAs:similarities and differences[J]. Funct Integr Genomics, 2005, 5(3): 129-135. DOI:10.1007/s10142-005-0145-2 |
| [23] |
Llave C, Xie Z, Kasschau KD, et al. Cleavage of Scarecrow like miRNA targets directed by a class of Arabidopsis miRNA[J]. Science, 2002, 297(5589): 2053-2056. DOI:10.1126/science.1076311 |
| [24] |
Reinhart BJ, Weinstein EG, Rhoades MW, et al. MicroRNAs in plants[J]. Genes Dev, 2002, 16(13): 1616-1626. DOI:10.1101/gad.1004402 |
| [25] |
Rhoades MW, Reinhart BJ, Lim LP, et al. Prediction of plant microRNA targets[J]. Cell, 2002, 110(4): 513-520. DOI:10.1016/S0092-8674(02)00863-2 |
| [26] |
Fujioka Y, Utsumi M, Ohba Y, et al. Location of a possible miRNA processing site in SmD3/SmB nuclear bodies in Arabidopsis[J]. Plant and Cell Physiol, 2007, 48(9): 1243-1253. DOI:10.1093/pcp/pcm099 |
| [27] |
Alejandra C, Jose LR. Post transcriptional gene regulation of salinity and drought responses by plant microRNAs[J]. Plant Cell & Environment, 2009, 339(4): 381-389. |
| [28] |
Olivier V. Origin, biogenesis, and activity of Plant MicroRNAs[J]. Cell, 2009, 136(4): 669-687. DOI:10.1016/j.cell.2009.01.046 |
| [29] |
Kong W, Zhao JJ, He LL, et al. Strategies for profiling microRNA expression[J]. Cell Physiol, 2009, 218(1): 22-25. DOI:10.1002/jcp.v218:1 |
| [30] |
Bartel DP. MicroRNAs:Genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281-297. DOI:10.1016/S0092-8674(04)00045-5 |
| [31] |
Tang GL, Reinhart BJ, Zamore PD. A biochemical framework for RNA silencing in plants[J]. Genes Dev, 2003, 17: 49-63. DOI:10.1101/gad.1048103 |
| [32] |
Bartel B, Bartel DP. MicroRNAs:at the root of plant development[J]. Plant Physiol, 2003, 132(2): 709-717. DOI:10.1104/pp.103.023630 |
| [33] |
Llave C, Xie ZX, Carrington JC. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA[J]. Science, 2002, 297(5589): 2053-2056. DOI:10.1126/science.1076311 |
| [34] |
Sunkar R, Zhu JK. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis[J]. Plant Cell, 2004, 16(8): 2001-2019. DOI:10.1105/tpc.104.022830 |
| [35] |
Bao N, Lye KW, Barton MK. MicroRNA binding sites in Arabido-psis class Ⅲ HD-ZIP mRNAs are required for methylation of the template chromosome[J]. Dev Cell, 2004, 7(5): 653-662. DOI:10.1016/j.devcel.2004.10.003 |
| [36] |
Liu B, Cao S. Loss of function OsDCL1 affects microRNAs accumulation and causes developmental defects in rice[J]. Plant Physiol, 2005, 139: 296-305. DOI:10.1104/pp.105.063420 |
| [37] |
Park W, Chen X. Carpel F. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana[J]. Current Bio, 2002, 12: 84-85. |
| [38] |
Lu X, Dun H, Lian C, et al. The role of peu-miR164 and its target PeNAC genes in response to abiotic stress in Populus euphratica[J]. Plant Physiol Biochem, 2017, 115: 418-438. DOI:10.1016/j.plaphy.2017.04.009 |
| [39] |
Laufs P, eaucelle A, Traas J. MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristem[J]. Development, 2004, 1: 4311-4322. |
| [40] |
Zhu JK. Plant salt tolerance[J]. Trends in Plant Sci, 2001, 6(2): 66-71. DOI:10.1016/S1360-1385(00)01838-0 |
| [41] |
Navarro L, Dunoyer P, Jay F, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling[J]. Science, 2006, 312(5772): 436-439. DOI:10.1126/science.1126088 |
| [42] |
Naya L, Paul S, Valdes-Lopez O, et al. Regulation of copper homeostasis and biotic interactions by microRNA 398b in common bean[J]. PLoS One, 2014, 9(1): e84416. DOI:10.1371/journal.pone.0084416 |
| [43] |
Sunkar R, Kapoor A, Zhu JK. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance[J]. Plant Cell, 2006, 18: 2051-2065. DOI:10.1105/tpc.106.041673 |
| [44] |
Gifford ML, Dean A, Gutierrez RA, et al. Cell-specific nitrogen responses mediate developmental plasticity[J]. Proc Natl Acad Sci USA, 2008, 105(2): 803-808. DOI:10.1073/pnas.0709559105 |
| [45] |
Fujii H, Chiou TJ, Lin SI, et al. A miRNA involved in phosphate-starvation response in Arabidopsis[J]. Curr Biol, 2005, 15(22): 2038-2043. DOI:10.1016/j.cub.2005.10.016 |
| [46] |
Chiou TJ, Aung K, Lin SI, et al. Regulation of phosphate homeostasis by microRNA in Arabidopsis[J]. Plant Cell, 2006, 18(2): 412-421. DOI:10.1105/tpc.105.038943 |
| [47] |
Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress induced miRNA[J]. Mol Cell, 2004, 14(6): 787-799. DOI:10.1016/j.molcel.2004.05.027 |
| [48] |
Zhao BT, Liang RQ, Ge LF, et al. Identification of drought-induced microRNAs in rice[J]. Biochem Biophys Res Commun, 2007, 354(2): 585-590. DOI:10.1016/j.bbrc.2007.01.022 |
| [49] |
Wang ST, Sun XL, Yoichiro H, et al. MicroRNA319 positively regulates cold tolerance by targeting OsPCF6 and OsTCP21 in rice(Oryza sativa L.)[J]. PLoS One, 2014, 9(3): e91357. DOI:10.1371/journal.pone.0091357 |
| [50] |
Liu HH, Tian X, Li YJ. Microarray based analysis of stress-regulated microRNAs in Arabidopsis thaliana[J]. RNA, 2008, 14(5): 836-843. DOI:10.1261/rna.895308 |
| [51] |
Ding D, Zhang LF, Wang H, et al. Differential expression of miRNAs in response to salt stress in maize roots[J]. Annals of Bot, 2009, 103(1): 29-38. DOI:10.1093/aob/mcn205 |
| [52] |
Xie FL, JR Stewart CN, Taki FA, et al. High-throughput deep sequencing shows that microRNAs play important roles in switch grass responses to drought and salinity stress[J]. Plant Biotechnol J, 2014, 12(3): 354-366. DOI:10.1111/pbi.2014.12.issue-3 |
| [53] |
Yang R, Zeng Y, Yi X, et al. reveals the important role of microRNAs in the halophyte Halostachys caspica[J]. Plant Biotechnol J, 2015, 13(3): 395-408. DOI:10.1111/pbi.12337 |
| [54] |
Sachin AG, Birendra Prasad S. Novel and conserved miRNAs in the halophyte Suaeda maritima identified by deep sequencing and computational predictions using the ESTs of two mangrove plants[J]. BMC Plant Biol, 2015, 15(1): 1-18. DOI:10.1186/s12870-014-0410-4 |
| [55] |
Xie FL, Wang QL, Sun RR, et al. Deep sequencing reveals important roles of microRNAs in response to drought and salinity stress in cotton[J]. J Exp Bot, 2015, 66(3): 789-804. DOI:10.1093/jxb/eru437 |
| [56] |
马风勇, 朱永兴, 石晓霞, 等. 植物microRNA抗逆性研究进展[J]. 西北农林科技大学学报:自然科学版, 2012, 40(5): 218-222. |
| [57] |
Zhang BH, Pan XP. Identification and characteristic of new plant microRNAs using EST analysis[J]. Cell Eesearch, 2005, 15: 336-360. |
| [58] |
Singh A, Roy S, Singh S, et al. Phytohormonal crosstalk modulates the expression of miR166/165s, target Class Ⅲ HD-ZIPs, and KANADI genes during root growth in Arabidopsis thaliana[J]. Sci Rep, 2017, 7(1): 3408-3412. DOI:10.1038/s41598-017-03632-w |
| [59] |
Zhu JF, Li WF, Yang WH, et al. Identification of microRNAs in Caragana intermedia by high-throughput sequencing and expression analysis of 12 microRNAs and their targets under salt stress[J]. Plant Cell Rep, 2013, 32(9): 1339-1349. DOI:10.1007/s00299-013-1446-x |
| [60] |
Qin Z, Chen J, Jin L, et al. Differential expression of miRNAs under salt stress in Spartina alterniflora leaf tissues[J]. J Nanosci Nanotechnol, 2015, 15(2): 1554-1561. DOI:10.1166/jnn.2015.9004 |
| [61] |
Shen JQ, Xie KB, Xiong LZ. Global expression profiling of rice microRNAs by one-tube stem-loop reverse transcription quantitative PCR revealed important roles of microRNAs in abiotic stress response[J]. Mol Genet Genomics, 2010, 10: 3-34. |
| [62] |
Jian X, Zhang L, Li G, et al. Identification of novel stress-regulated microRNAs from Oryza sativa L[J]. Genomics, 2010, 95: 47-55. DOI:10.1016/j.ygeno.2009.08.017 |
| [63] |
Tian YH, Tian YM, Luo XJ, et al. Identification and characterization of microRNAs related to salt stress in broccoli, using high-throughput sequencing and bioinformatics analysis[J]. BMC Plant Bio, 2014, 14: 226. DOI:10.1186/s12870-014-0226-2 |
| [64] |
Frazier TP, Sun G, Burklew CE, et al. Salt and drought stresses induce the aberrant expression of microRNA genes in Tobacco[J]. Mol Bio, 2011, 49(2): 159-165. DOI:10.1007/s12033-011-9387-5 |
| [65] |
Kazan K. Auxin and the integration of environmental signals into plant root development[J]. Annals of Bot, 2013, 112(9): 1655-1665. DOI:10.1093/aob/mct229 |
| [66] |
Jia XY, Wang WX, Ren LG, et al. Differential and dynamic regulation of miR398 in response to ABA and salt stress in Populus tremula and Arabidopsis thaliana[J]. Plant Mol Bio, 2009, 71(1/2): 51-59. |
| [67] |
Dongwon B, Hyun JC, Songhwa K. A role for Arabidopsis miR399f in salt, drought, and ABA signaling[J]. Mol Cells, 2016, 39(2): 111-118. DOI:10.14348/molcells.2016.2188 |
| [68] |
Paul S, Kundu A, Pal A. Identification and validation of conserved microRNAs along with their differential expression in roots of Vigna unguiculata grown under salt stress[J]. Plant Cell Tissue and Organ Culture, 2011, 105(2): 233-242. DOI:10.1007/s11240-010-9857-7 |
| [69] |
Higashi Y, Takechi K, Takano H, et al. Involvement of microRNA in copper deficiency-induced repression of chloroplastic Cu-Zn-superoxide dismutase genes in the moss Physcomitrella patens[J]. Plant Cell Physiol, 2013, 54(8): 1345-1355. DOI:10.1093/pcp/pct084 |
| [70] |
Lu S, Sun YH, Chiang VL. Stress responsive microRNAs in Populous[J]. Plant, 2008, 55(1): 131-151. |
| [71] |
Gharat SA, Shaw BP. Computational prediction and experimental validation of a novel miRNA in Suaeda maritima, a halophyte[J]. Genetics & Mole Res GMR, 2016, 15(1): 1-12. |
| [72] |
田鑫. 拟南芥胁迫诱导microRNA的分离和功能研究及microRNA启动子分析[D]. 泰安: 山东农业大学, 2009.
|
| [73] |
Muhammad A, Margaret YG, Ken W, et al. An insight into microRNA-156 role in salinity stress responses of Alfalfa[J]. Front Plant Sci, 2017, 8: 356-359. |
| [74] |
Xue T, Liu Z, Dai X, et al. Primary root growth in Arabidopsis thaliana is inhibited by the miR159 mediated repression of MYB33, MYB65 and MYB101[J]. Plant Sci, 2017, 262: 182-189. DOI:10.1016/j.plantsci.2017.06.008 |
| [75] |
Gao P, Bai X, Yang L, et al. Osa-miR393:A salinity and alkaline stress-related microRNA gene[J]. Mol Biol Rep, 2010, 12: 345-363. |
| [76] |
Jung HJ, Kang H. Expression and functional analyses of micro-RNA417 in Arabidopsis thaliana under stress conditions[J]. Plant Physiol Biochem, 2007, 45: 805-811. DOI:10.1016/j.plaphy.2007.07.015 |
| [77] |
Guo HS, Xie Q, Fei JF, et al. MicroRNA directs mRNA cleavage of the transcription factor NaCl to down regulate auxin signals for Arabidopsis lateral root development[J]. Plant Cell, 2005, 17: 1376-1386. DOI:10.1105/tpc.105.030841 |
| [78] |
Raman S, Greb T, Peaucelle A, et al. Interplay of miR164, CUP-SHAPED COTYLEDON genes and LATERAL SUPPRESSOR controls axillary meristem formation in Arabidopsis thaliana[J]. Plant, 2008, 55: 65-76. |
| [79] |
Ma HS, Liang D, Shuai P, et al. The salt and drought inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana[J]. J Exp Bot, 2010, 61(14): 4011-4019. DOI:10.1093/jxb/erq217 |
| [80] |
Liu TY, Huang TK, Tseng CY, et al. PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis[J]. Plant Cell, 2012, 24: 2168-2183. DOI:10.1105/tpc.112.096636 |
| [81] |
Carlsbecker A, Lee JY, Roberts CJ, et al. Cell signal ling by microRNA165/6 directs gene does dependent root cell fate[J]. Nature, 2010, 465: 316-321. DOI:10.1038/nature08977 |
| [82] |
Sakaguchi J, Watanabe Y. miR165/166 and the development of land plants[J]. Dev Growth Differ, 2012, 54: 93-99. DOI:10.1111/dgd.2012.54.issue-1 |
| [83] |
Debernardi JM, Mecchia MA, Vercruyssen L, et al. Post-transcriptional control of GRF transcription factors by microRNA miR396 and GIF co-activator affects leaf size and longevity[J]. Plant J, 2014, 79(3): 419-426. |
| [84] |
Zhao B, Ge L, Liang R, et al. Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor[J]. BMC Mol Biol, 2009, 10: 29-38. DOI:10.1186/1471-2199-10-29 |
| [85] |
Luan M, Xu M, Lu Y, et al. Expression of zma-miR169 miRNAs and their target ZmNF-YA genes in response to abiotic stress in maize leaves[J]. Gene, 2015, 55: 178-185. |
| [86] |
Jung JH, Seo YH, Seo PJ, et al. The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis[J]. Plant Cell, 2007, 19: 2736-2748. DOI:10.1105/tpc.107.054528 |