2. 宁波大学科学技术学院,宁波 315211
2. College of Science and Technology, Ningbo University, Ningbo 315211
核苷二磷酸激酶是一类高度保守的基因,其编码的NDPK在生物体内普遍存在。NDPK的大小一般在70-100 kD,在真核生物中几乎都是由两个六聚体组成的复合物,其单体是6个α-螺旋围绕着一个四股反向平行的β-折叠,只在一小部分的原核生物中以四聚体形式存在。NDPK最早于1953年在酵母和鸽子的组织中发现[1]。在植物中,第一个NDPK于1971年在豌豆中得到分离纯化[2],并于1991年公布了NDPK基因序列。在此后的研究中,从其他植物中发现了NDPK的广泛存在。
NDPK作为一个激酶,具有催化底物磷酸化的作用。NDPK通过一个兵乓机制首先将核苷三磷酸(Nucleoside triphosphate,NTP)上γ位的高能磷酸集团转移到其自身,再将高能磷酸集团转移到不同的核苷二磷酸(Nucleoside diphosphate,NDP)上,形成一个新的NTP,实现核苷二磷酸转化成为核苷三磷酸的过程,从而维持细胞体内核苷酸代谢的平衡。
NDPK还可以进行自身磷酸化,以达到变构蛋白结构,激活其酶活性的目的。大部分研究者认为,NDPK可能存在两个磷酸化位点:组氨酸磷酸化位点和丝氨酸磷酸化位点[3-5]。但也有研究者针对组氨酸残基和丝氨酸残基的自身磷酸化进行研究,发现组氨酸残基是NDPK唯一的自身磷酸化位点,实验中出现的丝氨酸/苏氨酸位点发生磷酸化是纯化该酶时化学处理所造成的实验误判[6]。
正因为NDPK的磷酸化及自身磷酸化作用,使得它与生物体的能量代谢、生物合成代谢息息相关。它为生物体提供能量代谢所需的ATP;半乳糖降解、乳糖合成、糖原合成所需的UTP;甘油磷脂合成、蛋白质糖基化所需的CTP;DNA合成、细胞信号转导、蛋白质延伸、细胞需氧呼吸中能量转换所需的GTP;为生物体核苷酸合成所需的NTPs。笔者对植物NDPK进行系统进化分析及功能进行综述,为今后植物核苷二磷酸激酶研究提供参考。
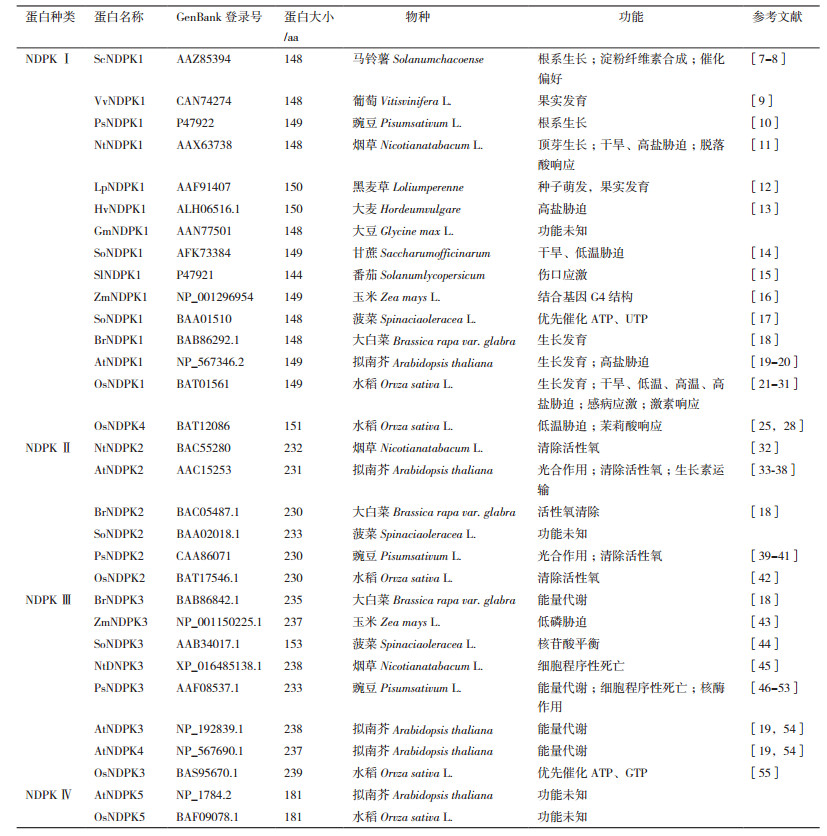
1 NDPK的系统进化分析本文整理了已发表的不同植物的31个NDPK蛋白质序列、功能等内容(表 1),用MEGA 6.06对这31个NDPK蛋白进行遗传分析,用邻近法(Neighbor-Joining,NJ)构建了分子系统进化树(图 1)。结果表明,来自不同物种的31个NDPK蛋白被分成4类。进化树上的聚类结果结合表 1中的功能,可以看到功能相近的NDPK亲缘关系较近,进化保守,聚为一簇。
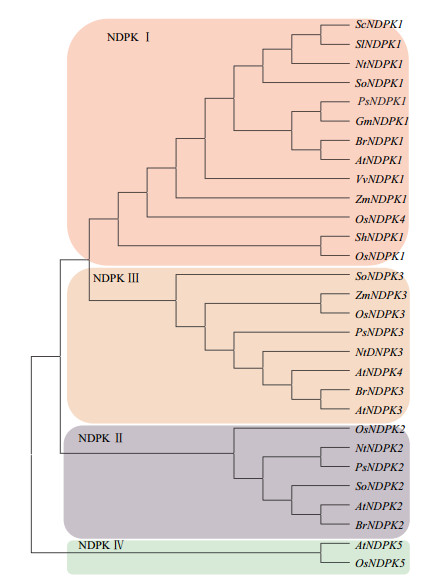
|
| 图 1 NDPK蛋白的系统进化树 |
植物NDPK Ⅰ是4种NDPK中含量最多[56]、活性最强的一个种类[57]。NDPK Ⅰ作为一个激酶,催化的底物存在多样性,既可以催化嘌呤也可以催化嘧啶,但其对底物存在偏好,会优先利用底物ATP[58]。但是有研究发现在其他的三磷酸中,它同样对底物存在偏好。马铃薯StNDPK1[7]、菠菜SoNDPK1[17]、杜氏盐藻DtNDPK1[59]均优先利用UTP进行反应。马铃薯StNDPK1还表现出对NTP/NDP比率的高度敏感,比率较小时,马铃薯StNDPK1酶活性受到抑制,当比率在2-3之间时,该酶活性最高[7]。
植物NDPK Ⅰ主要定位于细胞溶胶[7, 20, 59],在植物生长发育、非生物胁迫、感病应激、激素响应、DNA复制、淀粉纤维素合等方面起到重要作用。
2.1 参与生长发育在细胞分裂、分化快速的细胞中,NDPK Ⅰ提供植物细胞壁前体合成所需UTP,参与胚芽鞘、花、叶、幼苗、根尖、顶芽、果实等组织的生长。
NDPK Ⅰ对胚芽鞘的生长具有正向调节作用。过表达OsNDPK1的拟南芥表现出下胚轴长度增长、细胞数目增多[21];转入了反义OsNDPK1的水稻,胚芽鞘细胞的伸长受到抑制,植株表现出了生长缺陷,植株明显矮小[22]。
这种功能同样在分化活跃、分裂旺盛的组织中起到作用。拟南芥AtNDPK1在花序、叶片和根中表达量最高[19];烟草NtNDPK1在顶芽中表达量最高[11];大白菜BcNDPK1在幼苗、子叶和雌蕊中大量表达[18];葡萄VvNDPK1在果实发育早期大量表达[9];黑麦草LpNDPK1在种子萌发、颖果的发育时大量表达[12];过表达马铃薯StNDPK1的植株表现出根变长的性状[8];免疫印迹分析和免疫沉淀分析结果表明马铃薯StNDPK1大量存在于分生区和原形成层组织,如:根尖、叶片、块茎、雌蕊、愈伤组织[7]。此外还发现在马铃薯的愈伤组织中StNDPK1的表达不受细胞的年龄影响,而是与细胞的生长能力相关[7]。
2.2 参与非生物胁迫NDPK Ⅰ的表达还与非生物胁迫响应相关,在外界胁迫下,NDPK Ⅰ起到调节作用,但其具体响应机制尚未清晰。在干旱胁迫下,甘蔗幼苗中SoNDPK1从18 h起表达量急剧上升,并于30 h达到峰值,这表明甘蔗通过NDPK Ⅰ的表达做出对干旱胁迫的应答[14],同样表达上调也在烟草[11]和水稻叶[24, 31]中发现。在低温胁迫下,水稻叶片OsNDPK1表达上调、根部OsNDPK4的磷酸化水平提高[25]。在高温胁迫下,水稻颖果中OsNDPK1表达上调[26]。在热击胁迫下,甘蔗细胞培养物中SoNDPK1强烈表达[60]。在高盐胁迫下,豌豆的根部[10]、大麦的叶子[13]、水稻的根部[27]NDPK Ⅰ表达量上调,然而烟草幼苗中NtNDPK1在转录水平和翻译水平上均发生下调[11]。近期也有研究表明,在高盐胁迫下,拟南芥AtNDPK1的表达与病原体所诱导的促分裂蛋白激酶激酶(MAPKK)呈负相关[20]。这一实验结果表明AtNDPK1通过MAPK信号途径来进行对胁迫的调节。当受到机械损伤,番茄幼苗、茎、叶组织中SlNDPK1表达均发生上调[15]。
2.3 参与感病应激当外界病菌侵染时,NDPK Ⅰ参与植株对抗病原体防御机制。油菜根部感染根癌农杆菌24小时,NDPK Ⅰ含量增加了1.6倍[61],这一趋势同样发生在油菜感染黑斑菌时[62]。水稻感染白叶枯病菌或伯克霍尔德式菌后,OsNDPK1表达量显著上调[28]。过表达OsNDPK1的拟南芥植株相对于野生型,抗病基因PR1、NPR1表达量上调,表现出对灰葡萄孢真菌、丁香假单胞杆菌、番茄致病变种细菌的抗性增强。同时,BR信号转录调控因子Saur-AC1、转录因子BZR1、BES1表达量上升。这表明OsNDPK1通过正调控BR信号转导来增强植物抗病防御机制[21]。对细菌性条斑病菌JH01诱导的水稻抗病基因进行研究,发现OsNDPK4参与细菌性条斑病菌JH01诱导的水稻防卫反应[29]。
2.4 参与激素响应多种植物的NDPK Ⅰ可能参与激素调控机制或在激素信号途径中起到作用。经脱落酸处理后,烟草NtNDPK1表达量在1 h后到达顶峰,并维持了几小时[11]。水稻OsNDPK1受到水杨酸、茉莉酸、脱落酸、草甘膦的强烈诱导[13, 28, 30]。过表达OsNDPK1植株促进水杨酸积累基因PAD4和EDS1表达量上调,表现较高的水杨酸含量[21]。水稻OsNDPK4受茉莉酸诱导[28]。
2.5 参与DNA的复制玉米NDPK Ⅰ可以结合特殊结构的DNA,从而参与DNA的复制。DNA通常以双螺旋的结构存在,但在生物中DNA也以一些特殊的结构存在,如鸟苷酸-四联体结构(G-quadruplex DNA,G4 DNA)。G4 DNA是富含鸟苷酸(G)序列的四链形态的DNA二级结构,它在胞体内与其结合蛋白互相作用起到维持端粒稳定,参与DNA复制起始调控等作用。在植物中发现了第一个G4结合蛋白:ZmNDPK1。尽管在之前的研究中发现人类NM23-H2(NDPK同系物)也可以结合富含鸟嘌呤的序列[63],但是ZmNDPK1对于富含鸟嘌呤的序列结合较弱,具体的NDPK Ⅰ与G4结合的机制尚未清晰。实验仅表明ZmNDPK1除了含有一个酶活性位点,还含有一个G4结合位点,二者相互独立[16]。
2.6 参与淀粉与纤维素的合成马铃薯NDPK Ⅰ调节淀粉与纤维素的合成途径中碳元素的分布,从而参与淀粉和纤维素的合成。马铃薯StNDPK1正向调节纤维素的含量。在正义StNDPK1转基因植株中纤维素合成中间物尿苷二磷酸葡糖(Uridine diphosphate glucose,UDPG)和纤维素含量均增加[8]。StNDPK1反向调控淀粉的含量。淀粉合成关键酶ADP-蔗糖焦磷酸化酶在反义StNDPK1转基因植株中含量高;在正义StNDPK1转基因植株中含量少,同时伴随着酶的氧化程度高,表现出酶活降低[8]。因此,在反义转基因植株中积累了更多的淀粉。
3 NDPK Ⅱ功能植物NDPK Ⅱ主要定位于叶绿体中[64-65],在植物光合作用、清除活性氧和生长素调节机制这几个方面起到重要作用。
3.1 参与光合作用NDPK Ⅱ作为一个光调控蛋白激酶,通过与光敏色素相互作用,参与植物的光合作用。在20世纪90年代第一次发现NDPK活性与植物对红光[66]、蓝光[67]响应存在联系。随后的实验发现AtNDPK2基因与光敏色素A、光敏色素B互作,以响应植物对红光和远红光反应的应答[33, 68]。红光刺激黄化的豌豆PsNDPK2磷酸化水平上升[39]。燕麦的Ser598Ala突变体植株对光更敏感,研究者发现这是由于Ser598的磷酸化抑制了光信号传导途径中NDPK Ⅱ与光敏色素A的互作[69]。据此推断NDPK Ⅱ可能是植物光合作用的必要因素。在红光下,黄化燕麦中活化的光敏色素A与NDPK Ⅱ相互作用,刺激它的激酶活性,使自身磷酸化以及磷酸化作用增强[70],另一个实验表明黄化燕麦胚芽鞘中NDPK Ⅱ活性增加了约42%[57]。这表明NDPK Ⅱ是一个光调控蛋白激酶。拟南芥ndpk2突变体表现出对红光和远红光响应的部分缺失,子叶不能张开及绿化缺陷,这表明AtNDPK2是拟南芥光敏色素介导的光信号转导途径的正向调节因子[33-35]。
在黑暗中,豌豆幼苗PsNDPK2磷酸化受抑制,不再结合磷酸基团进行磷酸化[40]。将大白菜幼苗从黑暗环境转移到光照环境中,BcNDPK2表达急剧增加[18]。拟南芥AtNDPK2还与植物特有的Rho小G蛋白的ROP蛋白家族相互作用,可以激活鸟苷三磷酸酶(GTPase),有研究者认为AtNDPK2可能是光敏色素介导的信号与G蛋白介导信号中缺失的一环[36]。
3.2 参与清除活性氧光合作用是活性氧(Reactive oxygen species,ROS)和H2O2的一个重要来源,而定位在叶绿体上的NDPK Ⅱ参与MAPK级联反应,从而在清除活性氧中起到重要作用。NDPK Ⅱ与氧化胁迫呈正相关。经H2O2处理,水稻根部OsNDPK2表达上调[42]。转入AtNDPK2的苜蓿植株[71]、甘薯植株[72]、白杨植株[73]对氧化胁迫的耐受性增加。转入AtNDPK2的水稻植株,清除超氧化物和过氧化氢等活性氧自由基的基因OsAPX1,OsAPX2和OsSodB表达量增加[35],同时表现出对20% PEG的渗透压力、100 mmol/L NaCl的盐分胁迫、紫外线照射和臭氧处理有很高的耐受性[74]。转入AtAPX1的烟草BY-2细胞系,NtNDPK2与AtAPX1相互作用,调节ROS含量[32]。
研究者构建过氧化物酶SWPA2的启动子与AtNDPK2基因的表达载体转入甘薯中,发现该转基因植株对甲基紫精的耐受性增强,经甲基紫精(Methyl viologen,MV)处理,该转基因植株的3个抗氧化酶(过氧化氢酶、抗坏血酸盐酶、过氧化氢酶)活性均增加,表现出对低温、干旱胁迫耐受性增强[77]。这表明AtNDPK2可以有效地调节源自于环境压力所引起的过氧化物。同样的表达载体也转入到马铃薯中,转基因植株也出现同样的表型[76]。
通过酵母双杂交实验,发现AtNDPK2参与各种胁迫因子激活的信号途径,与SOS2蛋白激酶相互作用[38, 76]、诱导与压力信号转导途径相关的两个丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK):AtMPK3和AtMPK6的磷酸化[38]。有研究表明,它还与过氧化氢酶CAT2、CAT3相互作用[77],在豌豆[41]和拟南芥[37]中也发现NDPK可以直接与CAT亚型互作。过表达AtNDPK2的拟南芥植株,过氧化氢酶、过氧化物酶、硫氧还蛋白、硫氧还蛋白还原酶的表达均增加[78],表现出ROS、H2O2含量降低[38]。这表明NDPK Ⅱ通过调节细胞的氧化还原条件在ROS、H2O2介导的MAPK级联反应中起到作用[38]。
3.3 参与生长素传导NDPK Ⅱ还可能参与了生长素传导途径。拟南芥ndpk2突变表现出下胚轴的变短[33],对生长素极性运输抑制剂的更高敏感度,对生长素的运输增强,其中NDPK Ⅱ充当激素转运体的角色[34]。敲除AtNDPK2的植株表现出根及幼苗生长缓慢[38]。
4 NDPK Ⅲ功能植物NDPK Ⅲ主要定位于线粒体的膜间隙[47]、内膜[47]和叶绿体类囊体膜[79]中,主要参与能量代谢和其他核苷酸代谢酶间的互作、细胞的程序性死亡这几个方面起到重要作用。此外,在油菜中,它作为激酶还可以使自交不亲和关键因子SRK的激酶域磷酸化[81]。
4.1 参与能量代谢位于线粒体内膜的NDPK Ⅲ通过与腺嘌呤核苷酸转运体相互作用,参与细胞的能量代谢。Northern印迹分析表明:在豌豆的不同发育阶段,PsNDPK3表达量不同,与老叶相比在嫩叶中NDPK Ⅲ表达量更高[46],在生殖器官(花及豆荚)中表达比其在营养组织(根、叶)中高[47]。拟南芥快速分裂的花序和根部等对线粒体需求较大的组织中,AtNDPK3的表达量更高,拟南芥花发育后期的绒毡层、胚珠及花瓣中,AtNDPK4的转录水平增加[19]。在大白菜中,也发现此种现象[18]。这种结果可能是与线粒体呼吸活动的下降相关[19]。
免疫共沉淀实验发现豌豆线粒体膜上的PsNDPK3与线粒体内膜上腺嘌呤核苷酸转运体相互识别[48],以此来介导细胞质基质中ATP的合成和胞浆中ADP的交换。用呼吸代谢的产物蔗糖或葡萄糖处理拟南芥叶片后,AtNDPK3的表达上调,AtNDPK4转录水平略微受到的抑制[54]。有研究者提出AtNDPK4可能对AtNDPK3起到一个补充作用[19]。蔗糖诱导拟南芥基因AtWRKY4和AtWRKY34,在wrky4突变体中,葡萄糖诱导的AtNDPK3下调了了38%,而在wrky34突变体中,蔗糖处理后,AtNDPK3上调了31%[54]。
4.2 参与细胞程序性死亡位于线粒体膜间隙的NDPK Ⅲ通过与腺苷酸激酶(AK)相互作用,来参与细胞的程序性死亡[81]。热击处理烟草BY-2(Bright Yellow-2)细胞系,在细胞程序性死亡早期,烟草NtNDPK3活性受抑制,导致腺苷酸平衡的失调[45]。同样在豌豆中,也存在这一调节机制[49]。通过亲和层析纯化分离出维持豌豆细胞中腺苷酸的动态平衡的腺苷酸激酶,发现其与线粒体膜间隙中的PsNDPK3相互作用[50];重组酶的体外实验表明腺苷酸激酶刺激NDPK的活性,NDPK抑制腺苷酸激酶的活性[51]。同时cAMP以及Ca2+对二者均起到抑制作用[52]。
4.3 具有核酶活性豌豆NDPK Ⅲ可以作为核酶,裂解DNA。豌豆PsNDPK3可以裂解多种特殊结构的DNA,如超螺旋质粒DNA、高度结构化的RNA:tRNA、线粒体基因atp9 mRNA的3'-非翻译区(3'-UTR),这表明NDPK Ⅲ具有核酶活性,但是对核酸的结构有一定要求。ndpk3突变体(H117D,S69A)中PsNDPK3的核酶活性仍然存在,这表明NDPK Ⅲ的核酶活性和激酶活性相互独立,互不干扰[53]。
5 NDPK Ⅳ功能植物NDPK Ⅳ基因仅从拟南芥和水稻的基因组中发现。二者序列相似度达67%。均含有一个内质网保守信号HDEL序列,故预测其定位于内质网。关于其具体功能,尚未有研究。
6 结语NDPK除了作为激酶起到磷酸化及自身磷酸化作用维持植物体内核苷酸平衡外,NDPK Ⅰ主要参与植物生长发育、非生物胁迫、病菌应激、激素响应;NDPK Ⅱ主要参与光合作用、清除活性氧;NDPK Ⅲ主要参与能量代谢、细胞程序性死亡。对植物NDPK的研究已有多年,在大多数植物中均发现了NDPK的存在。尽管如此,目前的研究仍然集中在前3种NDPK,NDPK Ⅳ的研究目前仍停留在对其序列的生物信息学分析,对NDPK Ⅳ深入研究,可能发现NDPK的新功能。
NDPK除了起到以上的作用外,还有一些特殊的功能。例如,在玉米中发现可以结合DNA的G4结构NDPK Ⅰ;在马铃薯中发现参与淀粉和纤维素合成的NDPK Ⅰ;在拟南芥中发现参与生长素传导的NDPK Ⅱ;在豌豆中发现具有核酶活性的NDPK Ⅲ。此外,虽然有实验证明豌豆NDPK Ⅲ的核酶活性位点与其激酶活性位点相互独立,但其核酶活性位点尚未确定。NDPK在其他植物中是否也存在这些特殊的作用机制,需要进一步的研究。
值得注意的是,4种NDPK中NDPK Ⅰ在抵抗环境胁迫中起到重要作用、NDPK Ⅱ通过参与MAPK级联反应抵抗氧化胁迫,起到清除活性氧的功能。针对NDPK这类功能进行深入研究,有助于提高植物对抗环境胁迫,培育植物抗逆新品种。
| [1] | Krebs HA, Hems R. Some reactions of adenosine and inosine phosphates in animal tissues[J]. Biochim Biophys Acta, 1953, 12 (1/2): 172–180. |
| [2] | Edlund B. Purification of a nucleoside diphosphate kinase from pea seed and phosphorylation of the enzyme with adenosine(32 P)triphosphate[J]. Acta Chem Scand, 1971, 25 (4): 1370–1376. |
| [3] | Morera S, Chiadmi MG, Lascu I, et al. Mechanism of phosphate transfer by nucleoside diphosphate kinase:X-ray structures of the phosphohistidine intermediate of the enzymes from Drosophila and dictyostelium[J]. Biochemistry, 1995, 34 (35): 11062–11070. DOI:10.1021/bi00035a011 |
| [4] | Dorion S, Dumas F, Rivoal J. Autophosphorylation of Solanum chacoense cytosolic nucleoside diphosphate kinase on Ser117[J]. J Exp Bot, 2006, 57 (15): 4079–4088. DOI:10.1093/jxb/erl175 |
| [5] | Johansson M, Mackenziehose A, Andersson I, et al. Structure and mutational analysis of a plant mitochondrial nucleoside diphosphate kinase. Identification of residues involved in serine phosphorylation and oligomerization[J]. Plant Physiol, 2004, 136 (2): 3034–3042. DOI:10.1104/pp.104.044040 |
| [6] | Shen Y, Kim J, Song PS. Autophosphorylation of Arabidopsis nucleoside diphosphate kinase 2 occurs only on its active histidine residue[J]. Biochemistry, 2006, 45 (6): 1946–1949. DOI:10.1021/bi051868a |
| [7] | Dorion S, Rivoal J. Characterization of a cytosolic nucleoside diphosphate kinase associated with cell division and growth in potato[J]. Planta, 2006, 224 (1): 108–124. DOI:10.1007/s00425-005-0199-3 |
| [8] | Dorion S, Clendenning A, Rivoal J. Engineering the expression level of cytosolic nucleoside diphosphate kinase in transgenic Solanum tuberosum rootsb alters growth, respiration and carbon metabolism[J]. Plant J, 2017, 89 (5): 914–926. DOI:10.1111/tpj.2017.89.issue-5 |
| [9] | Martínez-Esteso MJ, Sellés-Marchart S, Lijavetzky D, et al. A DIGE-based quantitative proteomic analysis of grape berry flesh development and ripening reveals key events in sugar and organic acid metabolism[J]. J Exp Bot, 2011, 62 (8): 2521–2569. DOI:10.1093/jxb/erq434 |
| [10] | Kav NV, Srivastava S, Goonewardene L, et al. Proteome-level changes in the roots of Pisum sativum in response to salinity[J]. Ann Appl Biol, 2004, 145 (2): 217–230. DOI:10.1111/aab.2004.145.issue-2 |
| [11] | Zhou QY, Xie ZM, Zhang AG, et al. Cloning, expression and characterization of a nucleoside diphosphate kinase(NDPK)gene from tobacco[J]. Prog Nat Sci-Mater, 2007, 17 (8): 906–912. DOI:10.1080/10002007088537490 |
| [12] | Guo G, Lv D, Yan X, et al. Proteome characterization of developing grains in bread wheat cultivars(Triticum aestivum L.)[J]. BMC Plant Biol, 2012, 12 (1): 147–171. DOI:10.1186/1471-2229-12-147 |
| [13] | Fatehi F, Hosseinzadeh A, Alizadeh H, et al. The Proteome response of Hordeum spontaneum to salinity stress[J]. Cereal Res Commun, 2013, 41 (1): 78–87. DOI:10.1556/CRC.2012.0017 |
| [14] | 梁潘霞, 李杨瑞, 杨丽涛. 甘蔗核苷二磷酸激酶(NDPK1)基因克隆及表达分析[J]. 热带作物学报, 2012, 33(12): 2199–2205. DOI:10.3969/j.issn.1000-2561.2012.12.016 |
| [15] | Harris N, Taylor JE, Roberts JA. Isolation of a mRNA encoding a nucleoside diphosphate kinase from tomato that is up-regulated by wounding[J]. Plant Mol Biol, 1994, 25 (4): 739–742. DOI:10.1007/BF00029611 |
| [16] | Kopylov M, Bass HW, Stroupe ME. The maize(Zea mays L.)nucleoside diphosphate kinase1(ZmNDPK1)gene encodes a human NM23-H2 homologue that binds and stabilizes G-quadruplexDNA[J]. Biochemistry, 2015, 54 (9): 1743–1757. DOI:10.1021/bi501284g |
| [17] | Zhang J, Fukui T, Ichikawa A. A third type of nucleoside diphosphate kinase from spinach leaves:purification, characterization and amino-acid sequence[J]. Biochim Biophys Acta, 1995, 1248 (1): 19–26. DOI:10.1016/0167-4838(94)00222-3 |
| [18] | Shin DH, In JG, Lim YP, et al. Molecular cloning and characterization of nucleoside diphosphate(NDP)kinases from Chinese cabbage(Brassica campestris)[J]. Mol Cells, 2004, 17 (1): 86–94. |
| [19] | Hammargren J, Sundström J, Johansson M, et al. On the phylogeny, expression and targeting of plant nucleoside diphosphate kinases[J]. Physiol Plantarum, 2007, 129 (1): 79–89. DOI:10.1111/ppl.2007.129.issue-1 |
| [20] | Ovečka M, Takáč T, Komis G, et al. Salt-induced subcellular kinase relocation and seedling susceptibility caused by overexpression of Medicago SIMKK in Arabidopsis[J]. J Exp Bot, 2014, 65 (9): 2335–2350. DOI:10.1093/jxb/eru115 |
| [21] | 王凤茹, 董金皋, 司贺龙, 等. 水稻OsNDPK1基因在提高植物抗病性方面的应用: 中国, CN201210143843. 8[P]. 2013-11-13. |
| [22] | Pan L, Kawai M, Yano A, et al. Nucleoside diphosphate kinase required for coleoptile elongation in rice[J]. Plant Physiol, 2000, 122 (2): 447–452. DOI:10.1104/pp.122.2.447 |
| [23] | Yano A, Umeda M, Uchimiya H. Expression of functional proteins of cDNA encoding rice nucleoside diphosphate kinase(NDK)in Escherichia coli, and organ-related alteration of NDK activities during rice seed germination(Oryza sativa, L.)[J]. Plant Mol Biol, 1995, 27 (5): 1053–1058. DOI:10.1007/BF00037032 |
| [24] | Lee DG, Ahsan N, Lee SH, et al. An approach to identify cold-induced low-abundant proteins in rice leaf[J]. CR Biol, 2007, 330 (3): 215–225. DOI:10.1016/j.crvi.2007.01.001 |
| [25] | Chen JH, Tian L, Xu HF, et al. Cold-induced changes of protein and phosphoprotein expression patterns from rice roots as revealed by multiplex proteomic analysis[J]. Plant Omics J Plant, 2012, 5 (2): 194–199. |
| [26] | Lin SK, Chang MC, Tsai YG, et al. Proteomic analysis of the expression of proteins related to rice quality during caryopsis development and the effect of high temperature on expression[J]. Proteomics, 2005, 5 (8): 2140–2156. DOI:10.1002/(ISSN)1615-9861 |
| [27] | Kawasaki S, Borchert C, Deyholos M, et al. Gene expression profiles during the initial phase of salt stress in rice[J]. Plant Cell, 2001, 13 (4): 889–905. DOI:10.1105/tpc.13.4.889 |
| [28] | Cho SM, Shin SH, Kim KS, et al. Enhanced expression of a gene encoding a nucleoside diphosphate kinase 1(OsNDPK1)in rice plants upon infection with bacterial pathogens[J]. Mol Cells, 2004, 18 (3): 390–395. |
| [29] | 凌丹燕, 路梅. 细菌性条斑病菌JH01诱导水稻抗病均一化差减文库的构建[J]. 安徽农业科学, 2016(2): 188–191. |
| [30] | Ahsan N, Lee DG, Lee KW, et al. Glyphosate-induced oxidative stress in rice leaves revealed by proteomic approach[J]. Plant Physiol Bioch, 2008, 46 (12): 1062–1070. DOI:10.1016/j.plaphy.2008.07.002 |
| [31] | Salekdeh GH, Siopongco J, Wade LJ, et al. Proteomic analysis of rice leaves during drought stress and recovery[J]. Proteomics, 2002, 2 (9): 1131–45. DOI:10.1002/1615-9861(200209)2:9<1131::AID-PROT1131>3.0.CO;2-1 |
| [32] | Ishikawa T, Morimoto Y, Madhusudhan R, et al. Acclimation to diverse environmental stresses caused by a suppression of cytosolic ascorbate peroxidase in tobacco BY-2 cells[J]. Plant Cell Physiol, 2005, 46 (8): 1264–1271. DOI:10.1093/pcp/pci135 |
| [33] | Choi G, Yi H, Lee J, et al. Phytochrome signalling is mediated through nucleoside diphosphate kinase 2[J]. Nature, 1999, 401 (6753): 610–613. DOI:10.1038/44176 |
| [34] | Choi G, Kim JI, Hong SW, et al. A possible role for NDPK2 in the regulation of auxin-mediated responses for plant growth and development[J]. Plant Cell Physiol, 2005, 46 (8): 1246–1254. DOI:10.1093/pcp/pci133 |
| [35] | Seong ES, Guo J, Kim YH, et al. Regulations of marker genes involved in biotic and abiotic stress by overexpression of the AtNDPK2 gene in rice[J]. Biochem Bioph Res Co, 2007, 363 (1): 126–132. DOI:10.1016/j.bbrc.2007.08.147 |
| [36] | Shen Y, Han Y, Kim J, et al. Arabidopsis nucleoside diphosphate kinase-2 as a plant GTPase activating protein[J]. Bmb Rep, 2008, 41 (9): 645–650. DOI:10.5483/BMBRep.2008.41.9.645 |
| [37] | Fukamatsu Y, Yabe N, Hasunuma K. Arabidopsis NDK1 is a component of ROS signaling by interacting with three catalases[J]. Plant Cell Physiol, 2003, 44 (10): 982–989. DOI:10.1093/pcp/pcg140 |
| [38] | Moon H, Lee B, Choi G, et al. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants[J]. P Natl Acad Sci USA, 2003, 100 (1): 358–363. DOI:10.1073/pnas.252641899 |
| [39] | Hamada T, Tanaka N, Noguchi T, et al. Phytochrome regulates phosphorylation of a protein with characteristics of a nucleoside diphosphate kinase in the crude membrane fraction from stem sections of etiolated pea seedlings[J]. J Photoch Photobio B Bio, 1996, 33 (2): 143–151. DOI:10.1016/1011-1344(95)07236-5 |
| [40] | Tanaka N, Ogura T, Noguchi T, et al. Phytochrome-mediated light signals are transduced to nucleoside diphosphate kinase in Pisumsativum L. cv. Alaska[J]. J Photoch Photobio B, 1998, 45 (2-3): 113–121. DOI:10.1016/S1011-1344(98)00169-9 |
| [41] | Haque ME, Yoshida Y, Hasunuma K. ROS resistance in Pisum sativum cv. Alaska:the involvement of nucleoside diphosphate kinase in oxidative stress responses via the regulation of antioxidants[J]. Planta, 2010, 232 (2): 367–382. DOI:10.1007/s00425-010-1173-2 |
| [42] | 白晓娟, 刘丽娟, 张春华, 等. H2O2预处理对不同水稻品种Cd耐性的影响[J]. 中国水稻科学, 2010, 24(4): 391–397. |
| [43] | 丁艳, 韩卓, 王泽港, 等. 不同基因型玉米幼苗对低磷条件的响应[J]. 中国农学通报, 2011, 27(30): 32–34. |
| [44] | Zhang J, Fukui T, Ichikawa A. A third type of nucleoside diphosphate kinase from spinach leaves:purification, characterization and amino-acid sequence[J]. Biochim Biophys Acta, 1995, 1248 (1): 19–26. DOI:10.1016/0167-4838(94)00222-3 |
| [45] | Valenti D, Vacca RA, Pinto MCD, et al. In the early phase of programmed cell death in Tobacco Bright Yellow 2 cells the mitochondrial adenine nucleotide translocator, adenylate kinase and nucleoside diphosphate kinase are impaired in a reactive oxygen species-dependent manner[J]. Biochim Biophys Acta, 2007, 1767 (1): 66–78. DOI:10.1016/j.bbabio.2006.11.004 |
| [46] | EscobarGalvis ML, Håkansson G, Alexciev K, et al. Cloning and characterisation of a pea mitochondrial NDPK[J]. Biochimie, 1999, 81 (12): 1089–1096. DOI:10.1016/S0300-9084(99)00353-3 |
| [47] | Galvis MLE, Marttila S, HaKansson G, et al. Heat stress response in pea involves interaction of mitochondrial nucleoside diphosphate kinase with a novel 86-kilodalton protein[J]. Plant Physiol, 2001, 126 (1): 69–77. DOI:10.1104/pp.126.1.69 |
| [48] | Knorpp C, Johansson M, Baird AM. Plant mitochondrial nucleoside diphosphate kinase is attached to the membrane through interaction with the adenine nucleotide translocator[J]. Febs Letters, 2003, 555 (2): 363–366. DOI:10.1016/S0014-5793(03)01288-2 |
| [49] | Galvis MLE, Marttila S, HaKansson G, et al. Heat stress response in pea involves interaction of mitochondrial nucleoside diphosphate kinase with a novel 86-kilodalton protein[J]. Plant Physiol, 2001, 126 (1): 69–77. DOI:10.1104/pp.126.1.69 |
| [50] | Johansson M. The role of nucleoside diphosphate kinase in plant mitochondria[D]. Acta U Agr Sueciae, 2006. http://core.kmi.open.ac.uk/display/16388238 |
| [51] | Monika J, Jenni H, Eva U, et al. The activities of nucleoside diphosphate kinase and adenylate kinase are influenced by their interaction[J]. Plant Sci, 2008, 174 (2): 192–199. DOI:10.1016/j.plantsci.2007.11.005 |
| [52] | Hammargren J, Sundström J, Johansson M, et al. On the phylogeny, expression and targeting of plant nucleoside diphosphatekinases[J]. Physiol Plantarum, 2007, 129 (1): 79–89. DOI:10.1111/ppl.2007.129.issue-1 |
| [53] | Hammargren J, Salinas T, Maréchal-Drouard L, et al. The pea mitochondrial nucleoside diphosphate kinase cleaves DNA and RNA[J]. Febs Letters, 2007, 581 (18): 3507–3511. DOI:10.1016/j.febslet.2007.06.062 |
| [54] | Hammargren J, Rosenquist S, Jansson C, et al. A novel connection between nucleotide and carbohydrate metabolism in mitochondria:sugar regulation of the Arabidopsis, nucleoside diphosphate kinase 3a gene[J]. Plant Cell Rep, 2008, 27 (3): 529–534. DOI:10.1007/s00299-007-0486-5 |
| [55] | Kihara A, Saburi W, Wakuta S, et al. Physiological and biochemical characterization of three nucleoside diphosphate kinase isozymes from rice(Oryza sativa L.)[J]. Biosci Biotech Bioch, 2011, 75 (9): 1740–1745. DOI:10.1271/bbb.110285 |
| [56] | Manigbas NL, Park DS, Park SK, et al. Enhanced tolerance of transgenic rice overexpressing Arabidopsis thaliana nucleoside diphosphate kinase(AtNDPK2)against various environmental stresses[J]. Philipp Agric Sci, 2011, 94 (1): 29–37. |
| [57] | Hetmann A, Kowalczyk S. Nucleoside diphosphate kinase isoforms regulated by phytochrome A isolated from oat coleoptiles[J]. Acta Biochimica Poloni, 2009, 56 (1): 143–153. |
| [58] | Zrenner R, Stitt M, Sonnewald U, et al. Pyrimidine and purine biosynthesis and degradation in plants[J]. Annu Rev Plant Biol, 2006, 57 (1): 805–836. DOI:10.1146/annurev.arplant.57.032905.105421 |
| [59] | Anderca MI, Furuichi T, Muto S. Mitochondrial NDP kinase from Dunaliella tertiolecta(Chlorophyceae, Chlorophyta)[J]. Phycol Res, 2003, 51 (3): 147–153. DOI:10.1111/pre.2003.51.issue-3 |
| [60] | Dharmasiri S, Harrington HM, Dharmasiri N. Heat shock modulates phosphorylation status and activity of nucleoside diphosphate kinase in cultured sugarcane cells[J]. Plant Cell Rep, 2010, 29 (11): 1305–1314. DOI:10.1007/s00299-010-0917-6 |
| [61] | Cao TS, Srivastava S, Rahman MH, et al. Proteome-level changes in the roots of Brassica napus as a result of Plasmodiophora brassicae infection[J]. Plant Sci, 2008, 174 (1): 97–115. DOI:10.1016/j.plantsci.2007.10.002 |
| [62] | Sharma N, Rahman MH, Strelkov S, et al. Proteome-level changes in two Brassica napus lines exhibiting differential responses to the fungal pathogen Alternaria brassicae[J]. Plant Sci, 2007, 172 (1): 95–110. DOI:10.1016/j.plantsci.2006.07.016 |
| [63] | Postel EH. Multiple biochemical activities of NM23/NDP kinase in gene regulation[J]. J Bioenerg Biomer, 2003, 35 (1): 31–40. DOI:10.1023/A:1023485505621 |
| [64] | Lübeck J, Soll J. Nucleoside diphosphate kinase from pea chloroplasts:purification, cDNA cloning and import into chloroplasts[J]. Planta, 1995, 196 (4): 668–673. DOI:10.1007/BF01106759 |
| [65] | Bovet L, Meylan-Bettex M, Eggman T, et al. CDP phosphotransferase activity in spinach intact chloroplasts:Possible involvement of nucleoside diphosphate kinase Ⅱ[J]. Plant Physiol Bioch, 1999, 37 (37): 645–652. |
| [66] | Hamada T, Hasunuma K, Komatsu S. Phosphorylation of proteins in the stem section of etiolated rice seedling irradiated with red light[J]. Biol Pharm Bull, 1999, 22 (2): 122–126. DOI:10.1248/bpb.22.122 |
| [67] | Ito K, Hamada T, Hasunuma K. Blue light signal transmission to 15 kDa proteins in the crude membrane fraction from the stem section of etiolated pea seedlings[J]. J Photoch Photobio B, 1995, 28 (3): 223–227. DOI:10.1016/1011-1344(95)07114-H |
| [68] | Im YJ, Kim JI, Shen Y, et al. Structural analysis of Arabidopsis thaliana nucleoside diphosphate kinase-2 for phytochrome-mediated light signaling[J]. J Mol Biol, 2004, 343 (3): 659–670. DOI:10.1016/j.jmb.2004.08.054 |
| [69] | Kim JI, Song PS. Phytochrome phosphorylation modulates light signaling by influencing the protein-protein interaction[J]. Plant Cell, 2004, 16 (10): 2629–2640. DOI:10.1105/tpc.104.023879 |
| [70] | Hetmann A, Wujak M, Kowalczyk S. Protein transphosphorylation during the mutual interaction between phytochrome a and a nuclear isoform of nucleoside diphosphate kinase is regulated by red light[J]. Biochemistry, 2016, 81 (10): 1153–1162. |
| [71] | 王丽娜, 王丽艳, 刘景文, 等. AtNDPK2基因转化敖汉苜蓿及其耐盐性分析[J]. 激光生物学报, 2014, 23(1): 65–70. |
| [72] | 侯夫云, 赵兵, 王庆美, 等. 转AtNDPK2基因甘薯的耐盐性鉴定[J]. 山东农业科学, 2014(2): 29–31. |
| [73] | Kim YH, Kim MD, Choi YI, et al. Transgenic poplar expressing Arabidopsis NDPK2 enhances growth as well as oxidative stress tolerance[J]. Plant Biotechnol J, 2011, 9 (3): 334–347. DOI:10.1111/pbi.2011.9.issue-3 |
| [74] | Manigbas NL, Park DS, Park SK, et al. Enhanced tolerance of transgenic rice overexpressing Arabidopsis thaliana nucleoside diphosphate kinase(AtNDPK2)against various environmental stresses[J]. Philipp Agric Sci, 2011, 94 (1): 29–37. |
| [75] | Kim YH, Lim S, Yang KS, et al. Expression of Arabidopsis NDPK2 increases antioxidant enzyme activities and enhances tolerance to multiple environmental stresses in transgenic sweet potato plants[J]. Mol Breeding, 2009, 24 (3): 233–244. DOI:10.1007/s11032-009-9286-7 |
| [76] | Tang L, Kim MD, Yang KS, et al. Enhanced tolerance of transgenic potato plants overexpressing nucleoside diphosphate kinase2 against multiple environmental stresses[J]. Transgenic Res, 2008, 17 (4): 705–715. DOI:10.1007/s11248-007-9155-2 |
| [77] | Verslues PE, Batelli G, Grillo S, et al. Interaction of SOS2 with nucleoside diphosphate kinase 2 and catalases reveals a point of connection between salt stress and H2O2 signaling in Arabidopsis thaliana[J]. Mol Cell Biol, 2007, 27 (22): 7771–7780. DOI:10.1128/MCB.00429-07 |
| [78] | Yang KA, Moon H, Kim G, et al. NDP kinase 2 regulates expression of antioxidant genes in Arabidopsis[J]. P Jpn Acad B-Phys, 2003, 79 (3): 86–91. |
| [79] | Spetea C, Hundal T, Lundin B, et al. Multiple evidence for nucleotide metabolism in the chloroplast thylakoid lumen[J]. P Natl Acad Sci USA, 2004, 101 (5): 1409–1414. DOI:10.1073/pnas.0308164100 |
| [80] | Monika J, Jenni H, Eva U, et al. The activities of nucleoside diphosphate kinase and adenylate kinase are influenced by their interaction[J]. Plant Sci, 2008, 174 (2): 192–199. DOI:10.1016/j.plantsci.2007.11.005 |
| [81] | Matsushita Y, Suzuki T, Kubota R, et al. Isolation of a cDNA for a nucleoside diphosphate kinase capable of phosphorylating the kinase domain of the self-incompatibility factor SRK of Brassica campestris[J]. J Exp Bot, 2002, 53 (369): 765–767. DOI:10.1093/jexbot/53.369.765 |