2. 中国动物疫病预防控制中心,北京 102600
2. China Animal Disease Prevention and Control Center,Beijing 102600
核酸体外扩增是分子生物学、遗传学、医学等研究领域最常用的技术之一。其中聚合酶链式反应(polymerase chain reaction,PCR)以及在此基础上发展的多重PCR、单分子PCR、实时荧光定量PCR等技术使用最为广泛,但这些技术均依赖于控温准确的热循环仪器,限制了其在临床现场检测中的应用。核酸恒温扩增技术由于不需反复热变性,无需特殊仪器,反应速度更快,适合现场快速检测,在生命科学研究及相关诸多领域已经得到了广泛应用。
目前已有10余种核酸恒温扩增技术,其中重组酶聚合酶扩增(recombinase polymerase amplification,RPA)是2006年由英国公司TwistDx Inc研发的一种核酸恒温扩增技术[1]。RPA技术使用重组酶与引物结合形成的复合物能在模板上寻找同源序列,定位后就会引发链交换反应并启动DNA合成,对模板上的目标区域进行指数式扩增。RPA技术可以在25-42℃恒温条件下快速完成核酸扩增,产物可以通过探针法荧光定量进行实时监测,也可以与侧流层析试纸条、生物芯片、凝胶电泳等多种方法相结合进行检测[2-4]。目前基于RPA技术建立的检测方法在疾病诊断、食品安全检测、转基因作物检测、病原学检测等多个领域的应用越来越广泛。现就RPA技术的发展及其当前应用研究进展作一介绍。
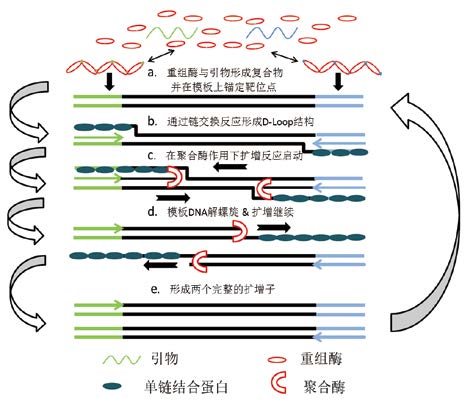
1 RPA技术的原理RPA技术包括3种关键组分,分别是重组酶(如T4 uvsX、E.coli recA等)、单链结合蛋白(如T4 gp32等)和链置换DNA聚合酶(如B. subtilis Pol I、S. aureus Pol等)。RPA技术的原理,见图 1[5]:a. 重组酶与长约30-35 nt的引物结合形成的复合物在双链DNA模板中寻找靶位点;b. 复合物在模板上定位后可以直接引发链交换反应形成D-Loop结构,单链结合蛋白随即结合被置换的DNA链,稳定形成的D-Loop结构并且防止引物解离;c. 重组酶-引物复合物主动水解体系中的ATP导致复合物构象改变,重组酶解离后引物3'端暴露并被DNA聚合酶识别,DNA聚合酶按照模板序列在引物3'末端添加相应碱基,DNA扩增启动;d. 链置换DNA聚合酶在延伸引物的同时继续解开模板的双螺旋DNA结构,DNA合成过程继续进行;e. 两条引物扩增完成即形成一个完整的扩增子。RPA扩增体系中还含有T4 uvsY和Carbowax20M等成分,可以改变重组酶-引物复合体解离及重新结合的可逆反应过程,使反应向更有利于RPA的进行[1]。同时,RPA体系中可以加入反转录酶将RNA作为模板合成DNA后再进行扩增,使RPA可以同时应用于RNA的检测[6, 7]。按照上述体系建立的RPA反应体系一般称为Basic-RPA。
|
|
图 1 RPA 反应的原理示意图
|
最初使用的RPA技术,即Basic-RPA的反应条件一般为37-39℃恒温20-40 min,然后通过琼脂糖凝胶电泳检测扩增产物。Basic-RPA反应具有很高的敏感性,Rohrman等[8]建立的Basic-RPA方法可以检测低至10拷贝的HⅣ病毒核酸。Basic-RPA的缺点是反应体系中存在一些可能影响DNA在琼脂糖凝胶中迁移的物质,可能导致电泳结果出现抹带等现象,因此扩增产物一般需要纯化后才可以进行电泳检测[9]。尽管如此,Basic-RPA仍有很大的实用价值,利用RPA可以常温扩增的特点与生物芯片结合,研究者建立了on-chip RPA检测方法,可以在20 min内特异性检测淋病奈瑟氏菌、沙门氏菌和耐甲氧西林金黄色葡萄球菌[2]。Sara Santiago-Felipe等[10]基于Basic-RPA建立的disc-based RPA技术可以高灵敏度检测多种病原体。
在Basic-RPA基础上,Piepenburg等[1]利用序列特异性荧光探针建立了可以实时监测荧光信号判断产物扩增情况的探针法RPA以及可以直接肉眼观察最终扩增结果的侧流层析试纸条RPA(lateral flow RPA,LF-RPA)。
2.1 探针法RPA与Basic-RPA的反应体系相比,探针法 RPA体系中一般含有核酸外切酶Ⅲ(exonuclease Ⅲ,即exo)和exo荧光探针(根据酶的名称命名为exo探针),通过检测荧光信号实时监测RPA产物的扩增情况。exo探针含有一个碱基模拟物四氢呋喃分子(tetrahydrofuran,THF),THF分子两侧分别带有荧光基团和淬灭基团,探针3'端带有防止探针延伸的阻断物。当探针与靶DNA结合形成双链杂合DNA结构后,exo Ⅲ作为一种DNA修复酶,识别THF位点并切割探针使荧光基团和淬灭基团分离从而产生荧光[11,12]。用exo探针的RPA反应不能使用凝胶电泳等方法进行终点定量,一般使用可以实时收集荧光信号的装置(如荧光定量PCR仪、ESEQuant Tube Scanner device、Twista®等)监测反应情况。此外,exo探针法RPA反应速度更快,一般在20 min内即可完成检测,灵敏度和特异性均很高[13-15]。
2.2 侧流层析试纸条RPA与Basic-RPA的反应体系相比,LF-RPA体系中加入了核酸外切酶Ⅳ(endonuclease Ⅳ,即nfo)和nfo探针(根据酶的名称命名为nfo探针),而且在反应中使用带有生物素或者地高辛等标记物的反向引物[16, 17]。nfo探针的5'末端带有荧光基团,3'端带有阻断物,探针序列中也带有一个THF分子。随着反应的进行,剪切后的探针与下游引物形成既带有探针荧光基团标记物又带有引物特殊标记物的双标记扩增子。这种双标记扩增产物可以使用凝胶电泳等终点定量方法和侧流层析法进行检测,目前主要使用的基于“夹心法”的侧流试纸条进行检测。侧流试纸条包括检测线和对照线,也有的只使用检测线,其原理是:检测线处使用相应的抗体来捕获引物所带有的标记物,胶体金颗粒标记的抗探针荧光基团抗体结合扩增子带有的荧光基团即可以观察到检测产物(也可以先捕获探针标记物,再结合引物标记物)[8]。LF-RPA的优点是经过37-39℃的恒温短时间扩增反应后,肉眼即可观察扩增产物在侧流试纸条上的检测结果,不需复杂仪器设备,适合现场快速检测。
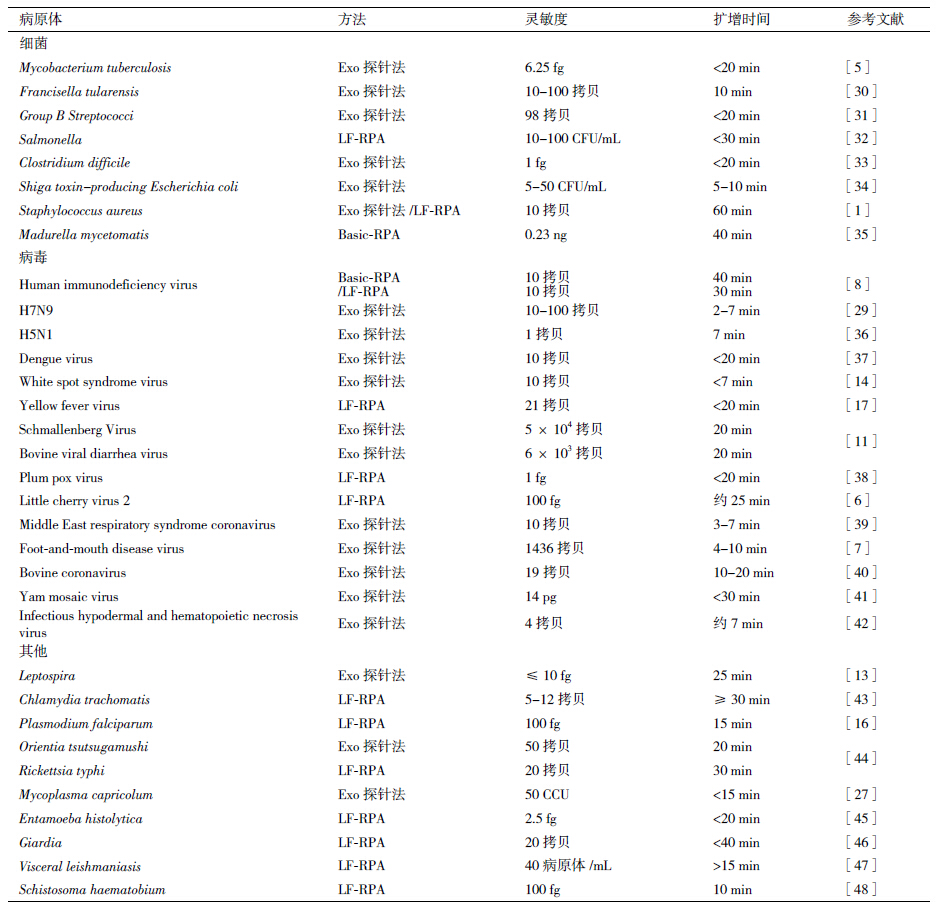
3 RPA技术与其他核酸恒温扩增技术的比较目前应用较多的核酸恒温扩增技术主要有:核酸依赖性扩增检测(nuclear acid sequence-based amplification,NASBA)、环介导恒温扩增(loop-me-diated isothermal amplification,LAMP)、链替代扩增(strand displacement amplification,SDA)、滚环扩增(rolling circle amplification,RCA)、依赖解旋酶的恒温基因扩增(helicase-dependent isothermal DNA amp-lification,HDA)及转录介导的扩增技术(transcrip-tion mediated amplification)等[18-21]。核酸恒温扩增技术与基于PCR的核酸扩增技术相比,具有高敏感性、高特异性、操作简便、反应时间短、不需复杂仪器设备等优点,在检验检疫、医学、法医等需要快速现场检测的一些领域实用性更强。以上扩增技术各有优缺点,RPA与这些技术相比,操作更简单,并且可以根据条件选择恰当的检测方法,目前在病原学检测领域应用特别广泛。RPA技术与其他核酸恒温扩增方法简要对比,见表 1[22],从表中可以看出RPA技术不需热变性因此反应时间更短,可以多通道同时检测多个靶基因,而且有多种方法可用来检测扩增产物。
RPA技术具有灵敏度高、特异性强、操作快速便捷等优点,而且可以实现定量分析,因此在疾病诊断和病原鉴定等许多领域具有广阔的应用前景。在癌症研究中,RPA技术可以用于癌症突变检测和抗癌药物筛选[23, 24]。RPA技术在病原学检测领域的研究尤为热门,目前已经建立了针对细菌、病毒、寄生虫等多种病原体的RPA检测方法(表 2)。RPA技术在维护人类公共卫生和生物反恐方面发挥着重要作用,如Euler等[12]研发的RPA检测板可以同时检测土拉弗朗西斯氏菌、鼠疫耶尔森氏菌、炭疽芽孢杆菌、天花病毒、裂谷热病毒、埃博拉病毒及马尔堡病毒等多种生物恐怖因子。在食品安全方面,RPA技术也应用广泛,如 Santiago-Felipe等[25]建立的RPA-ELISA方法可以同时检测食品中多种过敏原(榛子、花生、番茄、大豆及玉米)、病原微生物以及转基因成分;Chao等[26]建立的RPA方法可以检测玉米、水稻、棉花和大豆等作物中转基因成分。由于RPA技术对实验设备的要求非常低,使得该技术在经济条件差,资源不足的区域具有广阔的应用前景[7, 17, 27-29]。
RPA技术被称作有望替代PCR的核酸检测技术,主要优势在于不需温控仪器即可快速进行痕量DNA或RNA的特异性扩增,在临床检测和现场快速诊断方面具有显著的优越性。以RPA技术为基础建立的RPA-ELISA、on chip RPA等扩增技术可以高通量检测多种病原体,因此RPA技术在癌症突变检测、遗传病的定期和快速普查、转基因成分检测等领域也有广阔的应用前景。虽然目前RPA技术的检测成本高于PCR等其他核酸扩增技术,但随着RPA技术的进一步发展、完善以及生产工艺的改良,RPA技术有望成为常规的快速诊断手段,并在分子生物学、医学、遗传学等各个研究领域得到更加广泛的应用。
| [1] | Piepenburg O, Williams CH, Stemple DL, et al. DNA detection using recombination proteins[J]. PLoS Biology, 2006, 4(7): e204. |
| [2] | Kersting S, Rausch V, Bier FF, et al. Multiplex isothermal solid-phase recombinase polymerase amplification for the specific and fast DNA-based detection of three bacterial pathogens[J]. Microchimica Acta, 2014, 181(13-14): 1715–1723. |
| [3] | Xu C, Li L, Jin W, et al. Recombinase polymerase amplification(RPA)of CaMV-35S promoter and nos terminator for rapid detection of genetically modified crops[J]. International Journal of Molecular Sciences, 2014, 15(10): 18197–18205. |
| [4] | Shen F, Davydova EK, Du W, et al. Digital isothermal quantification of nucleic acids via simultaneous chemical initiation of recombinase polymerase amplification reactions on SlipChip[J]. Analytical Chemistry, 2011, 83(9): 3533–3540. |
| [5] | Boyle DS, McNerney R, Teng Low H, et al. Rapid detection of Mycobacterium tuberculosis by recombinase polymerase amplification[J]. PLoS One, 2014, 9(8): e103091. |
| [6] | Mekuria TA, Zhang S, Eastwell KC. Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification[J]. Journal of Virological Methods, 2014, 205(1): 24–30. |
| [7] | Abd El Wahed A, El-Deeb A, El-Tholoth M, et al. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus[J]. PLoS One, 2013, 8(8): e71642. |
| [8] | Rohrman BA, Richards-Kortum RR. A paper and plastic device for performing recombinase polymerase amplification of HⅣ DNA[J]. Lab Chip, 2012, 12(17): 3082–3088. |
| [9] | Wee EJ, Lau HY, Botella JR, et al. Re-purposing bridging flocculation for on-site, rapid, qualitative DNA detection in resource-poor settings[J]. Chemical Communications, 2015, 51(27): 5828–5831. |
| [10] | Santiago-Felipe S, Tortajada-Genaro LA, Morais S, et al. One-pot isothermal DNA amplification-Hybridisation and detection by a disc-based method[J]. Sensors and Actuators B:Chemical, 2014, 204(1): 273–281. |
| [11] | Aebischer A, Wernike K, Hoffmann B, et al. Rapid genome detection of schmallenberg virus and bovine viral diarrhea virus by use of isothermal amplification methods and high-speed real-time reverse transcriptase PCR[J]. Journal of Clinical Microbiology, 2014, 52(6): 1883–1892. |
| [12] | Euler M, Wang Y, Heidenreich D, et al. Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents[J]. Journal of Clinical Microbiology, 2013, 51(4): 1110–1117. |
| [13] | Ahmed A, van der Linden H, Hartskeerl RA. Development of a recombinase polymerase amplification assay for the detection of pathogenic Leptospira[J]. Int J Environ Res Public Health, 2014, 11(5): 4953–4964. |
| [14] | Xia X, Yu Y, Weidmann M, et al. Rapid detection of shrimp white spot syndrome virus by real time, isothermal recombinase polymerase amplification assay[J]. PLoS One, 2014, 9(8): e104667. |
| [15] | Boyle DS, Lehman DA, Lillis L, et al. Rapid detection of HⅣ-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification[J]. MBio, 2013, 4(2): e00135–e00113. |
| [16] | Kersting S, Rausch V, Bier FF, et al. Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis[J]. Malaria Journal, 2014, 13(1): 99. |
| [17] | Escadafal C, Faye O, Sall AA, et al. Rapid molecular assays for the detection of yellow fever virus in low-resource settings[J]. PLoS Neglected Tropical Diseases, 2014, 8(3): e2730. |
| [18] | Yan L, Zhou J, Zheng Y, et al. Isothermal amplified detection of DNA and RNA[J]. Molecular Biosystems, 2014, 10(5): 970–1003. |
| [19] | Fakruddin M, Mannan KS, Chowdhury A, et al. Nucleic acid amplification:Alternative methods of polymerase chain reaction[J]. Journal of Pharmaceutical Sciences, 2013, 5(4): 245–252. |
| [20] | Craw P, Balachandran W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics:a critical review[J]. Lab Chip, 2012, 12(14): 2469–2486. |
| [21] | Gill P, Ghaemi A. Nucleic acid isothermal amplification technol-ogies:a review[J]. Nucleosides Nucleotides Nucleic Acids, 2008, 27(3): 224–243. |
| [22] | Zaghloul H, El-Shahat M. Recombinase polymerase amplification as a promising tool in hepatitis C virus diagnosis[J]. World Journal of Hepatology, 2014, 6(12): 916–922. |
| [23] | Shin Y, Perera AP, Kim KW, et al. Real-time, label-free isothermal solid-phase amplification/detection(ISAD)device for rapid detection of genetic alteration in cancers[J]. Lab Chip, 2013, 13(11): 2106–2114. |
| [24] | Loo JF, Lau PM, Ho HP, et al. An aptamer-based bio-barcode assay with isothermal recombinase polymerase amplification for cytochrome-c detection and anti-cancer drug screening[J]. Talanta, 2013, 115(4): 159–165. |
| [25] | Santiago-Felipe S, Tortajada-Genaro LA, Puchades R, et al. Recombinase polymerase and enzyme-linked immunosorbent assay as a DNA amplification-detection strategy for food analysis[J]. Analytica Chimica Acta, 2014, 811(5): 81–87. |
| [26] | 徐潮, 李亮, 金芜军, 宛煜嵩. 荧光RPA技术检测转基因水稻科丰6号[J]. 分子植物育种, 2014, 12(5): 875–880. |
| [27] | Liljander A, Yu M, O'Brien E, et al. Field-applicable recombinase polymerase amplification assay for rapid detection of Mycoplasma capricolum subsp[J]. Journal of Clinical Microbiology, 2015, 53(9): 2810–2815. |
| [28] | Lillis L, Lehman D, Singhal MC, et al. Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HⅣ-1 DNA[J]. PLoS One, 2014, 9(9): e108189. |
| [29] | Abd El Wahed A, Weidmann M, Hufert FT. Diagnostics-in-a-Suitcase:Development of a portable and rapid assay for the detection of the emerging avian influenza A(H7N9)virus[J]. Journal of Clinical Virology, 2015, 69(3): 16–21. |
| [30] | Euler M, Wang Y, Otto P, et al. Recombinase polymerase amplific-ation assay for rapid detection of Francisella tularensis[J]. Journal of Clinical Microbiology, 2012, 50(7): 2234–2238. |
| [31] | Daher RK, Stewart G, Boissinot M, et al. Isothermal recombinase polymerase amplification assay applied to the detection of group B streptococci in vaginal/anal samples[J]. Clinical Chemistry, 2014, 60(4): 660–666. |
| [32] | Kim TH, Park J, Kim CJ, et al. Fully integrated lab-on-a-disc for nucleic acid analysis of food-borne pathogens[J]. Analytical Chemistry, 2014, 86(8): 3841–3848. |
| [33] | Tsaloglou MN, Watson RJ, Rushworth CM, et al. Real-time microfluidic recombinase polymerase amplification for the toxin B gene of Clostridium difficile on a SlipChip platform[J]. Analyst, 2015, 140(1): 258–264. |
| [34] | Murinda SE, Ibekwe AM, Zulkaffly S, et al. Real-time isothermal detection of Shiga toxin-producing Escherichia coli using recombinase polymerase amplification[J]. Foodborne Pathogens and Disease, 2014, 11(7): 529–536. |
| [35] | Ahmed SA, van de Sande WW, Desnos-Ollivier M, et al. Applica-tion of isothermal amplification techniques for the identification of Madurella mycetomatis, the prevalent agent of human mycetoma[J]. Journal of Clinical Microbiology, 2015, 53(10): 3280–3285. |
| [36] | Yehia N, Arafa AS, Abd El Wahed A, et al. Development of reverse transcription recombinase polymerase amplification assay for avian influenza H5N1 HA gene detection[J]. Journal of Virological Methods, 2015, 223: 45–49. |
| [37] | Teoh BT, Sam SS, Tan KK, et al. Early detection of dengue virus by use of reverse transcription-recombinase polymerase amplification[J]. Journal of Clinical Microbiology, 2015, 53(3): 830–837. |
| [38] | Zhang S, Ravelonandro M, Russell P, et al. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal Amplify-RP? using reverse transcription-recombinase polymerase amplification[J]. Journal of Virological Methods, 2014, 207(2): 114–120. |
| [39] | Abd El Wahed A, Patel P, Heidenreich D, et al. Reverse transcription recombinase polymerase amplification assay for the detection of middle east respiratory syndrome coronavirus[J]. PLoS Currents, 2013, 5: ecurrents. outbreaks. 62df1c7c75ffc96cd59034531e2e8364.. |
| [40] | Amer HM, Abd El Wahed A, Shalaby MA, et al. A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay[J]. Journal of Virological Methods, 2013, 193(2): 337–340. |
| [41] | Silva G, Bomer M, Nkere C, et al. Rapid and specific detection of Yam mosaic virus by reverse-transcription recombinase polymerase amplification[J]. Journal of Virological Methods, 2015, 15(1): 138–144. |
| [42] | Xia X, Yu Y, Hu L, et al. Rapid detection of infectious hypodermal and hematopoietic necrosis virus(IHHNV)by real-time, isothermal recombinase polymerase amplification assay[J]. Archives of Virology, 2015, 160(4): 987–994. |
| [43] | Krolov K, Frolova J, Tudoran O, et al. Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples[J]. J Mol Diagn, 2014, 16(1): 127–135. |
| [44] | Chao CC, Belinskaya T, Zhang Z, et al. Development of recombinase polymerase amplification assays for detection of Orientia tsutsugamushi or Rickettsia typhi[J]. PLoS Negl Trop Dis, 2015, 9(7): e0003884. |
| [45] | Nair G, Rebolledo M, White AC Jr, et al. Detection of entamoeba histolytica by recombinase polymerase amplification[J]. American Journal of Tropical Medicine and Hygiene, 2015, 93(3): 591–595. |
| [46] | Crannell ZA, Cabada MM, Castellanos-Gonzalez A, et al. Recombinase polymerase amplification-based assay to diagnose giardia in stool samples[J]. American Journal of Tropical Medicine and Hygiene, 2015, 92(3): 583–587. |
| [47] | Castellanos-Gonzalez A, Saldarriaga OA, Tartaglino L, et al. A novel molecular test to diagnose canine visceral Leishmaniasis at the point of care[J]. American Journal of Tropical Medicine and Hygiene, 2015, 3: 15–0145. |
| [48] | Rosser A, Rollinson D, Forrest M, et al. Isothermal recombinase polymerase amplification(RPA)of Schistosoma haematobium DNA and oligochromatographic lateral flow detection[J]. Parasites & Vectors, 2015, 8: 446. |