2.江南大学粮食发酵工艺与技术国家工程实验室,无锡 214122
2. National Engineering Laboratory for Cereal Fermentation Technology,Jiangnan University,Wuxi 214122
脂肪氧合酶(Lipoxygenase,LOX,EC1.13.11.12)是一类含有非血红素铁,能够专一催化氧化含有Z,Z-1,4-戊二烯结构的多元不饱和脂肪酸,形成具有共轭双键的脂肪酸氢过氧化物的双加氧酶[1]。LOX的催化反应过程如下:(1)连接共轭双键的C原子发生脱氢作用;(2)自由电子发生重排向+2或者-2位转移,同时在此过程中产生变位异构体;(3)在带有自由电子的C原子发生双加氧反应,并在此过程中产生手性异构体(图 1)。一般情况下,LOX的天然底物为亚油酸、亚麻酸和花生四烯酸,不同来源LOX对于同种底物的催化效率存在的差异[2]。

|
|
图 1
LOX 催化的反应过程[ |
LOX来源多样,在生物体内参与多种重要生命活动。1932年,Andre等[4]首次在大豆中发现了LOX,是不饱和脂肪酸氧化引起豆腥味形成的关键酶。LOX催化形成的脂肪酸氢过氧化物进一步酶解成茉莉酸等信号分子,调节破损植物细胞的程序性死亡、细胞性别、生长和发育及抵御外界胁迫等[5, 6]。LOX还分布于鼠[7]、兔[8]和人[9]等哺乳动物中,参与白细胞三烯等信号分子的合成,影响炎症、细胞程序性死亡、哮喘和心脏病等生理或病理过程[10, 11]。藻类[12]、真菌[13]及酵母[14]等真核微生物也是LOX的重要来源。在这些生物体中,LOX参与细胞间信号传导过程及具有抑菌作用的细菌内酯合成等[15, 16]。最近,人们在念珠藻(Nostoc punctiforme)[17, 18]和铜绿假单胞菌(Pseudomonas aeruginosa)[19]等原核生物中发现了LOX,但其生理功能尚不清楚[20]。
基于特殊的催化作用,LOX已在食品、化工、医药和造纸等工业应用或展现了较大的应用前景。LOX催化产生的脂肪酸氢过氧化物能够破坏β-胡萝卜素的双键结构,从而提高面粉白度。随着溴酸钾和过氧化苯甲酰化学增白剂的禁用,无毒、无害的LOX成为其最具竞争力的替代产品[21]。LOX合成的部分不饱和脂肪酸氢过氧化物经酶解等可生成不同香味化合物,较化学合成香料具有更高的商业价值[22]。LOX催化产生的不饱和脂肪酸氢过氧化物可用于涂料、洗涤剂、聚氯乙烯、染料等化工产品的生产。LOX将花生四烯酸转化为能抑制淋巴细胞增殖的前列腺素E2、D2和F2α等[23]。LOX能够降低造纸沉积物中的沥青含量,对纸浆进行漂白和脱墨[24]。
随着LOX应用领域的拓展,获得应用性能优良的LOX并实现其高效生产成为国内外相关研究的重要方向。对各来源LOX结构与功能的解析,是理性改造LOX的重要前提。目前,商品化的LOX主要来源于大豆提取,其批次稳定性易受大豆产地和同工酶的影响,不利于其应用推广[25]。基于质量稳定性、生产周期和成本方面的优势,发酵法生产是LOX工业化生产的首选方法。本文简要总结了典型LOX的结构、分子改造及发酵法生产的研究进展,旨为其后续应用性能改造及生产提供参考。
1 脂肪氧合酶的结构 1.1 脂肪氧合酶结构类型LOX广泛分布于动植物和原核生物,其分子结构类型多样。基于结构域组成,大致分为四类:(1)经典结构(图 2-A):LOX分子N端为多个反向平行的β-折叠组成的桶状结构域,分子量在25-30 kD;C端由α-螺旋组成的催化结构域,在催化活性中心含有一个非血红素铁,分子量在55-65 kD;植物和哺乳动物LOX一般为此类结构。(2)融合结构(图 2-B):具有该类结构的LOX的末端融合了其他酶分子。如珊瑚LOX N端融合一个丙二烯氧合酶分子,鱼腥藻LOX N端融合了具有过氧化氢酶特征的结构域[26]。(3)无N端β-折叠结构(图 2-C):已发现细菌LOX具有该类结构。如P. aeruginosa 42A2 LOX分子仅由α-螺旋组成,其N端α-螺旋形成“盖子”状结构,覆盖在底物结合区域上方(图 2-C)。(4)含锰离子结构:禾顶囊壳(Gaeumannomyces graminis)等少数真菌LOX催化活性中心含有一个锰离子[27]。目前尚无这类酶晶体结构的报道。

|
| 图 2 LOX 的结构 |
关于结构域功能的研究主要集中于具有经典结构的LOX。研究人员分别对该类LOX的N端β-折叠结构域与C端催化结构域的功能进行了深入分析。
LOX N端β-折叠结构与胰脂肪酶的C2结构域(亦称为PLAT结构域)相似,表明该β-折叠可能同样参与了酶与膜的结合[31, 32]。事实上,缺失或定点突变大豆[33]、兔子[34]、珊瑚[35]和人[36]等来源LOX的β-折叠结构域,不仅能改变酶与生物膜的结合能力,还能引起底物亲和力、转化数、结构稳定性、活性中心铁离子的可逆结合能力发生变化。这些结果表明,N端β-折叠结构域尽管对LOX催化活性是非必需的,但能一定程度上调节酶整体结构与催化活性中心。研究还发现,兔LOX可能通过N端β-折叠结构域随溶剂环境变化产生“摆动”来调节酶结构和活性[37]。软珊瑚(Gersemia fruticosa)LOX的晶体结构显示,β-折叠结构域保守序列FPCYRW中Trp107与其C端催化结构域中的Lys172之间存在一个阳离子-芳环作用,进而影响催化结构域底物结合口袋的构象[38]。上述研究从分子水平上揭示了N端β-折叠结构域调节LOX结构与活性的机制。
已知晶体结构的LOX中,催化活性中心均位于C端α-螺旋结构域,非血红素铁参与构成催化活性中心。由于LOX底物为疏水性不饱和脂肪酸,各来源酶的底物结合通道主要由亮氨酸、异亮氨酸、缬氨酸和苯丙氨酸等具有疏水性侧链的氨基酸残基组成[20]。但不同LOX的底物结合通道各具特点(图 3):兔子12/15-LOX底物结合区域呈较浅的“靴形”;柳珊瑚8R-LOX存在一个两头通透的“U形”底物结合通道;大豆LOX-1则形成“T形”底物结合通道。由于无法得到结合氧分子的LOX晶体结构,目前仅通过定点突变预测氧分子入口。兔12/15-LOX L367突变增加的空间位阻降低了氧分子扩散,表明该位点是可能的氧分子入口[39];大豆LOX-1中的氧分子扩散通道则受到Ile553调控[28];柳珊瑚8R-LOX的氧分子通道尚不清楚。
1.3 脂肪氧合酶结构与产物特异性的关系基于LOX催化反应的特点,其产物特异性主要包括位置异构体特异性和手性异构体特异性[40]。尽管尚无理论能够解释所有LOX的产物特异性,但针对真核生物LOX的相关研究已取得一定进展。
通过结构模拟确定了黄瓜LOX分子影响产物特异性的H608,将该氨基酸突变为缬氨酸后,催化产物由亚油酸13位氢过氧化物变为9位氢过氧化物[41]。序列比对分析揭示了哺乳动物LOX分子中存在与产物位置异构体特异性相关特征区域,如人体15-LOX 417-418位氨基酸等[42]。底物结合研究显示,哺乳动物15-LOX通过分子内底物结合口袋与底物(甲酯化的不饱和脂肪酸)疏水端结合,酶分子表面与底物亲水端的碱性氨基酸残基结合,进而控制位置异构体的比例[43]。因此,LOX催化产物的位置异构体类型可能受底物进入催化活性中心方式的影响,单点氨基酸可改变该产物特异性。目前,已在真核LOX的C端发现了与产物手性异构体相关的保守序列,命名为“coffa site”。LOX分子在该位点的保守氨基酸为丙氨酸和甘氨酸时,产物的手性异构体分别为S型和R型[44, 45, 46]。随着不同来源LOX底物通道的深入解析[20],其产物手性异构体特异性的分子机制将被进一步阐明。
近年来,部分结合底物或底物类似物的LOX晶体结构被解析,如来源于哺乳动物的15-LOX2与底物类似物[47]和8R-LOX与底物[48]的复合物晶体结构等,为LOX催化机制及产物特异性的研究提供了更精确的结构信息。
2 脂肪氧合酶的分子改造多数真核生物LOX具有较高的热稳定性,分子改造主要以提高酶催化效率为目标。如前所述,大豆等植物LOX的C端结构域为催化活性区域,N端β-折叠结构域对LOX活性有重要调控作用。研究显示,缺失N端β-折叠区域能使大豆LOX疏水区域的暴露增多、酶分子柔性增加,比酶活提高3倍,但热稳定性下降[49, 50]。在橄榄LOX-1的C端结构域中,分别将底物结合位点Phe277和Tyr280突变为侧链体较小的丙氨酸和异亮氨酸残基后,LOX-1突变体比酶活提高93倍[51]。
作者对P. aeruginosa BBE LOX热稳定性和比酶活进行了改造。结构分析显示,P. aeruginosa LOX N端30个氨基酸残基及分子内部201-206位氨基酸残基均为高柔性的loop结构(图 2-C)。通过缺失了N端前30个氨基酸,P. aeruginosa LOX 于50℃的半衰期较野生酶提高2.1倍,比酶活亦保持90%以上[52]。将该Gly201和Gly206之间的序列替换为刚性更强的PT linker,LOX热稳定性有进一步提高[52]。由于LOX底物主要为疏水性的不饱和脂肪酸,作者将疏水较强的自组装双亲短肽(图 4-A)融合至P. aeruginosa LOX N端,使其比酶活及50℃半衰期分别提高2.8倍和3.6倍,并指出寡聚化是融合酶热稳定性提高的重要原因之一[53](图 4-B)。
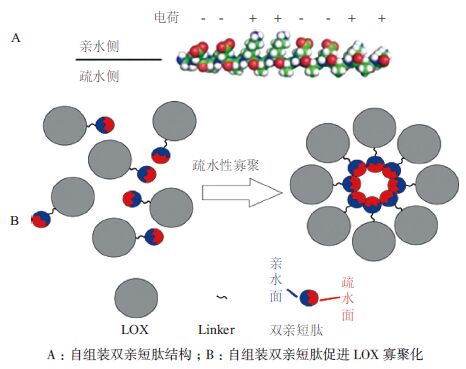
|
| 图 4 融合自组装双亲短肽提高LOX 热稳定的机制 |
目前,商品化的LOX主要从大豆中提取。由于大豆中存在多种LOX同工酶,其含量和种类随大豆的批次变化,导致LOX的产品质量不稳定[25]。与传统提取法相比,微生物发酵法能保证LOX产品批次稳定性的同时,产量更高、生产成本更低。由于产量极低或致病性等原因,自然环境中筛选得到的LOX生产菌,如寄生水霉(Saprolegnia parasi-tica)[54]、G. graminis[55]和P. aeruginosa[56]等,不适合工业生产。因此,构建高产重组菌成为发酵法生产LOX的关键。
真核生物LOX异源表达已有大量报道,但总体产酶水平不高。其中,大豆LOX在大肠杆菌(Escherichia coli)中的表达量最高(4.5 U/mL),但为胞内表达[57]。通过融合不同分泌信号肽,豌豆[58]、猪白细胞[59]和G. graminis[55]等来源的LOX可被酿酒酵母或毕赤酵母分泌至培养基中,但产量极低,远不能满足工业化生产要求。原核生物来源LOX的异源表达同样面临表达量低、难分泌至胞外等问题。尽管N. punctiforme[17]、P. aeruginosa[60]等多个原核生物LOX在大肠杆菌中表达,但未见产量的相关报道。最近报道显示,鱼腥藻(Anabaena sp. PCC 7120)LOX在枯草芽孢杆菌(Bacillus subtilis)168 nprB介导下可被B. subtilis WB800分泌至胞外,实现了LOX在食品级宿主中表达的突破[61]。但按标准酶活定义计算[57],其胞外LOX产量仅为0.01 U/mL,产酶水平有待进一步提高。作者的研究发现,P. aeruginosa LOX的天然信号肽可促进其在E. coli BL21(DE3)中分泌[40],经3 L罐表达条件优化,胞外LOX酶活达到8.3 U/mL[62]。然而,较低的产酶温度(20℃)是LOX的工业化生产亟待解决问题[62]。
由于发酵法产LOX的总体水平不高,从发酵液中提取LOX的报道较少。目前尚需多步柱纯化才能获得较高纯度的LOX,且酶活收率低,纯化技术仍停留于研究阶段。重组B. subtilis WB800分泌的LOX需经DEAE-Sephacel、Sephadex G-100和Ni-NTA agarose三步柱纯化才能达到电泳纯,酶活收率仅为5%[61]。在重组E. coli 中获得13.5倍纯化的LOX需经Q High Performance和Mono Q两步柱纯化,酶活收率也仅达到10%[40]。导致收率低的重要原因之一,可能是上述重组LOX的稳定性不佳[40]。因此,进一步提高LOX的稳定性,压缩纯化工艺流程是实现工业规模纯化LOX的关键。
4 结论随着应用研究的深入,LOX在食品加工、化工、医药等领域展现良好的应用前景。在此背景下,如何获得具有良好应用性能的LOX并实现其高效发酵生产,将是LOX后续研究的重点。由于目前尚无可供工业化生产的野生菌株,基于食品级表达系统(枯草芽孢杆菌、解脂亚洛酵母和米曲霉等)构建高效分泌LOX的重组菌是实现发酵法生产LOX最紧迫的任务之一。基于分泌效率的优势,原核生物来源的LOX较真核生物来源的LOX更适于通过基因工程菌进行工业化生产。然而,与大豆脂肪氧合酶相比,原核生物来源(如P. aeruginosa BBE和P. aeruginosa 42A2等)LOX的热稳定较差[40]。因此,加强对原核LOX热稳定性改造同样应引起足够重视。
| [1] | Nyyssola A, Heshof R, Haarmann T, et al. Methods for identifying lipoxygenase producing microorganisms on agar plates[J]. AMB Express, 2012, 2:17. |
| [2] | Coffa G, Brash AR. A single active site residue directs oxygenation stereospecificity in lipoxygenases:stereocontrol is linked to the position of oxygenation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(44):15579-15584. |
| [3] | Liavonchanka A, Feussner I. Lipoxygenases:occurrence, functions and catalysis[J]. Journal of Plant Physiology, 2006, 163(3):348-357. |
| [4] | Andre E, Hou KW. The presence of a lipid oxidase in soybean, Glycine soya[J]. Compte Rendu Acad Sci(Paris), 1932, 194:645-647. |
| [5] | Howe GA, Jander G. Plant immunity to insect herbivores[J]. Annual Review of Plant Biology, 2008, 59:41-66. |
| [6] | Mosblech A, Feussner I, Heilmann I. Oxylipins:structurally diverse metabolites from fatty acid oxidation[J]. Plant Physiol Biochem, 2009, 47(6):511-517. |
| [7] | Yu Z, Crichton I, Tang SY, et al. Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to COX-2 deletion in mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(17):6727-6732. |
| [8] | Aggarwal NT, Gauthier KM, Campbell WB. Endothelial nitric oxide and 15-lipoxygenase-1 metabolites independently mediate relaxation of the rabbit aorta[J]. Vascul Pharmacol, 2012, 56(1-2):106-112. |
| [9] | Skrzypczak-Jankun E, Jankun J, Al-Senaidy A. Human lipoxygenase:developments in its structure, function, relevance to diseases and challenges in drug development[J]. Current Medicinal Chemistry, 2012, 19(30):5122-5127. |
| [10] | Hersberger M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis:leukotrienes, lipoxins and resolvins[J]. Clinical Chemistry and Laboratory Medicine, 2010, 48(8):1063-1073. |
| [11] | Wymann MP, Schneiter R. Lipid signalling in disease[J]. Nature Reviews Molecular Cell Biology, 2008, 9(2):162-176. |
| [12] | Weinberger F, Lion U, Delage L, et al. Up-regulation of lipoxygenase, phospholipase, and oxylipin-production in the induced chemical defense of the red alga Gracilaria chilensis against epiphytes[J]. Journal of Chemical Ecology, 2011, 37(7):677-686. |
| [13] | Brodhun F, Feussner I. Oxylipins in fungi[J]. FEBS Journal, 2011, 278(7):1047-1063. |
| [14] | Shechter G, Grossman S. Lipoxygenase from baker’s yeast:purification and properties[J]. International Journal of Biochemistry, 1983, 15(11):1295-1304. |
| [15] | Joo YC, Oh DK. Lipoxygenases:Potential starting biocatalysts for the synthesis of signaling compounds[J]. Biotechnology Advances, 2012, 30(6):1524-1532. |
| [16] | Hughes DT, Sperandio V. Inter-kingdom signalling:communication between bacteria and their hosts[J]. Nature Reviews Microbio-logy, 2008, 6(2):111-120. |
| [17] | Koeduka T, Kajiwara T, Matsui K. Cloning of lipoxygenase genes from a cyanobacterium, Nostoc punctiforme, and its expression in Eschelichia coli[J]. Current Microbiology, 2007, 54(4):315-319. |
| [18] | Lang I, Gobel C, Porzel A, et al. A lipoxygenase with linoleate diol synthase activity from Nostoc sp. PCC 7120[J]. Biochemical Journal, 2008, 410(2):347-357. |
| [19] | Vance R, Hong S, Gronert K, et al. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(7):2135. |
| [20] | Ivanov I, Heydeck D, Hofheinz K, et al. Molecular enzymology of lipoxygenases[J]. Archives of Biochemistry and Biophysics, 2010, 503(2):161-174. |
| [21] | Casey R, West SI, Hardy D, et al. New frontiers in food enzymology:recombinant lipoxygenases[J]. Trends In Food Science & Technology, 1999, 10(9):297-302. |
| [22] | Buchhaupt M, Guder JC, Etschmann MM, et al. Synthesis of green note aroma compounds by biotransformation of fatty acids using yeast cells coexpressing lipoxygenase and hydroperoxide lyase[J]. Applied Microbiology and Biotechnology, 2012, 93(1):159-168. |
| [23] | Noverr MC, Toews GB, Huffnagle GB. Production of prostaglandins and leukotrienes by pathogenic fungi[J]. Infection and Immunity, 2002, 70(1):400-402. |
| [24] | 林影. 生物酶在造纸工业绿色制造中的应用[J]. 生物工程学报, 2014, 30(1):83-89. |
| [25] | Casey R, Hughes RK. Recombinant lipoxygenases and oxylipin metabolism in relation to food quality[J]. Food Biotechnology, 2004, 18(2):135-170. |
| [26] | Schneider C, Niisuke K, Boeglin WE, et al. Enzymatic synthesis of a bicyclobutane fatty acid by a hemoprotein lipoxygenase fusion protein from the cyanobacterium Anabaena PCC 7120[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(48):18941-18945. |
| [27] | Cristea M, Oliw EH. A G316A mutation of manganese lipoxygenase augments hydroperoxide isomerase activity:mechanism of biosynthesis of epoxyalcohols[J]. Journal of Biological Chemistry, 2006, 281(26):17612-17623. |
| [28] | Minor W, Steczko J, Boguslaw Stec O, et al. Crystal structure of soybean lipoxygenase L-1 at 1. 4 Å resolution[J]. Biochemistry, 1996, 35(33):10687-10701. |
| [29] | Gilbert NC, Niebuhr M, Tsuruta H, et al. A covalent linker allows for membrane targeting of an oxylipin biosynthetic complex[J]. Biochemistry, 2008, 47(40):10665-10676. |
| [30] | Garreta A, Carpenai Vilella X, Busquets Abió M, et al. Crystalliza-tion and resolution of the lipoxygenase of Pseudomonas aeruginosa 42A2 and phylogenetic study of the subfamilies of the lipoxygenases[M]// Muñoz-Torrero D. Recent Advances in Pharmaceutical Sci-ences, India:Transworld Research Network, 2011:247-273. |
| [31] | Corbin JA, Evans JH, Landgraf KE, et al. Mechanism of specific membrane targeting by C2 domains:localized pools of target lipids enhance Ca2+ affinity[J]. Biochemistry, 2007, 46(14):4322-4336. |
| [32] | Chahinian H, Sias B, Carriere F. The C-terminal domain of pancreatic lipase:functional and structural analogies with C2 domains[J]. Current Protein and Peptide Science, 2000, 1(1):91-103. |
| [33] | Dainese E, Angelucci CB, Sabatucci A, et al. A novel role for iron in modulating the activity and membrane-binding ability of a trimmed soybean lipoxygenase-1[J]. FASEB Journal, 2010, 24(6):1725-1736. |
| [34] | Walther M, Anton M, Wiedmann M, et al. The N-terminal domain of the reticulocyte-type 15-lipoxygenase is not essential for enzymatic activity but contains determinants for membrane binding[J]. Journal of Biological Chemistry, 2002, 277(30):27360-27366. |
| [35] | Oldham ML, Brash AR, Newcomer ME. Insights from the X-ray crystal structure of coral 8R-lipoxygenase:calcium activation via a C2-like domain and a structural basis of product chirality[J]. Journal of Biological Chemistry, 2005, 280(47):39545-39552. |
| [36] | Kulkarni S, Das S, Funk CD, et al. Molecular basis of the specific subcellular localization of the C2-like domain of 5-lipoxygenase[J]. Journal of Biological Chemistry, 2002, 277(15):13167. |
| [37] | Hammel M, Walther M, Prassl R, et al. Structural flexibility of the N-terminal beta-barrel domain of 15-lipoxygenase-1 probed by small angle X-ray scattering. Functional consequences for activity regulation and membrane binding[J]. Journal of Molecular Biology, 2004, 343(4):917-929. |
| [38] | Eek P, Järving R, Järving I, et al. Structure of a calcium-dependent 11R-Lipoxygenase suggests a mechanism for Ca2+ regulation[J]. Journal of Biological Chemistry, 2012, 287(26):22377-22386. |
| [39] | Saam J, Ivanov I, Walther M, et al. Molecular dioxygen enters the active site of 12/15-lipoxygenase via dynamic oxygen access channels[J]. Proceedings of the National Academy of Sciences of of the United States Of America, 2007, 104(33):13319. |
| [40] | Lu X, Zhang J, Liu S, et al. Overproduction, purification, and characterization of extracellular lipoxygenase of Pseudomonas aeruginosa in Escherichia coli[J]. Applied Microbiology and Biotechnology, 2013, 97(13):5793-5800. |
| [41] | Hornung E, Walther M, Kuhn H, et al. Conversion of cucumber linoleate 13-lipoxygenase to a 9-lipoxygenating species by site-directed mutagenesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(7):4192-4197. |
| [42] | Sloane DL, Leung R, Craik CS, et al. A primary determinant for lipoxygenase positional specificity[J]. Nature, 1991, 354(6349):149-152. |
| [43] | Gillmor SA, Villasenor A, Fletterick R, et al. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity[J]. Nature Structural Biology, 1997, 4(12):1003-1009. |
| [44] | Schneider C, Pratt DA, Porter NA, et al. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis[J]. Chemistry & biology, 2007, 14(5):473-488. |
| [45] | Coffa G, Brash A. A single active site residue directs oxygenation stereospecificity in lipoxygenases:stereocontrol is linked to the position of oxygenation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(44):15579. |
| [46] | Coffa G, Schneider C, Brash AR. A comprehensive model of positional and stereo control in lipoxygenases[J]. Biochemical and Biophysical Research Communications, 2005, 338(1):87-92. |
| [47] | Kobe MJ, Neau DB, Mitchell CE, et al. The structure of human 15-Lipoxygenase-2 with a substrate mimic[J]. Journal of Biological Chemistry, 2014, 289(12):8562-8569. |
| [48] | Neau DB, Bender G, Boeglin WE, et al. Crystal structure of a lipoxygenase in complex with substrate the arachidonic acid-binding site of 8R-lipoxygenase[J]. Journal of Biological Chemistry, 2014, 289(46):31905-31913. |
| [49] | Maccarrone M, Salucci ML, van Zadelhoff G, et al. Tryptic digestion of soybean lipoxygenase-1 generates a 60 kDa fragment with improved activity and membrane binding ability[J]. Biochemistry, 2001, 40(23):6819-6827. |
| [50] | Di Venere A, Salucci ML, van Zadelhoff G, et al. Structure-to-function relationship of mini-lipoxygenase, a 60-kDa fragment of soybean lipoxygenase-1 with lower stability but higher enzymatic activity[J]. Journal of Biological Chemistry, 2003, 278(20):18281-18288. |
| [51] | Palmieri-Thiers C, Alberti JC, Canaan S, et al. Identification of putative residues involved in the accessibility of the substrate-binding site of lipoxygenase by site-directed mutagenesis studies[J]. Archives of Biochemistry and Biophysics, 2011, 509(1):82-89. |
| [52] | Lu X, Liu S, Feng Y, et al. Enhanced thermal stability of Pseudomonas aeruginosa lipoxygenase through modification of two highly flexible regions[J]. Applied Microbiology and Biotechnology, 2013, 97(21):9419-9427. |
| [53] | Lu X, Liu S, Zhang D, et al. Enhanced thermal stability and specific activity of Pseudomonas aeruginosa lipoxygenase by fusing with self-assembling amphipathic peptides[J]. Applied Microbiology and Biotechnology, 2013, 97(21):9419-9427. |
| [54] | Herman RP, Hamberg M. Properties of the soluble arachidonic acid 15-lipoxygenase and 15-hydroperoxide isomerase from the oomycete Saprolegnia parasitica[J]. Prostaglandins, 1987, 34(1):129-139. |
| [55] | Cristea M, Engstrom K, Su C, et al. Expression of manganese lipoxygenase in Pichia pastoris and site-directed mutagenesis of putative metal ligands[J]. Archives of Biochemistry and Biophysics, 2005, 434(1):201-211. |
| [56] | Bae JH, Hou CT, Kim HR. Thermostable lipoxygenase is a key enzyme in the conversion of linoleic acid to trihydroxy-octadecenoic acid by Pseudomonas aeruginosa PR3[J]. Biotechnology and Bioprocess Engineering, 2010, 15(6):1022-1030. |
| [57] | Steczko J, Donoho GA, Dixon JE, et al. Effect of ethanol and low-temperature culture on expression of soybean lipoxygenase L-1 in Escherichia coli[J]. Protein Expression and Purification, 1991, 2(2-3):221-227. |
| [58] | Knust B, Wettstein D. Expression and secretion of pea-seed lipoxygenase isoenzymes in Saccharomyces cerevisiae[J]. Applied Microbiology and Biotechnology, 1992, 37(3):342-351. |
| [59] | Reddy RG, Yoshimoto T, Yamamoto S, et al. Expression, purifica-tion, and characterization of porcine leukocyte 12-lipoxygenase produced in the methylotrophic yeast, Pichia pastoris[J]. Biochemical and Biophysical Research Communications, 1994, 205(1):381-388. |
| [60] | Vidal-Mas J, Busquets M, Manresa A. Cloning and expression of a lipoxygenase from Pseudomonas aeruginosa 42A2[J]. Antonie Van Leeuwenhoek International Journal, 2005, 87(3):245-251. |
| [61] | Zhang C, Tao T, Ying Q, et al. Extracellular production of lipoxygenase from Anabaena sp. PCC 7120 in Bacillus subtilis and its effect on wheat protein[J]. Applied Microbiology and Biotechnology, 2012, 94(4):949-958. |
| [62] | 陆信曜, 徐智, 刘松, 等. 重组大肠杆菌生产脂肪氧合酶的发酵优化[J]. 食品科技, 2013, 5:31-36. |





