2. 广东省昆虫研究所 广东省野生动物保护与利用公共实验室 广东省农业害虫综合治理重点实验室,广州 510260
2. Guangdong Entomological Institute,Guangdong Public Laboratory of Wild Animal Conservation and Utilization,Guangdong Key Laboratory of Integrated Pest Management in Agriculture,Guangzhou 510260
纤维素是自然界中分布最广、含量最丰富的一种可再生性生物资源,纤维素的利用与生物转化对于解决目前世界能源危机以及粮食短缺、环境污染等问题具有十分重要的意义[1]。尽管许多微生物在纤维素类物质降解中起到了非常重要的作用,但降解缓慢,故目前纤维素的高效生物转化仍存在诸多亟待解决的问题。一些植食性昆虫与纤维素能源利用密切相关而受到关注,特别是木食性昆虫(Xylophagous insects)能通过纤维素酶高效利用纤维素类物质,从中获取足够营养维持自身生长发育的需要[2, 3],如食木蜚蠊、食木白蚁、天牛和木蜂等。其中,白蚁(蜚蠊目Blattodea:白蚁总科Termitoidae)是消化纤维素最有效的昆虫类群,其降解效率可达74%-99%[4, 5],而且每年消化的纤维素类物质总量巨大,被认为是地球上最小的高效生物反应器[6]。但是,白蚁被认为是完全依靠后肠共生微生物来完成纤维素的降解,白蚁具有内源性纤维素酶的观点虽有报道却长期受到质疑。直到1998年,栖北散白蚁(Reticulitermes speratus)唾液腺纤维素酶基因RsEG的克隆[7],人们才开始明确认识到白蚁内源性纤维素酶和共生微生物的外源性纤维素酶共同参与了纤维素的降解,内源性纤维素酶基因开始受到关注,特别是在白蚁中最大的类群白蚁科(Termitidae)[8]。白蚁高效利用纤维素的肠道生境条件和丰富的纤维素酶及其基因,为人类转化和利用生物质能的研究提供了可借鉴的模型,在纤维素的生物能源转化,以及环保型白蚁防治剂作用靶标的研究开发均具有重要意义[9]。为了推动相关研究的深入,本文对白蚁内源性纤维素酶基因克隆和表达等研究进展进行了综述。
1 白蚁消化系统和内源性纤维素酶基于低等白蚁肠道纤维素酶的分布,白蚁的双重纤维素消化系统(Dual cellulose-digesting system)首次被Nakashima等[10]提出,即源自白蚁自身的内源纤维素酶的消化系统和基于后肠共生微生物产生的纤维素酶的消化系统。其后,有观点认为白蚁的这两个纤维素消化系统其实是统一的,两者是连续发生并且共同作用[11, 12],反对者认为该观点的论据恰好是支持白蚁具有双重纤维素消化系统[13]的观点。白蚁纤维素消化应是内源性和共生微生物的纤维素酶协同作用,低等白蚁后肠的共生原生动物(厌氧鞭毛虫)在吞噬、降解经白蚁破碎的木质纤维素颗粒发挥了重要作用[14],而为数众多的高等白蚁则是内源性纤维素酶发挥了重要作用[6, 15],当然在巢体内的共生真菌[16]和白蚁后肠的共生细菌[11]分泌的外源性纤维素酶亦参与了纤维素的有效降解,以维持白蚁自身和共生微生物的生长。
一般认为将纤维素分解为葡萄糖需要3类纤维素酶,包括外切-β-1,4-葡聚糖酶(exo-1,4-β-D-glucanase,CBH,EC 3.2.1.91)、内切-β-1,4-葡聚糖酶(endo-1,4-β-glucanase,EG,EC 3.2.1.4)和β-葡萄糖苷酶(β-1,4-D-glucosidase,BG,EC 3.2.1.21)[17]。白蚁内源性纤维素酶包括内切-β-1,4-葡聚糖酶(EG)和β-葡萄糖苷酶(BG),主要由白蚁的唾液腺和中肠分泌产生。内切-β-1,4-葡聚糖酶主要作用于纤维素分子内部的非结晶区,随机水解β-1,4糖苷键,将长链纤维分子截断,产生带有非还原性末端的小分子纤维素和可溶性纤维寡聚糖;β-葡萄糖苷酶的主要作用是水解纤维二糖及低分子量的纤维寡糖生成葡萄糖。
2 白蚁内源性纤维素酶基因白蚁内源性的EG酶cDNA全长测序显示它们都存在一个催化结构域,并且这个结构域与其他动物的GHF9(糖基水解酶家族9)氨基酸序列的催化位点一致[10],这表明白蚁内源性的EG酶属于GHF9,每种白蚁都包括个别来自GHF9的EG酶基因和GHF1家族的BG酶基因,而白蚁共生微生物存在多个EG酶、BG酶和CBH酶基因,远大于白蚁的内源性纤维素酶基因。除了山林原白蚁(Hodotermopsis sjostedti)中肠的EST分析得到一个属于GHF3的基因外,目前报道的白蚁内源性的BG酶均属于GHF1[18]。美洲散白蚁(R. flavipes)的一内源性的EG酶基因经RNA干扰处理,白蚁甚至出现一定程度的死亡[19],说明内源性纤维素酶在白蚁消化甚至生长发育中起着不可或缺的作用。
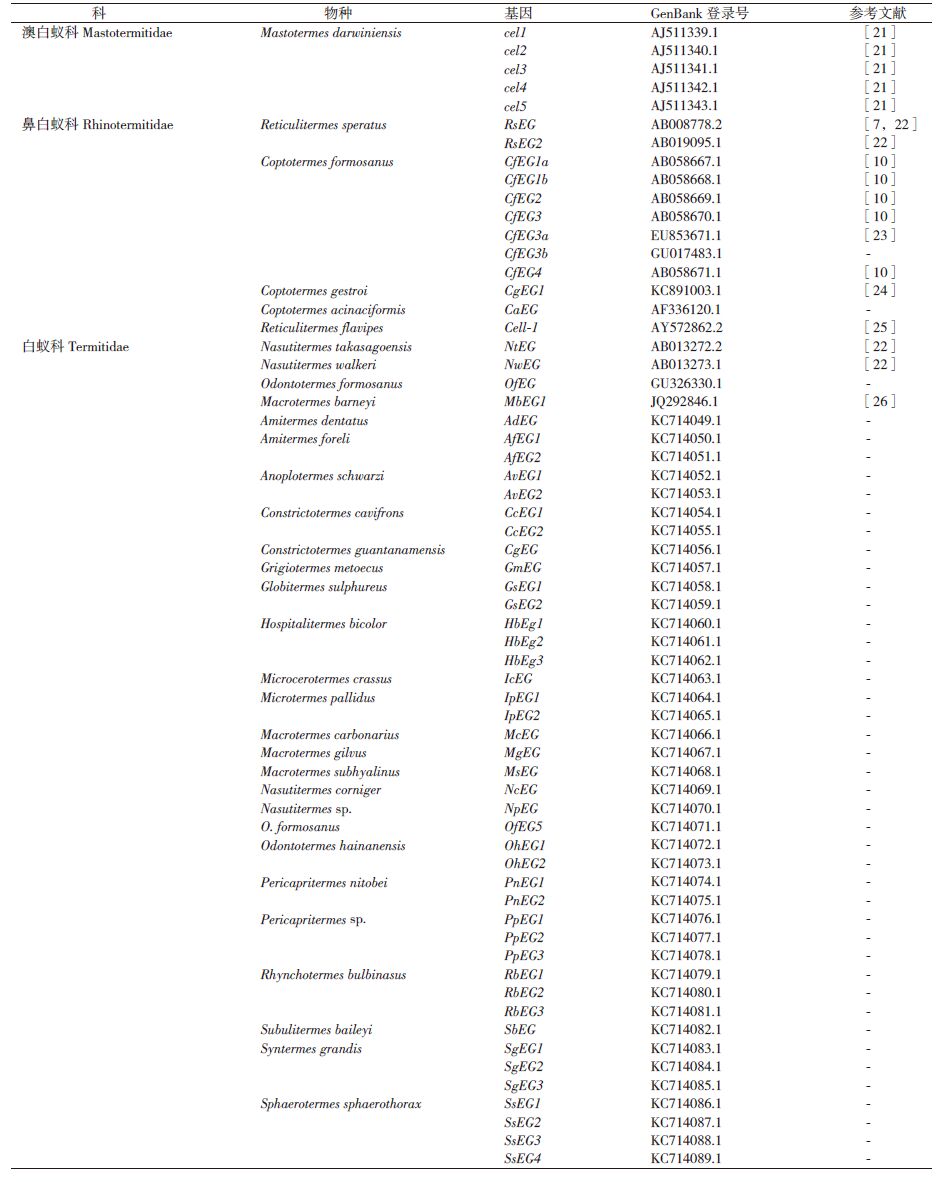
栖北散白蚁内源性的内切葡聚糖酶活性的发现[20],特别是其编码基因RsEG的克隆[7],使得白蚁自身能够产生纤维素酶得到广泛认可。根据美国国家生物技术信息中心的 GenBank数据,到目前为止已有4科18属33种白蚁的内源性纤维素酶基因克隆得到了cDNA全长序列,其中从32种白蚁中得到了62个完整的内源性EG酶基因cDNA编码序列,包括来自澳白蚁科和鼻白蚁科的6个低等白蚁种群,以及白蚁科的26个高等白蚁种群(表 1),每个白蚁种群的内切葡聚糖酶基因存在多个基因拷贝。
首个被克隆的β-葡萄糖苷酶基因是从恒春新白蚁(Neotermes koshunensis)中克隆的NkBG[27]。相比较于内切葡聚糖酶基因的研究,β-葡萄糖苷酶基因的研究较少,目前有11种白蚁的β-葡萄糖苷酶基因被克隆,获得了21个完整的内源性β-葡萄糖苷酶cDNA全长序列(表 2)。
多个基因拷贝的维持与定位被认为能够赋予原始基因新的功能、亚功能,同时保持每个基因拷贝功能的保守性[34]。白蚁的内源性纤维素酶基因也同时存在多个基因拷贝,这些同源基因组成一个基因家族,基因家族的成员彼此之间仅存在个别碱基的差异。虽然,白蚁内源性纤维素酶种内同源基因编码的纤维素酶共同作用于纤维素的降解,但每个种内同源基因编码的纤维素酶在纤维素的水解过程的功能意义仍有待研究。
3 白蚁内源性纤维素酶基因的体内表达白蚁内源性纤维素酶基因的表达场所主要是唾液腺和中肠。低等白蚁内源性纤维素酶基因主要在唾液腺表达,高等白蚁内源性纤维素酶基因主要在中肠表皮表达,即白蚁内源性纤维素酶基因在进化过程中的体内表达位点从唾液腺发展至中肠,但是培菌性的高等白蚁黑翅土白蚁(Odontotermes formosanus)内源性EG酶主要在唾液腺表达[15],木食性的高等白蚁高山象白蚁(Nasutitermes takasagoensis)内源性BG酶在中肠和唾液腺均有明显的表达[8, 31]。
低等白蚁如格斯特乳白蚁(Coptotermes gestroi)、恒春新白蚁和栖北散白蚁等,其唾液腺和前肠的纤维素酶活力存在明显的差异[35]。尽管前肠不是这些白蚁的分泌组织,但也存在较低的纤维素酶活力,这很有可能是在某种信号的诱导下,唾液腺表达分泌的纤维素酶随唾液流入到前肠,这种信号分子包括诱食信息素[36, 37]、抗生素[38]和蛋白类信息素[39]等。低等白蚁中肠纤维素酶可能由于中肠分泌的某些蛋白酶分解了一部分唾液腺分泌的纤维素酶,而导致较低的纤维素酶活力[40],而且在中肠内的分布也不均。
工业上应用于木质纤维素生物质能转化最广泛的里氏木霉菌(Trichoderma reesei)[41],是研究纤维素酶基因表达和分泌的模式系统。该系统存在多个表达调控因子,可对纤维素酶基因的表达进行连续的严谨控制[42, 43],但由于里氏木霉菌分泌的纤维素酶较为单一,其中85%都属于葡萄糖外切酶[44],而工业生物质能的转化普遍需要混合酶系。因此,如果将白蚁内源性纤维素酶在里氏木霉菌中表达将可以填补该系统中缺乏的葡萄糖内切酶及糖苷酶成分。
4 白蚁纤维素酶基因的体外表达与活性白蚁内源性纤维素酶在白蚁的纤维素降解过程中起着至关重要的作用,目前白蚁内源性纤维素酶基因在原核和真核宿主中已经成功实现超表达(表 3)。2004年,Lee等[45]利用昆虫杆状病毒表达系统表达出了桑天牛高活性内切酶重组蛋白,是昆虫纤维素酶第一次在体外表达有活性的蛋白。白蚁纤维素酶的外源表达开始很难实现,虽然通过刚果红平板法检测到高山象白蚁的NtEG基因的重组蛋白存在功能性表达[22],但首次在大肠杆菌(Escherichia coli)中实现超表达的白蚁纤维素酶基因的是4种白蚁[栖北散白蚁、高山象白蚁、台湾乳白蚁和短刀乳白蚁(C. acinaciformis)]的内切酶基因突变体[46]。随后,进行进一步的基因改组,又得到了PA68突变体酶,同样在大肠杆菌中实现超表达[47]。近年来,多种白蚁纤维素酶基因在原核生物大肠杆菌中成功表达[9, 23, 33, 48, 49]。相较于原核生物表达系统,真核生物表达系统能够表达更高活性的白蚁纤维素酶重组蛋白,栖北散白蚁的RsEG基因和高山象白蚁的NtEG基因在米曲霉(Aspergillus oryzae)中表达出高活性的纤维素酶重组蛋白,比活性分别高达1 200 U/mg和1 392 U/mg[50]。恒春新白蚁的NkEG基因也在米曲霉中成功表达出有活性的纤维素酶重组蛋白[51],白蚁纤维素酶基因在真菌中成功表达的还有毕赤酵母(Pichia pastoris)[52, 53]。与此同时,美洲散白蚁的RfBGlu-1基因在粉纹夜蛾(Trichoplusis ni)的Sf9细胞(一种昆虫表达系统的宿主细胞)中成功表达,并且以杆状病毒作为载体,重组的白蚁纤维素酶基因最大活性高达638 U/mg[28]。
近一个世纪以来,白蚁对木质纤维素降解的高效性吸引了许多学者的注意,但长期以来人们普遍认为白蚁完全依赖共生微生物来降解纤维素。随着新兴的高通量检测技术的发展,白蚁纤维素酶基因的相关研究得到了极大的关注,多种白蚁的内源性纤维素酶基因成功克隆,白蚁自身能够产生纤维素酶得到证实。低等白蚁肠道内源性纤维素酶在分解纤维素中发挥重要作用[54],而木食性高等白蚁纤维素的降解对内源性纤维素酶的依赖程度更高[6, 8, 15, 55]。当然高等白蚁后肠微生物在纤维素降解过程中也起着重要作用[56],同时高等白蚁后肠中的细菌群落的蛋白组学研究表明,相较于纤维素降解,后肠细菌产生的水解酶在新陈代谢(包括固氮作用、同化作用、异化作用和氨基酸的合成)中发挥更重要的作用[57]。最近的组学研究引起了关于白蚁生物学、生理学以及生物化学方面新信息的大爆炸,为生物技术进入新的既有的领域创造了空前的机会[57]。目前,只有两种白蚁的全基因组已经测得,包括低等白蚁内华达动白蚁(Zootermopsis nevadensis)[58]和高等白蚁纳塔尔大白蚁(Macrotermes natalensis)[59],这两种白蚁全基因组信息为更多白蚁基因组的组装提供了依据,以促进进化和生物过程相关基因的研究[60]。根据内华达动白蚁的全基因组序列分析,从中发现了大量与白蚁生长发育密切相关的基因[58]。在对格斯特乳白蚁转录组研究中发现新的内源性纤维素酶基因[61]。美洲散白蚁肠道宏转录组(Metatranscriptome)分析显示,白蚁具有消化半纤维素和木质素的漆酶基因和过氧化酶基因[62]。除了基于基因组学和转录组学的研究之外,利用高分辨率固体核磁13C NMR技术从代谢组学的角度分析13C标记的纤维素在山林原白蚁肠道中的降解过程,证实了内源性纤维素酶在纤维素消化过程中的重要性,并且表明后肠细菌主要作用于纤维糊精或纤维二糖的磷酸解作用[63]。
运用新的生物技术发现更多的纤维素酶基因,并从中选取能够在外源体系中表达高活性酶的基因,将能够解决工业生产中纤维素酶体外表达活性弱,产量低等问题。尽管模仿白蚁消化系统的想法也存在已久,但将这一高效纤维素分解系统在工业应用仍有诸多问题亟待解决。目前,仅有少数白蚁内源性纤维素酶能够在原核和真核生物表达系统中实现超表达。而对于生物质能的转化,需要的纤维素酶能够是低成本的大量表达,白蚁内源性纤维素酶在大肠杆菌中的表达大多只能得到少量的不可溶无水解酶活性的包涵体,目前在大肠杆菌能够以活性状态进行表达的白蚁内源性纤维素酶基因较少。因此,筛选体外高效表达并表达出活性状态的重组蛋白的基因克隆,一方面有必要对纤维素酶本身蛋白质序列进行改造以适应原核表达系统,同时寻求能够过度表达白蚁纤维素酶基因的真核表达系统,以实现白蚁内源性纤维素酶在工业生产上的应用。
| [1] | Carroll A, Somerville C. Cellulosic biofuels[J]. Annual Review of Plant Biology, 2009, 60(1):165-182. |
| [2] | Watanabe H, Tokuda, G. Animal Cellulases[J]. Cellular and Molecular Life Science, 2001, 58(9):1167-1178. |
| [3] | Watanabe H, Tokuda, G. Cellulolytic systems in insects[J]. Annual Review of Entomology, 2010, 55:609-632. |
| [4] | Prins RA, Kreulen DA. Comparative aspects of plant cell wall digestion in insects[J]. Animal Feed Science and Technology, 1991, 32(1-3):101-118. |
| [5] | Breznak JA, Brune A. Role of microorganisms in the digestion of lignocellulose by termite[J]. Annual Review of Entomology, 1994, 39:453-487. |
| [6] | Ohkuma M. Termite symbiotic systems:efficient bio-recycling of lignocelluloses[J]. Applied Microbiology and Biotechnology, 2003, 61(1):1-9. |
| [7] | Watanabe H, Noda H, Nakamura M, et al. A cellulase gene of termite origin[J]. Nature, 1998, 394(6691):330-331. |
| [8] | Tokuda G, Watanabe H, Hojo M, et al. Cellulolytic environment in themidgut of the wood-feeding higher termite Nasutitermes takasagoensis[J]. Journal of Insect Physiology, 2012, 58(1):147-154. |
| [9] | Zhang D, Lax AR, Bland JM, et al. Characterization of a new endogenous endo-β-1, 4-glucanase of Formosan subterranean termite(Coptotermes formosanus)[J]. Insect Biochemistry and Molecular Biology, 2011, 41(4):211-218. |
| [10] | Nakashima K, Watanabe H, Saitoh H, et al. Dual cellulosedigesting system of the wood-feeding termite, Coptotermes formosanus(Shiraki)[J]. Insect Biochemistry and Molecular Biology, 2002, 32(7):777-784. |
| [11] | Scharf ME, Karl ZJ, Sethi A, Boucias DG. Multiple levels of synergistic collaboration in termite lignocellulose digestion[J]. PLoS One, 2011, 6(7):e21709. |
| [12] | Zhou X, Smith JA, Oi FM, et al. Correlation of cellulase gene expression and cellulolytic activity throughout the gut of the termite Reticulitermes flavipes[J]. Gene, 2007, 395(1-2):29-39. |
| [13] | Tokuda G, Watanabe H, Lo N. Does correlation of cellulase gene expression and cellulolytic activity in the gut of termite suggest synergistic collaboration of cellulases?[J]. Gene, 2007, 401(1-2):131-134. |
| [14] | Brune A, Friedrich M. Microecology of the termite gut:structure and function on a microscale[J]. Current Opinion in Microbiology, 2000, 3(3):263-269. |
| [15] | Tokuda G, Lo N, Watanabe H, et al. Major alteration of the expression site of endogenous cellulases in members of an apical termite lineage[J]. Molecular Ecology, 2004, 13(10):3219-3228. |
| [16] | Aanen DK, Eggleton P, Rouland-Lefèvré C, et al. The evolution of fungus-growing termites and their mutualistic fungal symbionts[J]. Proceedings of the National Academy of Sciences, 2002, 99(23):14887-14892. |
| [17] | 孙建中, 陈春润. 昆虫与生物质能源利用:一个新的交叉学科前沿[J]. 昆虫知识, 2010, 47(6):1033-1042. |
| [18] | Yuki M, Moriya S, Inoue T, et al. Transcriptome analysis of the digestive organs of Hodotermopsis sjostedti, a lower termite that hosts mutualistic microorganisms in its hindgut[J]. Zoological Science, 2008, 25(4):401-406. |
| [19] | Zhou X, Wheeler MM, Oi FM, et al. RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA[J]. Insect Biochemistry and Molecular Biology, 2008, 38(8):805-815. |
| [20] | Watanabe H, Nakamura M, Tokuda G, et al. Site of secretion and properties of endogenous endo-β-1, 4-glucanase components from Reticulitermes speratus(Kolbe), a Japanese subterranean termite[J]. Insect Biochemistry and Molecular Biology, 1997, 27(4):305-313. |
| [21] | Li L, Fröhlich J, Pfeiffer P, et al. Termite gut symbiotic archaezoa are becoming living metabolic fossils[J]. Eukaryotic Cell, 2003, 2(5):1091-1098. |
| [22] | Tokuda G, Lo N, Watanabe H, et al. Metazoan cellulase genes from termites:intron/exon structures and sites of expression[J]. Biochimica et Biophysica Acta -Gene Structure and Expression, 1999, 1447(2-3):146-159. |
| [23] | Zhang D, Lax AR, Raina AK, et al. Differential cellulolytic activity of native-form and C-terminal tagged-form cellulase derived from Coptotermes formosanus and expressed in E. coli. [J]. Insect Biochemistry and Molecular Biology, 2009, 39(8):516-522. |
| [24] | Cairo JP, Oliveira LC, Uchima CA, et al. Deciphering the synergism of endogenous glycoside hydrolase families 1 and 9 from Coptotermes gestroi[J]. Insect Biochemistry and Molecular Biology, 2013, 43(10):970-981. |
| [25] | Scharf ME, Wu-Scharf D, Zhou X, et al. Gene expression profiles among immature and adult reproductive castes of the termite Reticulitermes flavipes[J]. Insect Molecular Biology, 2005, 14(1):31-44. |
| [26] | Ni J, Wu Y, Yun C, et al. cDNA cloning and heterologous expression of an endo-β-1,4-glucanase from the fungus-growing termite Macrotermes barneyi[J]. Archives of Insect Biochemistry and Physiology, 2014, 86(3):151-164. |
| [27] | Tokuda G, Saito H, Watanabe H. A digestiveb-glucosidase from the salivary glands of the termite, Neotermes koshunensis(Shiraki):distribution, characterization and isolation of its precursor cDNA by 5’- and 3’-RACE amplifications with degenerate primers[J]. Insect Biochemistry and Molecular Biology, 2002, 32(12):1681-1689. |
| [28] | Scharf ME, Kovaleva ES, Jadhao S, et al. Functional and translational analyses of a beta-glucosidase gene(glycosyl hydrolase family 1)isolated from the gut of the lower termite Reticulitermes flavipes[J]. Insect Biochemistry and Molecular Biology, 2010, 40(8):611-620. |
| [29] | Zhang D, Lax AR, Bland JM, et al. Hydrolysis of filter-paper cellulose to glucose by two recombinant endogenous glycosyl hydrolases of Coptotermes formosanus[J]. Insect Science, 2010, 17(3):245-252. |
| [30] | Zhang D, Lax AR, Henrissat B, et al. Carbohydrate-active enzymes revealed in Coptotermes formosanus(Isoptera:Rhinotermitidae)transcriptome[J]. Insect Molecular Biology, 2012a, 21(2):235-245. |
| [31] | Tokuda G, Miyagi M, Makiya H, et al. Digestive beta-glucosidases from the wood-feeding higher termite, Nasutitermes takasagoensis:Intestinal distribution, molecular characterization, and alteration in sites of expression[J]. Insect Biochemistry and Molecular Biology, 2009, 39(12):931-937. |
| [32] | Wu Y, Chi S, Yun C, et al. Molecular cloning and characterization of an endogenous digestive beta-glucosidase from the midgut of the fungus-growing termite Macrotermes barneyi[J]. Insect Molecular Biology, 2012, 21(6):604-614. |
| [33] | Bujang NS, Harrison NA, Su NY. Molecular cloning of five beta-glucosidases from four species of higher termites(Blattodea:Termitidae)[J]. Annals of the Entomological Society of America, 2014, 107(1):251-256. |
| [34] | Hahn MW. Distinguishing among evolutionary models for the maintenance of gene duplicates[J]. Journal of Heredity, 2009, 100(5):605-617. |
| [35] | Tokuda G, Lo N, Watanabe H. Marked variations in patterns of cellulase activity against crystalline- versus carboxymethyl-cellulose in the digestive systems of diverse, wood-feeding termites[J]. Physiological Entomology, 2005, 30(4):372-380. |
| [36] | Reinhard J, Kaib M. Food exploitation in termites:indication for a general feeding stimulating signal in labial gland secretion of Isoptera[J]. Journal of Chemical Ecology, 2001a, 27(1):189-201. |
| [37] | Reinhard J, Kaib M. Thin-layer chromatography assessing feeding stimulation by labial gland secretion compared to synthetic chemic-als in the subterranean termite Reticulitermes santonensis[J]. Journal of Chemical Ecology, 2001b, 27(1):175-187. |
| [38] | Lamberty M, Zachary D, Lanot R, et al. Insect immunity:Constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect[J]. Journal of Biological Chemistry, 2001, 276(6):4085-4092. |
| [39] | Matsuura K, Yashiro T, Shimizu K, et al. Cuckoo fungus mimics termite eggs by producing the cellulose-digesting enzyme β-glucosidase[J]. Current Biology, 2009, 19(1):30-36. |
| [40] | Fujita A, Shimizu I, Abe T. Distribution of lysozyme and protease, and amino acid concentration in the guts of a wood-feeding termite, Reticulitermes speratus(Kolbe):possible digestion of symbiont bacteria transferred by trophallaxis[J]. Physiological Entomology, 2001, 26(2):116-123. |
| [41] | Xu Q, Singh A, Himmel ME. Perspectives and new directions for the productionof bioethanol using consolidated bioprocessing of lignocellulose[J]. CurrentOpinion in Biotechnology, 2009, 20(3):364-371. |
| [42] | Seiboth B, Karimi RA, Phatale PA, et al. The putative protein met-hyltransferase LAE1 controlscellulase gene expression in Trichod-erma reeseimmi[J]. Molecular Microbiology, 2012, 84(6):1150-1164. |
| [43] | Portnoy T, Margeot A, Linke R, et al. The CRE1 carbon catabolite repressor of thefungus Trichoderma reesei:a master regulator of carbon assimilation[J]. BMC Genomics, 2011, 12:269. |
| [44] | Zou G, Shi S, Jiang Y, et al. Construction of a cellulase hyper-expressionsystem in Trichoderma reesei by promoter andenzyme engineering[J]. Microbial Cell Factories, 2012, 11:21. |
| [45] | Lee SJ, Kim SR, Yoon HJ, et al. cDNA cloning, expression, and enzymatic activity of a cellulase from the mulberry longicorn beetle, Apriona germari[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2004, 139(1):107-116. |
| [46] | Ni J, Takehara M, Watanabe H. Heterologous overexpression of a mutant termite cellulose gene in Escherichia coli by DNA shuffling of four orthologous parental cDNAs[J]. Bioscience, Biotechnology, and Biochemistry, 2005, 69(9):1711-1720. |
| [47] | Ni J, Tokuda G, Takehara M, et al. Heterologous expression and enzymatic characterization of β-glucosidase from the dry wood-eating termite, Neotermes koshunensis[J]. Applied Entomology and Zoology, 2007b, 42(3):457-463. |
| [48] | Zhang D, Allen AB, Lax AR. Functional analyses of the digestive β-glucosidase of Formosan subterranean termites(Coptotermes formosanus)[J]. Journal of Insect Physiology, 2012b, 58(1):205-210. |
| [49] | Hirayama K, Watanabe H, Tokuda G, et al. Purification and characterization of termite endogenous β-1, 4-endoglucanases produced in Aspergillus oryzae[J]. Bioscience, Biotechnology, and Biochemistry, 2010, 74(8):1680-1686. |
| [50] | Ni J, Takehara M, Miyazawa M, et al. Random exchanges of non-conserved amino acid residues among four parental termite cellulases by family shuffling improved thermostability[J]. Protein Engineering, Design and Selection, 2007a, 20(11):535-542. |
| [51] | Uchima CA, Tokuda G, Watanabe H, et al. Heterologous expression and characterization of a glucose-stimulated β-glucosidase from the termite Neotermes koshunensis in Aspergillus oryzae[J]. Applied Microbiology and Biotechnology, 2011, 89(6):1761-1771. |
| [52] | Uchima CA, Tokuda G, Watanabe H, et al. Heterologous expression in Pichia pastoris and characterization of an endogenous thermostable and high glucose-tolerant β-glucosidase from the termite Nasutitermes takasagoensis[J]. Applied and Environmental Microbiology, 2012, 78(12):4288-4293. |
| [53] | Uchima CA, Tokuda G, Watanabe H, et al. A novel glucose-tolerant β-glucosidase from the salivary gland of the termite Nasutitermes takasagoensis[J]. The Journal of General and Applied Microbiology, 2013, 59(2):141-145. |
| [54] | Watanabe H, Nakashima K, Saito H, et al. New endo-β-1, 4-glucan-ases from the parabasalian symbionts, Pseudotrichonympha grassii and Holomastigotoides mirabile of Coptotermes termites[J]. Cellular and Molecular Life Science, 2002, 59(11):1983-1992. |
| [55] | Tokuda G, Watanabe H, Matsumoto T, et al. Cellulose digestion in the wood-eating higher termite, Nasutitermes takasagoensis(Shiraki):distribution of cellulases and properties of endo-beta-1, 4-glucanase[J]. Zoological Science, 1997, 14(1):83-93. |
| [56] | Warnecke F, Luginbuhl P, Ivanova N, et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite[J]. Nature, 2007, 450(7169):560-565. |
| [57] | Burnum KE, Callister SJ, Nicora CD, et al. Proteome insights into the symbiotic relationship between a captive colony of Nasutitermes corniger and its hindgut microbiome[J]. The ISME Journal, 2011, 5(1):161-164. |
| [58] | Terrapon N, Li C, Robertson HM, et al. Molecular traces of alterna-tive social organizationin a termite genome[J]. Nature Communi-cations, 2014, 5:3636. |
| [59] | Poulsen M, Hu H, Li C, et al. Complementary symbiont contributions to plant decomposition in a fungus-farming termite[J]. Proceed-ings of the National Academy of Sciences, 2014, 111(40):14500-14505. |
| [60] | Scharf ME. Omic research in termites:an overview and aroadmap[J]. Frontiers in Genetics, 2015, 6:76. |
| [61] | Leonardo FC, da Cunha AF, da Silva MJ, et al. Analysis of the workers headtranscriptome of the Asian subterraneantermite, Coptotermes gestroi[J]. Bulletin of Entomological Research, 2011, 101(4):383-391. |
| [62] | Tartar A, Wheeler MM, Zhou XG, et al. Parallel metatranscriptome analyses of host and symbiont gene expression in the gut of the termite Reticulitermes flavipes[J]. Biotechnology for Biofuels, 2009, 2:25. |
| [63] | Tokuda G, Tsuboi Y, Kihara K, et al. Metabolomic profiling of 13C-labelled cellulose digestion in a lower termite:insights into gut symbiont function[J]. Proceeding B of the Royal Society, 2014, 281:20140990. |