b. University of Chinese Academy of Sciences, Beijing 10049, China
Early stages of pathogen infection in plants are usually associated with the production of phytohormones. Although there are some exceptions, jasmonate (JA) and ethylene signaling pathways are usually induced for necrotrophic pathogen resistance, while the salicylic acid pathway for resistance against biotrophic and hemibiotrophic pathogens (Glazebrook, 2005). In response to Alternaria alternata, a notorious necrotrophic fungal pathogen causing blown spot disease in leaves of Nicotiana species (Sun et al., 2014a), wild tobacco Nicotiana attenuata plants activate both jasmonate (JA) and ethylene signaling pathways to defend against this pathogen (Sun et al., 2014b, 2017; Li and Wu, 2016). Plants impaired in either JA (or ethylene) production or perception are highly susceptible to the fungus (Sun et al., 2014b, 2017), supporting the idea that host plants activate JA and ethylene signaling pathways for resistance against necrotrophic pathogens.
Patatin is a glycoprotein which contributes about 40% of the total soluble protein in potato tubers (Racusen, 1983). The protein has a conserved esterase motif (Gly-X-Ser-X-Gly) and has been shown to have lipid acyl hydrolase activity, catalyzing the nonspecific hydrolysis of phospholipids, glycolipids, sulfolipids and mono- and diacylglycerols (Rydel et al., 2003). Recently, it has been found that patatin-like proteins (PLPs) are present in many plant species and have been reported to be involved in plant-pathogen interactions. In Arabidopsis, AtPLP2 expression is strongly induced in leaves after Botrytis cinerea infection (La Camera et al., 2005). Furthermore, plants silenced for AtPLP2 show increased resistance to B. cinerea, whereas plants overexpressing AtPLP2 are more susceptible to this necrotrophic fungus (La Camera et al., 2005), indicating that AtPLP2 plays a negative role in resistance against B. cinerea by facilitating fungal colonization.
In this study, an A. alternata-elicited NaPLP was identified. The role of NaPLP in resistance against A. alternata and transcriptional regulation of JA, ethylene, and phytoalexin scopoletin biosynthetic genes were investigated in NaPLP-silenced plants through a virusinduced gene silencing (VIGS) technique. In addition, the transcriptional regulations of this gene by A. alternata, JA and, ethylene were investigated in WT and plants impaired in JA or ethylene signaling pathways.
2. Materials and methods 2.1. Plant and fungal materialSeeds of the 31st generation of an inbred line of N. attenuata were used as the wild type (WT) genotype. Stably transformed lines of ethylene-deficient (irACO), ethylene-insensitive (Ov-etr1), JAdeficient (irAOC), and JA-insensitive (irCOI1) plants have been previously generated (von Dahl et al., 2007; Kallenbach et al., 2012; Paschold et al., 2007) and were provided by Prof. Ian T. Baldwin (Max-Planck Institute for Chemical Ecology, Jena, Germany). Seed germination and plant growth were conducted as described in Krügel et al. (2002). A. alternata were grown and used for inoculation as described in Sun et al. (2014a).
| primers | Primer sequence 5'-3' | purpose | citation | Accession number |
| LOX3_F | TCGATCCTCACCACCCTCAT | NaLOX3 | Sun et al.(2017) | AY254349 |
| LOX3_R | CGGGATCATTCTCATCGGGG | NaLOX3 | Sun et al.(2017) | AY254349 |
| AOS_F | CGTACCCGATCGGTTCGTAG | NaAOS | Sun et al.(2017) | XM_019399851.1 |
| AOS_R | ACCAGCACCACGAAATCCTT | NaAOS | Sun et al.(2017) | XM_019399851.1 |
| JS142_F | CGAGTTATGGCTAATGGCAGC | NaF60H1 | Sun et al.(2014a, b) | KF771989 |
| JS143_R | AGCACTTCAGCCAAAGGACC | NaF60H1 | Sun et al.(2014a, b) | KF771989 |
| Sh33_Fr | GGATTCGGAGGTTTGTGGGT | NaACS1 | Sun et al.(2017) | AY426752 |
| Sh34_Rr | GAACGAGTGGTGAGTGAGGG | NaACS1 | Sun et al.(2017) | AY426752 |
| Sh39_Fr | CTATTGAATCTGATGTCAAGCTG | NaACO1 | von Dahl et al.(2007) | AY426756 |
| Sh40_Rr | TATGTAGTAGGGACACACGCTT | NaACO1 | von Dahl et al.(2007) | AY426756 |
The specific 387-bp PCR fragment used for silencing NaPLP (XM_019396434) was amplified by the primer pair (CJ07: 5ʹ-CATAAGCTTCCCTTCTCTGCTGCCAAAG-3ʹ and CJ08: 5ʹ-CATGGATCCGCAGCAACAACACCACCATC-3ʹ), and subsequently cloned into pTV00. To generate NaPLP-silenced plants (VIGS NaPLP), Agrobacterium tumefaciens (GV3101) cells carrying the above construct were combined with cells having pBINTRA, and were inoculated into leaves of N. attenuata. Green tissue of Phytoene desaturase (PDS)- silenced plants shows visible bleaching two to three weeks after transformation (Saedler and Baldwin, 2004; Wu et al., 2008), thus they were used as positive controls. When PDS-silenced plants began to bleach, the young rosette leaves of VIGS NaPLP and empty vector-transformed plants (EV plants) were collected for experiments. All VIGS experiments were repeated twice.
2.3. Methyl jasmonate (MeJA) and ethephon treatmentsMethyl jasmonate (MeJA) and ethephon treatments were conducted as described in Xu et al. (2018). Briefly, 1 mM MeJA (www.sigmaaldrich.com) and 5 mM ethephon (www.sangon.com) were prepared using distilled water, and sprayed with a fine mist individually or simultaneously on the source-sink transition leaves (young 0 leaves) of 35-day-old, rosette-staged N. attenuata plants until run off. The treated plants were immediately covered with a plastic bag, and harvested after 1, 3, 6, 12, 24, and 72 h. For control treatments, plants were sprayed with distilled water.
2.4. Real-time PCR assayTotal RNA of 4-5 replicate biological samples was extracted from a 1.5 × 1.5 cm2 area of leaf lamina with the inoculation site at the center. cDNA was synthesized from 500 ng total RNA with reverse transcriptase (Thermo Scientific). Real-time PCR was performed on a CFX Connect qPCR System (Bio-Rad) by using the iTaq Universal SYBR Green Supermix (Bio-Rad) and gene-specific primers (CJ05: 5'- TGCTGGGACATACACAGCAG 3ʹ; CJ06: 5'- CCAAGAGCGCGATAGATGGT 30) as described by Wu et al. (2013). Transcript abundance of NaActin 2 was not altered either in leaves inoculated with A. alternata at 1 and 3 dpi or in leaves treated with MeJA and ethephon, and thus was used as an internal standard (Xu et al., 2018).
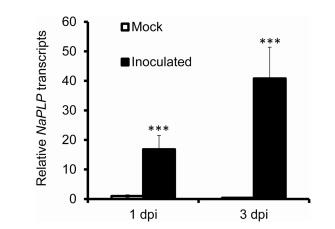
3. Results 3.1. A. alternata infection elicits NaPLP expressionTranscriptome analysis of N. attenuata source-sink transition leaves (0 leaves) inoculated with A. alternata revealed that a Patatin-like protein gene (NaPLP; Genebank accession number: XM_019396434) was highly elicited one day post inoculation (dpi). Sequence comparisons and alignments showed that this NaPLP protein has 65% sequence similarity to the potato tuber patatin and shares a strictly conserved serine hydrolase/lipase motif GXSXG (Fig. 1). To confirm the up-regulation of NaPLP expression after inoculation, its transcriptional levels were quantified by real-time PCR analysis at 1 and 3 dpi. Compared to mock controls, NaPLP transcripts increased to 16.8-fold at 1 dpi, and reached to 40.8-fold at 3 dpi (Fig. 2).

|
| Fig. 1 Comparison of patatin-like protein sequences. The amino acid sequence of NaPLP (Nicotiana attenuata, XP_019251979.1) was compared with AtPLP2 (Arabidopsis thaliana, AAM13304.1) and patatin (Solanum tuberosum, CAA25592.1). Consensus sequences are shaded with black and the serine hydrolase GXSXG motif is indicated in red font (bracket). |
|
| Fig. 2 Elicitation of NaPLP by A. alternata in WT plants. NaPLP transcripts were measured by real-time PCR in the source-sink transition leaves (0 leaves) of WT plants treated with mock or with A. alternata at 1 and 3 days post inoculation (dpi). All transcriptional levels were normalized with a housekeeping gene NaActin II. Values are means ± SE for five biological replicates. Asterisks indicate the level of significant difference between WT and transgenic plants with the same treatments (Student's t-test: ***, p < 0.005). |
To study the role of NaPLP in N. attenuata resistance to A. alternata, we silenced NaPLP via virus-induced gene silencing (VIGS). Compared with mock controls, transcripts of NaPLP were dramatically induced in young leaves of N. attenuata plants transformed with empty vector (EV) at 3 dpi; however, plants transformed with the NaPLP-silencing construct (VIGS NaPLP) showed a 92% reduction in NaPLP transcripts compared to EV plants with the same treatments, indicating effective silencing of the NaPLP (Fig. 3a).

|
| Fig. 3 Silencing NaPLP enhances plant resistance to A. alternata. a) Mean (±SE) NaPLP transcript levels were measured by real-time PCR in five biological replicates of young leaves of EV and VIGS NaPLP plants at 3 dpi. b) Mean (±SE) diameter of necrotic lesions of fifteen replicates of young leaves of EV and VIGS NaPLP plants at 6 dpi. Asterisks indicate the level of significant difference between EV and VIGS NaPLP plants (Student's t-test: *, p < 0.05; ***, p < 0.005). |
We measured the necrotic lesion diameter at 5 dpi in 0 leaves of VIGS NaPLP and EV plants to investigate whether NaPLP-silencing affects N. attenuata resistance to A. alternata. The lesion diameter in two independent VIGS experiments decreased significantly; the average diameter of lesions in EV plants was 6.14 ± 0.15 cm, whereas in VIGS NaPLP plants the average diameter was 4.56 ± 0.23 cm (Fig. 3b). This result indicates that NaPLP is a negative regulator of plant resistance to A. alternata.
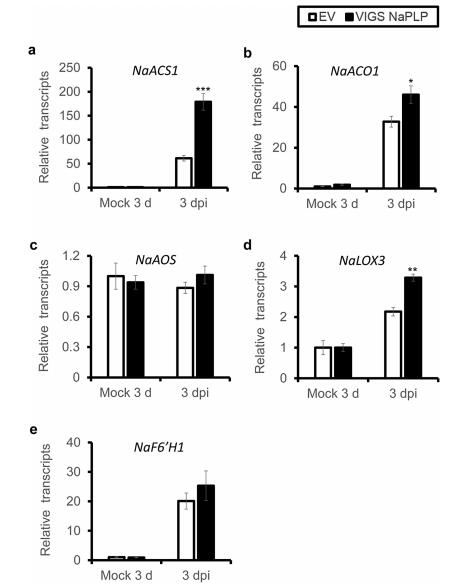
To further understand the role of NaPLP in plant resistance, we investigated the transcriptional levels of crucial genes for JA (NaAOS, NaLOX3), ethylene (NaACS1, NaACO1) and scopoletin (NaF6'H1) biosynthesis in VIGS NaPLP plants. Compared to EV plants, VIGS NaPLP plants had significantly higher transcriptional levels of NaACS1, NaACO1, and NaLOX3 (Fig. 4a, b, d); in contrast, NaAOS and NaF6'H1 were elicited to the same levels in EV and VIGS NaPLP plants (Fig. 4c, e). These results indicate that biosynthesis of JA and ethylene is enhanced in VIGS NaPLP plants, whereas scopoletin-based defense is not affected.
|
| Fig. 4 Effects of Silencing NaPLP on gene expression of JA and ethylene biosynthetic genes. Mean (±SE) NaACS1 (a), NaACO1 (b), NaAOS (c), NaLOX3 (d), NaF6'H1 (e) transcript levels were measured by real-time PCR in five biological replicates of young leaves of EV and VIGS NaPLP plants at 3 dpi. Asterisks indicate the level of significant difference between control and treated leaves (Student's t-test: *, p < 0.05; **, p < 0.01; ***, p < 0.005). |
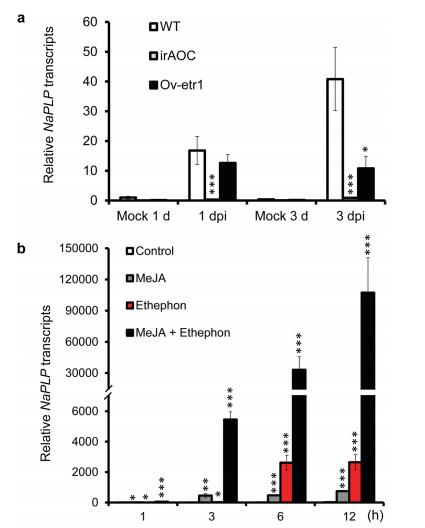
To test whether the elicitation of NaPLP expression by A. alternata is dependent on JA and ethylene signaling pathways, we investigated the expression of NaPLP in JA-deficient (irAOC) and ethylene insensitive (Ov-etr1) plants. The induction of NaPLP by A. alternata was completely abolished in irAOC plants at both 1 and 3 dpi (Fig. 5a), indicating that the elicitation of NaPLP expression is completely JA dependent. Ov-etr1 plants accumulated 75% of the NaPLP transcripts that WT had at 1 dpi, and only 26.4% at 3 dpi (Fig. 5a), suggesting that ethylene signaling is also required.
|
| Fig. 5 Elicitation of NaPLP transcripts by A. alternata in JA-deficient and ethyleneinsensitive plants; and by methyl jasmonate and ethephon in WT. a) NaPLP transcripts were measured by real-time PCR in the source-sink transition leaves (0 leaves) of WT, irAOC (JA-deficient) and Ov-etr1 (ethylene-insensitive) plants treated with mock or with A. alternata at 1 and 3 days post inoculation (dpi). b) Mean (±SE) NaPLP transcripts were measured by real-time PCR in five replicates of 0 leaves treated with water (as control), MeJA, and Ethephon individually and simultaneously at 1, 3, 6, and 12 h. Asterisks indicate the level of significant difference between control and treated leaves (Student's t-test: *, p < 0.05; **, p < 0.01; ***, p < 0.005). |
Because A. alternata-induced NaPLP expression was dependent on JA and ethylene signaling pathways, we reasoned that the transcriptional levels of NaPLP could be elicited by exogenous JA or ethylene treatments. NaPLP transcripts increased 12.4-fold one hour after MeJA treatment, and 457-fold and 474-fold at 3 and 6 h after treatment, respectively (Fig. 5b). Ethephon, a synthetic compound which decomposes into ethylene upon metabolism by plants, also increased NaPLP expression from 1.69-fold at 1 h, to 24.55-fold at 3 h, and 2619-fold at 6 h after treatment (Fig. 5b). Interestingly, co-treatment with MeJA and ethephon dramatically increased transcriptional levels of NaPLP, from 52.25-, 5457.13-, and 33129.40-fold after 1, 3 and 6 h treatments, respectively (Fig. 5b). These results indicate that NaPLP expression can be induced by JA and ethylene signaling both individually and synergistically.
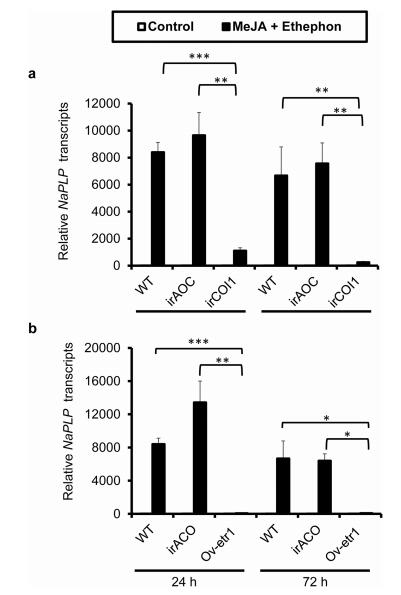
3.4. Synergistic induction of NaPLP is dependent on endogenous JA and ethylene signaling pathwaysTo test whether the synergistic induction of NaPLP by MeJA and ethephon was dependent on endogenous JA and ethylene signaling pathways, we treated the 0 leaves of WT, irAOC (JA-deficient), irCOI1 (JA-insensitive), irACO (ethylene-deficient), Ovetr1(ethylene-insensitive) plants with MeJA and ethephon simultaneously. The results revealed that NaPLP was synergistically induced in WT and irAOC plants at both 24 and 72 h, but not in irCOI1 plants after the same elicitation (Fig. 6a). Similarly, NaPLP was synergistically induced in WT and irACO plants but not in Ovetr1 plants (Fig. 6b). These results indicate that both JA and ethylene perception are required for the synergistic induction of NaPLP by MeJA and ethephon.
|
| Fig. 6 Synergistic induction of NaPLP by MeJA and ethephon is dependent on endogenous JA and ethylene signaling pathways. Mean (±SE) NaPLP transcripts were measured by real-time PCR in five biological replicates of 0 leaves in WT, irAOC (JA-deficient), irCOI1 (JA-insensitive), irACO (ethylene deficient), and Ov-etr1 (ethylene-insensitive) plants co-treated with MeJA and ethephon at 24 and 72 dpi. Asterisks indicate the level of significant difference between each genotypes co-treated with MeJA and ethephon (Student's t-test: *, p < 0.05; **, p < 0.01; ***, p < 0.005). |
Although patatin was initially identified as a major storage proteins, more and more reports indicate that patatin-like proteins exist widely in many plant species and play a role in plantpathogen interactions; for example, AtPLP2 in Arabidopsis confers susceptibility to necrotrophic fungus B. cinerea (La Camera et al., 2005). In this study, we also found that NaPLP is a susceptible factor in the N. attenuata - A. alternata pathosystem. Silencing NaPLP in N. attenuata plants increased the resistance to the pathogen A. alternata (Fig. 3). How NaPLP negatively regulates N. attenuata resistance to A. alternata remains unclear. We found that NaACS1, NaACO1 and NaLOX3 were elicited to a higher level in VIGS NaPLP plants at 3 dpi, which indicates that biosynthesis of JA and ethylene is enhanced in NaPLP-silenced plants. Lipid metabolism is known to play an important role in plant defense response, and the NaPLP protein shares 65% sequence similarity to the potato tuber patatin with a strictly conserved serine hydrolase/ lipase motif GXSXG (Fig. 1). This sequence similarity suggests that NaPLP may interfere with phospholipid-based signal transduction through nonspecific hydrolysis of phospholipids, which somehow directly or indirectly affects JA and ethylene biosynthesis, thereby reducing plant resistance to A. alternata.
JA and ethylene signaling pathways play central roles in plant defense against necrotrophic pathogens. In N. attenuata, they regulate a series of defense-related genes, including NaF6'H1, the gene encoding the key enzyme for phytoalexin scopoletin and scopolin biosynthesis (Sun et al., 2014b; Li and Wu, 2016); and NaPDR1 and NaPDR1-like, two pleiotropic drug resistance transporter genes required for resistance (Xu et al., 2018). NaPLP was highly elicited after A. alternata inoculation, and this induction was clearly dependent on JA and ethylene signaling pathways (Figs. 2 and 5). Indeed, exogenous treatments with either MeJA or ethephon induced NaPLP expression (Fig. 5). Interestingly, NaPLP transcripts were synergistically induced by MeJA and ethephon cotreatments, and this elicitation was completely dependent on endogenous JA and ethylene signaling pathways (Figs. 5 and 6). All these data indicate that the elicitation of NaPLP expression by A. alternata likely results from the synergistic induction of NaPLP by endogenous JA and ethylene signaling pathways. However, unlike other JA- and ethylene-regulated defense genes, silencing NaPLP increased plant resistance. Thus, this study provides a good example of a JA- and ethylene-regulated gene playing a negative role in pathogen resistance.
Taken together, our results indicate that we have identified an A. alternata-induced NaPLP gene, whose elicitation by the fungus likely results from JA and ethylene signaling pathways in a synergistic way, and unlike other JA- and ethylene-induced defense genes, it negatively affects plant resistance to the fungus possibly through suppression of JA and ethylene biosynthesis.
We thank Prof. Ian T. Baldwin (Max-Planck Institute for Chemical Ecology, Jena, Germany) for providing the seeds of transgenic plants (irACO, Ov-etr1, irAOC and irCOI1), and Biological Technology Open Platform of Kunming Institute of Botany (CAS) for greenhouse and instrument services. This project is supported by the NSFC (Grant No. 31670262), Key Project of Applied Basic Research Program of Yunnan (Grant No. 2014FA040), and 100-Oversea-Top-Talents Recruitment plan of Yunnan to Jinsong Wu.
Glazebrook J., 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol, 43: 205-227. DOI:10.1146/annurev.phyto.43.040204.135923 |
Kallenbach M., Bonaventure G., Gilardoni P.A., Wissgott A., Baldwin I.T., 2012. Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proc. Natl.Acad. Sci. U.S.A, 109: E1548-E1557. DOI:10.1073/pnas.1200363109 |
Krügel T. Lim M., Gase K., Halitschke R., Baldwin I.T., 2002. Agrobacteriummediated transformation of Nicotiana attenuata, a model ecological expression system.. Chemoecology, 12: 177-183. DOI:10.1007/PL00012666 |
La Camera S., Geoffroy P., Samaha H., Ndiaye A., Rahim G., Legrand M., Heitz T., 2005. A pathogen-inducible patatin-like lipid acyl hydrolase facilitates fungal and bacterial host colonization in Arabidopsis. Plant J, 44: 810-825. DOI:10.1111/tpj.2005.44.issue-5 |
Li J., Wu J., 2016. Scopolin, a glycoside form of the phytoalexin scopoletin, is likely involved in the resistance of Nicotiana attenuata against Alternaria alternata. J. Plant Pathol, 98: 641-644. |
Paschold A., Halitschke R., Baldwin I.T., 2007. Co(i)-ordinating defenses:NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J, 51: 79-91. DOI:10.1111/j.1365-313X.2007.03119.x |
Racusen D., 1983. Occurrence of patatin during growth and storage of potato-tubers.. Can. J. Bot. Revue Canadienne De Botanique, 61: 370-373. |
Rydel T.J., Williams J.M., Krieger E., Moshiri F., Stallings W.C., Brown S.M., Pershing J.C., Purcell J.P., Alibhai M.F., 2003. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad.. Biochemistry, 42: 6696-6708. DOI:10.1021/bi027156r |
Saedler R., Baldwin I.T., 2004. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. J. Exp. Bot, 55: 151-157. |
Sun H., Hu X., Ma J., Hettenhausen C., Wang L., Sun G., Wu J., Wu J., 2014a. Requirement of ABA signalling-mediated stomatal closure for resistance of wild tobacco to Alternaria alternata. Plant Pathol, 63: 1070-1077. DOI:10.1111/ppa.2014.63.issue-5 |
Sun H., Wang L., Zhang B., Ma J., Hettenhausen C., Cao G., Sun G., Wu J., Wu J., 2014b. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot, 65: 4305-4315. DOI:10.1093/jxb/eru203 |
Sun H., Song N., Ma L., Li J., Ma L., Wu J., Wu J., 2017. Ethylene signalling is essential for the resistance of Nicotiana attenuata against Alternaria alternata and phytoalexin scopoletin biosynthesis. Plant Pathol, 66: 277-284. DOI:10.1111/ppa.2017.66.issue-2 |
von Dahl C.C., Winz R.A., Halitschke R., Kuhnemann F., Gase K., Baldwin I.T., 2007. Tuning the herbivore-induced ethylene burst:the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J, 51: 293-307. DOI:10.1111/j.1365-313X.2007.03142.x |
Wu J., Wang L., Baldwin I.T., 2008. Methyl jasmonate-elicited herbivore resistance:does MeJA function as a signal without being hydrolyzed to JA?. Planta, 227: 1161-1168. DOI:10.1007/s00425-008-0690-8 |
Wu J., Wang L., Wunsche H., Ian I.T., 2013. Narboh D, a respiratory burst oxidase homolog in Nicotiana attenuata, is required for late defense responses after herbivore attack. J. Integr. Plant Biol, 55: 187-198. DOI:10.1111/jipb.2013.55.issue-2 |
Xu Z., Song N., Ma L., Fang D., Wu J., 2018. NaPDR1 and NaPDR1-like are essential for Nicotiana attenuata resistance to the fungal pathogen Alternaria alternata. Plant Divers, 40: 68-73. DOI:10.1016/j.pld.2018.01.001 |



