Plant synthesize fatty acids and store them as triacylglycerols (TAG) in seeds which subsequently utilize TAGs during seed germination and early seedling development for energy before seedlings are fully established and photosynthetically sustainable (Murphy, 1993; Quettier and Eastmond, 2009). In plant cells, TAGs are stored in a specialized intracellular organelle-like structure called oleosomes, or oil bodies, that are surrounded by a monolayer of phospholipids containing embedded proteins that stabilize their structures (Murphy, 1993). Mobilization of TAGs is initiated by the activity of seedling-specific TAG lipases which liberate fatty acids from TAG molecules. The free fatty acids can then be converted to acetyl-CoA units, which are the precursors of sugar synthesis, via the β-oxidation cycle in the peroxisome (Bradford, 1997; Kepczynski and Kepczynska, 1997). More than 200 unique fatty acids have been identified in plant species (Kelly, Quettier et al., 2011). These fatty acids are classified according to chain length, number of carbon atoms, type and arrangement of unsaturated bonds, and presence of functional groups (Badami and Patil, 1980; Quettier and Eastmond, 2009). This huge structural diversity means that seed oils often consist of a wide range of TAG species with different fatty acid combinations (Fatima, Snyder et al., 2012).
Depending on the rate of water uptake, the time course of germination and subsequent growth is divided into three phases. In phase Ⅰ, which is also called imbibition, water uptake is rapid; in phase Ⅱ, water uptake is much slower and reaches a plateau; in phase Ⅲ, water uptake increases (Bewley, 1997; Weitbrecht, Muller et al., 2011). During the germination process, membrane reorganization is an important event (Verkleij, Demaagd et al., 1982; Ishibashi, Koda et al., 2013). During phase Ⅲ, phosphatidylglycerol (PG) levels increase after seeds have germinated for one to three days at 25 ℃; at the same time, both monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) levels increase considerably. Taken together, these changes in phospholipids levels indicate that plastid biogenesis is achieved during this phase of germination. During this same phase, phosphatidic acid (PA) has been found to have the same level as in the membranes of non-germination cells. In contrast, phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylinositol (PI) levels increase first day after the beginning of imbibition and subsequently decrease to background levels by day 3 (Yu, Li et al., 2015). The hydrolysis of PC and PE which are catalyzed by PLD to lysophosphatidylcholine (lysoPC) disturbs the membrane structure, inducing the release of TAG from oil bodies, which is used as the source of energy for germination (Parthibane, Iyappan et al., 2012).
1-Butanol specifically stimulates PLD and functions as a transphosphatidylation substrate; tert-butanol, however, does not have the same effect (Munnik, Arisz et al., 1995). N-Acylethanolamines (NAEs) are fatty acid amides that are derived from an N-acylated phosphatidylethanolamine precursor, a minor membrane lipid constituent of plant and animal cells (Hayes, Stupak et al., 2013). Although these fatty acid amides have been identified and quantified in plant tissues, their significance and precise function in plant cells remains to be elucidated. NAE 12:0, a naturally occurring NAE in plants is a specific inhibitor of PLDα1 activity (Motes, Pechter et al., 2005; Jia, Tao et al., 2013). Seed germination is regulated by numerous chemicals derived from a multitude of metabolic pathways (Motes, Pechter et al., 2005; Zheng, Jia et al., 2012; Blancaflor, Kilaru et al., 2014). NAE 12:0 has a profound influence on Arabidopsis root cell development and early seedling growth. 1-Butanol, an antagonist of PLD-dependent PA production, was reported to induce defects in Arabidopsis by seedling development that were somewhat similar to effects induced by elevated levels of NAE. However, although 1-butanol and NAE 12:0 inhibit Arabidopsis seedling development, the severity, quality, and concentrations needed to invoke distinct cellular and developmental effects have been shown to be different between the two compounds. For example, 1-butanol, but not the inactive tert-butanol isomer, has been shown to inhibit Arabidopsis seed germination (Gardiner et al., 2003). In contrast, NAE 12:0 up to 500 μM did not significantly delay seed germination as monitored by radicle and cotyledon emergence (Gardiner, Collings et al., 2003; Motes, Pechter et al., 2005).
Seed storage oil metabolism is important for germination or seedling establishment in Arabidopsis (Quettier and Eastmond, 2009). Since NAE and 1-butanol appear to modulate PLD activity in vitro, and induce different effects on seed germination (Dhonukshe, Laxalt et al., 2003; Motes, Pechter et al., 2005), both compounds may induce different processes of TAG metabolism. To gain deeper insights into the mode of mobilization of TAG during NAE 12:0 or 1-butanol treatment, we conducted a detailed comparative analysis of the effect of NAE 12:0, DMSO, 1-butanol, and tert-butanol on Arabidopsis seed germination and fatty acid composition. Our data show that NAE and 1-butanol invoke different effects on mobilization of TAG and fatty acid composition as well as seed germination. 1-butanol induced the degradation of RNA in seeds and seedlings. Based on this result, we speculate that the large-scale degradation of RNA under the induction of 1-butanol may lead to a the lack of normal expression of most of the genes necessary for seed germination, including the genes needed for the mobilization of oil bodies, thus causing the delay of seed germination. To the best of our knowledge, we report for the first time that 1-butanol delays the mobilization of TAG.
2. Materials and methods 2.1. Plant materials and growth conditionsSeeds of Arabidopsis thaliana L. Heynh (ecotype Columbia) were sterilized for 2 min in 95% ethanol, 2 min in 5% bleach and then rinsed three times in sterile deionized water. The Arabidopsis seedlings used to detect the oil bodies with confocal laser scanning microscopy were planted in growth media consisting of MS salts supplemented with micronutrients, 0.05% morpholineethanesulfonic acid, 0.01% myo-inositol and 0.004 M each of pyridoxine, nicotinic acid, and thiamine. After adjusting the pH to 5.7, the media containing growth media supplemented with NAE 12:0, 1-butanol, tert-butanol, and the equivalent volume of dimethyl sulfoxide (DMSO) (used as a solvent for NAE) was poured onto plates (9 × 9 cm). For seed germination assays and fatty acid assays, seeds were planted in plates on filter paper floated on either sterile deionized water or water supplemented with NAE 12:0, 1-butanol, tert-butanol, or the equivalent volume of DMSO. DMSO and tert-butanol served as controls treatments (Blancaflor, Hou et al., 2003; Gardiner, Collings et al., 2003). After planting, plates were wrapped in aluminum foil and incubated for 48 h at 4 ℃. The aluminum foil was then removed and plates were transferred to a room with a photoperiod of 12 h light (120 mol/s·m2) and 12 h dark. Phenotype at 1, 3, and 5 days after removal from cold was observed using an Olympus SZ 61 stereomicroscope.
2.2. Confocal microscopyTo visually detect neutral lipids, seedlings (aged 1, 3-5 days) grown under 12-h-light/12-h-dark cycles were infiltrated with an aqueous solution of Nile Red (Sigma) (Siloto, Findlay et al., 2006). Images were obtained with a 100 × oil objective by confocal laser scanning microscopy using an Olympus FV 1000 system equipped with argon as an excitation source. Fluorescence was excited at 515 nm and collected with a 539-653 nm filter.
2.3. Lipid extraction and fatty acid methyl ester preparationApproximately 50 mg of seeds of each sample (n = four independent replicates) were placed in a hexane-washed, hand-held, group-glass homogenizer and boiled in 1 mL of isopropanol (80 ℃) for 10 min. The seeds were then cooled on ice for 5 min. Thereafter, 1 mL of hexane and 2 mL of 3:2 hexane:isopropanol (HIP) was added and the seeds were homogenized until completely pulverized. An additional 2 mL of 3:2 HIP was added and grinding continued. The homogenate was transferred to a screw-capped glass tube, and 2 mL of 3.3% (W/V) Na2SO4 was added, capped, and shaken for 2 min. The tubes were spun at 555 g for 2 min, and the upper organic phase was transferred to a new hexane-washed screw-capped tube. The aqueous phase was re-extracted with 4 mL of 7:2 HIP, capped, and shaken for 2 min. The tubes were spun again at 555 g for 2 min, and the upper organic phase added with the first extracted organic phase. The combined organic phases were evaporated to dryness in a heating block (37 ℃) under a gentle N2 stream. To isolate fatty acid methyl esters (FAMEs), 1.2 mL of HClmethanol (1.5 M HCl in methanol made fresh) was added to the dried lipid and incubated at 100 ℃ for 1 h. Then, 1 mL of double distilled water was added to quench the transesterification reaction. The FAMEs were then extracted with 2 mL of hexane. The samples were centrifuged as above and the upper organic phase containing the FAMEs transferred to a clean hexane-washed test tube. The aqueous phase was re-extracted with an additional 2 mL of hexane and centrifuged and the resulting upper phase transferred and combined with the previously collected organic phase. The combined organic phases containing the FAMEs were then dried down completely in 1 mL of hexane and transferred to gas chromatography vials and capped.
2.4. Data analysisStatistical analysis was performed using Origin 7.0 (Origin Lab Corporation, Northampton, MA, USA). For all quantitative measurements in this study, five replicates from each sampling time were analysed. The data were subjected to one-way ANOVA analysis of variance with SPSS 16.0.
3. Results 3.1. 1-Butanol, but not NAE12:0, retains oil bodiesOil bodies have been shown to release TAG to supply energy for seed germination and seedling establishment (Quettier and Eastmond, 2009). To visualize oil bodies in Arabidopsis seedlings, we stained Arabidopsis seeds germinated on wet filters containing 1-butanol, NAE 12:0, or their corresponding controls (DMSO and tert-butanol) with Nile Red during seedling growth from days 1-5 after imbibition (Fig. 1A). Confocal laser microscopy revealed the presence of red-stained spherical inclusions in seeds, which represent oil body accumulation. The oil bodies of seeds planted in NAE 12:0 were not significantly different from the oil bodies of seeds planted in plates water, DMSO, or tert-butanol. The number of oil bodies had substantially declined by day 2 and very few were evident by day 4 or 5 (Fig. 1A). In seeds treated with 1-butanol, oil bodies did not show any obvious change until day 4, and oil bodies were no longer evident by day 5, although by that point the radicle had emerged and the chloroplast had been formed (Fig. 1B, C). These results indicate that the NAE 12:0 did not affect the metabolism of oil bodies nor affect seed germination. Furthermore, these results suggest that the inhibition of seed germination by 1-butanol might be related to the inhibition of oil body metabolism.
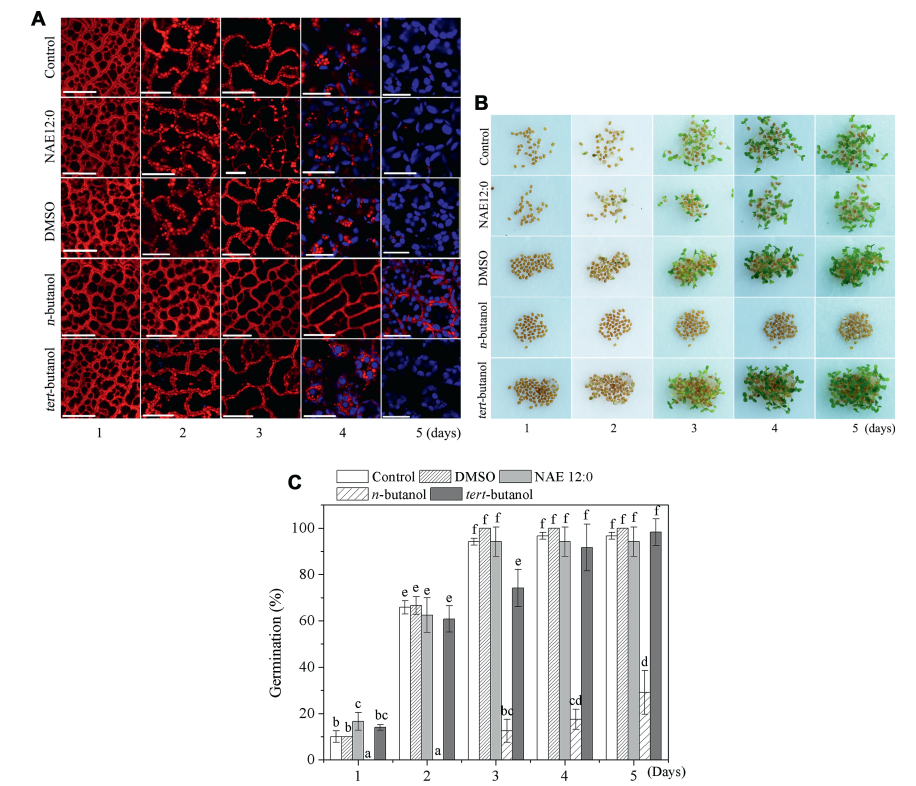
|
| Fig. 1 1-Butanol inhibited seed germination and mobilization of oil bodies in Arabidopsis. (A) Seedlings at different days after germination were stained with Nile red and analyzed by confocal microscopy. (B) Arabidopsis seeds were geminated in sterilized water with DMSO, NAE12:0, 1-butanol and tert-butanol, respectively. (C) Germination rate. Nile red was detected in the red channel and autofluorescence of chlorophyll was detected in the blue channel. Bar = 20 μm. |
In plant seeds, TAG is the major storage lipid in oil bodies and functions as a critical energy reserve during germination and seedling establishment (Bradford, 1997). The differential accumulation of oil bodies by 1-butanol-treated seedlings prompted us to investigate whether oil body accumulation coincided with changes in TAG content. We used gas chromatography-mass spectrometry (GC-MS) to analyze TAG content in dry seeds and seedlings treated with 1-butanol, NAE 12:0, DMSO, tert-butanol, and water. One day after germination, no significant change in TAG content of seedlings incubated in the four treatments were detected relative to the content in dry seeds. At day 3, the TAG content of seedlings treated with water, DMSO, NAE 12:0, and tert-butanol decreased significantly in comparison to that at day 1, while the TAG content of seedlings from the four treatment did not show obvious differences. Compared to dry seeds, TAG content five days after imbibition decreased in seedlings treated with water (28.49%), NAE 12:0 (31.59%), DMSO (27.26%), and tert-butanol (27.54%). In contrast, after 1-butanol incubation for 1 and 3 days, TAG content of decreased to 58.47% in comparison to that of dry seeds (Fig. 2). TAG degradation may provide energy for post-germinative growth. These results show that the 1-butanol-induced delay in TAG degradation rate coincides with 1-butanol-induced inhibition of seed germination. In contrast, the germination of seeds incubated in water, DMSO, NAE 12:0 and tert-butanol accompanies with the degradation of TAG. This change in TAG content coincided with that of oil bodies detected by confocal laser scanning microscopy.
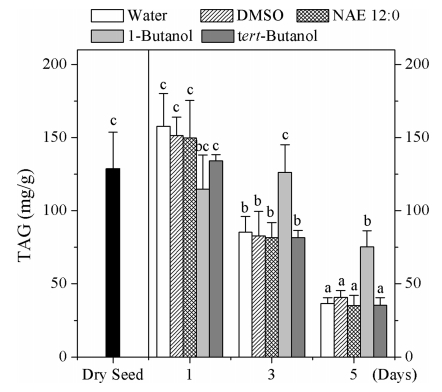
|
| Fig. 2 TAG content of in Arabidopsis dye seeds and seedlings incubated in water, DMSO, NAE 12:0, 1-buanol or tert-butanol. Data are shown as means ± SE (n = 4). Column with different letters differ significantly (P < 0.05). |
Oil breakdown is initiated during lipolysis when TAG in oil bodies is hydrolyzed to free fatty acids (FA) and glycerol (Quettier and Eastmond, 2009; Fatima, Snyder et al., 2012). We identified changes in FA content were identified among dry seeds and seedlings exposed to water, DMSO, NAE 12:0, 1-butanol and tert-butanol (Fig. 3).
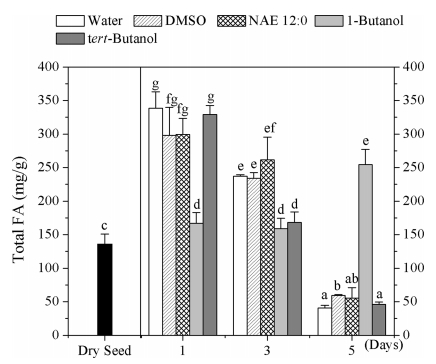
|
| Fig. 3 Total fatty acid content in Arabidopsis dye seeds and seedlings incubated in water, DMSO, NAE 12:0, 1-buanol, or tert-butanol. Data are shown as means ± SE (n = 4). Column with different letters differ significantly (P < 0.05). |
The total FA content of seedlings exposed to water, NAE and DMSO increased at day 1 in comparison to dry seeds, and then declined rapidly at day 3, and day 5, and declined to 12.01%, 19.69%, and 18.54%, respectively. These results indicate that during the early stages of germination, the FAs released from stored TAGs to provide carbon and metabolic energy for seedling development. Meanwhile, there were no obvious differences detected in the FA content of seedlings incubated in water, NAE, or DMSO. In 1-and 3- day-old seedlings, the total FAs content of seedlings exposed to 1- butanol showed a slight increase in contrast to dry seeds, whereas the content of FAs clearly increased at day 5, especially molecular species 16:0, 18:2, 18:3, 20:1 (ω-9) (Fig. 4). When compared with 1-butanol of the same concentration, the tert-butanol seedlings did not show considerable differences in overall fatty acid profiles in TAG with water treatment. These results indicated that1-butanol delayed seed germination at the same time at FA degradation delayed (See Fig. 5).

|
| Fig. 4 The content of major and engineered fatty acids of TAGs in seedlings of Arabidopsis dye seeds and seedlings incubated in water, DMSO, NAE 12:0, 1-buanol or tert-butanol. Data are shown as means ± SE (n = 4). |
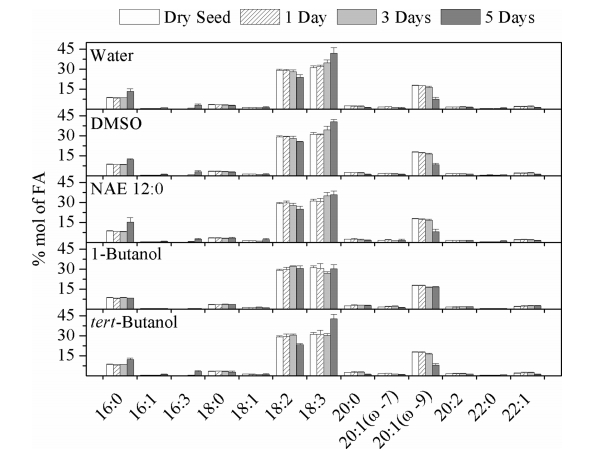
|
| Fig. 5 Profiles of major and engineered fatty acids of TAGs in seedlings of Arabidopsis dye seeds and seedlings incubated in water, DMSO, NAE 12:0, 1-buanol or tert-butanol. Data are shown as means ± SE (n = 4). |
Previously studies have shown that in seed oil, linoleic acid (18:2) and α-linolenic acid (18:3) accounted for roughly equal proportions of the total composition, at 28.32-29.98% and 29.68-32.46%, respectively. Eicosenoic acid (20:1ω-7) content was 17.47%e18.01%, followed by palmitic acid (16:0) at 8.46-8.76%. Oleic acid (18:1), palmitoleic acid (16:1), stearic acid (18:0), eicosenoic acid's isomer (20:1ω-7), 16:3, 20:2 and 22:1 were each present at < 4% of the total fatty acids (Fatima, Snyder et al., 2012).
We next asked whether 1-butanol and NAE12:0 exert differential effects on fatty acid composition. There was little variation among the seedlings incubated in water, DMSO, NAE12:0, or tert-butanol in the fatty acid compositions of the seed oils. Compared to dry seeds, all fatty acid species in seeds incubated in water, DMSO, NAE12:0, tert-butanol for 1 and 3 days remained unchanged; fatty acid molecular species palmitic acid (16:0), 16:3 and 18:3 increased, and molecular species 18:2, 20:0, and 20:1 (ω-9) decreased at day 5. In comparison to seedlings exposed to water, seedlings incubated in 1-butanol showed no obvious change over the 5-day observation period. These results suggest that the stage of seed germination is a main controlling factor in fatty acid composition.
4. DiscussionNAE and 1-butanol appear to modulate PLD activity in vitro and induce somewhat similar effects on seedling development (Austin-Brown and Chapman, 2002). NAE 12:0 and 1-butanol have been shown to have different effect on hypocotyl length, cotyledon area, first true leaf formation, and pattern of root swelling. NAE 12:0 is a more potent regulator of cellular organization, which in contrast to 1-butanol, translates into more profound effects on seedling morphology at micromolar concentrations. The differential effects of 1-butanol and NAE 12:0 on seedling morphology and cellular architecture are due in part to the differential modulation of specific PLD isoforms (Blancaflor, Hou et al., 2003; Gardiner, Collings et al., 2003; Motes, Pechter et al., 2005). 1-Butanol has been used by many researchers as a nonselective "inhibitor" of PLD action, because it blocks the transphosphatidylation reaction catalyzed by all PLD isoforms. NAE on the other hand is more specific, potently inhibiting PLDα, but not the PLD β, γ, or -δ isoforms (Austin-Brown and Chapman, 2002; Dhonukshe, Laxalt et al., 2003; Jia, Tao et al., 2013; Yu, Li et al., 2015; Chen, Yu et al., 2017). Given previous reports, although both NAE 12:0 and 1-butanol were known as antagonist of PLD activity, there were distinct effects on seed germination. Because NAEs are abundant in desiccated seeds and are rapidly hydrolyzed during seed imbibition and germination (Chapman, 2004), it is not surprising that NAE 12:0 does not inhibit seed germination. The lowest concentration of 1-butanol that has been found to induce a delay in seed germination is 0.025% (Austin-Brown and Chapman, 2002; Motes, Pechter et al., 2005).
When seeds germinate, the TAGs are degraded in order to produce a carbon sources that will fuel the embryo's postgerminative growth and allow it to become a photosynthetically active seedling with a root system and leaves (Quettier and Eastmond, 2009). In the present study, we show that 1-butanol, but not the inactive tert-butanol isomer, inhibited Arabidopsis seed germination, and that inhibition is accompanied by a retardation in the mobilization of TAG (Figs. 1-3). In contrast, NAE 12:0 did not affect mobilization of TAG, nor did it significantly delay seed germination as monitored by radicle and cotyledon emergence. In ungerminated seeds, oil bodies are composed of TAGs which are surrounded by a monolayer of phospholipids containing two kinds of embedded proteins, oleosin and caleosin. The analysis of changes in oil bodies and in the composition of oil storage at different germinating stages showed that oil degradation is progressive. The first step is the loss of oil body membrane integrity. There may be two reactions at the same time or one after the other, causing the disintegration of the oil body membrane. First, the cytoplasmic domain of oleosin, which is used as the structural protein of the oil body membrane, is hydrolyzed (Yang, Yu et al., 2011; Parthibane, Iyappan et al., 2012; Shrestha, Callahan et al., 2016); second, phospholipid of the oil body membrane are degraded, which may require PLD. After the disintegration of the oil body membrane, the TAG is exposed to TAG lipase and releases fatty acids (Quettier and Eastmond, 2009; ParthibaneIyappan et al., 2012). At the early stage of seed germination, a series of newly synthesized proteins (at least 3 new proteins, lipoxygenase, phospholipase and TAG lipase) can be detected on the phospholipid monolayer when the oil body begins to degrade (Hilhorst and Toorop, 1997; Hsiao, Haslam et al., 2014). The newly synthesized proteins begin mobilization of the stored TAG. Mobilization of TAG is initiated by the activity of seedlingspecific TAG lipases which liberate fatty acids from TAG molecules. 1-Butanol has been reported as an antagonist of PLDdependent phosphatidic acid production, and NAE has been shown to inhibit PLDα activity. The inhibition of PLD-mediated PA formation through application of 1-butanol or NAE alleviates imbibitional chilling injury in cucumber and pea seeds (Yu, Li et al., 2015) and increases desiccation tolerance in recalcitrant seeds. However, the specific and functional reorganization of lipid composition during germination occur on cell membranes (Chen, Yu et al., 2017). Furthermore, when PLDα1 and δ are suppressed, the germination level remained unchanged relative to wild type Arabidopsis. These findlings indicated that the retardation of mobilization by 1-butanol is not relative to the inhibition of PLD activity. Based on these results, we propose that 1-butanol inhibition of the mobilization of TAGs may affect activity of the TAG lipases. In recent years, a number of lipases have been cloned and characterized from plants, such as AtDAD1 (Yu, Zhu et al., 2006), SDP1, SDP 2, and SDP 3 (Cotter, Teaster et al., 2011; Yang, Yu et al., 2011; Shrestha, Callahan et al., 2016; Lunn, Wallis et al., 2017). We attempted to determine the expression pattern of these genes in seeds or seedlings incubated in 1-butanol, NAE, DMSO, tert-butanol, and water; however, RNA of the seeds incubated in 1- butanol is extremely degraded, therefore it was not possible to extract intact RNA that could be used for downstream gene expression detection. Based on this experimental result, we speculate that the large-scale degradation of RNA after 1-butanol treatment may lead to the lack of normal expression of most the necessary genes for seed germination, including the genes needed for the mobilization of oil bodies, thus causing the delay of seed germination. Therefore, other techniques may be needed to verify the effect of 1-butanol on the expression of genes involved in seed germination.
AcknowledgmentsThis research was supported by grants from the National Natural Science Foundation of China (31600215, 31600652, 31500272); Yunnan Applied Basic Research Projects-(2015FB171). Thanks doctor Buzhu Yu for date collection.
Abbreviations: TAG, triacylglycerols; NAE, N-Acylethanolamines; PLD, phospholipase D; PG, phosphatidylglycerol; MGDG, monogalactosyldiacylglycerol; DGDG, digalactosyldiacylglycerol; PC, Phosphatidylcholine; PE, phosphatidyletha-nolamine; PI, phosphatidylinositol; lysoPC, lysophosphatidylcholine; DMSO, dimethyl sulfoxide; GC-MS, gas chromatography-mass spectrometry; FA, fatty acid.
Austin-Brown S.L., Chapman K.D., 2002. Inhibition of phospholipase D alpha by N-acylethanolamines. Plant Physiol, 129(4), 1892-1898.
DOI:10.1104/pp.001974 |
||
Badami R.C., Patil K.B., 1980. Structure and occurrence of unusual fatty acids in minor seed oils. Prog. Lipid Res, 19(3-4), 119-153.
DOI:10.1016/0163-7827(80)90002-8 |
||
Bewley J.D., 1997. Seed germination and dormancy. Plant Cell, 9(7), 1055-1066.
|
||
Blancaflor E.B., Hou G., et al., 2003. Elevated levels of N-lauroylethanolamine, an endogenous constituent of desiccated seeds, disrupt normal root development in Arabidopsis thaliana seedlings. Planta, 217(2), 206-217.
|
||
Blancaflor E.B., Kilaru A., et al., 2014. N-Acylethanolamines:lipid metabolites with functions in plant growth and development. Plant J, 79(4), 568-583.
DOI:10.1111/tpj.12427 |
||
Bradford K.J., 1997. The hydrotime concept in seed germination and dormancy. Basic Appl. Asp. Seed Biol, 30, 349-360.
DOI:10.1007/978-94-011-5716-2 |
||
Chapman K.D., 2004. Occurrence, metabolism, and prospective functions of N-acylethanolamines in plants. Prog. Lipid Res, 43(4), 302-327.
DOI:10.1016/j.plipres.2004.03.002 |
||
Chen, H., Yu, X., et al., 2017. Phospholipase Dα1-mediated phosphatidic acid change is a key determinant of desiccation-induced viability loss in seeds. Plant Cell Environ. (n/a-n/a). https://www.ncbi.nlm.nih.gov/pubmed/28152567
|
||
Cotter M.Q., Teaster N.D., et al., 2011. N-acylethanolamine (NAE) inhibits growth in Arabidopsis thaliana seedlings via ABI3-dependent and -independent pathways. Plant Signal. Behav, 6(5), 671-679.
|
||
Dhonukshe P., Laxalt A.M., et al., 2003. Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell, 15(11), 2666-2679.
DOI:10.1105/tpc.014977 |
||
Fatima T., Snyder C.L., et al., 2012. Fatty acid composition of developing Sea Buckthorn (Hippophae rhamnoides L.) Berry and the transcriptome of the mature Seed. PLoS One, 7(4).
|
||
Gardiner J., Collings D.A., et al., 2003. The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis. Plant Cell Physiol, 44(7), 687-696.
DOI:10.1093/pcp/pcg095 |
||
Hayes A.C., Stupak J., et al., 2013. Identification of N-acylethanolamines in Dictyostelium discoideum and confirmation of their hydrolysis by fatty acid amide hydrolase. J. Lipid Res, 54(2), 457-466.
|
||
Hilhorst H.W.M., Toorop P.E., 1997. Review on dormancy, germinability, and germination in crop and weed seeds. Adv. Agron, 61(61), 111-165.
|
||
Hsiao A.S., Haslam R.P., et al., 2014. Gene expression in plant lipid metabolism in Arabidopsis seedlings. PloS One, 9(9).
|
||
Ishibashi Y., Koda Y., et al., 2013. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Ann. Bot, 111(1), 95-102.
DOI:10.1093/aob/mcs240 |
||
Jia Y., Tao F., et al., 2013. Lipid profiling demonstrates that suppressing Arabidopsis phospholipase Dδ retards ABA-promoted leaf senescence by attenuating lipid degradation. PLoS One, 8(6), e65687.
DOI:10.1371/journal.pone.0065687 |
||
Kelly A.A., Quettier A.L., et al., 2011. Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiol, 157(2), 866-875.
DOI:10.1104/pp.111.181784 |
||
Kepczynski J., Kepczynska E., 1997. Ethylene in seed dormancy and germination. Physiol. Plantarum, 101(4), 720-726.
|
||
Lunn D., Wallis J.G., et al., 2017. Overexpression of Seipin1 Increases Oil in Hydroxy-fatty Acid Accumulating seeds. Plant Cell Physio.
|
||
Motes C.M., Pechter P., et al., 2005. Differential effects of two phospholipase D inhibitors, 1-butanol and N-acylethanolamine, on in vivo cytoskeletal organization and Arabidopsis seedling growth. Protoplasma, 226(3-4), 109-123.
DOI:10.1007/s00709-005-0124-4 |
||
Munnik T., Arisz S.A., et al., 1995. G protein activation stimulates phospholipase D signaling in plants. Plant Cell, 7(12), 2197-2210.
DOI:10.1105/tpc.7.12.2197 |
||
Murphy D.J., 1993. Structure, function and biogenesis of storage lipid bodies and oleosins in plants. Prog. Lipid Res, 32(3), 247-280.
DOI:10.1016/0163-7827(93)90009-L |
||
Parthibane V., Iyappan R., et al., 2012. Serine/threonine/tyrosine protein kinase phosphorylates oleosin, a regulator of lipid metabolic functions. Plant Physiol, 159(1), 95-104.
DOI:10.1104/pp.112.197194 |
||
Quettier A.L., Eastmond P.J., 2009. Storage oil hydrolysis during early seedling growth. Plant Physiol. Biochem, 47(6), 485-490.
|
||
Shrestha P., Callahan D.L., et al., 2016. Reduced triacylglycerol mobilization during seed germination and early seedling growth in Arabidopsis containing nutritionally important polyunsaturated fatty acids. Front. Plant Sci, 7, 1402.
|
||
Siloto R.M., Findlay K., et al., 2006. The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell, 18(8), 1961-1974.
DOI:10.1105/tpc.106.041269 |
||
Verkleij A.J., Demaagd R., et al., 1982. Divalent-cations and chlorpromazine can induce non-bilayer structures in phosphatidic acid-containing model membranes. Biochim. Biophys. Acta, 684(2), 255-262.
DOI:10.1016/0005-2736(82)90014-1 |
||
Weitbrecht K., Muller K., et al., 2011. First off the mark:early seed germination. J. Exp. Bot, 62(10), 3289-3309.
DOI:10.1093/jxb/err030 |
||
Yang Y., Yu X., et al., 2011. ABI4 activates DGAT1 expression in Arabidopsis seedlings during nitrogen deficiency. Plant Physiol, 156(2), 873-883.
DOI:10.1104/pp.111.175950 |
||
Yu X.M., Li A.H., et al., 2015. How membranes organize during seed germination:three patterns of dynamic lipid remodelling define chilling resistance and affect plastid biogenesis. Plant Cell Environ, 38(7), 1391-1403.
DOI:10.1111/pce.2015.38.issue-7 |
||
Yu Z.Q., Zhu J., et al., 2006. Functional analysis of rice P0491-01 gene regulating anther development. Fen Zi Xi Bao Sheng Wu Xue Bao, 39(5), 467-472.
|
||
Zheng G.W., Jia Y.X., et al., 2012. o-Coumaric acid from invasive Eupatorium adenophorum is a potent phytotoxin. Chemoecology, 22(2), 131-138.
DOI:10.1007/s00049-012-0105-y |



