b. Key Laboratory of Tropical Forest Ecology, Xishuangbann1 Tropical Botanical Garden, Chinese Academy of Sciences, Menglun Town, Yunnan 666303, PR China;
c. Laboratory of Ecology and Evolutionary Biology, Yunnan University, Kunming, Yunnan 650091, PR China
Plant life history traits (growth, reproduction, and seeding) and adaptation (plasticity or differentiation) are influenced by the biotic and/or abiotic factors in heterogeneous environments (Miner et al., 2005; Matesanz et al., 2010). Spatial or temporal variation of ecological variables and/or microhabitat heterogeneity can affect plant morphological characteristics (plasticity) or differentiation of plant traits (Shen et al., 2008; Tétard-Jones et al., 2011; Herrera and Bazaga, 2013). Changes in plant traits are signals of plant adaptation and evolution in heterogeneous environments (Matesanz et al., 2010; Scheiner, 2013; Anderson et al., 2014; Leingärtner et al., 2014). Therefore, previous studies have focused on the effects of climate change on the abundance of species in heterogeneous environments (Valladares et al., 2007; Shimizu et al., 2011). Spatial-temporal variation in climatic variables (temperature, soil fertility, sunshine, rainfall, etc.) and resource limitation (Dorken and Barrett, 2004; Millal and Reich, 2011; Goh et al., 2013; Mou et al., 2013) in highly heterogeneous environments affect plant morphological characteristics and growth rates (Gómez-Aparicio et al., 2005). The limitations of ecological variables can play an important role in plant morphological, physiological, and anatomical trait development and adaptation under low resource conditions among different populations (Puijalon and Bornette, 2006; Gianoli and Valladares, 2010; Wang et al., 2014; Nascimbene and Marini, 2015). For instance, increasing elevation leads to limiting effects of multiple ecological variables (i.e. those related to soil, water, and temperature) which affect morphological plasticity and the level of adaptation of alpine species; however, this phenomenon has previously been poorly understood.
Significant variations in plant morphological traits (plasticity) is an important adaptation response for successful plant performance (Huber et al., 2009; Nicotra et al., 2010; Godoy et al., 2011) and natural selection for plant growth in a harsh environment (Richards et al., 2006; Nicotra et al., 2010). Morphological plasticity can be important in predicting population dynamics and plant evolutionary adaptations to different novel environments (Nicotra et al., 2010). Variation in climatic variables (temperature, light, rainfall, and other variables) along elevation gradients may be associated with enhanced life history characteristics of plants and their evolutionary responses (Aragon et al., 2012; Guerin et al., 2012; Leingärtner et al., 2014). This is because variation or limited resources (ecological variables) can be crucial for plant performance and survival (Nicotra et al., 2010). However, most previous research on plant morphological plasticity and survival has been carried out in controlled environments (Goh et al., 2013). Furthermore, to the best of our knowledge, no previous studies have focused on both morphological variation and adaptation of the both morph of plants in populations of distylous species at an elevational gradient that experience variations in climatic and ecological variables.
Primula nivalis is a spring-flowering herbaceous perennial distylous species, which grows in grassland and forest habitats at elevations of 1600-3000 m a.s.1. It has a wide distribution that includes Kazakhstan, Kyrgyzstan, Mongolia, Russia, Tajikistan, Turkmenistan, Uzbekistan and the northeastern and northwestern regions of the Xinjiang Uyghur Autonomous Region of China (Abdusalm, 2018). Plants flower from early May to late July, and fruits mature from July to mid-August, depending on elevation, with flowering and fruiting occurring later in high compared to low elevation populations.
Data from herbaria suggest that plant morphological traits differ significantly among specimens collected at different points along the elevational gradient, and plant height and leaf size decreases with increasing elevation. We hypothesized that elevational variation, climate change, and resource availability affects the morphological plasticity of both morph plants, and that this leads to adaptation in populations that occur at different elevations. To test this hypothesis, we used P. nivalis to address three questions: (1) Do morphological traits and biomass allocation of both morphs of P. nivalis plants differ at different elevations? (2) What is the relationship between ecological variables and morphological characteristics and the level of biomass allocation to both sexual morphs in each population? (3) What is the effect of a heterogeneous environment on the morphological plasticity of both morph plants at different elevations?
2. Material and methodsField experiments were conducted in the flowering season of 2014 in populations of P. nivalis at 17 different elevations (1650 m to 2704 m a.s.l.). The P. nivalis populations were natural populations growing in forests and grasslands in the Tianshan Mountains of the Kunas area of northwestern Xinjiang, China (Fig. 1). A transplanting experiment was carried out at an elevation of 1650 m a.s.l. during the flowering period in 2014 and 2015 (see below).
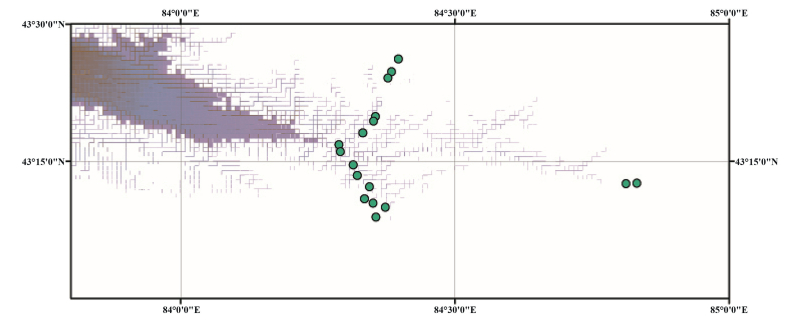
|
| Fig. 1 The observed population geographical distribution characteristics of Primula nivalis. |
To determine effects of elevational variation on morphological characteristics of P. nivalis during the flowering season of each population, in 2014, 30 individuals of long style (LS) and short style (SS) plants were randomly selected from 17 populations. The height of the above-ground part of the each individuals, the number of leaves, maximum leaf length, and length of scape in both the morph plants were recorded. To investigate the effects of elevational variations on resource allocation in LS and SS plants at each population were collected, and the root, scape, leaf, and inflorescence were separated, and oven-dried to constant mass at 80℃ for 48 h. After drying, each part of both morphs was weighed using a Sartorius BS210S electronic-balance (accurate to 0.0001 g). The sum of the weight of all individual parts was considered the total plant total biomass; and biomass allocation to each part (roots, scape, leaves and inflorescence) was calculated as T = (biomass of plant each part/plant total biomass) × 100%.
2.2. Transplantation experimentTo investigate the effects of variations in multiple ecological variables on plant morphological characteristics, we conducted transplanting experiments using P. nivalis populations at four different elevations (2, 704, 2, 423, 2, 013, and 1657 m a.s.l.). Thirty individual plants (both LS and SS morphs) were selected in each population, and dug out along the soil and placed in plastic bags to be transferred to lower elevations (1657 m a.s.l.) in 2014. In order to ensure the survival of the plants at the lower elevation (at which temperatures were higher), the plants were watered every second day. One year after plants were transplanted to the lower elevation, the plant height, number of leaves, and maximum leaf length was measured. The morphological plasticity and environmental adaptability of both sexual morph plants were also evaluated at the site of transplantation. The morphological plasticity index (MPI) was calculated following the formula from Ceplick (1995): MPI = (Xmaximum-Xminimum)/Xmaximum; where Xmaximum and Xminimum is the maximum or minimum measurement of each organ before and after transplanting both morph plants.
2.3. Data on ecological variablesIn this experiment, the data regarding climate variables included elevation, and geographic data (latitude and longitude), and this data was determined at each site using an electronic GPS. The climate variable data of temperature, precipitation, and annual sunshine were downloaded from http://www.worldclim.org. Soil samples were collected from each elevation site at a depth of 20 cm. The soil nutrient composition was evaluated at the Biogeochemical Laboratory of the Kunming Division of the Xishuangbanna Tropical Botanical Garden (XTBG), Chinese Academy of Sciences. Soil pH (1:2.5 v/v soil/water mixture; LY/T 1239-1999) for each population was measured using a digital pH meter (PHS-3C, Shanghai Leici Equipment Factory, China). Total N and C were measured using an elemental analyzer (Vario MAX CN, Elementar Analysensysteme GmBH, Germany). Concentrations of total potassium (K), total phosphorus (P), and full magnesium (Mg) were determined by digestive as well as inductively coupled plasmaeatomic emission spectrometry, LY/T (1254-1999 ICP-AES). The soil was extracted using HF-HClO4, and total nitrogen (N) concentrations were measured using molybdenum-antimony colorimeters. The concentrations of microelements (zinc (Zn), total copper (Cu), total iron (Fe), total manganese (Mn), and boron (B)) were investigated using the methods described by Zhang et al. (2011).
2.4. Data analysisData analyses were carried out using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The data on morphological characteristics and biomass allocation were arcsine transformed before statistical analysis to ensure homogeneity of variance. Non-linear data were square-root transformed. The relationships between elevation, morphological traits, and biomass of LS and SS plants were analyzed using bivariate correlations and linear models as y = b0 + b1 × x. The effect of multiple correlations of all the climatic variables (14 variables) on morphology and biomass allocation in both morph plants was evaluated by RDA (redundancy analysis) using R software. Generalized linear models (GLM) were used to analyze morphological characteristics, morphological plasticity indexes, and variation in different organs of LS and SS plants before and after plants were transplanted. Independent sample t-tests were used to compare differences in plant morphological characteristics and biomass of each part in both morph plants.
3. Results 3.1. Effects of elevation on plant morphological characteristics and resource allocationThere were significant variations in morphological characteristics of both morph plants at different elevations. Plant height and the length of the scape and leaves in both morph plants were significantly negatively correlated with elevation (Fig. 2). Plant height and the length of the scape and leaves decreased with increasing elevation. The size of each part of LS plants was larger than that of SS plants, and plant height (t = 3.380; P < 0.05), scape length (t = 4.423; P < 0.01), and leaf length (t = 4.233; P < 0.01) differed significantly between LS and SS plants.
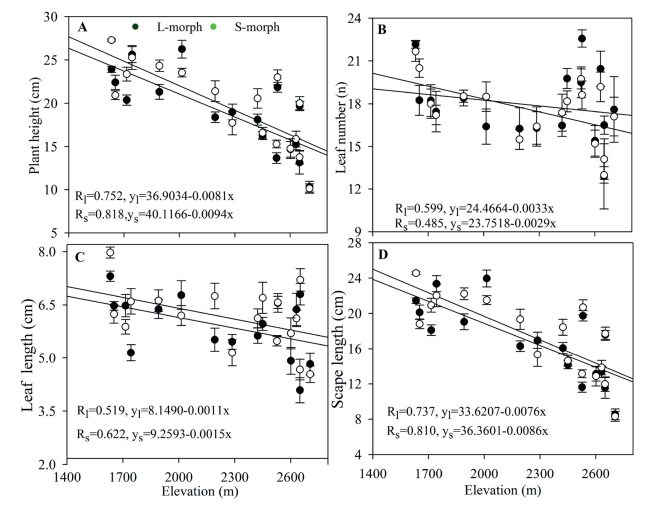
|
| Fig. 2 Variation in plant height (A), leaf number (B), leaf length (C), and scape length (D) for L- and S-morph individuals from 17 populations of Primula nivalis at different elevations. |
The biomass allocation in some components of both morph plants was significantly affected by changes in elevation. The resource allocation to reproductive organs and inflorescence biomass was not significantly correlated with elevation in either morph plant. The leaf biomass and root biomass of both morph plants were more strongly positively correlated with elevation, but scape biomass was negatively correlated with elevation (Fig. 3). Biomass allocation to roots, scapes, and leaves was significantly different between the two sexual morphs (Fig. 3). The biomass of each part of the LS plants was greater than that of each part of the SS plants.
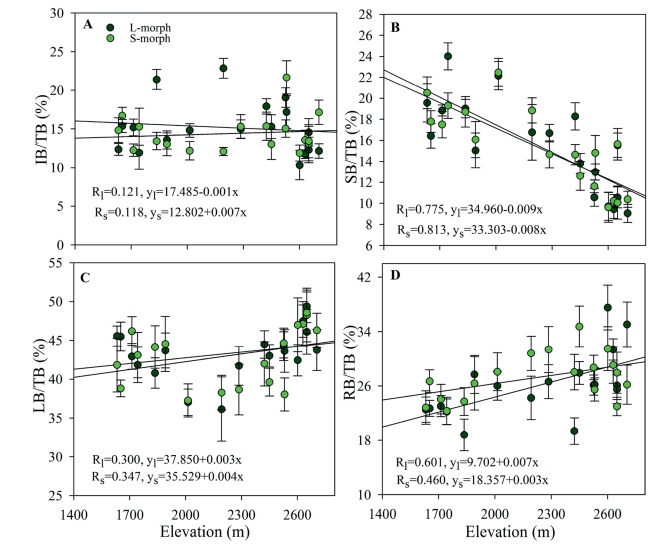
|
| Fig. 3 Variation in IB/TB (A), SB/TB (B), LB/TB (C), and RB/TB (D) for L- and S-morph individuals from 17 populations of Primula nivalis at different elevations. IB: inflorescence biomass; SB: scape biomass; LB: leaf biomass; RB: root biomass; and TB: total biomass. |
RDA results suggest that ecological variables can significantly affect plant morphological characteristics of both morph plants at different elevations. Almost all ecological variables examined affected the morphological characteristics of both morph plants. Mean temperature, annual sunshine, and rainfall were major variables which affected the plant morphological characteristics of LS plants (Fig. 4). Latitude was also a major variable for the differences in morphological characteristics of LS and SS plants. Soil characteristics, however, did not significantly affect the morphological characteristics of both morph plants (Fig. 4, Table 1).
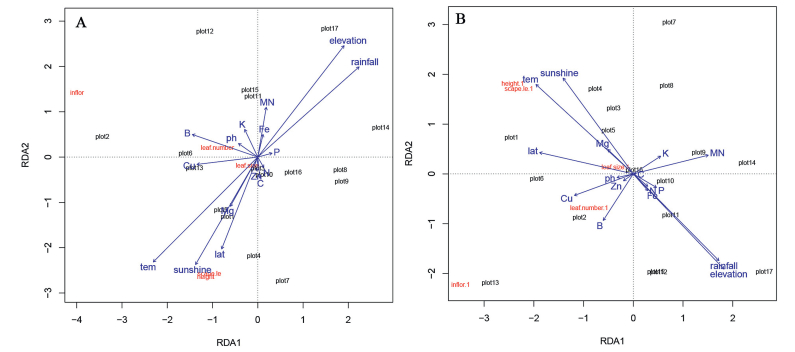
|
| Fig. 4 RDA analysis of plant morphological characteristics and ecological structure of 17 populations of Primula nivalis at different elevations. Analysis is based on variation among 16 ecological factors for (A) L-morph and (B) S-morph individuals. |
| L-morph | S-morph | ||||||||
| PC1 | PC2 | R2 | Pr(> r) | PC1 | PC2 | R2 | Pr(> r) | ||
| Elevation | 0.6127 | 0.7903 | 0.5874 | 0.005** | 0.6903 | -0.7235 | 0.648 | 0.002** | |
| Latitude | -0.3665 | -0.9304 | 0.2880 | 0.101 | -0.9752 | 0.2215 | 0.347 | 0.056 | |
| Temperature | -0.7063 | -0.7079 | 0.6484 | 0.002** | -0.7368 | 0.6762 | 0.653 | 0.001** | |
| Sunshine | -0.5017 | -0.8650 | 0.4561 | 0.009** | -0.5914 | 0.8064 | 0.529 | 0.007** | |
| Total rainfall | 0.7472 | 0.6646 | 0.5445 | 0.007** | 0.7030 | -0.7112 | 0.558 | 0.006** | |
| Soil pH | -0.8182 | 0.5749 | 0.0164 | 0.893 | -0.9748 | -0.2232 | 0.010 | 0.933 | |
| Total carbon | 0.1021 | -0.9948 | 0.0132 | 0.913 | 0.9994 | -0.0347 | 0.001 | 0.999 | |
| Total nitrogen | 0.4929 | -0.8701 | 0.0044 | 0.978 | 0.7590 | -0.6510 | 0.014 | 0.919 | |
| Total phosphorus | 0.9647 | 0.2633 | 0.0064 | 0.955 | 0.8629 | -0.5054 | 0.027 | 0.823 | |
| Total potassium | -0.4284 | 0.9036 | 0.0278 | 0.820 | 0.8398 | 0.5430 | 0.040 | 0.756 | |
| Full magnesium | 0.1680 | 0.9858 | 0.0754 | 0.576 | 0.9704 | 0.2415 | 0.224 | 0.166 | |
| Total iron | 0.2270 | 0.9739 | 0.0160 | 0.907 | 0.6571 | -0.7538 | 0.019 | 0.860 | |
| Total Mn | -0.4871 | -0.8734 | 0.0940 | 0.517 | -0.7220 | 0.6919 | 0.047 | 0.710 | |
| Copper | -0.9928 | -0.1196 | 0.1110 | 0.457 | -0.9392 | -0.3433 | 0.148 | 0.315 | |
| Zinc | -0.0712 | -0.9975 | 0.0057 | 0.960 | -0.8180 | -0.5753 | 0.005 | 0.962 | |
| Boron | -0.94541 | 0.32589 | 0.1418 | 0.335 | -0.5430 | -0.8397 | 0.1152 | 0.408 | |
| The * and ** is significantly difference at the 0.05 level and 0.05 level, respectively. | |||||||||
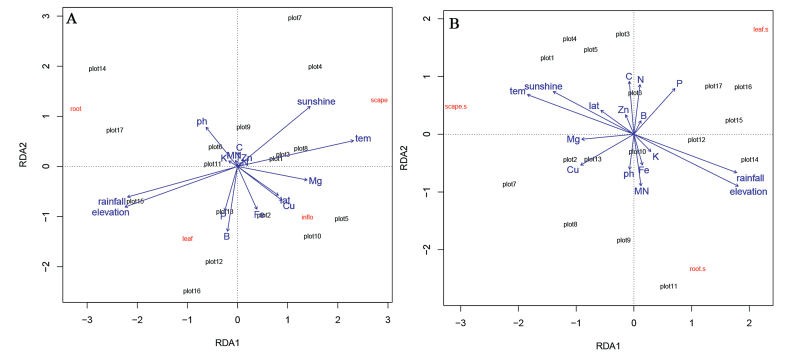
RDA analysis results suggest that all the evaluated variables, with the exception of soil characteristics, significantly affected resource allocation in both morph plants. Soil elements were only important with regard to the quality of resource allocation to individual components of both morph plants (Fig. 5, Table 2).
|
| Fig. 5 RDA analysis of plant biomass allocation and ecological structure of 17 populations of Primula nivalis at different elevations. Analysis is based on variation among 16 ecological factors for (A) L-morph and (B) S-morph individuals. |
| L-morph | S-morph | ||||||||
| PC1 | PC2 | R2 | Pr(> r) | PC1 | PC2 | R2 | Pr(> r) | ||
| Elevation | -0.9408 | -0.3390 | 0.564 | 0.002** | 0.8951 | -0.4460 | 0.7310 | 0.001** | |
| Latitude | 0.8171 | -0.5765 | 0.099 | 0.484 | -0.8107 | 0.5855 | 0.0894 | 0.529 | |
| Temperature | 0.9762 | 0.2175 | 0.557 | 0.005** | -0.9373 | 0.3486 | 0.6938 | 0.001** | |
| Sunshine | 0.7711 | 0.6367 | 0.353 | 0.035* | -0.8836 | 0.4683 | 0.4481 | 0.019* | |
| Total rainfall | -0.9635 | -0.2677 | 0.513 | 0.007** | 0.9368 | -0.3499 | 0.6475 | 0.001** | |
| Soil ph | -0.6256 | 0.7802 | 0.100 | 0.477 | -0.1226 | -0.9925 | 0.0674 | 0.613 | |
| Total carbon | 0.0982 | 0.9952 | 0.008 | 0.951 | -0.0863 | 0.9963 | 0.1515 | 0.297 | |
| Total nitrogen | 0.8872 | 0.4613 | 0.001 | 1.000 | 0.1218 | 0.9926 | 0.1346 | 0.353 | |
| Total phosphorus | -0.2772 | -0.9608 | 0.084 | 0.520 | 0.6683 | 0.7439 | 0.2030 | 0.206 | |
| Total potassium | -0.8466 | 0.5323 | 0.004 | 0.970 | 0.7001 | -0.7141 | 0.0314 | 0.808 | |
| Full magnesium | -0.2943 | 0.9557 | 0.002 | 0.986 | 0.1365 | -0.9906 | 0.1459 | 0.322 | |
| Total iron | 0.4126 | -0.9109 | 0.087 | 0.536 | 0.2673 | -0.9636 | 0.0548 | 0.679 | |
| Total Mn | 0.9816 | -0.1908 | 0.198 | 0.214 | -0.9954 | -0.0961 | 0.1477 | 0.321 | |
| Copper | 0.7952 | -0.6063 | 0.124 | 0.419 | -0.8643 | -0.5030 | 0.2034 | 0.192 | |
| Zinc | 0.7183 | 0.6958 | 0.001 | 0.994 | -0.3896 | 0.9210 | 0.0241 | 0.850 | |
| Boron | -0.1522 | -0.9883 | 0.169 | 0.279 | 0.4677 | 0.8839 | 0.0124 | 0.930 | |
| The * and ** is significantly difference at the 0.05 level and 0.05 level, respectively. | |||||||||
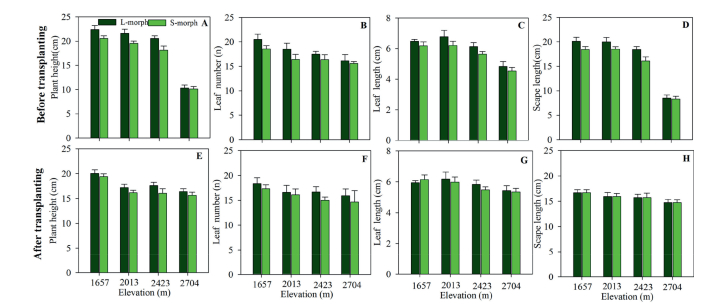
GLM results indicate that, before transplanting, the plant height, number of leaves, maximum length of leaves, and scape length in both morph plants were affected by elevation and sexual morph, but not by the interaction between elevation and sexual morph (Fig. 6 and Table 3). After transplanting P. nivalis plants from higher elevations (2, 704, 2, 423, 2013 m a.s.l.) to a lower elevation (and 1657 m a.s.l.), plant morphological characteristics were not significantly affected by elevation, sexual morph, nor the interaction between elevation and sexual morph (Fig. 6 and Table 3). However, the heterogeneous environment affected plant morphological characteristics in both morph plants. There was a significant difference between the morphological characteristics of the two sexual morphs before transplanting, but after transplanting, these differences became non-significant. This suggests that environmental heterogeneity is the main variable causing differences in plant morphology.
|
| Fig. 6 Variation in plant morphological characters (plant height, leaf number, leaf length, and scape length) for both sexual morphs of Primula nivalis plants from populations at different elevations. (A-D) Before transplanting; (E-H) after transplanting. |
| Plant height | Leaf number | Leaf length | Scape length | ||||||||
| MS | F | MS | F | MS | F | MS | F | ||||
| Before transplanting | |||||||||||
| Elevation (E) | 798.671 | 104.626** | 44.624 | 2.426* | 15.430 | 13.263** | 878.651 | 107.103** | |||
| Sexual morph (S) | 37.424 | 4.903* | 12.411 | .675 | 4.956 | 4.260* | 80.697 | 9.836** | |||
| E×S | 10.481 | 1.373 | 19.105 | 1.039 | .145 | .124 | 6.205 | .756 | |||
| Error | 7.634 | 18.391 | 1.163 | 8.204 | |||||||
| After transplanting | |||||||||||
| Elevation (E) | 3.402 | .584 | 38.058 | 2.024 | 2.540 | 2.360 | 8.519 | 1.437 | |||
| Sexual morph (S) | 3.503 | .601 | .026 | .001 | .103 | .096 | 2.784 | .470 | |||
| ExS | 7.629 | 1.309 | 12.358 | .657 | .390 | .362 | 4.304 | .726 | |||
| Error | 5.829 | 18.803 | 1.076 | 5.927 | |||||||
| The * and ** is significantly difference at the 0.05 level and 0.05 level, respectively. | |||||||||||
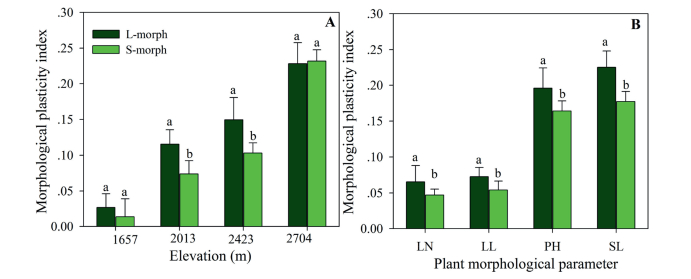
The MPI of individual plants of both morphs was positively affected by elevation (F3, 32 = 4.580, P < 0.01) and sexual morph (F1, 32 = 2.080, P < 0.05). The MPI of both morph plants increased with increasing elevation (Fig. 7A). The MPI of individual components of the plants of both sexual morphs was affected by plant organs (F1, 32 = 5.669, P < 0.01) and sexual morph (F1, 32 = 2.069, P < 0.05). In both sexual morphs of P. nivalis, the MPI of plant height and scape length was higher than that of the number and length of leaves (Fig. 7B). However, the MPI of LS plants was higher than that of SS plants (see Fig. 7).
|
| Fig. 7 Variation of morphological plasticity index for both sexual morph of Primula nivalis individuals at the population (A) and plant organ (B) level. LN: leaf number; LL: leaf length; PH: plant height; SL: scape length. |
Our study revealed that the morphological characteristics of P. nivalis plants varied between the sexual morphs and different elevational gradients. The morphological characteristics of both morphs of P. nivalis plants are crucial for predicting changes in different populations because inter-population (elevation) variation in ecological variables (climate change) decrease growth rates and adaptation (Hudson et al., 2011; Anderson et al., 2012). We found that an increase in elevation and associated shifts in ecological variables bring about changes in plant morphological traits. However, there are indications that SS plants have a higher adaptation potential than LS plants.
The growth-differentiation balance hypothesis (GDBH) suggests that there is a physiological trade-off between growth and secondary metabolism, and it predicts that more resources will be allocated to growth (Pizarro and Bisigato, 2010), or that related processes will differentiate over a range of environmental conditions (Xie et al., 2007; Frei et al., 2014). Although limiting ecological variables such as reduced resource allocation and soil nutrient heterogeneity in the different populations did not affect plant morphological adaptation, limiting ecological variables did influence biomass allocation to the different components of P. nivalis. Moreover, above-ground biomass was measured as an indication of overall plant growth and performance and found to be strongly influenced by the geographic distribution of P. nivalis. There was a consistent (and often strong) positive response of N to P ratios to increasing elevation in short style plant morphs. This is likely because improved nutrient availability leads to increases in leaf area growth and photosynthetic capacity (Wu et al., 2004).
4.2. Relationships between elevational climate variation and morphological plasticityVarying climatic conditions along elevational gradients also results in different selection pressures that shape inter-morph traits so that plants can adapt to a particular elevation. Previous studies have shown that different genotypes are found in locations that experience different annual weather conditions (Fusco and Minelli, 2010). P. nivalis, which has a large distribution in Xinjiang, may have adapted to short growing seasons at high elevations, and is likely to have evolved different morphological traits (Gugger et al., 2015). The adaptive hypothesis suggests that there is a strong correlation between plant morphological characteristics and fitness (adaptive significance) under different habitat environments (Dudley and Schmitt, 1996). For example, results from a previous transplant experiment suggest that there was no difference in plasticity between high and low elevation populations of three grassland species (Frei et al., 2014). Our transplanting data emphasized that specific maternal families of P. nivalis have growth benefits in low elevation populations. The ability to change their morphological characteristics is the most important adaptive strategy for P. nivalis in a heterogeneous environment.
We found evidence that indicates that the plasticity of morphological traits is greater in LS plants than in SS plants in response to environmental heterogeneity, and the LS plants were better adapted to high elevations (harsh environments) than SS plants. Trait plasticity is an advantage for plants experiencing great spatial habitat heterogeneity, and allows plants to maximize their fitness (Frei et al., 2014). In response to climate change and novel conditions, these plants responded through morphological plasticity and adapted through natural selection (Frei et al., 2014). Higher plasticity of plant height and scape length would be an advantage for P. nivalis experiencing greater spatial habitat heterogeneity under different environmental conditions. Therefore, environmental resource limitations in high elevation populations induced morphological plasticity, and this plasticity may be a critical component of the response of P. nivalis to changing environments.
4.3. Adaptations of both plant morphs and populationsAlpine systems have extreme temperature and water availability gradients associated with elevation, and adaptive plasticity is predicted to evolve when a species is subjected to environmental heterogeneity within the life span of the plants (Valladares et al., 2002). The plastic response to four ecological variables was adaptive for both morph plants in our experiment. The decreased plant size under increased elevation resulted in efficient use of available resources. Plasticity of morphological traits, such as height or biomass, may play a role in competitive interactions (Navas and Richard, 2005). The LS plants were significantly more plastic than SS plants in populations of P. nivalis located at different elevations. With the exception of plant height, morphological traits (scape length, root, and leaf biomass) were more plastic than other parameters in both plant morphs. The phenotypic variation among populations of S. candolleana along its distribution range was pointed out by Ramírez-Valiente et al. (2015). Our results support the idea that resource availability is limited in high elevation environments, and results in greater phenotypic plasticity (Bradshaw and Holzapfel, 2006; Gianoli and Valladares, 2010). Variation in ecological variables (resource limitation) can affect plant traits and the adaptive nature of morphological plasticity of P. nivalis; moreover, variation of plasticity in both plant morphs could be subjected to evolution by natural selection (Richards et al., 2006). Although several studies have shown that climate change can increase phenotypic plasticity within populations, we demonstrated the importance of environmental heterogeneity for distylous species, which can result in morphological plasticity mainly because of climate change, and rarely includes intra-specific differences (Lenoir and Svenning, 2013; Valladares et al., 2014).
AcknowledgmentsThe authors thank Parhat Sabit and Yusup Alim for help in the field. Lab work was carried out at the Xinjiang Key Laboratory of Grassland Resources and Ecology and at the Key Laboratory for Western Arid Region Grassland Resources and Ecology. This study was partially supported by the National Natural Science Foundation of China (NSFC, 31400279, 31860121) and Funded by the Scientific Research Program of the Higher Education Institution of Xinjiang (XJEDU2016I042).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2018.11.003.
Abdusalm A., 2018. Effect of habitat heterogeneity on floral trait differentiation level in distylous species Primula nivalis. Acta.Bot. Boreal.-Occident.Sin, 38, 158-165.
|
||
Anderson B.W., Mccauley S., Lewis G.P., et al., 2014. Impacts of a Poultry processing plant on the diversity of escherichia coli populations and transferability of tetracycline resistance genes in an Urban Stream in South Carolina. Water Air Soil Poll, 225, 20-30.
|
||
Anderson J.M., Horton P., Kim E.H., et al., 2012. Towards elucidation of dynamic structural changes of plant thylakoid architecture. Philos Trans R Soc Lond B Biol Sci, 367, 3515-3524.
DOI:10.1098/rstb.2012.0373 |
||
Aragon G., Martínez I., García A., 2012. Loss of epiphytic diversity along a latitudinal gradient in southern Europe. Sci. Total Environ, 426, 188-195.
DOI:10.1016/j.scitotenv.2012.03.053 |
||
Bradshaw W.E., Holzapfel C.M., 2006. Evolutionary response to rapid climate change. Science, 312, 1477-1478.
DOI:10.1126/science.1127000 |
||
Ceplick G.P., 1995. Genotypic variation and plasticity of clonal growth in relation to nutrient availability in Amphibromus scabrivalvis. J. Ecol, 83, 459-468.
DOI:10.2307/2261599 |
||
Dorken M.E., Barrett S.C.H., 2004. Phenotypic plasticity of vegetative and reproductive traits Blackwell Publishing, Ltd. in monoecious and dioecious populations of Sagittaria latifolia (Alismataceae):a clonal aquatic plant. J. Ecol, 92, 32-44.
DOI:10.1111/jec.2004.92.issue-1 |
||
Dudley S.A., Schmitt J., 1996. Testing the adaptive plasticity hypothesis:densitydependent selection on manipulated stem length in Impatiens capensis. Am. Nat, 147, 445-465.
DOI:10.1086/285860 |
||
Frei E.R., Ghazoul J., Pluess A.R., 2014. Plastic responses to elevated temperature in low and high elevation populations of three grassland species. PloS One, *(9), e98677.
DOI:10.1371/journal.pone.0098677 |
||
Fusco G., Minelli A., 2010. Phenotypic plasticity in development and evolution:facts and concepts. Philos. T. R. Soc, 365, 547-556.
DOI:10.1098/rstb.2009.0267 |
||
Godoy O., Saldaná A., Fuentes N., et al., 2011. Forests are not immune to plant invasions:phenotypic plasticity and local adaptation allow Prunella vulgaris to colonize a temperate evergreen rainforest. Biol. Invasions, 13, 1615-1625.
DOI:10.1007/s10530-010-9919-0 |
||
Gianoli E., Valladares F., 2010. Global change and the evolution of phenotypic plasticity in plants. Ann. NY. Acad. Sci, 1206, 35-55.
DOI:10.1111/nyas.2010.1206.issue-1 |
||
Goh C.H., Vallejos D.F.V., Nicotra A.B., et al., 2013. The impact of beneficial plantassociated microbes on plant phenotypic plasticity. J. Chem. Ecol, 39, 826-839.
DOI:10.1007/s10886-013-0326-8 |
||
Guerin G.R., Wen H.X., Lowe A.J., 2012. Leaf morphology shifts linked to climate change. Biol. Lett, 8, 882-886.
DOI:10.1098/rsbl.2012.0458 |
||
Gugger S., Kesselring H., Stöcklin J., et al., 2015. Lower plasticity exhibited by high-versus mid-elevation species in their phenological responses to manipulated temperature and drought. Ann. Bot. Lond, 116, 953-962.
|
||
Gómez-Aparicio L., Zamora R., Gómez J.M., 2005. The regeneration status of the endangered Acer opalus, subsp. granatense, throughout its geographical distribution in the Iberian Peninsula. Biol.Conserv, 121, 195-206.
DOI:10.1016/j.biocon.2004.04.019 |
||
Herrera C.M., Bazaga P., 2013. Epigenetic correlates of plant phenotypic plasticity:DNA methylation differs between prickly and nonprickly leaves in heterophyllous Ilex aquifolium (Aquifoliaceae) trees. Bot. J. Linn. Soc, 171, 441-452.
DOI:10.1111/boj.2013.171.issue-3 |
||
Huber H., Jacobs E., Visser E.J.W., 2009. Variation in flooding-induced morphological traits in natural populations of white clover (Trifolium repens) and their effects on plant performance during soil flooding. Ann. Bot. London, 103, 377-386.
DOI:10.1093/aob/mcn149 |
||
Hudson J.M.G., Henry G.H.R., Cornwell W.K., 2011. Taller and larger:shifts in Arctic tundra leaf traits after 16 years of experimental warming. Global Change Biol, 17, 1013-1021.
DOI:10.1111/gcb.2010.17.issue-2 |
||
Leingärtner A., Hoiss B., Krauss J., et al., 2014. Combined effects of extreme climatic events and elevation on nutritional quality and herbivory of alpine plants. PloS one, 9(4), e93881.
DOI:10.1371/journal.pone.0093881 |
||
Lenoir J., Svenning J.C., 2013. Latitudinal and elevational range shifts under contemporary climatecchange. Encyclopedia Biodiver, 599-611.
|
||
Matesanz S., Gianoli E., Valladares F., 2010. Global change and the evolution of phenotypic plasticity in plants. Ann. N.Y. Acad. Sci, 1206, 35-55.
DOI:10.1111/nyas.2010.1206.issue-1 |
||
Miner B.G., Sultan S.E., Morgan S.G., et al., 2005. Ecological consequences of phenotypic plasticity. Trends. Ecol. Evol, 20, 685-692.
DOI:10.1016/j.tree.2005.08.002 |
||
Millal R., Reich P.B., 2011. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Ann. Bot. Lond, 107, 455-465.
DOI:10.1093/aob/mcq261 |
||
Mou P., Jones R.H., Tan Z.Q., et al., 2013. Morphological and physiological plasticity of plant roots when nutrients are both spatially and temporally heterogeneous. Plant Soil, 364, 373-384.
DOI:10.1007/s11104-012-1336-y |
||
Navas M.L., Richard J.M., 2005. Can traits predict the competitive response of herbaceous Mediterranean species?. Acta Oecol, 27, 107-114.
DOI:10.1016/j.actao.2004.10.002 |
||
Nascimbene J., Marini L., 2015. Epiphytic lichen diversity along elevational gradients:biological traits reveal a complex response to water and energy. J. Biogeogr, 42, 1222-1232.
|
||
Nicotra A.B., Atkin O.K., Bonser S.P., et al., 2010. Plant phenotypic plasticity in a changing climate. Trends Plant Sci, 15, 684-692.
DOI:10.1016/j.tplants.2010.09.008 |
||
Pizarro L.C., Bisigato A.J., 2010. Allocation of biomass and photoassimilates in juvenile plants of six Patagonian species in response to five water supply regimes. Ann. Bot. Lond, 106, 297-307.
DOI:10.1093/aob/mcq109 |
||
Puijalon S., Bornette G., 2006. Phenotypic plasticity and mechanical stress:biomass partitioning and clonal growth of an aquatic plant species. Am. J. Bot, 93, 1090-1099.
DOI:10.3732/ajb.93.8.1090 |
||
Ramírez-Valiente J.A., Valladares F., Delgado A., et al., 2015. Understanding the importance of intrapopulation functional variability and phenotypic plasticity in Quercus suber. Tree Genet. Genomes, 11, 35.
DOI:10.1007/s11295-015-0856-z |
||
Richards C.L., Bossdorf O., Muth N.Z., et al., 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett, 9, 981-993.
DOI:10.1111/ele.2006.9.issue-8 |
||
Scheiner S.M., 2013. The genetics of phenotypic plasticity. ⅩⅡ. Temporal and spatial heterogeneity. Ecol. Evol, 3, 4596-4609.
DOI:10.1002/ece3.792 |
||
Shen H.H., Tang Y.H., Muraoka H., et al., 2008. Characteristics of leaf photosynthesis and simulated individual carbon budget in Primula nutans under contrasting light and temperature conditions. J. Plant Res, 121, 191-200.
DOI:10.1007/s10265-008-0146-z |
||
Shimizu K.K., Kudoh H., Kobayashi M.J., 2011. Plant sexual reproduction during climate change:gene function in nature studied by ecological and evolutionary systems biology. Ann. Bot. Lond, 108, 777-787.
|
||
Tétard-Jones C., Kertesz M.A., Phil R.F.P., 2011. Quantitative trait loci mapping of phenotypic plasticity and genotypeeenvironment interactions in plant and insect performance. Philos. Trans. R. Soc. B, 366, 1368-1379.
DOI:10.1098/rstb.2010.0356 |
||
Valladares F., Balaguer L., Martinez-Ferri E., 2002. Plasticity, instability and canalization:Is the phenotypic variation in seedlings of sclerophyll oaks consistent with the environmental unpredictability of Mediterranean ecosystems?. New Phytol, 156, 457-467.
DOI:10.1046/j.1469-8137.2002.00525.x |
||
Valladares F., Matesanz S., Guilhaumon F., et al., 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett, 17, 1351-1364.
DOI:10.1111/ele.12348 |
||
Valladares F., Gianoli E., Gómez J.M., 2007. Ecological limits to plant phenotypic plasticity. New Phytol, 176, 749-763.
|
||
Wang R., Yu G., He N., et al., 2014. Elevation-related variation in leaf stomatal traits as a function of plant functional type:evidence from Changbai Mountain, China. PloS One, 9(12), e115395.
DOI:10.1371/journal.pone.0115395 |
||
Wu R., Grissom J.E., McKeand S.E., et al., 2004. Phenotypic plasticity of fine root growth increases plant productivity in pine seedlings. BMC Ecol, 4, 14.
DOI:10.1186/1472-6785-4-14 |
||
Xie Y.H., Luo W.B., Ren B., et al., 2007. Morphological and physiological responses to sediment type and light availability in roots of the submerged plant Myriophyllum spicatum. Ann. Bot. Lond, 100, 1517-1523.
DOI:10.1093/aob/mcm236 |
||
Zhang X., Izaurralde R.C., Arnold J.G., et al., 2011. Comment on "modeling miscanthus in the soil and water assessment tool (SWAT) to Simulate Its water quality effects as a bioenergy crop". Environ. Sci. Technol, 45, 6211-6212.
DOI:10.1021/es201463x |



