b. Botany Department, and Biodiversity Research Centre, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada;
c. Department of Statistics, Colorado State University, Fort Collins, CO, 80523, USA
Quercus schottkyana is a dominant species of oak in the Asian evergreen broad-leaved forests in southwestern China and thus provides the major structural component for the forests of Yunnan Province. The species produces abundant acorns in most years, but even in years of high acorn production, seedlings of Q. schottkyana are uncommon. The maintenance of these forests and the biodiversity they support is a topic of critical importance to conservation biologists. A central question relates to an understanding of the factors that influence the survival of Q. schottkyana from acorn production to germination, seedling establishment and recruitment.
Seedling recruitment in forests results from the interaction of various processes such as acorn production, dispersal, predation, and seedling establishment. Given the number of variable processes, it is difficult to establish their relative importance to recruitment. Annual acorn production by Q. schottkyana is variable (Xia et al., 2016a). Local weather conditions influence acorn production, but the acorns produced are then exposed to a series of mortality risks. Firstly, a major pre-dispersal predator is weevils (Curculio, Coleoptera: Curculionidae) that infest acorns on the trees before the acorns fall to the ground (Maeto and Ozaka, 2003; Lombardo and McCarthy, 2008). Second, although acorns of Q. schottkyana are desiccation-sensitive (Pritchard et al., 2004; Xia et al., 2012a), they are typically dispersed from late September coincident with the beginning of the distinct dry season in our study area. Third, acorns of Q. schottkyana germinate slowly thus exposing them to a greater risk of desiccation and a higher risk of post-dispersal predation. In our region, bark beetles [Coccotrypes sp. (Coleoptera: Curculionidae)] are a primary post-dispersal predator for this oak (Xia et al., 2016b).
Many oak species show mast seeding which is the synchronous intermittent production of large seed crops in perennial plants (Kelly and Sork, 2002). The increase of acorn production in mast years permits oaks to maximize the probability of avoiding seed predation via predator satiation (predation satiation hypothesis, Janzen, 1971; Sork, 1993). The percentage germination of Q. schottkyana acorns is positively density-dependent, meaning that proportionately more acorns germinate with increasing acorn density (Xia et al., 2016a). Thus, under high levels of predation, variable acorn production in Q. schottkyana might be an evolved reproductive strategy.
Our previous study (Xia et al., 2016a) investigated correlations between acorn production and weevil populations, but did not consider the effects of other limiting factors such as desiccation and predation by bark beetles. Moreover, factors such as shade (sun light), drought and herbivory, which usually cause the death of oak seedlings (Lorimer et al., 1994; Gómez et al., 2003; Tyler et al., 2006; Gómez-Aparicio et al., 2008), may also contribute to poor seedling establishment of Q. schottkyana. Despite the dominance and importance of Q. schottkyana to the integrity of these southern Chinese forests and the region's biodiversity, factors that influence seedling establishment of this species have rarely been studied.
In this study, we tested if variable acorn production has a significant impact on the survival of acorns and on seedling establishment and recruitment. We monitored the survival of acorns in field plots that were artificially sown at a range of densities and monitored their fate through dispersal to seedling establishment and recruitment. We also tested the effects of herbivores on seedling survival by erecting fences around both natural and transplanted seedlings. By integrating data and results from our previous study (Xia et al., 2016a, b) and the current study for the same oak stand, we gain a better and more comprehensive understanding of the factors that limit seedling recruitment of Q. schottkyana.
2. Materials and methods 2.1. Study siteThe study site (approximately 1463 m2) is located in a natural pure stand of Q. schottkyana, just north of Kunming, China (25°01'N, 102°41'E). The region has a distinct wet season (May-October) and a dry season (November-April) with an average rainfall of 878 mm and 116 mm respectively, and average monthly temperatures of 19 ℃ and 12 ℃ respectively. The mean annual temperature for this region is 15.5 ℃ (Climatic Data Center, 2015).
2.2. Survival of acorns in different acorn densitiesThe first experiment tested if the probability of acorn survival to germination was affected by density of acorns. Throughout our nine-year study (Xia et al., 2016a), the annual acorn density produced ranged from 13 acorns∙m-2 to 759 acorns∙m-2. In the current experiment, we deliberately planted acorns at densities much higher than what we observed in the field to test if the reported relationships between acorn density and survival were consistent across a wider range of densities. Q. schottkyana acorns were collected on 5 November 2011 and stored at 10 ℃. On 12 January 2012, 29 locations were randomly selected throughout the study area. At each location two plots, each 25 cm × 25 cm, were established; one plot was fenced to a height of 25 cm with 1.5 × 1.5 mm wire mesh and the other plot was a control without fencing; paired plots were a minimum of 25 cm apart. The fences effectively excluded rodents but not bark beetles. All litter and acorns in the plots were gently brushed away, then the experimental acorns were placed directly on the soil surface. The litter was examined carefully and all acorns removed; the litter was then replaced evenly over the plots. Acorns were prepared in batches of 2, 5, 10, 15, 25, 50, 75, 100, 150, 250, 350 and 500 representing annual acorn densities of 32-8000 acorns∙m-2. Each acorn density was randomly assigned to sets of paired (fenced/unfenced) plots, with a single density in each pair of plots. For each set of paired plots, batches of 2-25 acorns were replicated three times and batches of 50-500 were replicated twice. Four levels of acorn density were used for the first experiment: low density, replicates of 2-10 acorns (32-160 acorns∙m-2); medium density, replicates of 15-50 acorns (240-800 acorns∙m-2); high density, replicates of 75-150 acorns (1200-2400 acorns∙m-2) and very high density, replicates of 250-500 acorns (4000-8000 acorns∙m-2). Prior to this experiment, we did not clean the weevil-infested acorns so that acorns used had the same status as those acorns that dispersed naturally on to the soil surface. Acorns were monitored and recorded on 7 May 2012, 2 September 2012, and 15 January 2013. During each recording, acorns were recorded as seedlings, germinated, sound (acorns of no apparent infestation by weevils or by bark beetles), partially eaten (by rodents), infested (by weevils or by bark beetles), rotten, or missing.
2.3. Survival of Quercus schottkyana seedlingsThe second experiment was done using natural populations of oak seedlings and transplanted seedlings. Because seedling density is naturally very low, we deliberately chose sites that had a few natural seedlings present. Four plots (each 1 m × 1 m) were chosen and were surrounded by a 50-cm high 1-cm-mesh fence and covered using the same 1-cm-mesh fence material. Monitoring of seedling survival began on 20 December 2010. Each of the four plots was paired with an unfenced control plot situated approximately 0.5 m away in areas that also had seedlings. Seedlings were never more than 20 cm high and they were seedlings from acorns produced before 2010.
An additional eight 1 m × 1 m plots (4 caged plots and 4 open plots close by) were set up under the canopy in places where there were no natural seedlings. At the same time, acorns that had been germinated in the lab were planted in a greenhouse (23 ℃, 45% relative humidity) for 1.5 years. On 14 April 2011 (before the wet season), 5 seedlings between 10 cm and 15 cm tall were transplanted from the greenhouse into each of the 16 plots. All 80 transplanted seedlings in both the fenced and open plots were labeled and survival was monitored every two to four weeks for 18 months. For each survey at solar noon (13:00-14:00), sunlight was measured at the center of each plot, 50 cm above ground level, using a solar meter (3415 FQF Quantum and Foot-Candle Meter, Spectrum Technologies, Inc., Plainfield, IL).
2.4. Data analysisWe used likelihood ratio tests on Generalized Linear Mixedeffects Model (GLMM) with binomial errors to determine if acorn viability responded on acorn density. In this model, the treatments (control or fenced) and acorn density were treated as the fixed effects and the plots were treated as the random effect. Likelihood ratio tests on a logistic regression model were used to determine how fences affected the survival of Q. schottkyana seedlings in the field. In this model, sunlight was used as a covariate. The survival curves for seedlings were analyzed by plotting number of survivors against time and then using the slope of the linear lines as a measure of relative death rates. All analyses were performed using R Development Core Team (2017).
3. Results 3.1. Acorns: effects of acorn density on acorn survivalAcorn status changed with time, acorn density, and treatments (±fences). On 7 May, about 82-86% of acorns were sound (i.e., no visible signs of mold or infestation), regardless of the treatment or acorn density (Fig. 1). At this time, bark beetles had not yet emerged and the remaining 14-18% of acorns were infested by weevils. On 2 September 2012, in both the control and the fenced plots, most acorns were infested by weevils and bark beetles, but the proportion of surviving acorns (includes sound acorns, germinated acorns, and seedlings) increased with increasing acorn density (P < 0.001, Figs. 1 and 2A). The percentage of surviving acorns in the fenced plots was higher than in control plots (P = 0.001). The percentage of surviving acorns was 2% for the lowest density (for both treatments), but it was 34% and 40% for the highest acorn density in the control and the fenced plots respectively (Fig. 1). At this time, in the control plots, only 0.6% of acorns in the very high density (i.e., 4000-8000 acorns∙m-2) survived to the seedling stage, but for the fenced plots, 0.6-2.3% of the high and very high-density acorns (1200-2400 acorns∙m-2) survived to become seedlings. By the third survey, 15 January 2013, any acorns that had not germinated to develop as seedlings, were infested and/or rotten. Seedlings only occurred in plots having high and very high densities (i.e., >12, 000 acorns∙m-2) but the percentages of the surviving seedlings were low (≤2.8%). For the low and medium acorn densities (i.e., < 800 acorns∙m-2), there were no seedlings established (Fig. 1). The GLM model for the January data showed the percentages of seedlings increased with acorn density (P < 0.001). The percentage of surviving seedlings in the fenced plots was significantly higher than in control plots (P = 0.001, Fig. 2B).
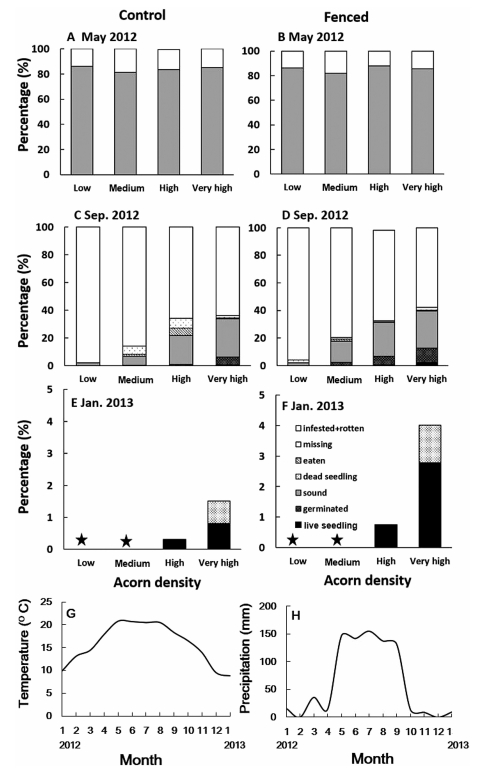
|
| Fig. 1 Status of acorns of Quercus schottkyana in different densities at different time intervals. Acorns in different densities were placed on the soil surface without (control) or with (fenced) fences. Four acorn densities were used: in each 25 cm × 25 cm plot, there were 2, 5 and 10 acorns (low density); 15, 25 and 50 acorns (medium density), 75, 100 and 150 acorns (high density) and 250, 350 and 500 acorns (very high density). Acorns in this experiment were produced in 2011. Field surveys were taken on 7 May 2012 (A, B), 2 September 2012 (C, D) and 15 January 2013 (E, F). Acorns were infested by weevils (pre-dispersal) or bark beetles (post-dispersal). Infested acorns were all rotten and dead in January and data of infestation are not included in the figure. Monthly mean temperatures (G) and monthly mean precipitation (H) during the experimental period are shown. Stars represent no survival of the acorns or seedlings observed. |
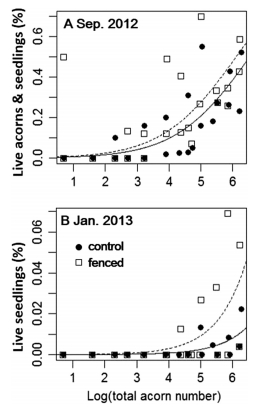
|
| Fig. 2 Relationship showing how the percentage of live acorns and seedlings changed with acorn densities in Sep. 2012 (A) and Jan. 2013 (B). The percentages of live acorns and seedlings for the control and fenced plots are shown by solid circles and open squares respectively. Curves are calculated from the GLM models. |
The first new seedlings to emerge naturally in the plots were observed on 1 April 2011. In the 16 plots, there was a total of only 12 new seedlings from acorns produced in 2010. The last new seedling in the plots was observed on 25 August 2011. At the final recording on 18 May 2012, only 7 seedlings were still alive and the average survival time for the other new seedlings that subsequently died was 130 days (range: 36-263 d).
For both the naturally established (n = 112 in total) and transplanted (n = 80) seedlings, fences significantly increased the probability of seedling survival (P < 0.05, Table 1). The death rate of naturally established seedlings (0.025 deaths per day, or 1 death in 40 days) in the fenced plots was consistent during the entire observation period (Fig. 3). In control plots, the death rate of natural seedlings was higher (0.194 deaths per day, or 1 death in 5.16 days) during the first 140 d (between 20 December 2010 and 9 May 2011), but it slowed thereafter (Fig. 3). In fenced plots, 81% of natural seedlings survived for 18 months compared with 37% in the control plots. For the transplanted seedlings, death rates followed the same trend: the death rate for fenced transplanted seedlings was 0.024 deaths per day (or 1 death in 41.7 days), which was similar to the fenced natural seedlings; the death rate for control transplanted seedlings was higher in the early stages but slowed later (Fig. 3). Our models show that sunlight (10-30.69 μmol.m-2.s-1) had no significant effect on survival of naturally established seedlings but had a negative effect on survival of the transplanted seedlings (P = 0.025, Table 1).
| Seedlings: treatment | Models | P (treatment) | P (sunlight) |
| Natural (fenced: n=64; control=48) | Death of seedlings=1.163-1.887×treatment | < 0.0001 | NS |
| Planted (fenced n=40; control=40) | Death of seedlings=-2.58-1.073×treatment+0.134×sunlight | 0.033 | 0.025 |
| NS: not significant. | |||
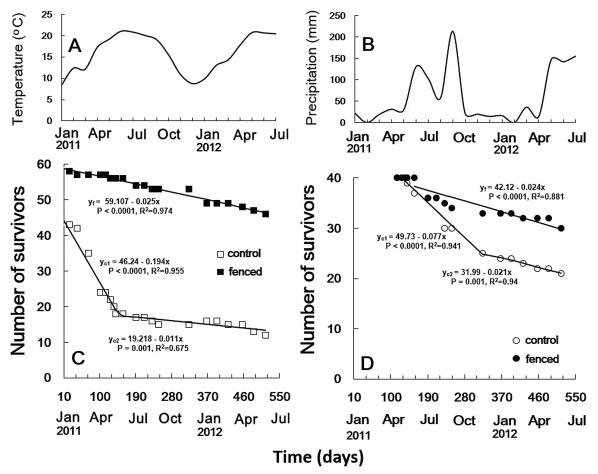
|
| Fig. 3 Time (days) of Q. schottkyana seedling survival in the study site from 20 December 2010 to 18 May 2012. A. monthly mean temperatures and B. monthly mean precipitation during the experimental period; C. natural seedlings, seedlings from acorns produced before 2010, and newly established seedlings in 2011 are included; D. transplanted seedlings. Open squares/circles show seedlings in the control plots and closed squares/circles show seedlings in the fenced plots. The survival curves of seedlings were plotted by linear regression (yf for the fenced seedlings, yc1 and yc2 for the control seedlings) and seedling death rate for given time was measured by the absolute value of the slope for each regression line. |
Plant populations in general are confronted by many factors that limit or regulate their numbers at various stages during their life cycle. The establishment phase is generally quite hazardous because, of the innumerable seeds produced by most species, few will actually germinate and establish as successful independent recruits to the population. This is true for oaks in general (Loftis, 1988) and Q. schottkyana in particular, and poor oak seedling establishment and survival is a crucial reason for the failure of oak regeneration. Variable acorn production, drought, and predation by insects (mainly weevils) results in poor seedling establishment of oaks. Having reached the seedling stage, however, many factors such as shade, drought, and herbivores sequentially limit oak seedlings (Lorimer et al., 1994; Gómez et al., 2003; Tyler et al., 2006; Gómez-Aparicio et al., 2008). For different oak species, the primary factor(s) that causes low survival of seedlings differs but effects of these factors are non-exclusive.
Q. schottkyana produces acorns annually from September to mid-late November. At our study site, production of Q. schottkyana ranged from 13 acorns∙m-2 (2013) to 759 acorns∙m-2 (2006) for the nine years from 2006 to 2014 (Xia et al., 2016a). High predation from insects (weevils and bark beetles) (Fig. 1) and desiccation (Xia et al., 2016b) led to the death of the majority of acorns, but the percentage of Q. schottkyana acorns germinating and becoming established seedlings is positively density-dependent, i.e., the rates of seedling establishment increased as the acorn density increased. Our study simulated the survival of acorns at four levels of acorn densities. However, in our earlier nine-year survey, natural acorn production of Q. schottkyana at our site never reached such highdensity levels (i.e., not more than 800 acorns∙m-2) (Xia et al., 2016a). Our experimental results showed that no seedlings established at acorn densities below 800 acorns∙m-2. The highest rate of successful seedling establishment was a mere 0.8% for the nonfenced acorns at very high densities (4000-8000 acorns∙m-2). In 2010, we observed a few acorns (0.28%) that survived and developed into seedlings. This means that with an acorn production of 261 acorns∙m-2∙yr-1, in an area of 3 m2 (783 acorns in total), only two acorns would successfully germinate and develop as seedlings, showing that the probability of successful seedling establishment and recruitment of Q. schottkyana is rare. In years of lower acorn production (5 out of 9 years recorded, Xia et al., 2016a), the number of successfully established seedlings was close to zero.
A surprising result was the increased probability of germination of acorns at higher densities. Previous studies, mostly carried out in the laboratory, have shown declines in germination with increasing seed density, both with increasing density of seeds of the same (Linhart, 1976; Waite and Hutchings, 1979) and of different species (Bergelson and Perry, 1989). Variation in percentage germination may be due to genetic factors, or to environmental factors such as small-scale differences in topography (Harper et al., 1965), resource level (Clauss and Venable, 2000), or seed density (Ellner, 1986). Seed density is known to have significant effects on timing of germination and emergence both inhibitory (Goldberg et al., 2001) and facilitative (Dyer et al., 2000; Bergelson and Perry, 1989) on herbaceous species, but we are not aware of such effects having been reported for trees or shrubs. However, the question of interest in this study was whether increasing acorn density can elicit a change in the probability of germinating, not on the timing. This positive density-dependent germination we observed must be due to the effects of acorns themselves on germination, i.e., acorns have an ability to detect other acorns. Possible mechanisms include increased local levels of CO2 with increasing seed density, or allelochemicals washed from seed coats. Inhibition by CO2 from respiration of germinating seeds is unlikely, because concentrations are unlikely to be high enough when seeds are placed on the soil surface. However, germination inhibition from surficial chemicals on seed coats is known from at least two grass genera: Avena (Naylor and Christie, 1956) and Aegilops (Dyer et al., 2000), providing some support for this mechanism, at least intraspecifically. Ongoing experiments are investigating this phenomenon. Lorimer et al. (1994) reported that Quercus rubra oak seedlings under mature oak forests have poor vigor and high mortality because of shade. They proposed that disturbances that reduce the understory could improve the survival probability of seedlings. In a 12-year survey Loftis (1988) showed that the survival rate of Q. rubra seedlings in a mature oak stand was < 10%. In a Mediterranean Quercus pyrenaica forest, seedlings have been shown to die mainly from herbivores (89% of dead seedlings) and desiccation (Gómez et al., 2003).
The results of our second experiment show that fences prevented seedling herbivores and effectively increased the probability of seedling survival in our site. Other factors such as strong sunlight, drought, and predation by various insects caused the death of seedlings in fenced plots. In control plots, the lower death rate after May and after November likely reflects lower predation pressure when vertebrates had a wider range of food resources available to them. Seedlings of Q. schottkyana are shade-tolerant but in low light conditions (such as 2% full light) their growth ceases (Cao et al., 2008). The study plots were in the understory and had the sunlight slightly stronger than the light compensation point of Q. schottkyana (6-10 μmol.m-2.s-1, Cao et al., 2008). However, Q. schottkyana seedlings in the plots were short and those established for several years were never more than 20 cm tall.
Acorns of many oak species germinate soon after dispersal, e.g., Quercus liaotungensis, Quercus petraea (Shaw, 1968; Sun et al., 2004; Tyler et al., 2006) and this is typically regarded as being an advantage for desiccation-sensitive acorns/seeds (Tweddle et al., 2003; Daws et al., 2005, 2006). These species usually occur in comparatively aseasonal and moist habitats (Tweddle et al., 2003) or disperse in the wet-season (Shaw, 1968; Pritchard et al., 2004; Sun et al., 2004; Daws et al., 2005). The moist climate in northwest Wales prevents Q. petraea acorn death due to water loss, and acorns on the ground germinate rapidly after dispersal (Shaw, 1968). Acorns of Q. schottkyana germinate slowly both in the field (current study) and in lab where there was sufficient water (Xia et al., 2015). In our study, we observed that germination and seedling emergence occurred between April and August during the wet season in the region. Intuitively, a delay of germination might be a better adaption for this species, allowing it to escape strong desiccation stress on vulnerable seedlings, because acorns of this species can survive quite long periods of drought (Xia et al., 2012). However, there is an inevitable trade-off because a longer delay of germination exposes the acorns to an increasingly higher risk of predation by bark beetles (Xia et al., 2016b).
As in other oak habitats worldwide (Tyler et al., 2006), we observed low recruitment rates of Q. schottkyana in our study area, potentially raising concerns about population decline. Many studies of oaks report a regeneration problem and base that conclusion on the rarity of seedlings (review by Tyler et al., 2006). However, lack of seedlings or recruits may suggest a regeneration problem, but cannot be used to confirm such a problem (Tyler et al., 2006). Seedling recruitment shows wide annual variation (Xia et al., 2016a) depending on the size of the acorn crop, rainfall, and other factors, and while recruitment rates may be low in any one year, they may be higher in other years or in other areas. Currently, there are insufficient quantitative data to conclude that we have a regeneration problem. The most significant gaps in our knowledge of seedling establishment and recruitment are data on survival rates among different age classes and size classes of Q. schottkyana and how various treatments, e.g., with/without fences, soil fertility levels, rainfall, length of wet/dry seasons etc., influence those rates. Longer term studies and monitoring of permanent plots is required to determine causes and rates of mortality within the seedling and sapling stages.
5. ConclusionsOur results show the rates of seedling establishment of Q. schottkyana were positively related to acorn production. Seedling establishment of Q. schottkyana was generally poor. Fig. 4, which is based on data from this study and our previous work (Xia et al., 2016a; b), summarizes the survival of acorns and seedlings of Q. schottkyana. Combined with our previous study, the current study shows that the major causes of low seedling establishment of Q. schottkyana at our study site were desiccation and predation, by both weevils and Coocotrypes sp., which killed 88% of acorns. Herbivores resulted in the death of seedlings and subsequently affected the establishment of seedlings of Q. schottkyana.
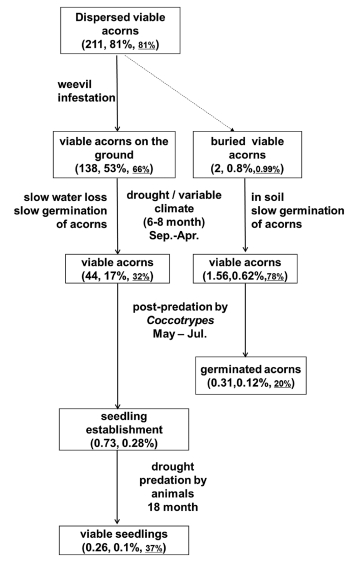
|
| Fig. 4 Fate of dispersed acorns. The numbers and percentages (x%, y%) of viable acorns∙m-2are shown in parentheses, where x% is the percentage of the original 100% and y% is the percentage of the previous box. For buried acorns, only the percentage of original 100% are shown. Data for the number of dispersed acorns in 2010 (n = 261) were obtained from Xia et al. (2016a) in the same Quercus schottkyana population. Because the initial viability of uninfested acorns was 81%, the total number of viable acorns∙m-2 at the beginning of the study was 211. |
This work was supported by the NSFC-Yunnan joint fund to support key projects (Nos. U1502231) and the National Natural Science Foundation of China (NSFC) (No. 31770358) to KX. We thank Jin-Jin Hu, Li Wang, and Qian Hu for their assistance in the field. We thank Wei-bang Sun for permission to work in the study site.
Bergelson J., Perry R., 1989. Interspecific competition between seeds:relative planting date and density affect seedling emergence. Ecology, 70, 1639-1644.
DOI:10.2307/1938097 |
||
Cao J.-X., Zhang G.-F., Zhang L., Li X.-H., Su W.-H., 2008. Acclimation of photosynthesis and growth to different growth light conditions for seedlings of Cyclobalanopsis glaucoides. Guihaia, 28, 126-129.
|
||
Clauss M.J., Venable D.L., 2000. Seed germination in desert annuals:an empirical test of adaptive bet hedging. Am. Nat, 155, 168-186.
DOI:10.1086/303314 |
||
Climatic Data Center, National Meteorological Information Center (CMA), 2015. China Meteorological Data Sharing Service System. Available at: . Accessed 2015. http://cdc.nmic.cn/home.do.
|
||
Daws M.I., Garwood N.C., Pritchard H.W., 2005. Traits of recalcitrant seeds in a semi-deciduous tropical forest in Panama:some ecological implications. Funct. Ecol, 19, 874-885.
DOI:10.1111/fec.2005.19.issue-5 |
||
Daws M.I., Garwood N.C., Pritchard H.W., 2006. Prediction of desiccation sensitivity in seeds of woody species:a probabilistic model based on two seed traits and 104 species. Ann. Bot, 97, 667-674.
DOI:10.1093/aob/mcl022 |
||
Dyer A.R., Fenech A., Rice K.J., 2000. Accelerated seedling emergence in interspecific competitive neighbourhoods. Ecol. Lett, 3(6), 523-529.
DOI:10.1046/j.1461-0248.2000.00187.x |
||
Ellner S., 1986. Germination dimorphisms and parent-offspring conflict in seed germination. J. Theor. Biol, 123(2), 173-185.
DOI:10.1016/S0022-5193(86)80151-5 |
||
Goldberg D.E., Turkington R., Olsvig-Whittaker L., Dyer A.R., 2001. Density dependence in an annual plant community:variation among life history stages. Ecol. Monogr, 71, 423-446.
DOI:10.1890/0012-9615(2001)071[0423:DDIAAP]2.0.CO;2 |
||
Gómez J.M., Garcıa D., Zamora R., 2003. Impact of vertebrate acorn and seedlingpredators on a Mediterranean Quercus pyrenaica forest. For. Ecol. Manag, 180, 125-134.
DOI:10.1016/S0378-1127(02)00608-4 |
||
Gómez-Aparicio L., Pérez-Ramos I.M., Mendoza I., Matías L., Quero J.L., Castro J., Zamora R., Marañón T., 2008. Oak seedling survival and growth along resource gradients in Mediterranean forests:implications for regeneration in current and future environmental scenarios. Oikos, 117, 1683-1699.
DOI:10.1111/j.1600-0706.2008.16814.x |
||
Harper J.L., Williams J.T., Sagar G.R., 1965. The behaviour of seeds in soil. Ⅰ. The heterogeneity of soil surfaces and its role in determining the establishment of plants from seed. J. Ecol, 53, 273-286.
DOI:10.2307/2257975 |
||
Janzen D.H., 1971. Seed predation by animals. Annu. Rev. Ecol. Systemat, 2, 465-492.
DOI:10.1146/annurev.es.02.110171.002341 |
||
Kelly D., Sork V.L., 2002. Mast seeding in perennial plants:why, how, where?. Annu. Rev. Ecol. Systemat, 33, 427-447.
DOI:10.1146/annurev.ecolsys.33.020602.095433 |
||
Linhart Y.B., 1976. Density-dependent seed germination strategies in colonizing versus non-colonizing plant species. J. Ecol, 64(1), 375-380.
DOI:10.2307/2258701 |
||
Loftis, D.L., 1988. Regenerating oaks on high-quality sites; an update. In: Workshop Proceedings Guidelines for Regenerating Appalachian Hardwood Stands, pp. 199-209.
|
||
Lombardo J.A., McCarthy B.C., 2008. Silvicultural treatment effects on oak seed production and predation by acorn weevils in southeastern Ohio. For. Ecol. Manag, 255, 2566-2576.
DOI:10.1016/j.foreco.2008.01.017 |
||
Lorimer C.G., Chapman J.W., Lambert W.D., 1994. Tall understory vegetation as a factor in the poor development of oak seedlings beneath mature stands. J. Ecol, 82, 227-237.
DOI:10.2307/2261291 |
||
Maeto K., Ozaka K., 2003. Prolonged diapauses of specialist seed-feeders makes predator satiation unstable in masting of Quercus crispula. Oecologia, 137, 392-398.
DOI:10.1007/s00442-003-1381-6 |
||
Naylor J.M., Christie L.A., 1956. The control of dormancy in wild oats. Natl. Weed Comm. Can. (Western section), 10, 56-59.
|
||
Pritchard H.W., Daws M.I., Fletcher B.J., Gaméné C.S., Msanga H., Omondi W., 2004. Ecological correlates of seed desiccation tolerance in tropical African dryland trees. Am. J. Bot, 9, 863-870.
|
||
R Development Core Team, 2017. R (3.3.3): a Language and Environment for Statistical Computing. Available at: https://www.r-project.org/.
|
||
Shaw M.W., 1968. Factors affecting the natural regeneration of sessile oak (Quercus Petraea) in North Wales:Ⅱ. acorn losses and germination under field conditions. J. Ecol, 56, 647-660.
DOI:10.2307/2258097 |
||
Sork V.L., 1993. Evolutionary ecology of mast-fruiting in temperate and tropical oaks (Quercus spp.). Vegetatio, 107/108, 133-147.
|
||
Sun S., Gao X., Chen L., 2004. High acorn predation prevents the regeneration of Quercus liaotungensis in the Dongling mountain region of North China. Restor. Ecol, 12, 335-342.
DOI:10.1111/rec.2004.12.issue-3 |
||
Tweddle J.C., Dickie J.B., Baskin C.C., Baskin J.M., 2003. Ecological aspects of seed desiccation sensitivity. J. Ecol, 91, 294-304.
DOI:10.1046/j.1365-2745.2003.00760.x |
||
Tyler C.M., Kuhn B., Davis F.W., 2006. Demography and recruitment limitations of three oak species in California. Q. Rev. Biol, 81, 127-152.
DOI:10.1086/506025 |
||
Waite S., Hutchings M.J., 1979. Comparative study of establishment of Plantago coronopus L. from seeds sown randomly and in clumps. New Phytol, 82, 575-583.
|
||
Xia K., Daws M.I., Hay F.R., Chen W.-Y., Zhou Z.-K., Pritchard H.W., 2012. A comparative study of desiccation responses of seeds of Asian evergreen oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. South Afr. J. Bot, 78, 47-54.
DOI:10.1016/j.sajb.2011.05.001 |
||
Xia K., Daws M.I., Zhou Z.K., Pritchard H.W., 2015. Habitat-linked temperature requirements for fruit germination in Quercus species:a comparative study of Quercus subgenus Cyclobalanopsis (Asian evergreen oaks) and Quercus subgenus Quercus. South Afr. J. Bot, 100, 108-113.
DOI:10.1016/j.sajb.2015.04.015 |
||
Xia K., Harrower W., Turkington R., Tan H.-Y., Zhou Z.-K., 2016a. Pre-dispersal strategies by Quercus schottkyana to mitigate the effects of weevil infestation of acorns. Sci. Rep, 6, 37520.
DOI:10.1038/srep37520 |
||
Xia K., Tan H.-Y., Hu J.-J., Zhou Z.-K., 2016b. Desiccation and post-dispersal infestation of acorns of Quercus schottkyana, a dominant evergreen oak in SW China. Plant Ecol, 217, 1369-1378.
DOI:10.1007/s11258-016-0654-1 |



