b. University of Chinese Academy of Sciences, 19 A Yuquan Road, Beijing 100049, China
Parasitic plants are important components of heterotrophic plants, and are widely distributed in various terrestrial ecosystems, with more than 4000 species worldwide (Press and Phoenix, 2005). Parasitic plants extract host resources via parasitic organs known as haustoria, leading to resource deprivation and often growth depression in their host plants (Westwood et al., 2010). Furthermore, they may have a strong impact on plant community structure and ecosystem stability by altering the competitive relationships between host and non-host plants (Press and Phoenix, 2005; Bardgett et al., 2006). Although parasitic plants differ dramatically in morphology as well as host dependence and preference, haustorium formation is the characteristic feature of all parasitic plants (Westwood et al., 2010). The evolution of haustoria in parasitic plant lineages signals the transition from autotrophic to parasitic habit. In addition, the haustorium is the sole parasitic plant structure necessary for resource extraction from host plants (Kuijt, 1969). Because of this, haustorium initiation and development is the key to understanding the processes that have led to the evolution of parasitic plants and how parasitic plants interact with host plants.
The family Orobanchaceae provides an excellent system for better understanding the evolution of plant parasitism (Westwood et al., 2010). Parasitic weed species such as Striga spp. and Orobanch spp., which are both members of family Orobanchaceae, cause agricultural losses of billions of dollars each year (Heide-Jørgensen, 2008). In addition, this family is the youngest (Naumann et al., 2013) and the only family among parasitic plants consisting of autotrophic plants (Lindenbergia spp.) as well as root parasitic plants representing the full spectrum of parasitism (Honaas et al., 2013).
Most early studies on Orobanchaceae have investigated the development of terminal haustoria in obligate weedy species, whereas studies in the development of lateral haustoria have primarily focused on a few facultative root hemiparasites (Bandaranayake and Yoder, 2013). All previous investigations addressed haustorium initiation and development after induction by host-derived signals or synthetic haustorium-inducing factors (HIFs). However, spontaneous haustorium initiation and development in the absence of specific host signals, which represents an important early evolutionary stage in parasitic plants, remains poorly understood.
Haustorium formation in the absence of host roots has been reported for many hemiparasitic species under field conditions (Musselman and Dickison, 1975; Westwood et al., 2010). We have previously found that some hemiparasitic Pedicularis species form abundant haustoria in the absence of hosts when cultivated in pots with a sterilized sand-soil mixture (Li et al., 2012, 2013). However, under strictly sterilized conditions such as in agar plates, spontaneous haustorium formation in these same species has been sparse and greatly delayed compared with those induced by host roots or synthetic HIFs (Atsatt et al., 1978; Yoder, 1997; Yoshida et al., 2016). A lack of suitable plant material with fast and frequent formation of spontaneous haustoria in vitro greatly hinders our understanding of haustorium initiation and development in the absence of host signals.
In our recent studies we found that Pedicularis kansuensis Maxim., a weedy facultative root hemiparasite rapidly spreading in grasslands of western China, can form abundant haustoria not only in sterilized soil without a host (Sui et al., 2014), but also in autoclaved water agar under strictly sterilized growth conditions (personal observations). However, it remains unclear whether the morphology and development of these haustoria differ substantially from other facultative root hemiparasites.
The objectives of this study were to characterize the morphology, position of occurrence, and temporal development of spontaneous haustorium formation in P. kansuensis. To better understand the factors that influence spontaneous haustorium formation, we tested whether sucrose amendment in plant growth medium increases spontaneous haustorium formation, as sucrose supply in growth medium was shown to play a role in formation of lateral roots (Little et al., 2005; Jain et al., 2007), where spontaneous haustoria mainly occur based on our preliminary observations. We then tested whether tactile stimuli (e.g., plant root contact with another seedling of the same species) increases spontaneous haustorium formation, as tactile stimuli were suggested to facilitate haustorium formation (Bandaranayake and Yoder, 2013). Knowledge obtained will serve as basis for a better understanding of spontaneous haustorium formation in root hemiparasitic plants, which will open up a new opportunity for investigations into haustorium initiation and development regulated by factors other than the known host signals, and may eventually contribute to unraveling the whole evolutionary process underlying the origin and evolution of haustoria in parasitic plants.
2. Materials and methods 2.1. Plant materials and seed germinationPedicularis kansuensis seeds were collected from Bayanbulak Grassland, Xinjiang, China in September, 2016. The seeds were pooled from different individual plants and stored in paper bags at 4 ℃ until used.
Seeds were surface sterilized by soaking in 70% ethanol for 5 min, then shaken in 5% sodium hypochlorite for 10 min. Seeds were rinsed thoroughly with sterilized reverse osmosis (RO) water before being transferred to 0.5% water Agar (Wako, Japan) plates for germination. The water agar had been previously autoclaved at 121 ℃ for 20 min and poured into 9 cm Petri dishes. Each agar plate contained 40e50 seeds. The plates were sealed with 3M membranes and incubated horizontally in a growth chamber at 18/25 ℃ (night/day). The photoperiod was 12 h light with 22.2 mmol photons m-2 s-1 illumination and 12 h dark. Most seeds germinated within one week.
2.2. Observation of morphological and temporal development of spontaneous haustoriaTo examine morphological and temporal development of spontaneous haustoria, we randomly selected ten agar plates after seeds had germinated. Each plate had 30-35 seedlings. Observation started four days after a majority of seeds germinated. Seedlings were checked daily until 15 days after germination. Observations and photographs were made using a stereomicroscope (Olympus SZX7, Japan) equipped with a digital camera. Spontaneous haustoria were distinguished from lateral roots by differences in origin and presence of haustorium hairs. Similar to induced haustoria in many facultative root hemiparasites, spontaneous haustoria arise from cortical parenchyma cells, whereas lateral roots are derived from pericycle cells (Musselman and Dickison, 1975).
Percentage of seedlings with spontaneous haustoria, number of haustoria, and number of lateral roots per seedling were recorded. The percentage of seedlings with spontaneous haustoria was calculated by dividing the number of total seedlings with those that produced spontaneous haustoria. Number of haustoria per seedling was calculated by dividing the total number of spontaneous haustoria per plate by the number of seedlings with haustoria.
2.3. Test for effect of sucrose amendment on spontaneous haustorium formationSix-day-old seedlings were transferred to 10 × 10 cm square water agar plates (0.8%) that were or were not amended with 2% sucrose (w/v; Xilong Science, China). Each plate had 10 seedlings. There were 8 replicates for each treatment. After incubation for two weeks under the same conditions as described above for seed germination, spontaneous haustorium formation and number of lateral roots were recorded under a stereomicroscope.
2.4. Spontaneous haustorium formation with or without root contact with a conspecific seedlingSix-day-old seedlings were transferred to 10 × 10 cm square water agar plates (0.8%) amended with 2% sucrose. To test if a single seedling can produce spontaneous haustoria and if tactile signals such as root contact promote spontaneous haustorium formation, either a single seedling or two P. kansuensis seedlings were grown in each plate. The two seedlings were put on top of each other to maximize root contact. Each treatment had 18 replicate plates. Newly-formed haustoria were observed daily and position of occurrence was recorded for plates with a single seedling. To facilitate root growth the plates were incubated vertically with the seedling roots pointing downwards for two weeks in a growth room equipped with incandescent tubes, with temperatures ranging from 16 to 28 ℃ and a photoperiod of 12 h.
2.5. Statistical analysisStudent's t-test was used in all analyses for comparisons between a treatment and the corresponding control to determine treatment effects, except that Fisher's exact test was used to compare frequency of spontaneous haustorium formation between singly grown seedlings and those grown with a conspecific seedling. Normality and equality of variance were verified using ShapiroeWilk and Levene's tests, respectively. Data were transformed when necessary using the appropriate transformations. Specifically, data for percentage of seedlings producing haustoria were arcsine transformed after dividing by 100, and data for number of haustoria and number of lateral roots per seedling were square-root transformed. The significance level for all tests was 0.05. All analyses were performed using the Statistical Product and Service Solution (SPSS) software (version 16.0; SPSS China, Shanghai, China).
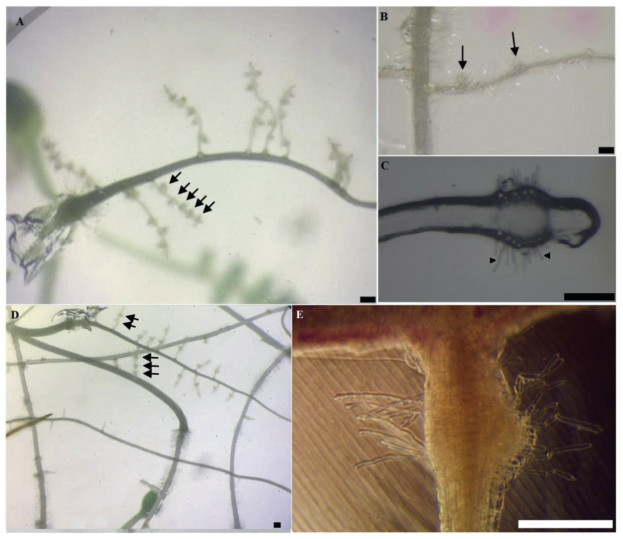
3. Results 3.1. Morphological and temporal development of spontaneous haustoriaSpontaneous haustoria formed by P. kansuensis were hemispherical swellings derived from cortical parenchyma cells. Most spontaneous haustoria had dense haustoium hairs (Fig. 1). A majority of spontaneous haustoria developed on lateral roots (Fig. 1A and B, D, and E), but were occasionally observed near the tip of a primary root (Fig. 1C). Quantitative analysis of position of occurrence for 18 singly grown seedlings showed that of the 183 haustoria formed over two weeks, 72% (132) of haustoria formed at the maturation zone of lateral roots, 18% (32) formed at the maturation zone of primary roots, and 10% (19) formed at the elongation zone near root tips. Great variation in capability of spontaneous haustorium formation was observed among seedlings, with some forming dozens of spontaneous haustoria while some did not form haustoria at all throughout the experiments (Fig. 1A and D).
|
| Fig. 1 Morphology and position of occurrence of spontaneous haustoria (visible as hemispherical swellings shown with arrows) in Pedicularis kansuensis. (A) Spontaneous haustoria formed on lateral rootlets. (B) Developing spontaneous haustoria along a lateral rootlet. (C) Spontaneous haustoria formed near the tip of a primary root, haustorial hairs shown with arrow heads; (D) Spontaneous haustoria formed more frequently on lateral rootlets than on primary roots. (E) Close-up of a spontaneous haustoria formed on a lateral rootlet. (Scale bar, 200 μm). |
When P. kansuensis seedlings were grown in water agar (0.5%), spontaneous haustoria were first observed six days after germination (Fig. 2). The proportion of seedlings with haustoria (Fig. 2A), total number of haustoria (Fig. 2B), and number of lateral roots per seedling (Fig. 2D) all increased with time. At 15 days after seed germination, an average of 28.8% of seedlings produced spontaneous haustoria (Fig. 2A), with a mean of 4 haustoria (Fig. 2C) and 9 lateral roots per seedling (Fig. 2D).

|
| Fig. 2 Temporal development of spontaneous haustoria and lateral roots by Pedicularis kansuensis in 0.5% water agar. (A) Percentage of seedlings with spontaneous haustoria. (B) Total number of spontaneous haustoria per plate. (C) Number of spontaneous haustoria per seedling with haustoria (D) number of lateral roots per seedling. Data are presented as mean ± SE (n = 10, each with 30-35 seedlings). |
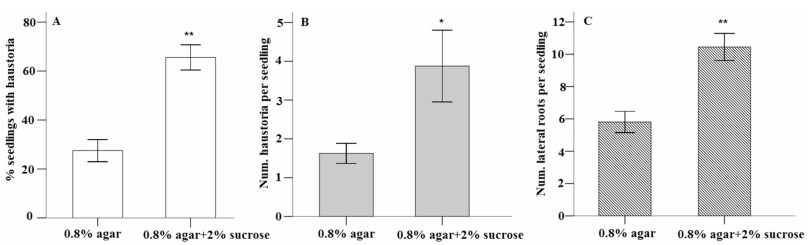
Sucrose amendment significantly increased the percentage of seedlings with spontaneous haustoria (P < 0.01, Fig. 3A), number of spontaneous haustoria per seedling with haustoria (P < 0.05, Fig. 3B), and number of lateral roots per seedling (P < 0.01, Fig. 3C). The average percentage of seedlings with spontaneous haustoria increased from 27% to 67% when the seedlings were grown in water agar (0.8%) amended with 2% sucrose. The average number of spontaneous haustoria per seedling increased from 1.6 to 3.9 and average number of lateral roots increased from 5.8 to 10.8.
|
| Fig. 3 Effect of sucrose amendment on spontaneous haustorium formation and lateral root development by Pedicularis kansuensis grown in water agar (0.8%) for two weeks. (A) Percentage of seedlings with spontaneous haustoria. (B) Number of spontaneous haustoria per seedling with haustoria. (C) Number of lateral roots per seedling. Data are presented as mean ± SE (n = 8, each with 10 seedlings, *P < 0.05 level, **P < 0.01 Student's t test.) |
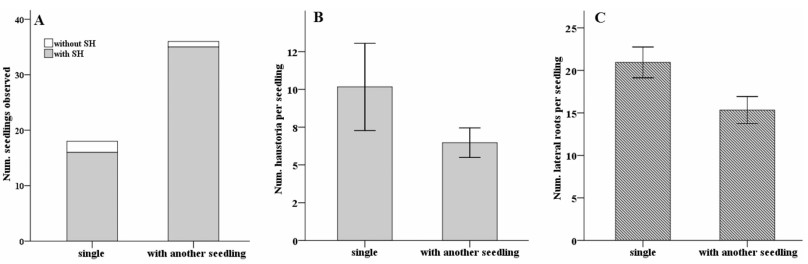
Seedlings of P. kansuensis developed spontaneous haustoria when grown singly in 0.8% agar amended with 2% sucrose (Fig. 4A). The occurrence rate of spontaneous haustoria was similar between singly grown seedlings and those stacked with another P. kansuensis seedling (P = 0.255 Fisher's exact test). Although not statistically significant, on average singly grown seedlings produced slightly more spontaneous haustoria (Fig. 4B) and lateral roots (Fig. 4C) than seedlings in contact with another P. kansuensis seedling.
|
| Fig. 4 Effect of root contact with a conspecific seedling on spontaneous haustorium formation and lateral root development by Pedicularis kansuensis in water agar (0.8%) amended with 2% sucrose. (A) Number of seedlings grown singly on an agar plate or stacked with another seedling observed for occurrence of spontaneous haustoria (SH), (B) number of spontaneous haustoria per seedling with haustoria. (C) Number of lateral roots per seedling. Data for number of spontaneous haustoria and lateral roots per seedling are presented as mean ± SE (n = 18, one single seedling per plate for control and two seedlings stacked together per plate for root contact treatment). |
Fast development of spontaneous haustoria in a high proportion of P. kansuensis seedlings was observed in all three independent in vitro experiments. Spontaneous haustoria on P. kansuensis roots were first observed six days after seed germination. Percentage of seedlings with spontaneous haustoria reached around 30% in water agar without sucrose amendment and more than 67% when amended with 2% sucrose within two to three weeks. This was in contrast to previous reports for other root hemiparasites, where in vitro spontaneous haustoria occurred much later and in a lower proportion of seedlings. For example, in Triphysaria versicolor and T. erianthus spontaneous haustoria were first observed 35 and 42 days after sowing (more than 4 weeks after germination), respectively, and it took more than 10 weeks for 30% of the seedlings to develop spontaneous haustoria (Yoder, 1997). In another root hemiparasite Castilleja exserta (previously known as Orthocarpus purpurascens), spontaneous haustoria were observed in 12% of seedlings with a mean of 1.3 haustoria per seedling 32 days after germination (Atsatt et al., 1978). To our knowledge, in all previously reported root hemiparasites spontaneous haustoria were observed in fewer than 2% of two-to three-week-old seedlings. The fast and abundant occurrence of spontaneous haustoria in P. kansuensis thus provides an excellent system for future research into endogenous factors regulating haustorium formation in root hemiparasites.
Similar to induced haustoria in other facultative root hemiparasitic plants (Musselman and Dickison, 1975; Westwood et al., 2010), spontaneous haustoria of P. kansuensis originated from cortical parenchyma cells and formed only at lateral positions on the root axis. A majority of spontaneous haustoria were observed on lateral roots and developed dense haustoium hairs. However, unlike haustoria reported for other facultative root hemiparasites in Orobanchaceae, very few spontaneous haustoria in P. kansuensis formed near the root tip of a primary root. Anatomical investigations of spontaneous haustoria are required for a more detailed comparison between spontaneous and induced haustoria to detect any difference in haustorium differentiation.
Great variation among individual P. kansuensis seedlings was observed in terms of their capability for spontaneous haustorium formation. This was not surprising as the seeds were from a seed pool collected from different plants. According to research with Triphysaria species, there is heritable variation in how haustorium development responds to HIFs (Jamison and Yoder, 2001). In vitro experiments with T. versicolor have shown that spontaneous haustorium formation also has a genetic component (Yoder, 1997). Specifically, the proportion of F1 seedlings with spontaneous haustoria has been shown to be higher in plants if their parents formed spontaneous haustoria than if their parents did not. The ecological causes and significance of the great variation in capability for haustorium formation remains unknown. Testing whether seedlings with higher haustorium formation capability perform better under certain circumstances might also reveal if this trait leads to trade-offs with traits that are better adapted for different circumstances. In other words, the great variation in capability for haustorium formation may be related to adaptation to microenvironments.
Although sucrose amendment has been shown to have a strong influence on lateral root initiation (Little et al., 2005; Jain et al., 2007), its effect on haustorium formation has seldom been reported for root hemiparasitic plants. However, distinct haustorium induction protocols in terms of sucrose supply among different root hemiparasites have been suggested. Although sucrose supply in growth medium for haustorium induction of Triphysaria species is a common practice (Yoder, 1997; Jamison and Yoder, 2001; Bandaranayake et al., 2010), sucrose has been purposely avoided for optimal haustorium induction for Phtheirospermum japonicum (Ishida et al., 2016; Cui et al., 2018), indicating a significant but controversial role of sucrose in haustorium formation by those hemiparasites. The marked increase in spontaneous haustorium formation after sucrose amendment suggests significant roles of sucrose in haustorium initiation for P. kansuensis seedlings. Increased lateral root number by sucrose may partially explain the promoting effect of sucrose amendment on haustorium formation, as a majority of spontaneous haustoria occurred on lateral roots. However, sucrose may also directly regulate spontaneous haustorium formation as a signal. Further studies are required to unravel the underlying mechanisms of sucrose-mediated enhancement on spontaneous haustorium formation in P. kansuensis.
Single seedlings of P. kansuensis developed spontaneous haustoria in vitro in a similar manner as those grown with a second seedling of the same species. This suggests that tactile stimulation by root contact or signal for presence of another plant is not required for spontaneous haustorium formation. Endogenous factors seem to be regulating spontaneous haustorium formation in P. kansuensis. These unknown endogenous factors and the relevant mechanisms that regulate spontaneous haustorium formation require further research, especially because they may contribute to our understanding of haustorium initiation and development in early evolutionary stages of parasitic plants.
5. ConclusionsThis study demonstrated fast and abundant spontaneous haustorium formation in P. kansuensis. These spontaneous haustoria resembled induced haustoria of other facultative root hemiparasitic plants in many ways. Spontaneous haustoria of P. kansuensis were first observed six days after seed germination and were developed in vitro on singly grown seedlings without any known haustorium-inducing stimuli. Sucrose supply markedly increased spontaneous haustorium formation and lateral root number. Because spontaneous haustorium initiation does not require any known host-derived signals, P. kansuensis seedlings may provide an excellent system for future investigations into new mechanisms for haustorium formation in root hemiparasitic plants.
AcknowledgementsThe authors would like to thank Dr. Yanyan Liu from Key Laboratory of Biogeography and Bioresource in Arid Land, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences for collecting Pedicularis kansuensis seeds. The research was financially supported by the Natural Science Foundation of China (31370512, U1303201, and 31400440), Natural Science Foundation of Yunnan Province (2016FB059), and funding for Airong Li from The Youth Innovation Promotion Association of Chinese Academy of Sciences and the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2014HB047).
Atsatt P.R., Hearn T.F., Nelson R.L., et al., 1978. Chemical induction and repression of haustoria in Orthocarpus purpurascens (Scophulariaceae). Ann. Bot, 42, 1177-1184.
DOI:10.1093/oxfordjournals.aob.a085559 |
||
Bandaranayake P.C.G., Filappova T., Tomilov A., et al., 2010. A single-electron reducing quinone oxidoreductase is necessary to induce haustorium development in the root parasitic plant Triphysaria. Plant Cell, 22, 1404-1419.
DOI:10.1105/tpc.110.074831 |
||
Bandaranayake, P.C.G., Yoder, J.I., 2013. Haustorium initiation and early development. In: Joel, D.M., Gressel, J., Musselman, L.J. (Eds.), Parasitic Orobanchaceae. Springer, Berlin, pp. 61-74.
|
||
Bardgett R.D., Smith R.S., Shiel R.S., et al., 2006. Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature, 439, 969-972.
DOI:10.1038/nature04197 |
||
Cui S., Wada S., Tobimatsu Y., et al., 2018. Host lignin composition affects haustorium induction in the parasitic plants Phtheirospermum japonicum and Striga hermonthica. New Phytol, 218(2), 710-723.
DOI:10.1111/nph.15033 |
||
Heide-Jørgensen, H.S., 2008. Parasitic Flowering Plants. Brill Academic, Leiden, NL.
|
||
Honaas L.A., Wafula E.K., Yang Z., et al., 2013. Functional genomics of a generalist parasitic plant:laser microdissection of host-parasite interface reveals hostspecific patterns of parasite gene expression. BMC Plant Biol, 13, 9.
DOI:10.1186/1471-2229-13-9 |
||
Ishida J.K., Wakatake T., Yoshida S., et al., 2016. Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates the haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell, 28, 1795-1814.
DOI:10.1105/tpc.16.00310 |
||
Jain A., Poling M.D., Karthikeyan A.S., et al., 2007. Differential effects of sucrose and auxin on localized Pi deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol, 144, 232-247.
DOI:10.1104/pp.106.092130 |
||
Jamison D.S., Yoder J.I., 2001. Heritable variation in quinone-induced haustorium development in the parasitic plant Triphysaria. Plant Physiol, 125, 1870-1879.
DOI:10.1104/pp.125.4.1870 |
||
Kuijt J., 1969. The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA. |
||
Li A.R., Li Y.J., Smith S.E., et al., 2013. Nutrient requirements differ in two Pedicularis species in the absence of a host plant:implication for driving forces in the evolution of host preference of root hemiparasitic plants. Ann. Bot, 112, 1099-1106.
DOI:10.1093/aob/mct179 |
||
Li A.R., Smith F.A., Smith S.E., et al., 2012. Two sympatric root hemiparasitic Pedicularis species differ in host dependency and selectivity under phosphorus limitation. Funct. Plant Biol, 39, 784-794.
DOI:10.1071/FP12159 |
||
Little D.Y., Rao H.Y., Oliva S., et al., 2005. The putative high-affinity nitrate transporter NRT2., 1 represses lateral root initiation in response to nutritional cues. Proc. Natl. Acad. Sci. USA, 102(38), 13693-13698.
|
||
Musselman L.J., Dickison W.C., 1975. The structure and development of the haustorium in parasitic Scrophulariaceae. Bot. J. Linn. Soc, 70, 183-212.
DOI:10.1111/boj.1975.70.issue-3 |
||
Naumann J., Salomo K., Der J.P., et al., 2013. Single-copy nuclear genes place haustorial Hydnoraceae within Piperales and reveal a Cretaceous origin of multiple parasitic angiosperm lineages. PLoS One, 8, e79204.
DOI:10.1371/journal.pone.0079204 |
||
Press M.C., Phoenix G.K., 2005. Impacts of parasitic plants on natural communities. New Phytol, 166, 737-751.
DOI:10.1111/j.1469-8137.2005.01358.x |
||
Sui X.L., Li A.R., Chen Y., et al., 2014. Arbuscular mycorrhizal fungi:potential biocontrol agents against the damaging root hemiparasite Pedicularis kansuensis?. Mycorrhiza, 24, 187-195.
DOI:10.1007/s00572-013-0528-5 |
||
Westwood J.H., Yoder J.I., Timko M.P., et al., 2010. The evolution of parasitism in plants. Trends Plant Sci, 15, 227-235.
DOI:10.1016/j.tplants.2010.01.004 |
||
Yoder J.I., 1997. A species specific recognition system directs haustorium development in the parasitic plant Triphysaria (Scrophulariaceae). Planta, 202, 407-413.
DOI:10.1007/s004250050144 |
||
Yoshida S., Cui S., Ichihashi Y., et al., 2016. The haustorium, a specialized invasive organ in parasitic plants. Annu. Rev. Plant Biol, 67, 643-667.
DOI:10.1146/annurev-arplant-043015-111702 |



