b. University of Chinese Academy of Sciences, Beijing 100049, China;
c. Key Laboratory of Urban Environment and Health, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
Positive associations in plant communities have received increasing attention (Bertness and Callaway, 1994; Callaway, 1995), and the importance of positive associations has been shown particularly in communities where there are harsh abiotic conditions and where plants are often found in clumps within a matrix of bare ground (Callaway et al., 2002; Tirado and Pugnaire, 2005). Alpine cushion plants, which usually have a low, compact structure and form dwarf, much-branched cushions, were originally discovered in the Alps at high elevations inhabiting cold ecosystems (Raunkiaer, 1934). Similar to other alpine species, alpine cushion plants are confronted with limiting factors such as low temperatures, short growing seasons, excessive radiation, constant wind, and unstable substrates in their remarkable habitats (Billings and Mooney, 1968; Körner, 2003). Alpine cushions are pioneer plants that form positive associations with other organisms in the same community.
Alpine cushion plants affect plant communities in a number of ways. Firstly, alpine cushion plants contribute to the richness of plant communities. For instance, in the high Andean mountains alpine cushions act as ecosystem engineers, introducing new species into the community and increasing species richness at the entire community level (Badano et al., 2006; Cavieres and Badano, 2009). Secondly, alpine cushion plants modify the diversity of plant communities. Research involving the types of facilitation effects of different cushion species has shown that the presence of alpine cushion plants leads to higher community diversity (Badano and Cavieres, 2006). Cushion plants also prevent phylogenetic diversity from decreasing at a global scale (Butterfield et al., 2013). Thirdly, alpine cushion plants alter the composition of plant communities. Native cushion plants have facilitation effects on invasive plants in stressful alpine habitats (Cavieres et al., 2008). Moreover, compared with adjacent open areas, areas with alpine cushion are associated with a greater abundance of arthropod species (Molenda et al., 2012; Molina-Montenegro et al., 2006). By altering microhabitats, alpine cushions act as microclimatic shelters, providing shelter from wind, moderated soil temperatures, and greater soil moisture; in stressful alpine environments, these factors may play a role in plant establishment and recruitment of other species within communities (Arroyo et al., 2003; Cavieres et al., 2005). In the Sino-Himalayas, cushion plant positive associations increase with elevation; in addition, the increased positive effects of older and larger cushion plants may be related to improved nutrient availability associated with cushions (Yang et al., 2010, 2017). Furthermore, the strength of the positive effects of cushion plant association varies with the underlying soil nutrient levels (Chen et al., 2014, 2015).
Despite extensive evidence that alpine cushion plants positively affect the distribution and performance of macrofauna, few reports have investigated whether and how alpine cushion plants affect rhizospheric microbes. Alpine cushion plants have been shown to improve the condition of arbuscular mycorrhiza. For example, native plant species growing within dominant cushion plants in the Andes Mountains harbor much higher mycorrhizal associations than the same species growing on bare ground (Casanova-Katny et al., 2011). Alpine cushion plants are also considered potential selective forces on the process of structuring bacterial and fungal communities (Roy et al., 2013).
In alpine environments, differences in soil microbial communities appear to be amplified by alpine cushion habitat modification and environmental stress. The positive effects of alpine cushions on microbes have been shown to increase in stressful, acidic and nutrient-restricted environments (Roy et al., 2013). However, in arid Trans-Himalayan habitats, alpine cushion plants reduce the bacterial diversity in the rhizospheric soils (Rehakova et al., 2015). In addition to plant species, microbial community structure and composition can be influenced by soil pH and by season (Geremia et al., 2016; Siles and Margesin, 2016; Zhang et al., 2015; Lazzaro et al., 2015). However, few studies have examined how seasons affect microbial communities in the rhizospheres of alpine cushion plants; indeed, to our knowledge, all research on the effects of alpine cushion plants on microbes have been conducted during the vegetation growing season.
Taken together, these studies prompted us to conjecture that the positive effects of alpine cushion plants on rhizospheric bacterial communities are seasonal. The goals of the present study were, therefore, twofold. First, we tested whether alpine cushion plants affected the surrounding soil environment. Specifically, we examined whether the rhizospheric soil of two alpine cushion plants (Arenaria polytrichoides, Chionocharis hookeri) differed in physicochemical properties (pH, C/N ratio, etc.) as compared with soil from bare ground. Second, we asked whether alpine cushion plants altered soil bacterial diversity seasonally. Specifically, we used high throughput sequencing of bacterial 16S ribosomal RNA gene to assess bacterial diversity and composition of the soil of rhizospheres of alpine cushion plants as compared to soil of bare ground in the vegetation growing season and the vegetation non-growing season.
2. Materials and methods 2.1. Study sitesThe study sites are located on an east-facing slope in the Lakaka Pass on Baima Snow Mountain, Deqen County, Yunnan Province, southwest China (28°22′15″ N, 98°59′16″ E). A humid summer monsoon dominates the study area's climate. The vegetation growing season commonly starts in May at the snow-melt and ends in September when the summer monsoon ceases (Yang and Sun, 2009). Annual precipitation recorded from 2010 to 2013 at the nearest meteorological station (28°23′ N, 99°01′ E, 10 km from the study site, elevation 4290 m), was 600.0 mm, with 536.2 mm of rainfall between May and September. The annual average air temperature recorded from 2010 to 2013 was -0.1 ℃. The difference in mean temperature between the coldest and the warmest month was around 15.5 ℃. Mean wind speed is 2.9 m-3.1 m per second (Wang, 2006).
We studied two species of alpine cushion plants: A. polytrichoides (Caryophyllaceae) and C. hookeri (Boraginaceae). Both species are long-lived perennial herbs that form dwarf and muchbranched cushions, and they mainly survive at high elevations in the Sino-Himalaya (Fig. S1). The presence of A. polytrichoides and C. hookeri commonly increases species richness in alpine plant communities. They strengthen the facilitation effect on non-cushion alpine plants in the severe habitats of plant communities in the Himalayan Hengduan Mountains (Chen et al., 2015). The study sites were situated near the mountaintop at 4780 m elevation, where A. polytrichoides and C. hookeri occur simultaneously.
2.2. Soil samplingSoil sampling of the study sites was carried out in early September 2011 during the vegetation growing season and in late October 2011 outside the vegetation growing season (i.e., the vegetation non-growing season), respectively. Twelve plots were chosen during each season, including four plots vegetated by A. polytrichoides, four by C. hookeri, and four plots of bare ground. In each vegetated plot, five cushions with a diameter of 15 cm were collected. Soils attached to the cushion taproots were sampled and pooled to represent the rhizospheric microhabitat. In each bare ground plot, five soil cores with a diameter of 10 cm were sampled from 2 to 15 cm below the surface and pooled together to represent the bulk soil microhabitat. In total, 24 soil samples were gathered and stored at -80 ℃.
2.3. Soil physicochemical propertiesEleven feature parameters of soil samples were characterized, including C, N, P, K, S, ammonia nitrogen [NH4+--N], nitrate nitrogen [NO3- -N], available phosphorus [PO43--P], pH, soil moisture content and the C/N ratio. Soil moisture content was measured immediately after sampling by the gravimetric method (Black et al., 1965). Ammonium and nitrates were extracted from fresh soil samples by the Bremner method (Bremner, 1965), and afterwards their concentrations were measured with a flow injection analysis (Lachat Instrument, USA) following the manufacturer's instructions. To characterize the other physicochemical parameters, soils were first freeze-dried at -46 ℃. Soil samples were then finely ground by hand and sieved through a 150 mm mesh sift. Soil pH was measured on a pH Meter (Fisher Scientific, USA). Total C, N, S data of soils was obtained with a flame photometric detector (Elementar Analysensysteme GmbH, Germany), while total P and K were determined with an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Perkin-Elmer Optima 7000DV, USA) by means of a strong acid soil digestion method (Lee et al., 2006). The available phosphorus of soils was extracted by the Bray 1 method (Bray and Kurtz, 1945), and then measured by flow injection analysis following the manufacturer's instructions. The C/N ratio was computed after C and N content had been determined.
2.4. DNA extraction and 16S rRNA gene amplificationTotal soil DNA for each sample was extracted from 500 mg of freeze-dried soil using the FastDNA Spin for soil kit (MP Biomedicals, France). The total soil DNA solutions were purified using the MinElute PCR Purification Kit (Qiagen, Germany). Concentrations of the total soil DNA were quantified on a SpectraMax M5 (Molecular Devices, USA) using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Invitrogen, USA). Extracted DNA was stored at -20 ℃. The manufacturers' instructions were followed in all the experimental procedures.
The universal primers 338F and 533R were used to amplify a ~150 bp fragment covering the hypervariable V3 region of the bacterial 16S rRNA gene (Huse et al., 2008). A unique eight-base error-correcting barcode was added to the pair of primers that was used to amplify a particular soil DNA (Table S1) (Hamady et al., 2008). All amplifications were performed in threefold 25 ml reactions, while another reaction was used as a negative control. Each reaction system contained 12.5 μl of 2× DreamTaq Green PCR Master Mix (Thermo Scientific, USA), 10.25 μl of deionized nucleasefree water, 0.5 μl of each appropriate primer (at a final concentration of 0.4 μM), 0.25 μl of bovine serum albumin (BSA, at a final concentration of 6 mM) (Takara, Japan) and 20 ng of DNA template. For the controlled reaction, 1 μl of nuclease-free water was used instead of the soil DNA. The PCRs were performed under a thermal profile comprising an initial denaturation step at 95 ℃ for 3 min, followed by 35 cycles of 95 ℃ for 30 s, 55 ℃ for 30 s and 72 ℃ for 30 s, and a final extension step at 72 ℃ for 5 min. Soil DNA products of threefold reactions were pooled, visualized on 1.5% agarose gels, and purified with a MinElute PCR Purification Kit (Qiagen, Germany). Concentrations of the purified amplification products were also quantified on a SpectraMax M5 (Molecular Devices, USA) using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Invitrogen, USA).
2.5. Sequencing and processingThe purified amplification products were pooled in equal DNA mass into one library to be sequenced. The paired-end sequencing was performed on Illumina HiSeq2000 on 2013 20 July (BGI tech, China) (Degnan and Ochman, 2012). The sequencing data was processed using the Quantitative Insights Into Microbial Ecology pipeline (QIIME, version 1.7.0) (Caporaso et al., 2010). The processing of the sequencing data contained the following steps. Initial base-calling and quality filtering of paired-end reads were performed. Barcodes and primers were trimmed. Sequences that contained undetermined bases were removed. Since the clean data had been produced, sequences were clustered into operational taxonomic units (OTUs) using a 97% similarity threshold, which corresponds to the taxonomic level of species for organisms (Turnbaugh et al., 2010). Putative chimeras and singletons were removed from the OTU table in this stage. Taxonomic assignment was carried out according to the Greengenes OTU database provided by the website of http://greengenes.lbl.gov/.
In total, 1, 111, 342 effective reads were clustered into 24, 588 bacterial OTUs at the 97% similarity threshold. For the subsequent analyses, such as computing the alpha and beta diversity, the number of the minimal effective reads in all samples within the dataset was adopted as an even sequencing depth so as to equitably compare traits of the bacterial communities in soils. Thus, the even sequencing depth used for the subsequent analysis of the dataset was 31, 290 reads (Table S1). The rarefactions of the alpha diversity were also conducted under the even sequencing depth. Principal coordinates analyses (PCoA) of the weighted UniFrac distances based on the OTU tables were performed to estimate the variations in beta diversity among microhabitats (Lozupone et al., 2007). The analysis procedure followed the tutorials on the website of the pipeline (http://qiime.org). The main scripts that used in the processing procedure on QIIME were shown in Table S2.
2.6. Statistical analysisNot all the data of the soil physicochemical properties coincided with the normal distribution and homogeneity of variance, and the samples in each group were small (n = 4). We therefore performed the Kruskal-Wallis nonparametric test using the analysis software Predictive Analytics Suite Workstation Statistics (PASW Statistics, version 19.0.0) to examine the difference of the soil traits between microhabitats. In order to take the evenness of each bacterial species observed into consideration, we used the Shannon-Wiener indices to assess the alpha diversity of the bacterial communities. The difference of the Shannon-Wiener indices among microhabitats was evaluated using the One-Way ANOVA followed by the Student-Newman-Keuls' multiple comparison test. In consideration of the enormous number of bacterial species and their relative abundances, PCoA combined with weighted UniFrac was used to determine the beta diversity of bacterial communities (Lozupone et al., 2011). Independent samples t-test was performed on PASW to test the dissimilarity of the relative abundance of bacterial phyla between microhabitats. Spearman's correlation was performed on PASW to test if the relative abundance of bacterial phyla was related to season or microhabitats, respectively. To identify the abiotic properties that may be important in building the bacterial communities and to reveal the possible relationships between bacterial community composition and the soil properties, canonical correspondence analysis (CCA) was performed in R (Version 3.0.1) with the community ecology package 'vegan (2.0-8)' (Oksanen et al., 2013). All significant differences are at the p < 0.05 level, unless otherwise stated.
The representative sequences of the clusters were deposited in the NCBI public database (https://www.ncbi.nlm.nih.gov/nuccore/?term?MG781119:MG781151) under the accession number of MG781119 - MG781151.
3. Results 3.1. Soil propertiesAll soils sampled were acidic. The soil pH was significantly different between rhizospheres and bare ground during the vegetation non-growing season. The rhizospheres of A. polytrichoides and C. hookeri had significantly higher contents of total C, N, S and ammonia nitrogen, soil moisture and C/N ratio than the bare ground in both seasons (Fig. 1). The bare ground had distinctly higher K content than the rhizospheres of the two cushions in both seasons. The total P content, available phosphorus, and nitrate nitrogen was not notably different among microhabitats in either season.
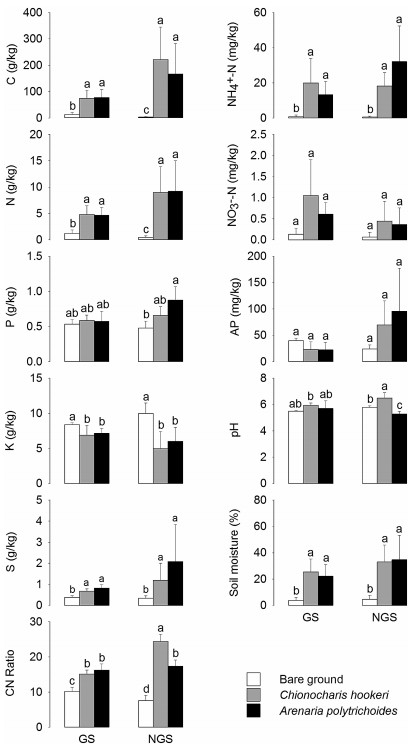
|
| Fig. 1 Physicochemical properties of soils sampled from the rhizosphere of cushion plants and bare ground. GS, the vegetation growing season. NGS, the vegetation nongrowing season. Soil carbon (C), C/N Ratio, soil potassium (K), soil nitrogen (N), ammonia nitrogen [NH4+ -N], nitrate nitrogen [NO3- -N], available phosphorus (AP), soil phosphorus (P), soil sulfur (S), soil pH and moisture content of soil samples from each site are shown. Different letters above bars indicate significant differences according to Student-Newman-Keuls comparisons at a significance level of 0.05. |
Shannon indices indicated that neither the rhizospheres of the plant cushions nor the bare ground had significantly different bacterial alpha diversities between the vegetation growing and the non-growing season. The rhizosphere of C. hookeri harbored significantly higher bacterial alpha diversities than did the bare ground in the vegetation growing season, and it harbored significantly higher bacterial alpha diversities than the rhizosphere of A. polytrichoides in the vegetation non-growing season (Fig. 2). The rhizosphere of A. polytrichoides cushions had nearly the same bacterial alpha diversities as the bare ground in both the seasons. Rarefaction curves showed the dynamics of Shannon indices, chao1, observed species, and PD whole trees with the sequencing depths (Fig. S2). PCoA patterns showed that bacterial communities beneath bare ground both in and outside the vegetation growing season mainly gathered in the third quadrant, whereas those in the rhizosphere of cushions distributed in the other quadrants. The first axis explained 38.44% of the overall variation observed, and the first two axes, in total, explained 62.02% of the overall variation (Fig. 3).
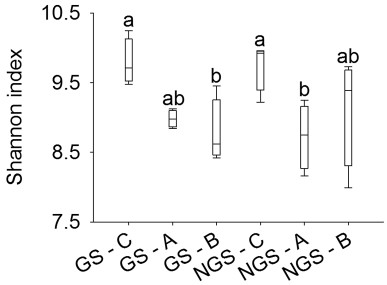
|
| Fig. 2 Differences in bacterial ShannoneWiener indices at an even sequencing depth of 31, 290 sequences (n = 4, F = 4.137, p = 0.011, p < 0.05). Alpha diversities of bacterial communities were calculated on the basis of the OTU tables. Different letters above bars indicate significant differences according to Student-Newman-Keuls comparisons. A, Arenaria polytrichoides; B, bare ground; C, Chionocharis hookeri; GS, the plant growing season. NGS, the plant non-growing season. |
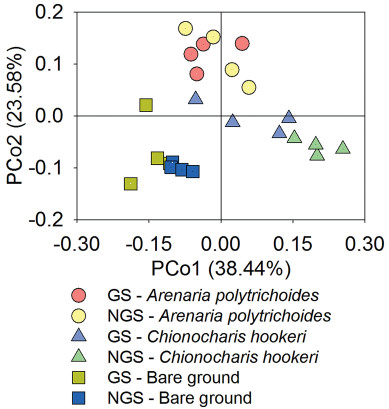
|
| Fig. 3 PCoA of bacterial communities. The two axes, in total, explain 62.02% of the overall variations. GS, the plant growing season. NGS, the plant non-growing season. |
In all soils, Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi and AD3 were the five most abundant bacterial phyla, together accounting for 76.80%e92.17% of the overall bacterial abundance, as illustrated in Fig. 4. In the vegetation growing season, the rhizosphere of A. polytrichoides and C. hookeri together harbored significantly more Proteobacteria, Actinobacteria, Bacteroidetes and TM7, while the bare ground harbored significantly more Chloroflexi, AD3, WPS-2 and Gemmatimonadetes than the cushions. Outside the growing season, the rhizosphere of cushions harbored significantly more Proteobacteria, while the bare ground harbored significantly more Chloroflexi and Gemmatimonadetes. In the rhizosphere of A. polytrichoides, the relative abundance of bacterial phyla showed no variation with seasons. In the rhizosphere of C. hookeri, Bacteroidetes, Cyanobacteria and TM7 were mostly found outside the vegetation growing season. The bare ground had much higher relative abundance of AD3 in the vegetation growing season than outside it. In contrast, the phyla Bacteroidetes and TM7 mainly emerged beneath the bare ground outside the vegetation growing season.

|
| Fig. 4 Bacterial community composition detected in soil samples. A, Arenaria polytrichoides; B, bare ground; C, Chionocharis hookeri; GS, the vegetation growing season; NGS, the vegetation non-growing season. Relative abundance is presented in terms of a percentage of the total effective sequences in the sample, classified by QIIME at a confidence threshold of 97%. "Low abundance" refers to taxa with a maximum abundance of less than 1% in any sample. |
Nine environmental properties with variance inflation factors (VIF) less than 10 were included in the CCA biplot (Fig. 5). Results showed that the total S was the only parameter which had a significant effect on the distribution of the bacterial communities (p = 0.005, p < 0.01). The first axis explained 12.01%, while the second axis explained 6.11% of the overall variation observed.
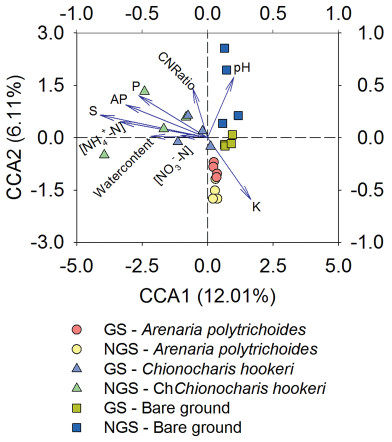
|
| Fig. 5 CCA of the bacterial communities and soil properties in and outside the vegetation growing season. GS, the vegetation growing season; NGS, the vegetation non-growing season; AP, available phosphorus; CNRatio, soil carbon/soil nitrogen; K, soil potassium; NH4+-N, soil ammonia nitrogen content; NO3--N, soil nitrate nitrogen content; P, total soil phosphorus; pH, the soil pH; S, soil sulfur. Arrows indicate the directions and magnitudes of soil properties associated with bacterial community composition. Each symbol represents the bacterial community from a specific soil sample. |
Spearman's correlation tests revealed that almost all the bacterial phyla were significantly correlated with the microhabitats, i.e., the rhizosphere of cushions and the bare ground. Four phyla were significantly correlated with seasons, i.e., the vegetation growing and non-growing season, including Bacteroidetes, Cyanobacteria, AD3, and TM7. Bacteroidetes, AD3 and TM7 significantly correlated with the microhabitats and the seasons simultaneously (Table S3).
4. Discussion 4.1. Cushions harbor higher soil nutrientsAbiotic factors such as soil pH and soil nutrient availability have been identified as key determinants of the richness and composition of microbial communities (Fierer and Jackson, 2006; Lauber et al., 2008). Consequently, soil properties are the principal abiotic factors which require examination when studying microbes in the rhizosphere of plants. However, only a few studies have examined the soil properties in the rhizosphere of alpine cushion plants. Furthermore, to our knowledge, no research has reported the soil properties in the rhizosphere of alpine cushion plants outside the vegetation non-growing season.
In this study, we characterized eleven soil properties in the rhizosphere of two alpine cushion plants and the bare ground both in and outside the vegetation growing season. We found that the rhizosphere of cushions held significantly higher total soil C, N, S, ammonia nitrogen, moisture content and C/N ratio than the bare ground did both during and outside the vegetation growing season. Our finding that rhizospheres of alpine cushion plants had higher soil C, N, and soil moisture than bare ground supports previous research (Casanova-Katny et al., 2011; Rehakova et al., 2015; Roy et al., 2013); similarly, soil moisture patterns in the vegetation growing season were also in accord with previous research (Roy et al., 2013; Yang et al., 2010). The low, compact stature and thick canopy of cushions and the microclimate conditions that they generate may explain these physicochemical properties.
Soil pH is a key factor that impacts soil bacterial communities (Shahnavaz et al., 2012). Although previous studies reported that the soil pH of rhizospheres of cushion plants during the vegetation growing season are lower than bare ground bulk soil (Roy et al., 2013), we found that outside of the vegetation growing season soil pH was significantly higher in C. hookeri cushions and lower in the A. polytrichoides cushions. The difference in soil pH between cushions may be due to plant root exudates, which can remarkably lower the soil pH of the rhizosphere (Dakora and Phillips, 2002). Interestingly, previous studies that have examined the association between alpine cushion plants and rhizospheric microbes have all been located in arid mountain regions with dry summer and rainy winter or where all seasons lack precipitation. In contrast, our study area has humid summers and dry winters. The hydrothermal conditions in our study area may be much more suitable for alpine plants and rhizospheric microbes to flourish.
According to the soil properties that we examined, the rhizospheres of alpine cushion plants generally held more main mineral nutrients and soil moisture than the bare ground did. The different levels of positive effects of the two species of cushion plants are assumed to be the reason why the variations in physicochemical properties between microhabitats outside the vegetation growing season are greater than in the vegetation growing season.
4.2. Cushions harbor different bacterial diversities in the vegetation non-growing seasonFew studies have focused on the microbes in the rhizospheres of alpine cushion plants (Casanova-Katny et al., 2011; Rehakova et al., 2015; Roy et al., 2013). Furthermore, studies that have reported the microbes in the rhizosphere of alpine cushion plants were conducted in the vegetation growing season. In contrast to those studies, we compared seasonal differences in bacterial community diversity in the rhizospheres of two alpine cushion plants. Although bacterial diversities in the rhizospheres of cushion plants and in the soil of bare ground were not significantly different during the growing and non-growing seasons, bacterial communities identified in the rhizosphere of C. hookeri exhibited significantly greater richness and diversity than beneath the bare ground in the vegetation growing season. Moreover, the rhizospheres of C. hookeri had significantly higher bacterial diversity than the rhizospheres of A. polytrichoides did in the vegetation non-growing season.
Structures and composition of the bacterial communities did coincide partly to previous research in that the dominant phylum was Actinobacteria in some plots (Rehakova et al., 2015), such as the rhizosphere of C. hookeri in both seasons and the bare ground outside the vegetation growing season. However, the dominant phylum was Proteobacteria in the rhizosphere of A. polytrichoides in both seasons and beneath the bare ground in the vegetation growing season (Table S4). We also found that the two most abundant genera beneath A. polytrichoides and bare ground were Candidatus Solibacter and Rhodoplanes in the both seasons. The most abundant genera underneath the cushions of C. hookeri in the vegetation growing season were the same as those of A. polytrichoides, whereas Mycobacterium and Rhodoplanes were dominant outside the vegetation growing season. In a previous study that used cultivation methods, the most abundant bacterial genera found under the alpine cushion species Thylacospermum caespitosum were totally different (including Streptomyces, Arthrobacter, and Paenibacillus) (Rehakova et al., 2015). Whether the differences in bacterial genera abundance are due to differences in host plants or due to techniques remain unclear.
Associations between the bacterial diversities and the two alpine cushion plants were different under similar soil nutrients, especially in the vegetation non-growing season. The effect of alpine plants on microbes may be species-dependent, whereas, except for sulfur content, the physicochemical soil properties did not have significant effects on bacterial communities. The correlations between the relative abundance of all bacterial phyla and the microhabitats suggest that bacterial communities beneath alpine cushion plants are precisely assembled and affected by alpine cushion plants. Bacteria share the same nutrient pools in the rhizosphere as alpine cushion plants. Microbes have been detected competing for soil amino acids, which are potentially a significant nitrogen source in alpine tundra (Lipson and Monson, 1998). Other resources, such as soil moisture and the majority of nutrients, remain at low levels in the soil of bare ground, which does not produce positive effects on bacterial or botanical communities (Dakora and Phillips, 2002). In contrast, pioneer cushion plants are thought to have positive effects on ground surface differentiation and topsoil development.
5. ConclusionsIn conclusion, the alpine cushion plants had positive effects on the rhizospheric bacterial communities, even though the strength of the effect varied in different cushion species. Cushion species and the soil sulfur content are probably the major factors driving the spatial distribution and structure of soil bacterial communities in the alpine communities dominated by cushion plants.
AcknowledgmentsThis study was supported by the National Key Research and Development Program of China (Grant No. 2017YFC0505200, to Hang Sun), the Major Program of National Natural Science Foundation of China (Grant No. 31590823, to Hang Sun), the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDA20050203, to Hang Sun), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB15020302, to Jianqiang Su) and the Yunnan Applied Basic Research Project (Grant No. 2018FA015, to Yang Yang). Editors and anonymous reviewers are thanked for their helpful comments on our writing.
Appendix A. Supplementary dataSupplementary data to this article can be found online at pld.2018.09.003" target="_blank">https://doi.org/10.1016/j.pld.2018.09.003.
Arroyo M.T.K., Cavieres L.A., Penaloza A., Arroyo-Kalin M.A., 2003. Positive associations between the cushion plant Azorella monantha (Apiaceae) and alpine plant species in the Chilean Patagonian Andes. Plant Ecol, 169(1), 121-129.
DOI:10.1023/A:1026281405115 |
||
Badano E.I., Cavieres L.A., 2006. Ecosystem engineering across ecosystems:do engineer species sharing common features have generalized or idiosyncratic effects on species diversity?. J. Biogeogr, 33(2), 304-313.
DOI:10.1111/jbi.2006.33.issue-2 |
||
Badano E.I., Jones C.G., Cavieres L.A., Wright J.P., 2006. Assessing impacts of ecosystem engineers on community organization:a general approach illustrated by effects of a high-Andean cushion plant. Oikos, 115(2), 369-385.
DOI:10.1111/oik.2006.115.issue-2 |
||
Bertness M.D., Callaway R., 1994. Positive interactions in communities. Trends Ecol. Evol, 9(5), 191-193.
DOI:10.1016/0169-5347(94)90088-4 |
||
Billings W.D., Mooney H.A., 1968. The ecology of arctic and alpine plants. Biol. Rev, 43(4), 481-529.
|
||
Black C.A., Evans D.D., White J.L., Ensminger L.E., Clark F.E., 1965. Methods of Soil Analysis:Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling. Inc, Madison, Wisconsin, American Society of Agronomy. |
||
Bray R.H., Kurtz L.T., 1945. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci, 59(1), 39-45.
DOI:10.1097/00010694-194501000-00006 |
||
Bremner, J.M., 1965. Inorganic forms of nitrogen. In: Black, C.A. (Ed.), Methods of Soil Analysis. American Society of Agronomy, Madison, Wisconsin, pp. 1191-1205.
|
||
Butterfield B.J., Cavieres L.A., Callaway R.M., Cook B.J., Kikvidze Z., Lortie C.J., et al., 2013. Alpine cushion plants inhibit the loss of phylogenetic diversity in severe environments. Ecol. Lett, 16(4), 478-486.
DOI:10.1111/ele.12070 |
||
Callaway R.M., 1995. Positive interactions among plants. Bot. Rev, 61(4), 306-349.
DOI:10.1007/BF02912621 |
||
Callaway R.M., Brooker R., Choler P., Kikvidze Z., Lortie C.J., Michalet R., et al., 2002. Positive interactions among alpine plants increase with stress. Nature, 417(6891), 844-848.
DOI:10.1038/nature00812 |
||
Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., et al., 2010. QⅡME allows analysis of high-throughput community sequencing data. Nat. Methods, 7(5), 335-336.
DOI:10.1038/nmeth.f.303 |
||
Casanova-Katny M.A., Torres-Mellado G.A., Palfner G., Cavieres L.A., 2011. The best for the guest:high Andean nurse cushions of Azorella madreporica enhance arbuscular mycorrhizal status in associated plant species. Mycorrhiza, 21(7), 613-622.
DOI:10.1007/s00572-011-0367-1 |
||
Cavieres L., Quiroz C., Molina-Montenegro M., 2008. Facilitation of the non-native Taraxacum officinale by native nurse cushion species in the high Andes of central Chile:are there differences between nurses?. Funct. Ecol, 22(1), 148-156.
|
||
Cavieres L.A., Badano E.I., 2009. Do facilitative interactions increase species richness at the entire community level?. J. Ecol, 97(6), 1181-1191.
DOI:10.1111/jec.2009.97.issue-6 |
||
Cavieres L.A., Quiroz C.L., Molina-Montenegro M.A., Munoz A.A., Pauchard A., 2005. Nurse effect of the native cushion plant Azorella monantha on the invasive non-native Taraxacum officinale in the high-Andes of central Chile. Perspect. Plant Ecol. Evol. Systemat, 7(3), 217-226.
DOI:10.1016/j.ppees.2005.09.002 |
||
Chen J., Schöb C., Zhou Z., Gong Q., Li X., Yang Y., et al., 2015. Cushion plants can have a positive effect on diversity at high elevations in the Himalayan Hengduan Mountains. J. Veg. Sci, 26(4), 768-777.
DOI:10.1111/jvs.12275 |
||
Chen J., Yang Y., Stocklin J., Cavieres L.A., Peng D., Li Z., et al., 2014. Soil nutrient availability determines the facilitative effects of cushion plants on other plant species at high elevations in the south-eastern Himalayas. Plant Ecol. Divers, 8(2), 199-210.
|
||
Dakora F.D., Phillips D.A., 2002. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil, 245(1), 35-47.
DOI:10.1023/A:1020809400075 |
||
Degnan P.H., Ochman H., 2012. Illumina-based analysis of microbial community diversity. ISME J, 6(1), 183-194.
DOI:10.1038/ismej.2011.74 |
||
Fierer N., Jackson R.B., 2006. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. U. S. A, 103(3), 626-631.
DOI:10.1073/pnas.0507535103 |
||
Geremia R.A., Puşcaş M. Zinger L., Bonneville J.M., Choler P., 2016. Contrasting microbial biogeographical patterns between anthropogenic subalpine grasslands and natural alpine grasslands. New Phytol, 209(3), 1196.
DOI:10.1111/nph.13690 |
||
Hamady M., Walker J.J., Harris J.K., Gold N.J., Knight R., 2008. Error-correcting barcoded primers allow hundreds of samples to be pyrosequenced in multiplex. Nat. Methods, 5(3), 235.
DOI:10.1038/nmeth.1184 |
||
Huse S.M., Dethlefsen L., Huber J.A., Welch D.M., Relman D.A., Sogin M.L., 2008. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet, 4(11).
|
||
Körner, C., 2003. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Springer Berlin Heidelberg.
|
||
Lauber C.L., Strickland M.S., Bradford M.A., Fierer N., 2008. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem, 40(9), 2407-2415.
DOI:10.1016/j.soilbio.2008.05.021 |
||
Lazzaro A., Hilfiker D., Zeyer J., 2015. Structures of microbial communities in alpine soils:seasonal and elevational effects. Front. Microbiol, 6(1330).
|
||
Lee C.S., Li X.D., Shi W.Z., Cheung S.C., Thornton I., 2006. Metal contamination in urban, suburban, and country park soils of Hong Kong:a study based on GIS and multivariate statistics. Sci. Total Environ, 356(1-3), 45-61.
DOI:10.1016/j.scitotenv.2005.03.024 |
||
Lipson D.A., Monson R.K., 1998. Plant-microbe competition for soil amino acids in the alpine tundra:effects of freeze-thaw and dry-rewet events. Oecologia, 113(3), 406-414.
DOI:10.1007/s004420050393 |
||
Lozupone C., Lladser M.E., Knights D., Stombaugh J., Knight R., 2011. UniFrac:an effective distance metric for microbial community comparison. ISME J, 5(2), 169-172.
DOI:10.1038/ismej.2010.133 |
||
Lozupone C.A., Hamady M., Kelley S.T., Knight R., 2007. Quantitative and qualitative b diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol, 73(5), 1576-1585.
DOI:10.1128/AEM.01996-06 |
||
Molenda O., Reid A., Lortie C.J., 2012. The alpine cushion plant Silene acaulis as foundation species:a bug's-eye view to facilitation and microclimate. PLoS One, 7(5).
|
||
Molina-Montenegro O., Badano E.I., Cavieres L.A., 2006. Cushion plants as microclimatic shelters for two ladybird beetles species in alpine zone of central Chile. Arctic Antarct. Alpine Res, 38(8), 224-227.
|
||
Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O'Hara, R.B., et al., 2013. Vegan: Community Ecology Package. R Package Version 2.0-8.
|
||
Raunkiaer C., 1934. The Life Forms of Plants and Statistical Plant Geography. Clarendon press. Clarendon press, |
||
Rehakova K., Chronakova A., Kristufek V., Kuchtova B., Capkova K., Scharfen J., et al., 2015. Bacterial community of cushion plant Thylacospermum ceaspitosum on elevational gradient in the Himalayan cold desert. Front. Microbiol, 6.
|
||
Roy J., Albert C.H., Ibanez S., Saccone P., Zinger L., Choler P., et al., 2013. Microbes on the cliff:alpine cushion plants structure bacterial and fungal communities. Front. Microbiol, 4.
|
||
Shahnavaz B., Zinger L., Lavergne S., Choler P., Geremia R.A., 2012. Phylogenetic clustering reveals selective events driving the turnover of bacterial community in alpine tundra soils. Arctic Antarct. Alpine Res, 44(2), 232-238.
DOI:10.1657/1938-4246-44.2.232 |
||
Siles J.A., Margesin R., 2016. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in alpine forest soils:what are the driving factors? Microbial. Ecol, 72(1), 207-220.
|
||
Tirado R., Pugnaire F.I., 2005. Community structure and positive interactions in constraining environments. Oikos, 111(3), 437-444.
DOI:10.1111/oik.2005.111.issue-3 |
||
Turnbaugh P.J., Quince C., Faith J.J., McHardy A.C., Yatsunenko T., Niazi F., et al., 2010. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc. Natl. Acad. Sci. U. S. A, 107(16), 7503-7508.
DOI:10.1073/pnas.1002355107 |
||
Wang, Y., 2006. Yunnan Mountain Climate. Yunnan Science and Technology, Kunming. CN (in Chinese).
|
||
Yang Y., Chen J., Schöb C. Sun H., 2017. Size-mediated interaction between a cushion species and other non-cushion species at high elevations of the Hengduan Mountains, SW China. Front. Plant Sci, 8.
|
||
Yang Y., Niu Y., Cavieres L.A., Sun H., 2010. Positive associations between the cushion plant Arenaria polytrichoides (Caryophyllaceae) and other alpine plant species increase with altitude in the Sino-Himalayas. J. Veg. Sci, 21(6), 1048-1057.
DOI:10.1111/j.1654-1103.2010.01215.x |
||
Yang Y., Sun H., 2009. The bracts of Saussurea velutina (Asteraceae) protect inflorescences from fluctuating weather at high elevations of the Hengduan Mountains, Southwestern China. Arctic Antarct. Alpine Res, 41(4), 515-521.
DOI:10.1657/1938-4246-41.4.515 |
||
Zhang Y., Cong J., Lu H., Li G., Xue Y., Deng Y., et al., 2015. Soil bacterial diversity patterns and drivers along an elevational gradient on Shennongjia Mountain, China. Microbial. Biotechnol, 8(4), 739-746.
DOI:10.1111/mbt2.2015.8.issue-4 |


