b. University of Chinese Academy of Sciences, Beijing 100049, China
Orchidaceae is one of the largest and most diverse families of flowering plants, with more than 28, 000 accepted species spanning 763 genera (Christenhusz and Byng, 2016). Those species are absent only from polar and desert regions but are particularly abundant in the wet tropics worldwide (Chase, 2005). However, many orchids are locally distributed and generally rare (Waterman and Bidartondo, 2008). China, with its small tropical area and large desert region, has relatively few orchids (Luo et al., 2003). The most recent common ancestor of extant orchids lived in the Late Cretaceous, and the dramatic radiation of orchids began shortly after the mass extinctions at the Cretaceous-Tertiary boundary (65.5 Myr ago) (Ramírez et al., 2007). Orchidaceae appears to have undergone one significant acceleration of net species diversification in the orchidoids, and two accelerations in the upper epidendroids (Givnish et al., 2015). This rapid speciation and high species diversity is likely linked to the family's specialized pollination syndromes, symbiotic associations with mycorrhizal fungi, colonization of epiphytic habitats, and Crassulacean Acid Metabolism (Gravendeel et al., 2004; Silvera et al., 2009; Givnish et al., 2015). Due to the important ecological and evolutionary significance of orchids, research has long been conducted on their pollination biology and associations with mycorrhizal fungi (Waterman and Bidartondo, 2008; Fay and Chase, 2009).
As fascinating and highly popular plants, orchids are valued for their exquisite flowers and long floral lifespan. These plants exhibit great diversity in floral form, size, color, fragrance, and texture (Fig. 1). Commercial production has greatly expanded and become a very profitable industry. Many species, such as those of Cymbidium, Paphiopedilum, and Phalaenopsis, are cultivated for the enjoyment of their flowers (Hew and Yong, 2004). In fact, some members of Cymbidium have been cultivated in China for more than 1000 years (Chen and Luo, 2003; Luo et al., 2003). Orchids currently account for a significant share of the world's flower trade, with annual sales of more than $4 billion (U.S.). Some plants are also used as food and traditional medicine in many countries (Arditti, 1992). For example, the dried seed pods of vanilla (especially Vanilla planifolia) are commercially important as a flavoring in baking, as well as for perfume manufacturing and aromatherapy (Lubinsky et al., 2008). Gastrodia elata is one of three orchids listed in the earliest known Chinese Materia Medica, and is used for treating headaches, dizziness, tetanus, and epilepsy (Tsaia et al., 2011). However, because they are economically valuable to floral and pharmaceutical industries, and have suffered great losses in habitat, many species are becoming rare (Luo et al., 2003; Liu et al., 2015). All known orchid species are protected by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (Luo et al., 2003). In addition, their life histories, including interactions with mycorrhizal fungi, specialized pollinators, and host trees, make many orchid species particularly vulnerable to environmental changes and human disturbance (Fay and Chase, 2009). Therefore, the physiology of orchids requires further study, which is important for appropriately utilizing and conserving species resources while increasing our understanding of the evolution of orchid species diversity (Hew and Yong, 2004; Zhang et al., 2015b, c).

|
| Fig. 1 Flowers of nine Paphiopedilum species. a, P. charlesworthii; b, P. armeniacum; c, P. tigrinum; d, P. wardii; e, P. delenatii; f, P. micranthum; g, P. appletonianum; h, P. malipoense; i, P. bellatulum. |
Orchids have complex life histories and diversified adaptation strategies; consequently, researchers have paid much more attention to orchid pollination and orchid-mycorrhizal fungi interactions than to orchid physiology. Here, we review advances in orchid biology made in photosynthetic physiology; physiological adaptations to light, water, and temperature extremes; as well as strategies for nutrient acquisition and utilization. Our objective is to summarize the main findings on orchids, which may provide guidance for future investigations.
2. Important biological characteristics of orchidsOrchids are mostly long-lived, evergreen or deciduous herbs. Some individual plants, such as those of Cypripedium calceolus, can live 30-100 years (Kull, 1999). The pre-flowering vegetative period for most species usually lasts for four to seven years, but can be even longer (Kull, 1999; Wang et al., 2010). This longevity may be attributed to their inherently slow growth and reduced photosynthetic capacity (Schmidt and Zotz, 2002; Shefferson, 2006).
Orchids usually grow according to one of two patterns. For monopodial orchids, the stem emerges from a single bud, elongating and producing leaves from the apex each year. Sympodial orchids develop a series of adjacent shoots that continue to grow until they bloom and are finally replaced (Arditti, 1992; Sailo et al., 2014). The orchid life form can be terrestrial, epiphytic, lithophytic, or saprophytic. The epiphytic orchids, living in tree canopies or on rocks, exhibit many differences from soil-grown terrestrial orchids in their roots, stems, and leaves. For example, the roots of terrestrial orchids are frequently ground-dwelling, thick, and fleshy, with a storage function. Epiphytic orchids have modified aerial roots that are sometimes more than 1 m long. They also feature a velamen that consists of dead cells (Fig. 2). This velamen covers the entire root except the tip, and functions in rapidly absorbing moisture and nutrients from the surrounding humid atmosphere (Benzing, 1990; Zotz and Winkler, 2013).
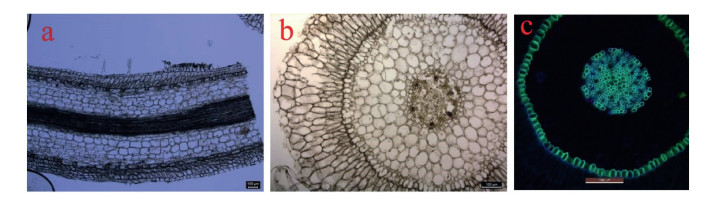
|
| Fig. 2 Root anatomy of Dendrobium officinale. a, longitudinal section; b, cross section; c, fluorescence microstructure. |
Like most monocots, orchids generally have simple leaves with parallel veins, although some species in the subfamily Vanilloideae show a reticulate venation. However, epiphytic orchids are characterized by thick and succulent leaves with thick cell walls, cuticles, and a small substomatal chamber, and they have smaller stomata than terrestrial species (Table 1; Arditti, 1992; Guan et al., 2011; Sailo et al., 2014). The stomata are slightly sunken into the leaf epidermis in Paphiopedilum (Fig. 3) but extrude outside the leaf surface in Cypripedium (Guan et al., 2011). One or more internodes of the stems from some orchids (e.g., Cymbidium, Cattleya, or Dendrobium) thicken to form pseudobulbs that can store nutrients and water during dry periods (Arditti, 1992; Yang et al., 2016).
| Functional traits | Function | Difference between two life forms |
| Leaf | ||
| Leaf mass per unit area | Water availability and energy exchange | Epiphytic > Terrestrial |
| Leaf thickness | Water availability | Epiphytic > Terrestrial |
| Leaf epidermal thickness | Water conservation | Epiphytic > Terrestrial |
| Degree of leaf succulence | Water conservation | Epiphytic > Terrestrial |
| Saturated water content | Water conservation | Epiphytic > Terrestrial |
| Epidermal conductance | Water loss | Epiphytic < Terrestrial |
| Water loss rate | Water balance | Epiphytic > Terrestrial |
| Vessel diameter | Water transport | Epiphytic > Terrestrial |
| Crassulacean acid metabolism | Water utilization | Occurs only in epiphytic species |
| Pseudobulb | ||
| Relative water content | Water conservation | Epiphytic > Terrestrial |
| Ratio of leaf area to pseudobulb dry weight | Water balance | Epiphytic > Terrestrial |
| Area of water storage cell | Water storage | Epiphytic > Terrestrial |
| Root | ||
| Velamen radicum | Water and nutrient uptake | Very common in epiphytic orchids than in terrestrial orchid |
| Ratio of velamen radicum thickness to root semi-diameter | Water and nutrient uptake | Epiphytic > Terrestrial |
| Number of xylem conduit | Water transport | Epiphytic > Terrestrial |
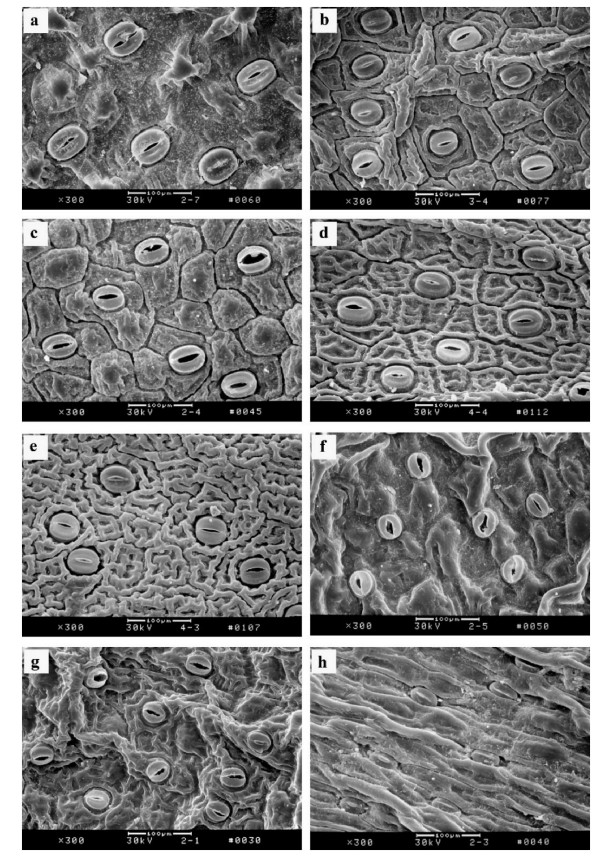
|
| Fig. 3 Leaf epidermal structures of Paphiopedilum species. a, P. malipoense; b, P. micranthum; c, P. armeniacum; d, P. emersonii; e, P. hangianum; f, P. concolor; g, P. bellatulum; h, P. hirsutissimum. Scale bars = 100 μm. |
Although orchid flowers are bilaterally symmetric, the inferior ovary or pedicel usually rotates 180° so that the labellum goes on the lower part of the flower to form a platform for pollinators. In Paphiopedilum, the shape of the lip staminode and petal, as well as the width of the petal, are phylogenetically conserved, while flower color is significantly convergent among species (Zhang et al., 2016). The seeds are numerous and extremely small (Arditti, 1992), and exhibit various anatomies (Fig. 4). Compared with terrestrial species, epiphytic Paphiopedilum species have larger embryos and a smaller percentage of air space. Those larger embryos may ensure more successful seedling establishment while the higher amount of air space in terrestrial species may increase their seed buoyancy and enable them to disperse over longer distances (Zhang et al., 2015a). However, due to the lack of endosperm, most orchids are thought to begin their life cycles aided by mycorrhizal fungi that provide the seeds with the nutrients necessary for germination. Thus, orchid species are myco-heterotrophic (MH) during germination (Arditti, 1992; Merckx, 2013).
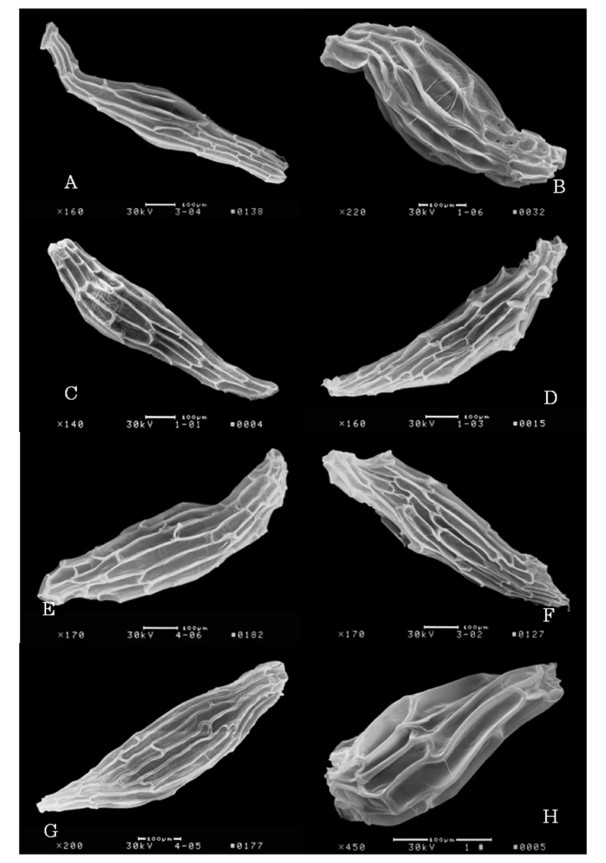
|
| Fig. 4 Seed anatomies of eight Paphiopedilum species. A, P. malipoense; B, P. armeniacum; C, P. micranthum; D, P. bellatulum; E, P. emersonii; F, P. concolor; G, P. rhizomatosum; H, P. dianthum. |
When plants achieve a certain size after a specific period of vegetative growth, they bloom under suitable temperature and light conditions. For example, most Phalaenopsis species and hybrids must be exposed to relatively cool temperatures, i.e., < 28 ℃, to trigger elongation of the spike (Lee and Lin, 1984). Cypripedium flavum, an alpine orchid, requires two years from floral bud formation to flowering. In the first year, two new buds are formed at the lateral base of the two-year-old bud. They then differentiate seven to nine spires before becoming dormant in winter. In the second year, one of those younger buds develops into a floral bud that then produces flowers and fruits in the third year (Weng et al., 2002). These observations are evidence of significant differences in life histories and flowering strategies among orchids.
3. Photosynthesis 3.1. Photosynthetic pathways in orchidsPhotosynthesis is the main way that many orchids acquire carbon. However, saprophytic species, which comprise a small proportion of the Orchidaceae, are myco-heterotrophic (Zhang et al., 2015c). With respect to photosynthetic pathways, green plants can be divided into three groups: C3, C4, or Crassulacean Acid Metabolism (CAM). Approximately 10% of all orchid species in Panama and Costa Rica belong to the CAM group, being most prevalent at low elevations and within the epiphytic clade, whereas C3 photosynthesis is the ancestral state (Silvera et al., 2009). Winter et al. (1983) have proposed that Cymbidium canaliculatum and Cymbidium madidum are CAM and C3 plants, respectively, based on δ13C values. However, Hocking and Anderson (1986) have reported that leaf extracts from those species show substantial pyruvate phosphate dikinase (PPD) activity. This enzyme is usually absent or is only slightly active in the leaves of C3 and CAM plants. The synthesis of phosphoenolpyruvate through the action of PPD is considered an essential adjunct to the C4 pathway. Therefore, these results seem to suggest that C4 photosynthesis occurs in those two Cymbidium orchids. However, one would need a complete analysis to demonstrate this, perhaps by studying the transfer of label from carbon-4 of C4 acids to carbon-1 of phosphoglyceric acid (Edwards and Walker, 1983).
Most orchids, especially species with thin leaves, assimilate CO2 through the C3 pathway. Those plants have fewer layers of smaller mesophyll cells and a larger number of stomata than the thickleaved species. They also have high CO2 compensation points and active glycolic acid activity, all of which are characteristics of plants with high rates of photorespiration. The thick-leaved orchids usually have features typical of CAM plants, such as leaf and cell succulence, diurnal fluctuations in titratable acidity and nocturnal CO2 fixation, and inverted stomatal physiology. Those genera include Vanilla, Cattleya, Thunia, Coelogyne, Laelia, Dendrobium, Calanthe, Bulbophyllum, Aerides, Phalaenopsis, Aranda, and Aranthera (Hew and Yong, 2004; Kerbauy et al., 2012; Sailo et al., 2014). As with other CAM plants, thick-leaved orchids have four typical phases of gas exchange (Hew and Yong, 2004). For example, no net gas exchange is observed in the leaves of Aranda from 9 am to 12 noon, but CO2 uptake begins after mid-day, and the rate increases with time. Those leaves also have two peaks of CO2 uptake: approximately 7 pm and 3 am. In addition, distinct regions of the same plant may utilize different photosynthetic pathways and varying degrees of CAM expression depending upon water availability (Rodrigues et al., 2013). Epiphytic orchids, many of which are CAM plants, grow on rock or tree trunks in tropical and subtropical forests where water deficits are frequent (Silvera et al., 2005). Thus, CAM plants can adapt to drought stress and high temperature (Kerbauy et al., 2012).
Because the ability of plants to transport water from root to leaf (hydraulic conductivity) is relatively lower in orchids than in other angiosperms, orchids utilize a variety of mechanisms to reduce water loss. Compared with terrestrial orchids, epiphytic CAM orchids usually grow under conditions where the volume of substrates is limited because of the scouring action of frequent rainfall. Furthermore, because those regions have relatively high temperatures, the potential for daytime evaporation is elevated. Stomatal closure is a very efficient strategy for minimizing water losses during the daytime. At night, the relative air humidity is very high there, which may lead to a low rate of evaporation and prompts those orchids to open the stomata for CO2 uptake. Photosynthetic carbon gain is optimized in some orchids that are facultative CAM plants but also induces C3 photosynthesis under well-watered conditions (Kerbauy et al., 2012). For example, Dendrobium officinale exhibits a typical CAM pattern when the content of substrate water is diminished (Zhang et al., 2014), but those plants reveal a concomitance of C3 and CAM patterns when re-watered. A shorter lightedark cycle leads to a C3 pattern alone. Consequently, substrate moisture and the lightedark cycle are inducible factors for switching between C3 and CAM patterns in that species (Zhang et al., 2014). Thus, the CAM pathway is an important strategy by which many epiphytic orchids prevent water loss and acclimate to fluctuation in water availability.
3.2. Photosynthetic limitations in C3 orchidsMost orchids have lower photosynthetic capacities when compared with other angiosperms. This capacity can be affected by stomatal conductance (gs), mesophyll conductance (gm), and biochemical factors (Grassi and Magnani, 2005). In angiosperms, biochemical limitations tend to be the main constraint (Carriqui et al., 2015). In contrast, the photosynthetic capacities of Cypripedium and Paphiopedilum species are more strongly limited by gm than by biochemical factors or gs (Yang et al., 2018). However, the three deciduous Cypripedium species show significantly higher photosynthetic capacities, gs, and gm than the three evergreen Paphiopedilum species (Yang et al., 2018). Higher values for gs in Cypripedium are independent of stomatal density but mainly affected by a larger stomatal apparatus area and smaller pore depths. Furthermore, the low levels of gm in Paphiopedilum are caused by much thicker cell walls and a reduced surface area for mesophyll cells and chloroplasts exposed to intercellular airspace per unit of leaf area. In that genus, cell wall resistance is responsible for approximately 50% of total mesophyll resistance. As wall thickness increases, the contribution of cell wall resistance to total resistance also rises (Terashima et al., 2011). A reduction in gm increases the resistance of CO2 conductance to the chloroplasts, causing chloroplast CO2 concentrations to decline, thereby restricting CO2 assimilation (Carriqui et al., 2015; Yang et al., 2018).
3.3. Regulation of photosynthesis in C3 orchidsThe light reactions of photosynthesis convert solar energy into chemical energy in the form of NADPH and ATP, which are utilized for CO2 assimilation. In photosynthesis, NADPH and ATP are mainly synthesized by linear electron transport from water to NADP+. The production ratio of ATP/NADP by linear electron transport is approximately 1.29 (Allen, 2002), which cannot satisfy the ratio of 1.5 required by the CalvineBenson cycle. Therefore, supplemental mechanisms for ATP synthesis are needed. In C3 plants, the process of photorespiration can increase that ratio up to 1.6. In addition, alternative electron transport, including cyclic electron flow (CEF) around photosystem I (PSI) and the waterewater cycle, contributes to this compensation of ATP synthesis. In our recent study, four Cymbidium species show little CEF activation under low light (Fig. 5B), suggesting that other mechanisms, such as the malate valve and the Mehler reaction, can maintain the energy balance when electron flow is low. Under intense irradiance, those four species have significant CEF activity. This is especially true for Cymbidium faberi, which has the highest electron flow through PSII (ETRII) (Fig. 5A). By comparison, the species with the lowest ETRII, Cymbidium lowianum, shows the least CEF activity. These results indicate that CEF is required for energy balance under high PPFD. The low levels of ETRII and CEF in C. lowianum are accompanied by a high level of non-photochemical quenching in PSII (NPQ) under more intense light (Fig. 5C). Because NPQ activation is based on lumenal acidification, which is dependent upon photosynthetic electron flow and functioning of chloroplast ATP synthase, activity by that enzyme in C. lowianum is the lowest among the four species.
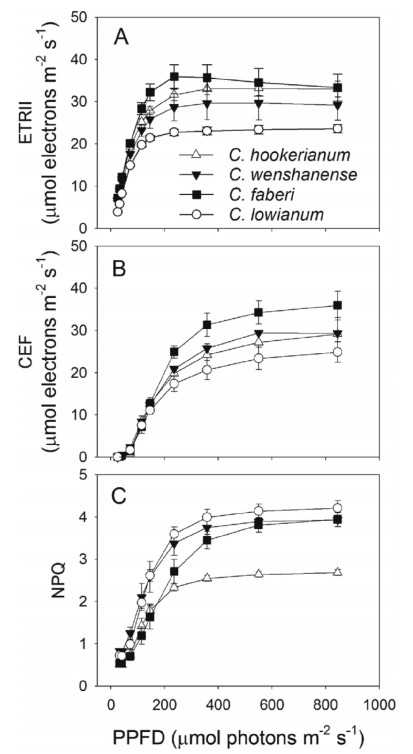
|
| Fig. 5 A, Light-intensity dependence of photosynthetic electron flow through PSII (ETRII); B, cyclic electron flow around PSI (CEF); and C, non-photochemical quenching in PSII (NPQ) for the leaves of four Cymbidium species. |
The restriction on CO2 assimilation can increase the production of reactive oxygen species (ROS) that cause photoinhibition. Under such conditions, photorespiration and CEF are important for alleviating photoinhibition, and proton motive force (pmf) must also be rapidly formed to protect PSI and PSII against excess light energy (Huang et al., 2015). The generation of ΔpH eases PSII photoinhibition by activating NPQ and stabilizing the oxygen-evolving complex (Huang et al., 2016). Meanwhile, lumenal acidification slows electron transfer from PSII to PSI via Cyt b6/f (Tikkanen and Aro, 2014), thereby preventing the over-reduction of PSI reaction centers and diminishing the production of superoxide and singlet oxygen within the thylakoid membrane to protect PSI activity (Kanazawa et al., 2017). When the leaves of Bletilla striata are transferred from darkness to light, CEF stimulation plus the low activity of chloroplastic ATP synthase contributes to rapid formation of high pmf (Huang et al., 2018). During photosynthetic induction, the performance of CEF is finely regulated to coordinate the activity of chloroplastic ATP synthase, optimizing photosynthesis and photoprotection.
4. Light requirement and adaption in orchidsIn orchids, a universal pattern of light requirement exists for individual species. Because they live in forests, the photosynthesis and growth of most orchids require a low level of irradiance (Zhang et al., 2007; Chang et al., 2011). However, specific light requirements for each species may depend on nutritional mode, life form, developmental stage, and habitat.
Myco-heterotrophic orchid species are usually light-independent because they acquire carbon through heterotrophic exploitation of mycorrhizal fungi rather than through photosynthesis. Even though such plants harbor a certain amount of photosynthetic pigment, e.g., chlorophyll (Chl) a and xanthophylls, they are photochemically ineffective (Cameron et al., 2009). Nevertheless, for species that are partially myco-heterotrophic (PMH), their reliance upon nearby fungi is governed by light availability, i.e., low levels lead to strong myco-heterotrophy while higher irradiance drives orchids toward autotrophy (Preiss et al., 2010). Species with different life forms also exhibit different requirements for light. For example, Cymbidium tracyanum, occurring in the tree canopy as an epiphyte, is more tolerant of intense irradiance than the closely related Cymbidium sinense found on shady forest floors (Kuang and Zhang, 2015). When light intensity exceeds the amount necessary for photosynthesis, e.g., after seasonal leaf-shedding by the host tree in a tropical dry forest, epiphytic orchids in the newly exposed canopy show considerable photoprotective plasticity to cope with such stress (Rosa-Manzano et al., 2015). Furthermore, in canopies with a high spatio-temporal heterogeneity in light environments, orchids may display a strategy for light interception that is commonly observed for plants that typically grow in low-light environments (Ventre-Lespiaucq et al., 2017).
Light can inhibit the seed germination of many terrestrial, and even some epiphytic, species (Rasmussen et al., 2015). Thus, in vitro germination for most orchids are conducted in the dark (Huang and Hu, 2001). Germination under darkness may help avoid seedling desiccation that might be a fatal consequence of exposure to naturally high light. Orchids may also have different light requirements at various developmental stages after germination. For Phalaenopsis hybrids, a relatively lower light intensity helps seeding whereas stronger light promotes seedling growth, with an even higher intensity recommended for the induction of flower spikes (Wang, 1995).
Orchid growth is also affected by light quality. Stomatal opening and photosynthesis by C. flavum is highly induced by mixed blue and red light rather than by pure blue or red light. However, because guard cell chloroplasts are lacking in the closely related Paphiopedilum species, stomatal opening is stimulated by specific blue light during photosynthetic induction (Zhang et al., 2011). Red or far-red light usually promotes the vegetative growth of seedlings in flask or greenhouse cultivation, whereas blue light elevates Chl production (Islam et al., 1999; Lee et al., 2017). In a natural habitat, orchids growing beneath a canopy are inescapably subjected to a reduction and alteration of light quality due to reflection and selective absorption within the upper canopy. When compared with a more open habitat, the lower canopy generally has a higher level of green light but lower levels of both red light and its proportion to far-red light (Caldwell and Pearcy, 1994). The photosynthetic apparatus of an orchid growing on a forest floor can acclimate to such light environments by various means, such as modifying levels of Chl and the ratio of Chl a to b to maintain coordination between PSII and PSI (Zhang et al., 2007).
Like other plants, orchids can be classified as short-day, longday, or day-neutral (Hew and Yong, 2004). The impact of photoperiod on orchid vegetative growth is species-specific, and species and hybrids within a genus may have different responses. Some orchids require a short day for flower initiation, whereas others, such as species within Cymbidium and Phalaenopsis, show no response or an ambiguous response to photoperiod, probably because of the influence of growth temperature (Lopez and Runkle, 2005). The practice of night interruption can be used to stimulate flowering by long-day orchids and improve flower quality in commercial cultivation (Kim et al., 2011). Although a relatively high light intensity can increase photosynthesis during that interruption, the photosynthesis rate and PSII activity in those plants during the daytime may decline due to a leaf-nitrogen deficiency. Therefore, night interruption accompanied by additional fertilization is recommended (Kim et al., 2015).
Most orchids can adapt, in a species-specific manner, to a broad range of light environments in different habitats. In a dry forest, wide fluctuations in irradiance levels are generally caused by the phenology of the individual host tree. Orchids from such a habitat may demonstrate higher plasticity than those from a more humid forest (Rosa-Manzano et al., 2017). Some species are capable of adjusting, via morphological and physiological changes, to a wide range of environments. For example, Cypripedium guttatum, the only species in that genus occurring in both the Old and the New World, is found in both open and shady habitats where irradiance can vary from 22 to 76% of full sunlight (Zhang et al., 2007). Tropical orchids that are adapted to full sunlight also do well after being subjected to 75% shading (Pires et al., 2012). However, when compared with plants in other herbaceous families, orchids have a very low rate of leaf turnover, which might put them at risk if they encounter a sudden change in growth irradiance because turnover rate plays an important role in light acclimation (Ishii and Ohsugi, 2011). One extreme example is Pleione aurita, an orchid that produces only one leaf per growing season. As a result, the mature leaf of P. aurita cannot photosynthesize optimally under new lighting conditions, due to this structural restriction. Therefore, that sole, inefficient leaf ultimately leads to a decrease in annual carbon gain (Zhang et al., 2017). A comprehensive survey of vascular epiphytes in a lowland forest has revealed that most orchid species and individual plants grow within the intermediate stratum (Zotz and Schultz, 2008), which means that they have a moderate light requirement, i.e., a maximum of approximately 50% of full sunlight (Zhang et al., 2007, 2017). Canopy closure caused by forest succession has an adverse impact on the reproduction of the understory C. calceolus, and the practice of selective tree harvesting has been proposed to ensure that a brightly lit forest floor is available for the conservation of this rare orchid (Hurskainen et al., 2017). In contrast, epiphytic orchids on isolated trees are confronted, post-logging, with a harsher microclimate characterized by more intense light and increased drought conditions, and their seedling establishment is severely restricted when compared with epiphytic orchids growing on trees in a closed-canopy forest (Werner and Gradstein, 2008). Thus, the requirements of orchids for light are highly complex.
5. Adaptation of orchids to temperature extremesPlants exhibit different degrees of physiological tolerance to environmental stresses, but members within the Orchidaceae can occur in habitats from tropical to temperate zones (Arditti, 1992). Their growth often responds to an optimum temperature at which the rate of progress toward a particular developmental event is maximal. Temperatures that exceed either end of that optimum range may have a negative effect on growth and development. For example, night-time CO2 absorption by Phalaenopsis is inhibited at temperatures above 25 ℃ or below 15 ℃ (Arditti and Pridgeon, 1997).
5.1. Response and acclimation to low temperatureOrchids originating from tropical or sub-tropical areas tend to be sensitive to chilling stress and genera such as Phalaenopsis can hardly survive in regions where severe, long-term chilling occurs naturally. Low temperatures may lead to many symptoms of stress, such as leaf-yellowing, defoliation, or a reduced rate of growth. Leaf-pitting in Phalaenopsis can be induced at temperatures of 2-7 ℃, with the amount of pitting depending upon the duration of exposure and the physiological age of the leaf tissue. At these temperatures, mature leaves are less susceptible than young leaves (Sheehan and McConnell, 1980). Anatomical studies have revealed that pitting is the result of mesophyll cell collapse, which initially occurs in cells between the large vascular bundles. Severely damaged areas are characterized by extensive collapse and those cells are always surrounded by hypertrophical cells (Sheehan, 1983).
Plants may decrease their photosynthetic activities at low temperatures due to the depression of Rubisco activity and RuBP regeneration. As a result, the excess excitation energy may induce the production of a large amount of ROS, which can damage the photosynthetic apparatus (Asada, 1999). Some photoprotection mechanisms are also activated during periods of chilling stress, such as NPQ and CEF. Three Paphiopedilum species present significant PSII photoinhibition when they are exposed to 4 ℃, but their PSI activities are not susceptible to combined chilling-light stress for 8 h (Yang et al., 2017). Compared with Paphiopedilum purpuratum, both Paphiopedilum armeniacum and Paphiopedilum micranthum are less impaired because they have relatively higher CEF activity that alleviates PSII photoinhibition and protects PSI activity in stressed leaves. Similarly, stimulation of CEF capacity is also important for easing chilling-induced PSII photoinhibition in two Cymbidium species (Li and Zhang, 2016). However, even the most sensitive species, P. purpuratum, is not very vulnerable to shortterm chilling treatment because its PSI activity remains stable. This is probably not due to CEF activation but rather to the inhibition of electron transport from PSII to PSI. The latter scenario is largely responsible for preventing excess electron flow to PSI, thereby allowing the amount of active PSII to be balanced and the capacity of the PSI electron acceptors left undisturbed (Tikkanen et al., 2014).
During periods of growth and development, a trade-off usually exists among the governing physiological processes. Although low temperatures may hinder plant development, a concomitant decline in the growth rate can be compensated by a longer growth period. For example, shoot growth in Cymbidium sazanami 'Otome' may be delayed and slowed during a cold winter, causing the primary shoots to be smaller and mature later. However, the total number of primary shoots will not be affected by such conditions. Consequently, shoots can produce a similar number of leaves if the growing season is relatively long (Kako et al., 1976). Furthermore, for evergreen orchids that display greater temperature homeostasis of photosynthesis, some species, such as those in Pleiones and Bletilla, employ an escape strategy by up-regulating photosynthetic efficiency and fixing more carbon during the warm season, then shedding their leaves and roots, leaving a dormant pseudobulb to survive in the cold conditions (Cribb and Butterfield, 1999).
5.2. Response and acclimation to high temperatureModerately high temperatures usually favor plant growth, but extremely high temperatures may impair physiological processes. Exposure to high temperatures may cause cellular membranes to weaken and ion leakage to occur, as manifested by tissue necrosis (Jones, 1992). For example, the optimum temperature for photosynthesis by C. flavum is approximately 20 ℃. When plants are transferred from their usual alpine habitat to a lower elevation, their leaves exhibit decreases in rate of photosynthesis, stomatal conductance, transpiration, and carboxylation efficiency. Reduced gs values measured at that lower elevation may retard the diffusion of CO2 into the leaf, which further exacerbates the depression of photosynthetic capacity (Zhang et al., 2005). The optimum temperature for photosynthesis is generally below 30 ℃, while that for respiration occurs just below the temperature at which enzymes are heat-inactivated, i.e., > 45 ℃. When plants are exposed to a temperature above this photosynthetic optimum, their photosynthetic rates are depressed while that of respiration continues to increase. This may lead to an imbalance between carbon-fixation and consumption, which directly affects vegetative growth and indirectly influences flowering through decreases in plant size and nutrient supply (Iersel, 2003). Enhanced expression of relevant enzymes and metabolites plays an important role in protecting cells against high-temperature stress (Law et al., 2001). In Phalaenopsis, the oxidative damage caused by elevated temperatures may decrease photochemical efficiency as malondialdehyde levels and lipoxygenase activity increase. Meanwhile, the activities of dehydroascorbate reductase, glutathione peroxidase, and glutathione-Stransferase in the leaf and root; glutathione reductase in the leaf; and guaiacol peroxidase in the root are induced significantly at 40 ℃ when compared with 25 ℃, suggesting that these enzymes have roles in thermal protection (Mohammad et al., 2005).
The optimum temperature varies among developmental processes. Some species require a relatively low temperature for flower induction, and high temperature delays the development of floral buds (Sinoda et al., 1984). This temperature requirement for flower induction reflects an important natural adaptation to seasonal change in the growing environment (Arditti, 1992). In Odontioda, a high percentage of the inflorescences abort at a daily temperature of 26 ℃ or 29 ℃ for 12 h or 24 h, respectively. Although inflorescences can be initiated at those warmer temperatures, many of them do not continue to develop any further. The percentage of the plant that initiates inflorescences and develops opening flowers is the greatest at a temperature of 14 ℃-17 ℃ (Blanchard, 1993). In Dendrobium, flower initiation only occurs when mature pseudobulbs are exposed to temperatures of 7.5 ℃-20.0 ℃. Decreasing temperatures caused by cool rain may promote the flowering of these orchids in their natural habitats. Temperature signals usually affect floral development and morphogenesis by influencing hormone levels (Arditti and Pridgeon, 1997). In Phalaenopsis hybrida, this temperature regulation depends upon an optimal concentration of endogenous gibberellin in the tip of the flowering shoot. Such shoots have a lower amount of the hormone when grown at 30/25 ℃ (day/night) than at 25/20 ℃ (Su et al., 2001). These findings have been used to devise strategies for commercial production of orchids. For example, temperature manipulation is used to control and synchronize flowering time for Cymbidium, Dendrobium, and Phalaenopsis (Chen et al., 1994; Hew and Yong, 2004). Future investigations to verify other mechanisms that underlie temperature acclimation in orchids would be of great importance for the conservation and cultivation of rare species.
6. Water relations in orchidsMembers of Orchidaceae are more successful than any other lineage in colonizing tree crowns, with at least 70% of the species in this family being canopy-adapted, and approximately 2/3 of all epiphytes being orchids (Benzing, 1990; Zhang et al., 2015c). The epiphytic orchids benefit from intense irradiance and relatively little competition but are confronted with limited supplies of nutrients and, especially, water (Zotz and Hietz, 2001). To cope with such challenges, these orchids develop suites of anatomical and physiological adaptations to improve the uptake and internal storage of water, as well as to reduce its loss (Table 1).
6.1. Fast water uptakeFast water uptake from the atmosphere is an important strategy for survival in the tree canopy. The velamen radicum is a unique dead structure on the root surfaces of most epiphytic orchids. One of its most important roles is the absorption of water (Benzing, 1990; Zotz and Winkler, 2013) after rainfall is captured and immobilized. It generally takes more than one hour for the velamen radicum of many orchids to fill with water (Dycus and Knudson, 1957). However, Zotz and Winkler (2013) have shown that this structure, when dry, can take up moisture within seconds. The volume of the velamen radicum varies among species and is positively linked with higher initial rate of uptake. However, because all velamina become saturated very rapidly, such differences may not be very important functionally.
6.2. Storage of waterThe water stored within the organs is key to maintaining the whole-plant water balance during periods of drought. For most orchids, the leaves and pseudobulbs act as those storage organs. Values for leaf mass per unit area (LMA), leaf thickness, and saturated water content (SWC) are important functional traits when characterizing leaf water storage capacity. In Cymbidium, the epiphytic species have greater ability than the terrestrial species to tolerate drought because they have higher LMA, leaf thickness, and SWC (Table 1; Zhang et al., 2015b). Large leaf epidermal cells also contribute to water storage. In some orchids, the amount of water stored in those epidermal cells can account for up to 80% of the entire leaf volume (Pridgeon and Stern, 1982). For Paphiopedilum species growing in karst habitats, the adaxial epidermis cells have significantly larger volume than the abaxial cells, and the leaves are thick, fleshy, and contain more water than plants of those species growing in other environments (Guan et al., 2011).
The pseudobulb, an adaptively unique stem of many orchids, serves as a buffer against drought stress because of its ability to retain water (Ng and Hew, 2000). During a period of drought, the presence of pseudobulbs may slow the usual reductions in leaf water content and water potential (He et al., 2013). We have found significant differences in water-related traits and the physiological responses of pseudobulbs to drought between epiphytic and terrestrial orchids. Compared with terrestrial C. sinense, epiphytic C. tracyanum has larger water storage cells and a higher relative water content (Table 1). Those features may contribute to the maintenance of normal physiological functioning for longer periods of time under a water deficit. As expected, C. tracyanum can quickly utilize water stored in the pseudobulb when exposed to drought stress, and leafless pseudobulbs help sustain long-term leaf photosynthesis (our unpublished data). Many epiphytic orchids buffer transpiration to extend stomatal conductance and photosynthesis for more than 20 d when responding to a soilmoisture deficit (Sinclair, 1983). For example, Dimerandra emarginata can maintain a normal leaf water content for 23 d in the absence of rain (Zotz, 1999). Although Dendrobium chrysotoxum and D. officinale have thicker leaves and upper cuticles when compared with Dendrobium chrysanthum and Dendrobium crystallinum, the latter two compensate for that by having higher SWCs in their pseudobulbs (Yang et al., 2016). This indicates that the strategies for maintaining water balance differ among orchid genera and species.
6.3. Reduction of water lossAs part of their strategies for reducing water loss, the epiphytic species in Cymbidium have a thicker epidermis and require more time to dry saturated leaves to 70% relative water content when compared with terrestrial species in that genus (Table 1). These traits make the epiphytes more drought-tolerant (Zhang et al., 2015b). Physiological and proteomic analyses of an epiphytic and a terrestrial orchid have found that the former has greater ability to maintain a carbon balance under drought stress and it also responds more effectively to abscisic acid in the leaves (Li et al., 2018). The stomata of P. armeniacum are slightly sunken into the leaf epidermis; this specific structure may reflect an adaptation to periodic water deficiencies in limestone habitats (Zhang et al., 2011).
Velamen radicum appears to be effective in reducing water loss (Zotz and Winkler, 2013). Orchids growing in drier habitats usually have thicker velamina (Sanford and Adanlawo, 1973). Water retention volume also increases with the size of that velamen radicum (Luttge, 1989). Zotz and Winkler (2013) have reported that, for most orchids, water is retained for more than 1 h in the velamen radicum. An epiphytic orchid, C. tracyanum, has a higher radio of velamen thickness to root thickness, along with larger-diameter xylem conduits than those of terrestrial C. sinense (Table 1). These findings indicate that epiphytic orchids have greater capacity to conserve water and avoid the negative effects of drought based on their root functioning (Li et al., 2018).
In many orchid species, CAM plays a critical role in improving carbon gains and reducing water losses (Kerbauy et al., 2012). In addition to CAM regulation in the leaves (described above), CAM photosynthesis also occurs in the stems, fruits, and flowers of some orchids, and in the aerial roots of epiphytic plants (Hew and Yong, 2004; Motomura et al., 2008; Kerbauy et al., 2012; Rodrigues et al., 2013). Although pseudobulbs lack stomata, Hew et al. (1998) have discovered chlorophylls and PEPC and Rubisco activities in the pseudobulbs of Oncidium goldiana. This implies that, in some cases, pseudobulbs may be capable of some CAM activity (Winter et al., 1983; Hew et al., 1998; Rodrigues et al., 2013). This process recycles the respiratory CO2 generated by the voluminous underlying parenchyma of pseudobulbs (Ng and Hew, 2000). Pseudobulbs alone are apparently unable to assimilate carbon via CAM because the stomata are absent. When the entire shoot of Laelia anceps is illuminated during the light period, the leaf assimilates carbon then as well as during the next dark period. However, when those pseudobulbs are exposed to darkness during the light period, the leaf assimilates carbon only at night (Ando and Ogawa, 1987). This suggests that the pseudobulbs influence the uptake of CO2 by the leaf under both light and dark conditions.
7. Nutrients of orchids 7.1. Nutrient uptake and utilizationNutrients are important factors that control plant growth and development. For example, nitrogen-deficiency can decrease protein synthesis, growth rates, and productivity (Amâncio and Stulen, 2004). Terrestrial orchids obtain nutrients mainly from the soil, while sources for epiphytic orchids can also include atmospheric dry/wet depositions, solid substrates (such as bark or litter), and nitrogen fixation by microorganisms (Benzing, 1990; Reich et al., 2003). For two bromeliad species, atmospheric-nitrogen provides as much as 77-80% of that element to small individuals when compared with soil-derived nitrogen, which contributes 64-72% of leaf-nitrogen to large plants (Reich et al., 2003). Dischidia major derives 39% of its carbon from ant-related respiration, and 29% of its nitrogen supply comes from the debris deposited by ants inhabiting its cavities (Treseder et al., 1995).
Roots are the main organ for absorbing nutrients. Plants can regulate nutrient acquisitions by altering root architecture and morphology. Increasing the root biomass, specific root length, and number of fine roots can improve the absorption of nitrogen and other nutrients (López-Bucio et al., 2003). Orchid roots are usually thick and succulent, produce a large biomass, but have few root hairs (Hew and Yong, 2004). The presence of fungal mycelia can increase the absorbing surface area of those roots (Dearnaley and Cameron, 2017). The velamen radicum may also compensate for the lack of root hairs and help epiphytic orchids quickly absorb mineral nutrients from fog and rainwater (Zotz and Winkler, 2013). Elevating nitrogen concentration decreases the number of root cells and the thickness of velamen radicum in Laelia purpurata (Júnior et al., 2013).
The most common forms of nitrogen absorbed by plants are nitrate nitrogen (NO3-) and ammonium nitrogen (NH4+). High activities by nitrate reductase (NR) and glutamine synthetase (GS) can enhance the assimilation of NO3- and NH4+. The uptake of NO3- is highest at the root tip, and obviously decreases with increasing distance from the root tip due to the presence of fibrous layers in the older root tissue that can hinder the process. In contrast, NH4+ can be taken up by any part of the root, but it mainly occurs at the mature zone (Colmer and Bloom, 1998). Epiphytic and terrestrial orchids can absorb both NO3- and NH4+, but the absorption rate for the former is higher in terrestrial orchids while that of the latter is higher in epiphytic orchids. Both NR and GS are present in orchid roots and leaves, although the former enzyme is more active in the roots and less so in the leaves. The reverse is true for GS (Hew et al., 1993; Hew and Yong, 2004). When provided with 75% or 100% NH4+, plants of Phalaenopsis are smaller and tend to show a decrease in the width of the top leaf and less whole-plant leaf spread as the level of NO3- declines from 100% to 0%. Spiking is delayed and the spiking rate is reduced when those plants receive more than 50% NH4+. As the ratio between NO3- and NH4- rises, flowers become increasingly larger (Wang and Chang, 2017). Flower buds and flowers of C. sinense form normally when plants are treated with NO3- at 1 or 10 mmol L-1, but no flower buds form regardless of the level of NH4+ treatment (Pan and Chen, 1994). These results suggest that orchids have a preference for nitrogen forms.
Although the effects of nitrogen on orchid growth under cultivation conditions have been confirmed by some studies, the demand for that nutrient is relatively low (Mou et al., 2012; Wang and Chang, 2017). Within a certain range, increasing the nitrogen concentration can promote vegetative growth, and increase the number of flowers produced by genera such as Phalaenopsis, Cattleya, and Dendrobium. However, flowering is delayed when the nitrogen concentration is too high (Bichsel et al., 2008; Wang and Chang, 2017). The demand for nitrogen by plants of P. armeniacum is higher at the vegetative growth stage but lower at the reproductive stage. When abundant nitrogen is available, this orchid is propagated primarily by seeds. However, asexual reproduction dominates when nitrogen supply is limited (Mou et al., 2012). Therefore, these reports demonstrate that the effect of nitrogen on orchid growth varies by species and developmental stage. Whereas reports have shown how low nutrient availability affects plants in epiphytic habitats, the conclusions on how the relative limitations of nitrogen and phosphorus influence epiphytes growing in the wild remain ambiguous (Zotz and Hietz, 2001; Wanek and Zotz, 2011).
Deposition of atmospheric-nitrogen is an important environmental issue. Increasing the levels of nitrogen compounds in the atmosphere may affect species diversity and ecosystem functioning due to acid rain, eutrophication, and direct toxic effects (Amâncio and Stulen, 2004). Because they are largely dependent on atmospheric sources for nutrients, epiphytes are more susceptible to such depositions, especially those of NHX compounds. The δ15N values measured from the leaves, pseudobulbs, and roots of Laelia speciosa are higher at sites exposed to industrial and vehicular activities than in oak forests (Díaz-Álvarez et al., 2016). Performance by those plants is optimal at doses of up to 20 kg N ha yr-1, but toxic effects are observed at doses of 40 and 80 kg N ha yr-1 (Díaz-Álvarez et al., 2015). However, few studies have examined the responses of orchids to nitrogen-deposition and it remains unclear whether the continuous rise in those depositions is beneficial or harmful to epiphytic orchids.
7.2. Mycorrhizal nutrients of orchidsMycorrhizal fungi play an important role in the life history of orchids. The success of seed germination depends upon the nutrients supplied by fungal symbionts. At the adult stage, some orchids produce green leaves and become putatively autotrophic. However, many achlorophyllous species remain fully MH. Aphyllorchis and Gastrodia are the largest genera of full myco-heterotrophs (Merckx, 2013). In contrast, PMH orchids, commonly terrestrial, obtain nutrients via their own photosynthesis and their mycobionts, and they include many green-leaved species (Gebauer and Meyer, 2003; Preiss et al., 2010). Occasionally, however, achlorophyllous MH variants are found in some PMH species, such as Epipactis and Cephalanthera (Selosse et al., 2004).
Compared with autotrophic plants, the adaptation and evolution of MH and PMH plants are usually accompanied by changes in morphology and genetics, including reduced leaf size and the loss of expression by photosynthesis-related genes (Barrett et al., 2014). Most MH orchids grow in deeply shaded habitats and are mainly colonized by the specialized ectomycorrhizal fungi of neighboring trees (Merckx, 2013; Selosse et al., 2004). A common tripartite mycorrhizal network - autrotropic trees, fungi, and MH plants - is vital to those MH species in acquiring nutrition and is critically important during the early stages of forest succession and tree recruitment (Selosse et al., 2006). However, only a few studies have focused on nutrient flow back to the fungal partner from MH plants (Cameron et al., 2006). Some MH orchids associate with litterand wood-decaying fungi, including Eulophia zollingeri and Cyrtosia septentrionalis (Martos et al., 2009).
The PMH plants are part of a transition away from autotrophy to MH status, usually dwelling in habitats with higher irradiance where they remain chlorophyllous (Preiss et al., 2010; Merckx, 2013). These plants display various levels of specificity to their fungal hosts. Members of Tulasnellaceae and Ceratobasidiaceae are very common in the mycorrhizae of PMH orchids (Hock, 2012), but mutualisms also exist between Atractiellomycetes and orchids in tropical regions (Kottke et al., 2010). As an exception, some perennial green species of Cephalanthera and Epipactis that are albino specimens can survive up to 14 years. They may share similar mycobionts with the green individuals but are usually maladapted due to low fitness during the vegetative or reproductive phases (Gonneau et al., 2014).
The transfer of nutrients from mycorrhizal fungi to their symbiotic orchids has already been demonstrated (Cameron et al., 2006; Dearnaley and Cameron, 2017). For some autotrophic orchids, the adult plants still obtain carbon, nitrogen, phosphorus, and other nutrients through mycorrhizal fungi (Zimmer et al., 2007). Organic matter, such as bark, can be decomposed by fungal mycelia to provide nutrients to epiphytes. Thus, fungal-based mixed-heterotrophic plants have higher levels of δ15N and nitrogen than autotrophic plants. When the orchid root cells digest the fungal mycelium, those resulting compounds are then incorporated into the orchid biomass (Smith and Read, 1997).
Elemental studies utilizing radioisotope and stable isotope tracing have expanded our understanding about the source-sink relationships among plants, fungi, and the environments (Cameron et al., 2006; Mayor et al., 2009). A field survey in 1960 is the first to confirm that Monotropa hypopitys is nourished by neighboring trees through fungal mycelia, based on traces of 14C-labeled glucose and 32P-labeled phosphate (Björkman, 1960). The transfer of carbon and nitrogen from trees or substrates to orchids (MH, PMH, or putatively autotrophic) through fungi has been documented by the application of radiocarbon and stable isotope methods (McKendrick et al., 2000). The enrichment of 13C in MH plants can be explained by these species tapping into a carbon source that is an alternative to the atmospheric-CO2 utilized for photosynthesis by autotrophic plants. Meanwhile, the 15N enrichment in MH plants is probably due to receiving compounds enriched in 15N when compared with surrounding autotrophic plants that share the same mycorrhizal fungi (Merckx, 2013). Many fungi are enriched in the heavy isotopes 13C and 15N in comparison to autotrophic plants from the same habitat due to their specific physiology (Mayor et al., 2009). However, enrichment of heavy isotopes in fungi is not uniform but, instead, is specific to certain functional and taxonomic fungal groups.
8. PerspectivesA good understanding of orchid physiology is essential for orchid conservation and utilization. However, because of their long life-history and slow growth rate, only the physiology of a small portion of the species within Orchidaceae has been studied. Furthermore, we summarize some important questions that remain unanswered and must be addressed in future research.
(1) Orchids usually have a long vegetative phase, a slow growth rate, and low photosynthetic potential. Shortening the period of vegetative growth is an important concern for orchid breeders and growers. Improving our knowledge about the mechanisms underlying slow growth and low photosynthetic rates is still a long-term task. In addition, while some orchids bloom continuously throughout the year, others require more than two years of recovery before reblooming. This raises questions about how the costs of construction and maintenance (i.e., respiration), as well as water supply, affect such flowering behavior and floral lifespan. However, information is still lacking about the mechanism of flower induction and floral organ development in most orchids. More extensive research is needed to develop a commercially viable method for controlling flowering in economically important orchids such as Paphiopedilum, Oncidium, and Dendrobium (Hew and Yong, 2004).
(2) The effects of nitrogen on orchid growth under cultivation conditions have been confirmed but it is still unclear how limited supplies of nitrogen and phosphorus might affect development for wild plants of epiphytic orchids (Zotz and Hietz, 2001; Wanek and Zotz, 2011). Moreover, because the epiphytic forms rely heavily upon atmospheric sources of nutrients, they are more susceptible to nitrogen deposition. The potential trade-off between benefiting and harming those plants as levels of atmospheric-nitrogen continue to rise is another important topic for future investigations.
(3) New information about the correlations between mycorrhizal fungi and orchid plants has expanded our understanding about the symbiotic relationships among G. elata, Armillaria mellea, and Mycena osmundicola, and results from those studies have been used to promote the artificial cultivation of Gastrodia. However, mycorrhizal fungi are still rarely applied in commercial cultivation of orchids, especially for autotrophs.
(4) Genome sequencing has been completed for several orchids, including Phalaenopsis equestris (Cai et al., 2014), and new techniques are being widely used for molecular ecology, stable isotopes, and computer visualization in plant sciences. These tools will provide new insight into orchid physiology.
AcknowledgementsThis work is financially supported by the National Natural Science Foundation of China (31670342, 31370362) and the Natural Science Foundation of Yunnan Province (2018FA016).
Allen J., 2002. Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell, 110, 273-276.
DOI:10.1016/S0092-8674(02)00870-X |
||
Amâncio S., Stulen I., 2004. Nitrogen Acquisition and Assimilation in Higher Plants. Kluwer Academic Publishers, Dordrecht. |
||
Ando T., Ogawa M., 1987. Photosynthesis of leaf blades in Laelia anceps Lindl. is influenced by irradiation of pseudobulb. Photosynthetica, 21, 588-590.
|
||
Arditti J., 1992. Fundamentals of Orchid Biology. John Wiley & Sons, New York. |
||
Arditti J., Pridgeon A.M., 1997. Orchid Biology:Reviews and Perspectives Ⅶ. Kluwer Academic Publishers, Dordrecht. |
||
Asada K., 1999. The water-water cycle in chloroplasts:scavenging of active oxygens and dissipation of excess photons. Plant Physiol. Plant Mol. Biol, 50, 601-639.
DOI:10.1146/annurev.arplant.50.1.601 |
||
Barrett C.F., Freudenstein J.V., Li J., et al., 2014. Investigating the path of plastid genome degradation in an early-transitional clade of heterotrophic orchids, and implications for heterotrophic angiosperms. Mol. Biol. Evol, 31, 3095-3112.
DOI:10.1093/molbev/msu252 |
||
Benzing D.H., 1990. Vascular Epiphytes:General Biology and Related Biota. Cambridge University Press, Cambridge UK. |
||
Bichsel R.G., Starman T.W., Wang Y.T., 2008. Nitrogen, phosphorus, and potassium requirements for optimizing growth and flowering of the Nobile dendrobium as a potted orchid. Hortscience, 43, 328-332.
|
||
Björkman E., 1960. Monotropa hypopitys L.- an epiparasite on tree roots. Physiol. Plantarum, 13, 308-327.
DOI:10.1111/ppl.1960.13.issue-2 |
||
Blanchard M.G., 1993. Effects of Temperature on Growth and Flowering of Two Phalaenopsis and Two Odontioda Orchid Hybrids. Master of Science thesis. Michigan State University, East Lansing, MI, USA. |
||
Cai J., Liu X., Vanneste K., et al., 2014. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet, 47, 65-72.
|
||
Caldwell M.M., Pearcy R.W., 1994. Exploitation of Environmental Heterogeneity by Plants. Academic Press, San Diego. |
||
Cameron D.D., Leake J.R., Read D.J., 2006. Mutualistic mycorrhiza in orchids:evidence from plantefungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytol, 171, 405-416.
DOI:10.1111/nph.2006.171.issue-2 |
||
Cameron D.D., Preiss K., Gebauer G., et al., 2009. The chlorophyll-containing orchid Corallorhiza trifida derives little carbon through photosynthesis. New Phytol, 183, 358-364.
DOI:10.1111/j.1469-8137.2009.02853.x |
||
Carriqui M., Cabrera M., Conesa M.A., et al., 2015. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ, 38, 448-460.
DOI:10.1111/pce.2015.38.issue-3 |
||
Chang W., Zhang S.B., Li S.Y., et al., 2011. Ecophysiological significance of leaf traits in Cypripedium and Paphiopedilum. Physiol. Plantarum, 141, 30-39.
DOI:10.1111/ppl.2011.141.issue-1 |
||
Chase M.W., 2005. Classification of Orchidaceae in the age of DNA data. Curtis's Bot. Mag, 22, 2-7.
DOI:10.1111/curt.2005.22.issue-1 |
||
Chen S.C., Luo Y.B., 2003. Advances in some plant groups in China 1. A retrospect and prospect of orchidology in China. Acta Bot. Sin, 45(Suppl. l), 2-20.
|
||
Chen W.S., Liu H.Y., Liu Z.H., et al., 1994. Gibberellin and temperature influence carbohydrate content and flowering in Phalaenopsis. Physiol. Plantarum, 90, 391-395.
DOI:10.1111/ppl.1994.90.issue-2 |
||
Christenhusz M.J.M., Byng J.W., 2016. The number of known plant species in the world and its annual increase. Phytotaxa, 261, 201-217.
DOI:10.11646/phytotaxa.261.3 |
||
Colmer T.D., Bloom A.J., 1998. A comparison of NH4+ and NO3- net fluxes along roots of rice and maize. Plant Cell Environ, 21, 240-246.
DOI:10.1046/j.1365-3040.1998.00261.x |
||
Cribb P., Butterfield I., 1999. The Genus Pleione. Royal Botanic Gardens, Kew. |
||
Dearnaley J.D., Cameron D.D., 2017. Nitrogen transport in the orchid mycorrhizal symbiosis-further evidence for a mutualistic association. New Phytol, 213, 10-12.
DOI:10.1111/nph.14357 |
||
Díaz-Álvarez E.A., Lindig-Cisneros R., de la Barrera E., 2015. Responses to simu-lated nitrogen deposition by the neotropical epiphytic orchid Laelia speciosa. Peer J, 3, e1021.
DOI:10.7717/peerj.1021 |
||
Díaz-Álvarez E.A., Reyes-García C., de la Barrera E., 2016. A δ15N assessment of nitrogen deposition for the endangered epiphytic orchid Laelia speciosa from a city and an oak forest in Mexico. J. Plant Res, 129, 863-872.
DOI:10.1007/s10265-016-0843-y |
||
Dycus A.M., Knudson L., 1957. The role of the velamen of the aerial roots of orchids. Bot. Gaz, 119, 78-87.
DOI:10.1086/335966 |
||
Edwards G.E., Walker D.A., 1983. C3, C4:Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis. Blackwell Scientific Publication, London. |
||
Fay M.F., Chase M.W., 2009. Orchid biology:from Linnaeus via Darwin to the 21st century. Ann. Bot, 104, 359-364.
DOI:10.1093/aob/mcp190 |
||
Gebauer G., Meyer M., 2003. 15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol, 160, 209-223.
DOI:10.1046/j.1469-8137.2003.00872.x |
||
Givnish T.J., Spalink D., Ames M., et al., 2015. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proc. R. Soc. B, 282, 1814.
|
||
Gonneau C., Jersakova J., de Tredern E., et al., 2014. Photosynthesis in perennial mixotrophic Epipactis spp. (Orchidaceae) contributes more to shoot and fruit biomass than to hypogeous survival. J. Ecol, 102, 1183-1194.
DOI:10.1111/jec.2014.102.issue-5 |
||
Grassi G., Magnani F., 2005. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ, 28, 834-849.
DOI:10.1111/pce.2005.28.issue-7 |
||
Gravendeel B., Smithson A., Slik F.J.W., et al., 2004. Epiphytism and pollinator specialization:drivers for orchid diversity. Philos. Trans. R. Soc. B, 359, 1523-1535.
DOI:10.1098/rstb.2004.1529 |
||
Guan Z.J., Zhang S.B., Guan K.Y., et al., 2011. Leaf anatomical structures of Paphiopedilum and Cypripedium and their adaptive significance. J. Plant Res, 124, 289-298.
DOI:10.1007/s10265-010-0372-z |
||
He, J., Norhafis, H., Qin, L., 2013. Responses of green leaves and green pseudobulbs of CAM orchid Cattleya laeliocattleya Aloha Case to drought stress. J. Bot. Article ID 710539.
|
||
Hew C.S., Lim L.Y., Low C.M., 1993. Nitrogen uptake by tropical orchids. Environ. Exp. Bot, 33, 273-281.
DOI:10.1016/0098-8472(93)90073-O |
||
Hew C.S., Koh K.T., Khoo G.H., 1998. Pattern of photoassimilate portioning in pseudobulbous and rhizomatous terrestrial orchids. Environ. Exp. Bot, 40, 93-104.
DOI:10.1016/S0098-8472(98)00024-0 |
||
Hew C.S., Yong J.W.H., 2004. The Physiology of Tropical Orchids in Relation to the Industry. World Scientific Publishing, Singapore. |
||
Hock B., 2012. Fungal Associations. Springer, Berlin, Heidelberg. |
||
Hocking C.G., Anderson J.W., 1986. Survey of pyruvate, phosphate dikinase activity of plants in relation to the C3, C4 and CAM mechanisms of CO2 assimilation. Phytochemistry, 25, 1537-1543.
DOI:10.1016/S0031-9422(00)81205-4 |
||
Huang J.L., Hu H., 2001. Seed germination requirements of Cypripedium flavum in axenic culture. Acta Bot. Yunnanica, 23, 105-108.
|
||
Huang W., Zhang S.B., Hu H., 2015. Photorespiration plays an important role in the regulation of photosynthetic electron flow under fluctuating light in tobacco plants grown under full sunlight. Front. Plant Sci, 6, 621.
|
||
Huang W., Yang Y.J., Hu H., et al., 2016. Evidence for the role of cyclic electron flow in photoprotection for oxygen-evolving complex. J. Plant Physiol, 194, 54-60.
DOI:10.1016/j.jplph.2016.02.016 |
||
Huang W., Quan X., Zhang S.B., et al., 2018. In vivo regulation of proton motive force during photosynthetic induction. Environ. Exp. Bot, 148, 109-116.
DOI:10.1016/j.envexpbot.2018.01.001 |
||
Hurskainen S., Jäkäläniemi A., Ramula S., et al., 2017. Tree removal as a management strategy for the lady's slipper orchid, a flagship species for herb-rich forest conservation. For. Ecol. Manag, 406, 12-18.
DOI:10.1016/j.foreco.2017.09.056 |
||
Iersel M.W.V., 2003. Short-term temperature change affects the carbon exchange characteristics and growth of four bedding plant species. J. Am. Soc. Hortic. Sci, 128, 100-106.
|
||
Ishii H., Ohsugi Y., 2011. Light acclimation potential and carry-over effects vary among three evergreen tree species with contrasting patterns of leaf emergence and maturation. Tree Physiol, 31, 819-830.
DOI:10.1093/treephys/tpr079 |
||
Islam M.O., Islam O.M., Matsui S., et al., 1999. Effects of light qualify on seed germination and seedling growth of Cattleya orchids in vitro. J. Jpn. Soc. Hortic. Sci, 68, 1132-1138.
DOI:10.2503/jjshs.68.1132 |
||
Jones H.G., 1992. Plants and Microclimate. Cambridge University Press, New York. |
||
Júnior J.M., Rodrigues M., De Castro E.M., et al., 2013. Changes in anatomy and chlorophyll synthesis in orchids propagated in vitro in the presence of urea. Acta Sci. Agron, 35, 65-72.
|
||
Kako S., Oono H., Sakakibara K., 1976. Studies on growth and flowering of Cymbidium., 5. Effects of temperature on growth of vegetative shoots and flower buds differentiation. Abstr. Jpn. Soc. Hort. Sci. Autumn Meet, 236-237.
|
||
Kanazawa A., Ostendorf E., Kohzuma K., 2017. Chloroplast ATP synthase modulation of the thylakoid proton motive force:implications for photosystem Ⅰ and photosystem Ⅱ photoprotection. Front. Plant Sci, 8, 719.
DOI:10.3389/fpls.2017.00719 |
||
Kerbauy, G. B., Takahashi, C. A., Lopez, A. M., et al., 2012. Crassulacean acid metabolism in epiphytic orchids: current knowledge, future perspectives. In: Najafpour, M. M. (Ed. ), Applied Photosynthesis. InTech, Rijeka.
|
||
Kim Y.J., Lee H.J., Kim K.S., 2011. Night interruption promotes vegetative growth and flowering of Cymbidium. Sci. Hortic, 130, 887-893.
DOI:10.1016/j.scienta.2011.08.031 |
||
Kim Y.J., Yu D.J., Rho H., et al., 2015. Photosynthetic changes in Cymbidium orchids grown under different intensities of night interruption lighting. Sci. Hortic, 186, 124-128.
DOI:10.1016/j.scienta.2015.01.036 |
||
Kottke I., Suarez J.P., Herrera P., et al., 2010. Atractiellomycetes belonging to the 'rust' lineage (Pucciniomycotina) form mycorrhizae with terrestrial and epiphytic neotropical orchids. Proc. R. Soc. B, 277, 1289-1298.
DOI:10.1098/rspb.2009.1884 |
||
Kuang M.L., Zhang S.B., 2015. Physiological response to high light in Cymbidium tracyanum and C. sinense. Plant Divers. Res, 37, 55-62.
|
||
Kull T., 1999. Cypripedium calceolus L. J. Ecol, 87, 913-924.
DOI:10.1046/j.1365-2745.1999.00407.x |
||
Law R.D., Crafts-Brandner S.J., Salvucci M.E., 2001. Heat stress induces the synthesis of a new form of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase in cotton leaves. Planta, 214, 117-125.
DOI:10.1007/s004250100592 |
||
Lee H.B., An S.K., Lee S.Y., et al., 2017. Vegetative growth characteristics of Phalaenopsis and Doritaenopsis plants under different artificial lighting sources. Hortic. Sci. Technol, 35, 21-29.
|
||
Lee N., Lin G.M., 1984. Effect of temperature on growth and flowering of Phalaenopsis white hybrid. J. Chin. Soc. Hortic. Sci, 30, 223-231.
|
||
Li J.W., Zhang S.B., 2016. Differences in the responses of photosystems Ⅰ and Ⅱ in Cymbidium sinense and C. tracyanum to long-term chilling stress. Front. Plant Sci, 6, 1097.
|
||
Li J.W., Chen X.D., Hu X.Y., et al., 2018. Comparative physiological and proteomic analyses reveal different adaptive strategies by Cymbidium sinense and C. tracyanum to drought. Planta, 247, 69-91.
DOI:10.1007/s00425-017-2768-7 |
||
Liu Q., Chen J., Corlett R.T., et al., 2015. Orchid conservation in the biodiversity hotspot of southwestern China. Conserv. Biol, 29, 1563-1572.
DOI:10.1111/cobi.12584 |
||
Lopez R.G., Runkle E.S., 2005. Environmental physiology of growth and flowering of orchids. Hortscience, 40, 1969-1973.
|
||
López-Bucio J., Cruz-Ramírez A., Herrera-Estrella L., 2003. The role of nutrient., availability in regulating root architecture. Curr. Opin. Plant Biol, 6, 280-287.
|
||
Lubinsky P., Bory S., Hernandez J.H., et al., 2008. Origins and dispersal of cultivated vanilla (Vanilla planifolia Jacks. [Orchidaceae]). Econ. Bot, 62, 127-138.
DOI:10.1007/s12231-008-9014-y |
||
Luo Y.B., Jia J.S., Wang C.L., 2003. A general review of the conservation status of Chinese orchids. Biodivers. Sci, 11, 70-77.
|
||
Luttge U., 1989. Vascular Plants as Epiphytes:Evolution and Ecophysiology. Springer, Heidelberg. |
||
Martos F., Dulormne M., Pailler T., et al., 2009. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol, 184, 668-681.
DOI:10.1111/nph.2009.184.issue-3 |
||
Mayor J.R., Schuur E.A.G., Henkel T.W., 2009. Elucidating the nutritional dynamics of fungi using stable isotopes. Ecol. Lett, 12, 171-183.
DOI:10.1111/ele.2009.12.issue-2 |
||
McKendrick S.L., Leake J.R., Read D.J., 2000. Symbiotic germination and development of myco-heterotrophic plants in nature:transfer of carbon from ectomycorrhizal Salix repens and Betula pendula to the orchid Corallorhiza trifida through shared hyphal connections. New Phytol, 145, 539-548.
DOI:10.1046/j.1469-8137.2000.00592.x |
||
Merckx V.S., 2013. Mycoheterotrophy:the Biology of Plants Living on Fungi. Springer, New York. |
||
Mohammad B.A., Hahn E.J., Paek K.Y., 2005. Effects of temperature on oxidative stress defense systems, lipid peroxidation and lipoxygenase activity in Phalaenopsis. Plant Physiol. Biochem, 43, 213-223.
DOI:10.1016/j.plaphy.2005.01.007 |
||
Motomura H., Ueno O., Kagawa A., et al., 2008. Carbon isotope ratios and the variation in the diurnal pattern of malate accumulation in aerial roots of CAM species of Phalaenopsis (Orchidaceae). Photosynthetica, 46, 531-536.
DOI:10.1007/s11099-008-0090-0 |
||
Mou Z.M., Yan N., Li S.Y., et al., 2012. Nitrogen requirements for vegetative growth, flowering, seed production, and ramet growth of Paphiopedilum armeniacum(Orchid). Hortscience, 47, 585-588.
|
||
Ng C.K.Y., Hew C.S., 2000. Orchid pseudobulbs-'false' bulbs with a genuine importance in orchid growth and survival! Sci. Hortic, 83, 165-172.
DOI:10.1016/S0304-4238(99)00084-9 |
||
Pan R.C., Chen J.X., 1994. Effects of nitrate-nitrogen and ammonium-nitrogen on growth and development in Cymbidium sinense. Acta Bot. Yunnanica, 16, 285-290.
|
||
Pires M.V., de Almeida A.F., Abreu P.P., et al., 2012. Does shading explain variation in morphophysiological traits of tropical epiphytic orchids grown in artificial conditions? Acta Physiol. Plant, 34, 2155-2164.
|
||
Preiss K., Adam I.K.U., Gebauer G., 2010. Irradiance governs exploitation of fungi:fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proc. R. Soc. B, 277, 1333-1336.
DOI:10.1098/rspb.2009.1966 |
||
Pridgeon A.M., Stern W.L., 1982. Vegetative anatomy of Myoxanthus (Orchidaceae). Selbyana, 7, 55-63.
|
||
Ramírez S.R., Gravendeel B., Singer R.B., et al., 2007. Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature, 448, 1042-1045.
DOI:10.1038/nature06039 |
||
Rasmussen H.N., Dixon K.W., Jersakova J., et al., 2015. Germination and seedling establishment in orchids:a complex of requirements. Ann. Bot, 116, 391-402.
DOI:10.1093/aob/mcv087 |
||
Reich A., Ewel J.J., Nadkarni N.M., et al., 2003. Nitrogen isotope ratios shift with plant size in tropical bromeliads. Oecologia, 137, 587-590.
DOI:10.1007/s00442-003-1386-1 |
||
Rodrigues M.A., Matiz A., Cruz A.B., et al., 2013. Spatial patterns of photosynthesis in thin-and thick-leaved epiphytic orchids:unravelling C3-CAM plasticity in an organ-compartmented way. Ann. Bot, 112, 17-29.
DOI:10.1093/aob/mct090 |
||
Rosa-Manzano E., Andrade J.L., García-Mendoza E., et al., 2015. Photoprotection related to xanthophyll cycle pigments in epiphytic orchids acclimated at different light microenvironments in two tropical dry forests of the Yucatan Peninsula, Mexico. Planta, 242, 1425-1438.
DOI:10.1007/s00425-015-2383-4 |
||
Rosa-Manzano E., Andrade J.L., Zotz G., et al., 2017. Physiological plasticity of epiphytic orchids from two contrasting tropical dry forests. Acta Oecol, 85, 25-32.
DOI:10.1016/j.actao.2017.09.006 |
||
Sailo N., Rai D., De L.C., 2014. Physiology of temperate and tropical orchids-an overview. Int. J. Sci. Res, 3, 3-7.
|
||
Sanford W.W., Adanlawo I., 1973. Velamen and exodermis characters of West African epiphytic orchids in relation to taxonomic grouping and habitat tolerance. Bot. J. Linn. Soc, 66, 307-321.
DOI:10.1111/boj.1973.66.issue-4 |
||
Schmidt G., Zotz G., 2002. Inherently slow growth in two Caribbean epiphytic species:a demographic approach. J. Veg. Sci, 13, 527-534.
DOI:10.1111/j.1654-1103.2002.tb02079.x |
||
Selosse M.A., Faccio A., Scappaticci G., et al., 2004. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb. Ecol, 47, 416-426.
|
||
Selosse M.A., Richard F., He X.H., et al., 2006. Mycorrhizal networks:des liaisons dangereuses? Trends Ecol. Evol, 21, 621-628.
|
||
Sheehan, T. J., 1983. Recent advances in botany, propagation, and physiology of orchids. In: Janick, J. (Ed. ), Horticultural Reviews, vol. 5. John Wiley & Sons, Inc., Hoboken.
|
||
Sheehan, T. J., McConnell, D. B., 1980. Mesophyll cell collapse of Phalaenopsis Bl. In: Proc. 9th World Orchid Conf., Bangkok, Thailand.
|
||
Shefferson R.P., 2006. Survival costs of adult dormancy and the confounding influence of size in lady's slipper orchids, genus Cypripedium. Oikos, 115, 253-262.
DOI:10.1111/oik.2006.115.issue-2 |
||
Silvera K., Santiago L.S., Winter K., 2005. Distribution of crassulacean acid metabolism in orchids of Panama:evidence of selection for weak and strong modes. Funct. Plant Biol, 32, 397-407.
DOI:10.1071/FP04179 |
||
Silvera K., Santiago L.S., Cushman J.C., et al., 2009. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiol, 149, 1838-1847.
DOI:10.1104/pp.108.132555 |
||
Sinclair R., 1983. Water relations of tropical epiphytes Ⅱ. Performance during droughting. J. Exp. Bot, 34, 1664-1675.
DOI:10.1093/jxb/34.12.1664 |
||
Sinoda K., Hara M., Aoki M., 1984. Growth and flowering control in Dendrobium. 4. Cold treatments and flowering. Abstr. Jpn. Soc. Hort. Sci. Spring Meet, 364-365.
|
||
Smith S.E., Read D.J., 1997. Mycorrhizal Symbiosis. Academic Press, San Diego. |
||
Su W.R., Chen W.S., Koshioka M., et al., 2001. Changes in gibberellin levels in the flowering shoot of Phalaenopsis hybrida under high temperature conditions when flower development is blocked. Plant Physiol. Biochem, 39, 45-50.
DOI:10.1016/S0981-9428(00)01218-3 |
||
Terashima I., Hanba Y.T., Tholen D., et al., 2011. Leaf functional anatomy in relation to photosynthesis. Plant Physiol, 155, 108-116.
DOI:10.1104/pp.110.165472 |
||
Tikkanen M., Aro E.M., 2014. Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci, 19, 10-17.
DOI:10.1016/j.tplants.2013.09.003 |
||
Tikkanen M., Mekala N.R., Aro E.M., 2014. Photosystem Ⅱ photoinhibition-repair cycle protects photosystem Ⅰ from irreversible damage. Biochim. Biophys. Acta, 1837, 210-215.
DOI:10.1016/j.bbabio.2013.10.001 |
||
Treseder K.K., Davidson D.W., Ehleringer J.R., 1995. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature, 375, 137-139.
DOI:10.1038/375137a0 |
||
Tsaia C.F., Huang C.L., Lind Y.L., et al., 2011. The neuroprotective effects of an extract of Gastrodia elata. J. Ethnopharmacol, 138, 119-125.
DOI:10.1016/j.jep.2011.08.064 |
||
Ventre-Lespiaucq A.B., Delgado J.A., Ospina-Calderón N.H., et al., 2017. A tropical epiphytic orchid uses a low-light interception strategy in a spatially heterogeneous light environment. Biotropica, 49, 318-327.
DOI:10.1111/btp.2017.49.issue-3 |
||
Wanek W., Zotz G., 2011. Are vascular epiphytes nitrogen or phosphorus limited? A study of plant 15N fractionation and foliar N:P stoichiometry with the tank bromeliad Vriesea sanguinolenta. New Phytol, 192, 462-470.
DOI:10.1111/nph.2011.192.issue-2 |
||
Wang Y., Ma Y., Dai S., 2010. The molecular mechanism in regulation of flowering in ornamental plants. Chin. Bull. Bot, 45, 641-653.
|
||
Wang Y.T., 1995. Phalaenopsis orchid light requirement during the induction of spiking. Hortscience, 30, 59-61.
|
||
Wang Y.T., Chang Y.C.A., 2017. Effects of nitrogen and the various forms of nitrogen on Phalaenopsis orchid-a review. HortTechnology, 27, 44-149.
|
||
Waterman R.J., Bidartondo M.I., 2008. Deception above, deception below:linking pollination and mycorrhizal biology of orchids. J. Exp. Bot, 59, 1085-1096.
DOI:10.1093/jxb/erm366 |
||
Weng E.S., Hu H., Li S.Y., et al., 2002. Differentiation of flower bud of Cypripedium flavum. Acta Bot. Yunnanica, 24, 222-228.
|
||
Werner F.A., Gradstein S.R., 2008. Seedling establishment of vascular epiphytes on isolated and enclosed forest trees in an Andean landscape, Ecuador. Biodivers. Conserv, 17, 3195-3207.
DOI:10.1007/s10531-008-9421-5 |
||
Winter K., Wallace B.J., Stocker G.C., et al., 1983. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia, 57, 129-141.
DOI:10.1007/BF00379570 |
||
Yang S.J., Sun M., Yang Q.Y., et al., 2016. Two strategies by epiphytic orchids for maintaining water balance:thick cuticles in leaves and water storage in pseudobulbs. AoB Plants, 8, plw046.
DOI:10.1093/aobpla/plw046 |
||
Yang Y.J., Chang W., Huang W., et al., 2017. The effects of chilling-light stress on photosystems Ⅰ and Ⅱ in three Paphiopedilum species. Bot. Stud, 58, 53.
DOI:10.1186/s40529-017-0208-4 |
||
Yang Z.H., Huang W., Yang Q.Y., et al., 2018. Anatomical and diffusional determinants inside leaves explain the difference in photosynthetic capacity between Cypripedium and Paphiopedilum, Orchidaceae. Photosynth. Res, 136, 315-328.
DOI:10.1007/s11120-017-0466-8 |
||
Zhang F.P., Zhang J.J., Yan Y., et al., 2015a. Variations in seed micromorphology of Paphiopedilum and Cypripedium (Cypripedioideae, Orchidaceae). Seed Sci. Res, 25, 395-401.
DOI:10.1017/S0960258515000276 |
||
Zhang F.P., Huang J.L., Zhang S.B., 2016. Trait evolution in the slipper orchid Paphiopedilum (Orchidaceae) in China. Plant Signal. Behav, 11, e1149668.
DOI:10.1080/15592324.2016.1149668 |
||
Zhang S.B., Hu H., Zhou Z.K., et al., 2005. Photosynthetic performances of transplanted Cypripedium flavum plants. Bot. Bull. Acad. Sin, 46, 307-313.
|
||
Zhang S.B., Hu H., Xu K., et al., 2007. Flexible and reversible responses to different irradiance levels during photosynthetic acclimation of Cypripedium guttatum. J. Plant Physiol, 164, 611-620.
DOI:10.1016/j.jplph.2006.02.012 |
||
Zhang S.B., Guan Z.J., Chang W., et al., 2011. Slow photosynthetic induction and low photosynthesis in Paphiopedilum armeniacum are related to its lack of guard cell chloroplast and peculiar stomatal anatomy. Physiol. Plantarum, 142, 118-127.
DOI:10.1111/ppl.2011.142.issue-2 |
||
Zhang S.B., Dai Y., Hao G.Y., et al., 2015b. Differentiation of water-related traits in terrestrial and epiphytic Cymbidium species. Front. Plant Sci, 6, 260.
|
||
Zhang S.B., Chen W.Y., Huang J.L., et al., 2015c. Orchid species richness along elevational and environmental gradients in Yunnan, China. PLoS One, 10, e0142621.
DOI:10.1371/journal.pone.0142621 |
||
Zhang W., Huang W., Zhang S.B., 2017. The study of a determinate growth orchid highlights the role of new leaf production in photosynthetic light acclimation. Plant Ecol, 218, 997-1008.
DOI:10.1007/s11258-017-0747-5 |
||
Zhang Z.J., He D.X., Niu G.H., et al., 2014. Concomitant CAM and C3 photosynthetic pathways in Dendrobium officinale plants. J. Am. Soc. Hortic. Sci, 139, 290-298.
|
||
Zimmer K., Hynson N.A., Gebauer G., et al., 2007. Wide geographical and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. New Phytol, 175, 166-175.
DOI:10.1111/nph.2007.175.issue-1 |
||
Zotz G., 1999. What are backshoots good for? Seasonal changes in mineral, carbohydrate and water content of different organs of the epiphytic orchid, Dimerandra emarginata. Ann. Bot, 84, 791-798.
DOI:10.1006/anbo.1999.0983 |
||
Zotz G., Hietz P., 2001. The physiological ecology of vascular epiphytes:current knowledge, open questions. J. Exp. Bot, 52, 2067-2078.
DOI:10.1093/jexbot/52.364.2067 |
||
Zotz G., Schultz S., 2008. The vascular epiphytes of a lowland forest in Panamaspecies composition and spatial structure. Plant Ecol, 195, 131-141.
DOI:10.1007/s11258-007-9310-0 |
||
Zotz G., Winkler U., 2013. Aerial roots of epiphytic orchids:the velamen radicum and its role in water and nutrient uptake. Oecologia, 171, 733-741.
DOI:10.1007/s00442-012-2575-6 |



