It has been ~400 million years since plants colonized land and the coevolution of plants and insects has last 350 million years (Gatehouse, 2002; Rodriguez and Redman, 2008). Roughly half of all insect species, the most speciose group of animals on the planet, are herbivores. These insect herbivores can be classified by range of host plant species (i.e., specialists or generalists) and mode of feeding (i.e., chewing or piercing-sucking). Specialist herbivores feed on restricted sets of related plant species, whereas generalists feed on relatively wide ranges of unrelated plant species (Lankau, 2007). For instance, the fall armyworm (Spodoptera frugiperda) is a chewing generalist that feeds on maize and rice (Burtet et al., 2017; Ray et al., 2016), whereas the rice brown planthopper (BPH, Nilaparvata lugens) is a piercing-sucking specialist commonly found on rice plants (Cheng et al., 2013).
To survive, plants have equipped themselves with physical and chemical weapons. According to whether the defense is inducible or not, plant defenses are categorized into constitutive or induced defenses. Constitutive defenses consist of physical barriers, such as cuticles and thorns, as well as chemical defensive metabolites whose concentrations do not generally change. Induced defenses are conferred by secondary metabolites which accumulate upon herbivore infestation and serve as powerful chemical weapons against insects (Wu and Baldwin, 2010). Induced defenses are initiated by perception of wounding, elicitors derived from insect feeding, or oviposition, and followed by the activation of a complex regulatory network which mediates the biosynthesis of secondary metabolites, which are toxic, antidigestive, or repellent to herbivores. Because induced defense is a sophisticated system that controls the balance between defense and growth, it has received much attention. For instance, the defensive functions of trypsin protein inhibitors (TPIs) and 17-hydroxygeranyllinalool diterpene glycosides have been demonstrated in the wild tobacco Nicotiana attenuata, a plant in which both compounds are highly induced by the lepidopteran insect Manduca sexta (Ethinh et al., 2012; Heiling et al., 2010; Wang and Wu, 2013). Glucosinolates (GSs) are potent chemical weapons produced in crucifers, such as Arabidopsis thaliana, and their concentrations increase after caterpillar Spodoptera littoralis feeding (Hopkins et al., 2009; Schweizer et al., 2013). How dicotyledons defend against herbivores is relatively well understood, especially in Arabidopsis and tobacco, and has been reviewed elsewhere (Stam et al., 2013; Wang and Wu, 2013; Wu and Baldwin, 2010). In comparison, however, little is known about the defense responses of monocotyledons against insect herbivores.
Maize (Zea mays) and rice (Oryza sativa), two staple crop plants, provide food for most of the world. In recent years, substantial progress has been made in understanding the defense responses of maize and rice against insects. Here we review how two model monocotyledonous species, rice and maize, perceive herbivory, and how plant defense in these species is regulated by R geness and phytohormone signaling, including jasmonic acid (JA), salicylic acid (SA), and ethylene. Previous studies on defense-related secondary metabolites in rice and maize are also summarized.
2. Insect-derived elicitors recognized by rice and maize upon herbivory and oviposition 2.1. Elicitors from lepidopteran larvaeDefense is costly. Consequently, plants need to distinguish herbivore feeding from mechanical wounding. Various plant species, including rice and maize, are able to recognize elicitors derived from herbivore oral secretions (OS) or oviposition fluids (Wu and Baldwin, 2010). Among the elicitors recognized by rice and maize, the best characterized are fatty acid-amino acid conjugates (FACs). OS from a generalist caterpillar Mythimna separata feeding on maize contain more than ten kinds of FACs, the most abundant of which is the hydroxylated FAC volicitin (Qi et al., 2016b). Applying M. separata OS to maize wounds induces elevated levels of the hormones JA and its derivative JA-Ile (JA-isoleucine conjugate, which acts as the real jasmonate signal to elicit JA responses) (Qi et al., 2016b). Applying synthetic volicitin to maize induces volatiles that function as an indirect defense, attracting the predators of insects (Alborn et al., 1997). In addition to volicitin, maize is able to perceive several other insect-derived elicitors, including caeliferin and inceptin, although different maize lines show variations in their responses to these elicitors (Schmelz et al., 2009). Rice can also recognize elicitors from the OS of various herbivores such as Spodoptera mauritia, Mythimna loreyi, and Parnara guttata and in response accumulates higher levels of JA/JA-Ile and/or defenserelated secondary metabolites compared with wounding alone (Fukumoto et al., 2013; Shinya et al., 2016). Some generalist insects, such as S. frugiperda, feed on various dicots as well as monocots, including rice and maize. It would be interesting to study the common and specific components of the elicitors in the OS of specialist and generalist, and more importantly, how they are specifically perceived.
2.2. Elicitors from piercing-sucking herbivoresCurrently, little is known about the interactions between piercing-sucking insects and monocots. Brown planthopper (BPH, N. lugens) and maize leaf aphid (Rhopalosiphum maidis) obtain nutrients by using their stylets to suck phloem sap from rice and maize, respectively. Soon after punching their stylets into plant tissues, BPHs and aphids secrete gelling saliva, which quickly solidifies, protecting the stylets and preventing phloem sieve elements from clogging (Shangguan et al., 2018; Walling, 2000). Recent studies have shown that BPH saliva contains a mucin-like protein (NlMLP), the C-terminus of which is recognized by rice plants and induces cell death, callose deposition, and defense responses related to the JA signaling pathway (Shangguan et al., 2018). Although aphid feeding does not inflict much wounding, strong transcriptome changes have been identified in different maize lines (Betsiashvili et al., 2015; Meihls et al., 2013; Song et al., 2017). These findings suggest that elicitors from piercing-sucking insects can be perceived by the host plants and induce defenserelated responses.
Some proteins in the saliva of piercing-sucking insects function as suppressors of host defense. A salivary endo-β-1, 4-glucanase (NlEG1) from BPH has endoglucanase activity and actively degrades cellulose in rice (Ji et al., 2017).
2.3. Elicitors from ovipositionBoth female white-backed planthopper (Sogatella furcifera) and BPH lay eggs inside rice tissues. Rice plants recognize signals (perhaps lipids) on the surface of white-backed planthopper eggs and thereafter accumulate benzyl benzoate, which kills the eggs (Yang et al., 2013). BPH infestation of rice by female adults rather than nymphs induces the expression of OsJMT1, a gene that encodes a jasmonate methyl transferase which converts JA to volatile jasmonate. This difference in OsJMT1 induction may be caused by female BPH oviposition. If so, this suggests that the defense responses of rice plants are activated after the perception of certain molecules on BPH eggs (Qi et al., 2016a). Compared with eggs laid inside rice tissues (S. furcifera and BPH), eggs laid on the leaf surface are also recognized by maize to trigger defense. In some maize landraces but not commercial cultivars, oviposition by the moths of stemborer Chilo partellus induces release of volatiles that not only attract egg parasitic wasps (Trichogramma bournieri) but also attract larval parasitoids (Cotesia sesamiae), suggesting that some maize landraces can anticipate the hatching of herbivore eggs (Tamiru et al., 2011).
3. Signaling regulation of herbivore resistance in rice and maizeThe mitogen-activated protein kinase (MAPK) cascade is a conserved eukaryotic signaling pathway that regulates numerous cellular responses. Activation of the MAPK cascade is one of the earliest signaling events in response to herbivore infestation (Fig. 1A) (Chang and Karin, 2001; Hettenhausen et al., 2015). In tomato and N. attenuata, MAPKs have been found to regulate early signaling of herbivore resistance by controlling the JA and SA biosynthesis (Kandoth et al., 2007; Wu et al., 2007). Similarly, rice OsMPK3 has been found to positively regulate the defense response against rice striped stemborer (SSB, Chilo suppressalis) by modulating JA biosynthesis (Wang et al., 2013). Whether MAPKs are involved in maize defense against insects remains unknown.
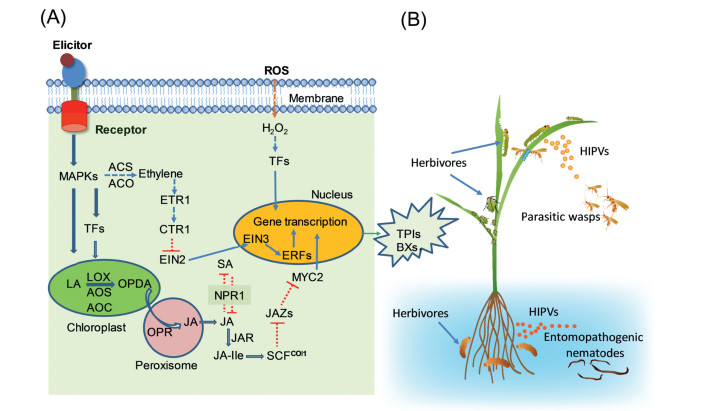
|
| Fig. 1 A working model of defense responses in an herbivore-attacked plant. (A) Herbivore-derived elicitors are perceived by unidentified receptors on the plasma membranes, triggering rapid activation of MAPKs followed by biosynthesis of phytohormones, JA, JA-Ile, and ethylene. After several steps of signaling transduction, transcription factors (MYC2 and ERFs, for instance) regulate the accumulation of non-volatile secondary metabolites (such as TPIs in rice and BXs in maize), which function as direct defenses against herbivores. (B) Herbivory-induced plant volatiles recruit natural enemies above ground (such as parasitic wasps) and below ground (entomopathogenic nematodes, for instance) to indirectly defend plants against herbivores. Solid lines indicate reported pathways in monocots, dashed lines indicate unconfirmed pathways inferred from findings in dicots. |
JA biosynthesis starts from chloroplastic membrane-released ɑlinolenic acid, which is further catalyzed by LIPOXYGENASE (LOX), ALLENE OXIDE SYNTHASE (AOS), and ALLENE OXIDE CYCLASE (AOC) to form 12-oxo-phytodienoic acid (OPDA) in the chloroplast. OPDA is then transported into the peroxisome and further transformed into JA by 12-OXOPHYTODIENOATE REDUCTASE (OPR) and three rounds of β-oxidation. After being released into the cytosol, JA is conjugated with the amino acid isoleucine (Ile) to generate JA-Ile under the catalyzation by JASMONATE RESISTANT (JAR) (Fig. 1A) (Zhang et al., 2017b). In dicotyledonous plants, the central role of the JA signaling pathway in plant defense against herbivores, especially lepidopteran caterpillars, has been well documented (Browse and Howe, 2008; Wu and Baldwin, 2009). However, the function of the JA pathway in monocots still requires much research. SSB feeding elevates JA levels in rice, and silencing the JA pathway reduces both SSB herbivory-induced TPI activity and SSB resistance (Zhou et al., 2009). Similarly, transgenic rice plants expressing the antisense of OsPLDa4/5 (encoding enzymes for phospholipases which supply substrate for JA after two reaction steps) have shown reduced JA levels and decreased resistance to SSB (Qi et al., 2011). Rice Oshpl3 mutants carry a mutation in the hydroperoxide lyase (HPL) gene, which functions in the production of green leaf volatiles (GLVs). This mutant exhibits decreased emission of GLVs due to its impaired capacity to catabolize hydroperoxylinoleic acid, which is a common substrate for JA and GLV biosynthesis. However, Oshpl3 mutants have been shown to have elevated JA, probably because of increased hydroperoxylinoleic acid contents, and increased resistance to SSB (Tong et al., 2012). Transgenic rice plants with reduced JA levels produce smaller amounts of SSB-induced volatiles and thus attract fewer larval parasitoids (Apanteles chilonis) (Qi et al., 2011). JA signaling also positively regulates rice resistance to belowground herbivores: When the generalist cucumber beetle (Diabrotica balteata) and the more specialized rice water weevil (Lissorhoptrus oryzophilus), two belowground insects feed on rice roots, root JA levels increased; moreover, application of MeJA (methyl jasmonate) to rice roots reduced the growth of these insects, while the roots of transgenic rice plants impaired in JA biosynthesis were highly damaged (Lu et al., 2015).
In maize, MeJA treatment reduces the performance of Asian corn borer (Ostrinia furnacalis). This may be related to MeJAinduced proteins, including pathogenesis-related protein 1 (PR1) and thioredoxin M-type chloroplastic precursor (TRXM), as the recombinant proteins of PR1 and TRXM have been shown to inhibit the development of Asian corn borer larvae and pupae (Zhang et al., 2015). Maize mutants lacking OPR7/OPR8 have extremely reduced JA contents, and beet armyworms (Spodoptera exigua) consume more tissues on those plants and grow better than do those on wild-type plants (Yan et al., 2012). Similarly, ZmLOX10 is involved in maize JA biosynthesis. Zmlox10 mutants showed strongly reduced wounding-induced JA levels as well as reduced volatile emissions and attractiveness to larval parasitoid wasps (Cotesia marginiventris). Furthermore, S. exigua has been found to grow larger on Zmlox10 mutants than on wild-type maize plants (Christensen et al., 2013). These findings indicate that JA mediates direct and indirect defense responses in maize.
The role of JA signaling in monocot resistance to piercingsucking insects is rather complex. In the wild rice Oryza rufipogon and Oryza nivara carrying the R gene Bph6, BPH feeding increases the levels of JA and JA-Ile, and exogenous application of MeJA results in an increased BPH death rate, probably owing to Bph6induced cell death (Guo et al., 2018). Compared with wild-type rice plants, Oshpl3 mutants emit higher levels of BPH-induced volatiles and are more attractive to the egg parasitoid Anagrus nilaparvatae, probably due to increased JA levels in the mutants (Tong et al., 2012). Similarly, volatile emission and A. nilaparvatae attraction increased after rice was treated with exogenous MeJA (Lou et al., 2005). In addition, Cis-12-oxo-phytodienoic acid (OPDA) has been suggested to have a signaling function, as transgenic rice plants with elevated OPDA, rather than JA, have been shown to be more resistant to BPH (Guo et al., 2014).
The SA pathway has also been implicated in the defense of monocots against insects. SA receptors NPR1 and NPR3/NPR4 play opposite roles in transcriptional regulation during plant resistance to pathogens (Ding et al., 2018). OsNPR1 is mainly localized in cytoplasm and shuttles into the nucleus after redox changes induced by elevated SA levels. Overexpression of OsNPR1 reduces rice defense against white-backed planthoppers (Yuan et al., 2007). Whether NPR3/NPR4 exist in monocots, and what their functions may be, remain unknown. Silencing the JA pathway increases BPH herbivoryinduced SA levels in rice plants, leading to increased ROS (reactive oxygen species) and elevated resistance to BPH (Zhou et al., 2009). Wild rice plants (O. rufipogon and O. nivara) carrying the R gene Bph6 exhibit increased SA levels after BPH feeding, and exogenous application of SA increases rice resistance to BPH (Guo et al., 2018). Furthermore, the application of SA has been found to induce TPI accumulation in rice leaves (Wang et al., 2011). How SA signaling regulates the defense of monocots still needs to be explored.
Several lines of evidence have indicated that ethylene (ETH) is important for plant defense against insects in monocots (Fig. 1A). ETH signaling activation starts from the perception by ETH receptors, including ETR1 (ETHYLENE RESPONSE 1), leading to dephosphorylation of EIN2 (ETHYLENE INSENSITIVE 2) and its cleavage. The C-terminal fragment of EIN2 is translocated into the nucleus and results in stabilization and accumulation of transcription factor EIN3 (ETHYLENE INSENSITIVE 3), and consequently, induces transcription of ERFs (ETHYLENE RESPONSIVE ELEMENT BINDING FACTORS) and other ethylene-responsive genes (Fig. 1A) (Yang et al., 2015). SSB feeding and simulated M. separata herbivory induces ETH production in rice and maize, respectively (Lu et al., 2011; Qi et al., 2011, 2016b), indicating that ETH signaling may regulate herbivore resistance in these plants. In the maize inbred line Mp708, a maize insect resistance1 (mir1) gene was identified that encodes a cysteine (Cys) proteinase, named Mir1-Cys protease, which disrupts the peritrophic matrix of S. frugiperda and thus reduces insect growth (Pechan et al., 2002). Feeding the corn leaf aphid (R. maidis) with an artificial diet containing Mir1-Cys protease has demonstrated that Mir1-Cys protease is toxic to R. maidis (Louis et al., 2015). Blocking ETH synthesis or perception in Mp708 reduces Mir1-Cys protease accumulation, causing maize plants to become more susceptible to S. frugiperda and R. maidis (Harfouche et al., 2006; Louis et al., 2015). In contrast, ethephon (an ETH donor) treatment increases mir1 transcription levels, increasing maize plant resistance to R. maidis (Louis et al., 2015). These findings suggest that the ETH pathway is involved in maize resistance against S. frugiperda and R. maidis, although more mechanistic studies are still needed to understand how ETH regulates maize defenses.
In rice, antisense expression of OsACS2 reduces ETH production, and in these transgenic rice plants, SSB-elicited TPI activity and volatile emission decreased. Reduced ETH levels in these plants also leads to decreased resistance to SSB, whereas applying ACC (L-aminocyclopropane-L-carboxylic acid), the precursor of ETH, restores rice resistance (Lu et al., 2014). In contrast, silencing OsACS2 increases direct and indirect defense against BPH (Lu et al., 2014). Yet little is known about how ETH regulates rice herbivore resistance. Rice silenced for ethylene responsive factor 3 (OsERF3) show compromised resistance to SSB, probably due to decreased JA levels. In contrast, OsERF3-silenced rice is more resistant to BPH, which has been associated with increased H2O2 production mediated by MAPKs (Lu et al., 2011). These findings suggest that ethylene signaling has contrasting and herbivore-specific effects on rice defense against insects.
In addition to JA, SA, and ETH, gibberellins (GAs) have been suggested to regulate herbivore resistance in rice. Rice GA-deficient (sd-1) and -excessive (eui) mutants exhibit reduced and increased resistance to BPH female adults, respectively. Similarly, in transgenic rice plants overexpressing OsWRKY70, GA levels decrease and BPH attraction increases. Exogenous application of GA to these OsWRKY70-overexpressing plants restores BPH resistance (Li et al., 2015a). The rice DELLA gene OsSLR1 also positively regulates BPHinduced levels of JA, ETH, and H2O2 by modulating the transcript levels of OsMPK3 and OsWRKY24/45/53/70 (Zhang et al., 2017a). GA-regulated rice herbivore resistance may be regulated by crosstalk between GA, SA, and ROS, or GA-regulated constitutive secondary cell wall components; however, the mechanisms underlying this regulation still need to be explored.
Many transcription factors located downstream of hormonal signaling pathways play key roles in plant adaptation to stresses (Fig. 1A) (Yang et al., 2016). In rice, OsWRKY70 physically interacts with W-box motifs in its own promoter leading to self-activation. Upon attack by SSB, OsWRKY70 functions in prioritizing defense over growth by positively and negatively regulating JA and GA biosynthesis, respectively. OsWRKY70 is regulated by OsMPK3 and OsMPK6, and further positively modulates the levels of JA, ETH, and TPI activity (Li et al., 2015a). In contrast, OsWRKY53 physically interacts with OsMPK3 and OsMPK6, suppressing their activity. Thus, OsWRKY53 negatively regulates SSB-induced JA, JA-Ile, ETH, and TPI activity as well as rice resistance to SSB (Hu et al., 2015).
Kauralexins are a group of maize diterpenoid phytoalexins that accumulate in response to Ostrinia nubilalis infestation. These compounds also show significant O. nubilalis antifeedant activity (Schmelz et al., 2011). Maize ZmWRKY79 physically binds to the Wboxes or WLE cis-elements in the promoters of terpenoid phytoalexin biosynthetic genes (An2 and ZmTPS6) in maize, regulating the expression of these genes (Fu et al., 2018). An AP2/ERF type transcription factor EREB58 (GRMZM2G381441) has been shown to bind the promoter of TPS10 and mediate jasmonate-induced biosynthesis of sesquiterpenes (Li et al., 2015b). EREB58 is specifically induced by simulated M. separata herbivory, but not by wounding (Qi et al., 2016b).
4. Defensive secondary metabolitesControlled by the signaling network, secondary metabolites are the foremost line of defense against insects. However, little is known about which metabolites have anti-insect functions in monocots.
4.1. VolatilesVolatile blends released from a plant can comprise up to hundreds of components. The major function of volatiles is to attract natural enemies of the herbivores, a phenomenon called indirect defense (Fig. 1B). Some plant volatiles also confer direct defense. The most common volatiles can be classified into three groups: GLVs, aromatic compounds, and terpenes (Dicke, 2009). GLVs include C6 alcohols, aldehydes, and esters that are produced through the lipoxygenase pathway. GLVs are quickly released upon tissue damage or herbivory. In rice, antisense expression of phospholipases D α4 and α5 have been shown to decrease herbivoryelicited GLVs and resistance against BPH and SSB, while application of GLVs complements BPH and SSB resistance, indicating that GLVs regulate rice direct resistance or they are toxic to these insects (Qi et al., 2011). Similarly, Oshpl3 mutants, which are impaired in GLV biosynthesis, exhibit reduced direct and indirect resistance against BPH (Tong et al., 2012).
In maize, indole, a volatile aromatic compound which decreases insect food consumption and survival rate, has been found to be part of the direct defense against S. littoralis (Veyrat et al., 2016). Interestingly, indole also functions as a priming agent. Applying indole to maize elevates herbivory-induced JA and JA-Ile contents. In addition, indole biosynthesis mutants have shown that airborne indole can prime neighboring maize plants to release herbivoryinduced mono- and homoterpenes (Erb et al., 2015). Another well-studied aromatic compound is methyl salicylate (MeSA), which has been shown to be a critical mobile signal for tobacco systemic acquired resistance (Park et al., 2007). Both rice and maize release MeSA after sensing insect feeding or oviposition (Kollner et al., 2010; Qi et al., 2011; Tamiru et al., 2011). Application of MeSA to maize strongly repels leafhoppers (Cicadulina storeyi) (Oluwafemi et al., 2011). Field experiments have also shown that MeSA decreases aphid density in wheat (Pettersson et al., 1994). These findings suggest that MeSA regulates direct defenses of monocotyledonous plants against piercing-sucking insects.
The key structural element for terpenes is isoprene (C5), and according to their structures, there are monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), and a few homoterpenes (C11and C16) derived from sesquiterpenes and diterpenes (Chen et al., 2011). In rice, S-linalool (monoterpene) and (E)-β-caryophyllene (sesquiterpene) are produced by OsLIS and OsCAS, respectively. (E)-β-caryophyllene attracts both parasitoids and BPH in rice fields and field studies have shown that OsLIS-silenced rice plants are infested by BPH more frequently and attract fewer BPH predators and parasitoids. Moreover, OsLIS-silenced rice plants attract fewer chewing herbivores (Cnaphalocrocis medinalis) (Xiao et al., 2012). Similar results have been found in maize. For instance, the north American maize lines (F584, A654 and F7001) impaired in (E)-β-caryophyllene production attract fewer Heterorhabditis megidis (an entomopathogenic nematode and the natural enemy of Diabrotica virgifera virgifera); consequently, D. v. virgifera larvae cause more damage to these plants (Fig. 1B). Application of (E)-β-caryophyllene to these maize lines restores reduced resistance in the field (Rasmann et al., 2005). By contrast, the South American maize landrace Braz1006 releases eightfold of (E)-β-caryophyllene than does the inbred line Delprim, owing to the higher TPS23 transcript levels, and making Braz1006 more attractive to egg parasitoid (C. sesamiae) of stemborer (C. partellus) (Tamiru et al., 2017). Likewise, maize landraces C-2101, B-3016, and H-2034 also release (E)-β-caryophyllene and other terpenes ((E)-4, 8-Dimethyl-1, 3, 7, nonatriene (DMNT) and (E, E)-4, 8, 12-Trimethyl1, 3, 7, 11-tridecatetraene (TMTT)) after sensing C. partellus oviposition. While these volatiles attract larval parasitoids of C. sesamiae, the commercial maize varieties WS505 and PH4 only release trace amount of these volatiles and do not attract C. sesamiae (Tamiru et al., 2011).
4.2. Non-volatile defense-related metabolitesIn rice plants, feeding of the chewing herbivores S. mauritia and P. guttata induces accumulation of phenolamides, p-coumaroylputrescine and feruloylputrescine. Bioassays using diets containing phenolamides suggest that phenolamides are toxic to BPH, but not to S. mauritia and P. guttata (Alamgir et al., 2016). Diterpene phytoalexins momilactone A and B also accumulate in rice after S. frugiperda, S. mauritia, and M. loreyi feeding, but they are not induced by P. guttata, suggesting that herbivory by different insects activates different signaling pathways (Alamgir et al., 2016; Shinya et al., 2016).
S. littoralis infestation on maize seedlings leads to a marked local increase of 1, 3-benzoxazin-4-ones, phospholipids, N-hydroxycinnamoyltyramines, azealic acid, and tryptophan, but bioassays have indicated that N-hydroxycinnamoyltyramines (e.g., p-coumaroyltyramine) are unlikely to be toxic to S. littoralis (Marti et al., 2013).
The biosynthetic pathway of benzoxazinoids (BXs), a group of compounds known to play a role in maize defense against insect herbivores, has been well characterized (Wouters et al., 2016). The first step of the BX biosynthesis pathway is catalyzed by the BX1 enzyme, which converts indole-3-glycerol phosphate into indole in the chloroplasts. The following steps are carried out by 13 enzymes, including cytochrome P450-dependent monooxygenases (BX2-to BX5), UDP-glucosyltransferases (BX8-9), 2-oxoglutarate-dependent dioxygenases (BX6, and BX13), and O-methyltransferases (BX7, BX10, BX11, BX12, and BX14) (Wouters et al., 2016). Several lines of evidence have indicated the importance of BXs in maize defense against multiple feeding guilds (Glauser et al., 2011; Handrick et al., 2016; Meihls et al., 2013; Wouters et al., 2016). BXs are commonly stored as glucosides in vacuoles of undamaged maize cells, but hydrolyzed to unstable aglucones upon leaf damage by herbivores. BXs have been shown to be toxic to European corn borer (O. nubilalis) and Asian corn borer (O. furnacalis), the major maize pests feeding on stems. An artificial diet containing DIMBOA (2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one) increases mortality and developmental times of both Asian corn borer and European corn borer (Campos et al., 1989; Yan et al., 1999). DIMBOA also reduces the performance of generalist S. littoralis but not S. frugiperda, as S. frugiperda glycosylates DIMBOA to DIMBOA-Glc to reduce the toxicity; however, HDMBOA is toxic to both S. littoralis and S. frugiperda and cannot be glycosylated by either herbivore (Glauser et al., 2011). Although HDMBOA-Glc is more toxic to chewing caterpillars than DIMBOA-Glc is, DIMBOA-Glc but not HDMBOA-Glc is involved in callose induction by maize leaf aphid (R. maidis) (Meihls et al., 2013). How BX biosynthesis and catabolism are regulated still needs further investigation.
5. R gene-mediated herbivore resistanceR genes play critical roles in plant resistance to pathogens (Dodds and Rathjen, 2010). However, little is known about whether, and how, R geness are involved in planteinsect interactions. In tomato, the R genes Mi-1 confers resistance to aphids, whiteflies, and nematodes (Kaloshian, 2004). In monocotyledonous plants, R genesmediated resistance has only been found in rice so far: More than 25 R geness related to rice defense against BPH have been found (Cheng et al., 2013; Zhao et al., 2016). For instance, BPH14, which is expressed in vascular bundles, was identified by a map-base cloning approach, and found to encode a coiled-coil, nucleotide-binding and leucine-rich repeat (CC-NB-LRR) protein (Du et al., 2009). The CC and NB domains of BPH14 activate the SA signaling pathway to increase reactive oxygen species without leading to cell death (Hu et al., 2017). BPH14 forms homocomplexes that activate the expression of WRKY46 and WRKY72, transcription factors that bind to the promoters of the receptor-like cytoplasmic kinase gene RLCK281 and the callose synthase genes to activate callose deposition, leading to BPH resistance (Hu et al., 2017). BPH1 and BPH9 are two alleles and they encode a nucleotide-binding and leucinerich repeat (NLR)-containing protein which are localized in the endomembrane system and overexpression of BPH1/9 caused a cell death phenotype; BPH1/9 activates both SA and JA signaling pathways and confers both antixenosis and antibiosis to BPH (Zhao et al., 2016). The coding region of BPH1/9 shows a high level of diversity between different rice germplasms, which may serve as a repository for generating allele diversity (Zhao et al., 2016). Another recently discovered R genes is BPH6, which was found in the wild rice O. rufipogon and O. nivara. BPH6 confers broad resistance against BPH and white-backed planthoppers without sacrificing yield (Guo et al., 2018). Elevation of BPH6 expression increases exocytosis and BPH6 participates in cell wall maintenance and reinforcement by controlling callose deposition. Furthermore, BPH6 regulates not only JA and SA, but also the cytokinin (CK) signaling pathway, which positively regulates the expression of phytoalexin biosynthesis genes and accordingly the accumulation of phytoalexins to defend against BPH and white-backed planthoppers (Guo et al., 2018).
6. PerspectivesDespite a wide spectrum of protection methods, insects and pathogens still cause at least 15% of world crop loss (Oerke, 2006). The still increasing human population will require an equally increasing amount of food. While maize and rice are staple food crops for human consumption, maize has also become both a major biofuel crop and the most important animal feed (http://www.fao.org/worldfoodsituation/csdb/en/). Modern maize and rice may have lost some defense genes during domestication (de Lange et al., 2014; Guo et al., 2018; Rasmann et al., 2005; Tamiru et al., 2011), but their wild relatives still harbor strong resistance and could be important resources for identifying new insect-resistant genes, such as R geness. For instance, it was found that the defense-related genes showed greater expression levels in teosinte (ancestor of modern maize) than in maize, and this was correlated with the greater teosinte resistance to S. frugiperda (Szczepaniec et al., 2013). Furthermore, BPH6, an important R genes regulating resistance to BPH, exists only in wild rice not in modern rice (Guo et al., 2018). Elucidating the molecular regulation of signaling networks that control rice and maize response to herbivory is of great importance for understanding the interactions between these monocots and insects, which is critical for designing new pest control strategies. The large numbers of maize and rice varieties can also be used for identification of important regulatory elements and metabolites conferring resistance to insects.
AcknowledgementsThe Wu lab is partly supported by the National Natural Science Foundation of China (Nos. 31772179, U1502263, 31600213, 31470369, and 31770301).
Alamgir K.M., Hojo Y., Christeller J.T., et al., 2016. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ, 39, 453-466.
DOI:10.1111/pce.v39.2 |
||
Alborn H.T., Turlings T.C.J., Jones T.H., et al., 1997. An elicitor of plant volatiles from beet armyworm oral secretion. Science, 276, 945-949.
DOI:10.1126/science.276.5314.945 |
||
Betsiashvili M., Ahern K.R., Jander G., 2015. Additive effects of two quantitative trait loci that confer Rhopalosiphum maidis (corn leaf aphid) resistance in maize inbred line Mo17. J. Exp. Bot, 66, 571-578.
DOI:10.1093/jxb/eru379 |
||
Browse J., Howe G.A., 2008. New weapons and a rapid response against insect attack. Plant Physiol, 146, 832-838.
DOI:10.1104/pp.107.115683 |
||
Burtet L.M., Bernardi O., Melo A.A., et al., 2017. Managing fall armyworm, Spodoptera frugiperda (Lepidoptera:Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Manag. Sci, 73, 2569-2577.
DOI:10.1002/ps.2017.73.issue-12 |
||
Campos F., Atkinson J., Arnason J.T., et al., 1989. Toxicokinetics of 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one (DIMBOA) in the European corn-borer, Ostrinia nubilalis (Hubner). J. Chem. Ecol, 15, 1989-2001.
DOI:10.1007/BF01207432 |
||
Chang L.F., Karin M., 2001. Mammalian MAP kinase signalling cascades. Nature, 410, 37-40.
DOI:10.1038/35065000 |
||
Chen F., Tholl D., Bohlmann J., et al., 2011. The family of terpene synthases in plants:a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J, 66, 212-229.
DOI:10.1111/tpj.2011.66.issue-1 |
||
Cheng X.Y., Zhu L.L., He G.C., 2013. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant, 6, 621-634.
DOI:10.1093/mp/sst030 |
||
Christensen S.A., Nemchenko A., Borrego E., et al., 2013. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J, 74, 59-73.
DOI:10.1111/tpj.2013.74.issue-1 |
||
de Lange E.S., Balmer D., Mauch-Mani B., et al., 2014. Insect and pathogen attack and resistance in maize and its wild ancestors, the teosintes. New Phytol, 204, 329-341.
DOI:10.1111/nph.13005 |
||
Dicke M., 2009. Behavioural and community ecology of plants that cry for help. Plant Cell Environ, 32, 654-665.
DOI:10.1111/pce.2009.32.issue-6 |
||
Ding Y., Sun T., Ao K., et al., 2018. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell, 173, 1454-1467.
DOI:10.1016/j.cell.2018.03.044 |
||
Dodds P.N., Rathjen J.P., 2010. Plant immunity:towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet, 11, 539-548.
|
||
Du B., Zhang W.L., Liu B.F., et al., 2009. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. U. S. A, 106, 22163-22168.
DOI:10.1073/pnas.0912139106 |
||
Erb M., Veyrat N., Robert C.A.M., et al., 2015. Indole is an essential herbivoreinduced volatile priming signal in maize. Nat. Commun, 6, 6237.
DOI:10.1038/ncomms7237 |
||
Ethinh S., Gális I., Baldwin I., 2012. UVB radiation and HGL-DTGs provide durable resistance against mirid (Tupiocoris notatus) attack in field-grown Nicotiana attenuata plants. Plant Cell Environ, 36, 590-606.
|
||
Fu J., Liu Q., Wang C., et al., 2018. ZmWRKY79 positively regulates maize phytoalexin biosynthetic gene expression and is involved in stress response. J. Exp. Bot, 69, 497-510.
DOI:10.1093/jxb/erx436 |
||
Fukumoto K., Alamgir K.M., Yamashita Y., et al., 2013. Response of rice to insect elicitors and the role of OsJAR1 in wound and herbivory-induced JA-Ile accumulation. J. Integr. Plant Biol, 55, 775-784.
DOI:10.1111/jipb.v55.8 |
||
Gatehouse J.A., 2002. Plant resistance towards insect herbivores:a dynamic interaction. New Phytol, 156, 145-169.
DOI:10.1046/j.1469-8137.2002.00519.x |
||
Glauser G., Marti G., Villard N., et al., 2011. Induction and detoxification of maize 1, 4-benzoxazin-3-ones by insect herbivores. Plant J, 68, 901-911.
DOI:10.1111/j.1365-313X.2011.04740.x |
||
Guo H.M., Li H.C., Zhou S.R., et al., 2014. Cis-12-oxo-phytodienoic acid stimulates rice defense response to a piercing-sucking insect. Mol. Plant, 7, 1683-1692.
DOI:10.1093/mp/ssu098 |
||
Guo, J., Xu, C., Wu, D., et al., 2018. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. https://doi.org/10.1038/s41588-018-0039-6.
|
||
Handrick V., Robert C.A., Ahern K.R., et al., 2016. Biosynthesis of 8-O-methylated benzoxazinoid defense compounds in maize. Plant Cell, 28, 1682-1700.
|
||
Harfouche A.L., Shivaji R., Stocker R., et al., 2006. Ethylene signaling mediates a maize defense response to insect herbivory. Mol. Plant Microbe Interact, 19, 189-199.
DOI:10.1094/MPMI-19-0189 |
||
Heiling S., Schuman M.C., Schoettner M., et al., 2010. Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell, 22, 273-292.
DOI:10.1105/tpc.109.071449 |
||
Hettenhausen C., Schuman M.C., Wu J.Q., 2015. MAPK signaling:a key element in plant defense response to insects. Insect Sci, 22, 157-164.
DOI:10.1111/ins.2015.22.issue-2 |
||
Hopkins R.J., van Dam N.M., van Loon J.J.A., 2009. Role of glucosinolates in insectplant relationships and multitrophic interactions. Annu. Rev. Entomol, 54, 57-83.
DOI:10.1146/annurev.ento.54.110807.090623 |
||
Hu L.F., Ye M., Li R., et al., 2015. The rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of mitogen-activated protein kinase activity. Plant Physiol, 169, 2907-2921.
|
||
Hu L., Wu Y., Wu D., et al., 2017. The coiled-coil and nucleotide binding domains of brown planthopper resistance14 function in signaling and resistance against planthopper in rice. Plant Cell, 29, 3157-3185.
DOI:10.1105/tpc.17.00263 |
||
Ji R., Ye W.F., Chen H.D., et al., 2017. A salivary endo-beta-1, 4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol, 173, 1920-1932.
DOI:10.1104/pp.16.01493 |
||
Kaloshian I., 2004. Gene-for-gene disease resistance:bridging insect pest and pathogen defense. J. Chem. Ecol, 30, 2419-2438.
DOI:10.1007/s10886-004-7943-1 |
||
Kandoth P.K., Ranf S., Pancholi S.S., et al., 2007. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemi n-mediated defense response against herbivorous insects. Proc. Natl. Acad. Sci. U. S. A, 104, 12205-12210.
DOI:10.1073/pnas.0700344104 |
||
Kollner T.G., Lenk C., Zhao N., et al., 2010. Herbivore-induced SABATH methyltransferases of maize that methylate anthranilic acid using s-adenosyl-lmethionine. Plant Physiol, 153, 1795-1807.
DOI:10.1104/pp.110.158360 |
||
Lankau R.A., 2007. Specialist and generalist herbivores exert opposing selection on a chemical defense. New Phytol, 175, 176-184.
DOI:10.1111/nph.2007.175.issue-1 |
||
Li R., Zhang J., Li J.C., et al., 2015a. Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. Elife, 4, e04805.
DOI:10.7554/eLife.04805 |
||
Li S.Y., Wang H., Li F.Q., et al., 2015b. The maize transcription factor EREB58 mediates the jasmonate-induced production of sesquiterpene volatiles. Plant J, 84, 296-308.
DOI:10.1111/tpj.12994 |
||
Lou Y.G., Du M.H., Turlings T.C.J., et al., 2005. Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the Parasitoid Anagrus nilaparvatae. J. Chem. Ecol, 31, 1985-2002.
DOI:10.1007/s10886-005-6072-9 |
||
Louis J., Basu S., Varsani S., et al., 2015. Ethylene contributes to mir1-mediated maize defense against the phloem-sap sucking insect Rhopalosiphum maidis. Plant Physiol, 169, 313-324.
DOI:10.1104/pp.15.00958 |
||
Lu J., Ju H.P., Zhou G.X., et al., 2011. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J, 68, 583-596.
DOI:10.1111/j.1365-313X.2011.04709.x |
||
Lu J., Li J.C., Ju H.P., et al., 2014. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol. Plant, 7, 1670-1682.
DOI:10.1093/mp/ssu085 |
||
Lu J., Robert C.A.M., Riemann M., et al., 2015. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol, 167, 1100-1116.
DOI:10.1104/pp.114.252700 |
||
Marti G., Erb M., Boccard J., et al., 2013. Metabolomics reveals herbivore-induced metabolites of resistance and susceptibility in maize leaves and roots. Plant Cell Environ, 36, 621-639.
DOI:10.1111/pce.12002 |
||
Meihls L.N., Handrick V., Glauser G., et al., 2013. Natural variation in maize aphid resistance is associated with 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell, 25, 2341-2355.
DOI:10.1105/tpc.113.112409 |
||
Oerke E.C., 2006. Crop losses to pests. J. Agric. Sci, 144, 31-43.
DOI:10.1017/S0021859605005708 |
||
Oluwafemi S., Bruce T.J.A., Pickett J.A., et al., 2011. Behavioral responses of the leafhopper, cicadulina storeyi China, a major vector of maize streak virus, to volatile cues from intact and leafhopper-damaged maize. J. Chem. Ecol, 37, 40-48.
DOI:10.1007/s10886-010-9891-2 |
||
Park S.W., Kaimoyo E., Kumar D., et al., 2007. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science, 318, 113-116.
DOI:10.1126/science.1147113 |
||
Pechan T., Cohen A., Williams W.P., et al., 2002. Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc. Natl. Acad. Sci. U. S. A, 99, 13319-13323.
DOI:10.1073/pnas.202224899 |
||
Pettersson J., Pickett J.A., Pye B.J., et al., 1994. Winter host component reduces colonization by bird-cherry-oat aphid, Rhopalosiphum padi (L.) (homoptera, aphididae), and other aphids in cereal fields. J. Chem. Ecol, 20, 2565-2574.
DOI:10.1007/BF02036192 |
||
Qi J.F., Zhou G.X., Yang L.J., et al., 2011. The chloroplast-localized phospholipases d alpha 4 and alpha 5 regulate herbivore-induced direct and indirect defenses in rice. Plant Physiol, 157, 1987-1999.
DOI:10.1104/pp.111.183749 |
||
Qi J.F., Li J.C., Han X., et al., 2016a. Jasmonic acid carboxyl methyltransferase regulates development and herbivory-induced defense response in rice. J. Integr. Plant Biol, 58, 564-576.
DOI:10.1111/jipb.v58.6 |
||
Qi J.F., Sun G.L., Wang L., et al., 2016b. Oral secretions from Mythimna separata insects specifically induce defence responses in maize as revealed by highdimensional biological data. Plant Cell Environ, 39, 1749-1766.
DOI:10.1111/pce.12735 |
||
Rasmann S., Kollner T.G., Degenhardt J., et al., 2005. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature, 434, 732-737.
DOI:10.1038/nature03451 |
||
Ray S., Basu S., Rivera-Vega L.J., et al., 2016. Lessons from the far end:caterpillar frass-induced defenses in maize, rice, cabbage, and tomato. J. Chem. Ecol, 42, 1130-1141.
DOI:10.1007/s10886-016-0776-x |
||
Rodriguez R., Redman R., 2008. More than 400 million years of evolution and some plants still can't make it on their own:plant stress tolerance via fungal symbiosis. J. Integr. Plant Biol, 59, 1109-1114.
|
||
Schmelz E.A., Engelberth J., Alborn H.T., et al., 2009. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. U. S. A, 106, 653-657.
DOI:10.1073/pnas.0811861106 |
||
Schmelz E.A., Kaplan F., Huffaker A., et al., 2011. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. U. S. A, 108, 5455-5460.
DOI:10.1073/pnas.1014714108 |
||
Schweizer F., Fernandez-Calvo P., Zander M., et al., 2013. Arabidopsis basic helixloop-helix transcription factors myc2, myc3, and myc4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell, 25, 3117-3132.
DOI:10.1105/tpc.113.115139 |
||
Shangguan X., Zhang J., Liu B., et al., 2018. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiol, 176, 552-565.
DOI:10.1104/pp.17.00755 |
||
Shinya T., Hojo Y., Desaki Y., et al., 2016. Modulation of plant defense responses to herbivores by simultaneous recognition of different herbivore-associated elicitors in rice. Sci. Rep. UK, 6, 32537.
DOI:10.1038/srep32537 |
||
Song J., Liu H., Zhuang H.F., et al., 2017. Transcriptomics and alternative splicing analyses reveal large differences between maize lines B73 and Mo17 in response to aphid Rhopalosiphum padi infestation. Front. Plant Sci, 8, 1738.
DOI:10.3389/fpls.2017.01738 |
||
Stam J.M., Kroes A., Li Y., et al., 2013. Plant interactions with multiple insect herbivores:from community to genes. Annu. Rev. Plant Biol, 65, 689-713.
|
||
Szczepaniec A., Widney S.E., Bernal J.S., et al., 2013. Higher expression of induced defenses in teosintes (Zea spp.) is correlated with greater resistance to fall armyworm, Spodoptera frugiperda. Entomol. Exp. Appl, 146, 242-251.
DOI:10.1111/eea.12014 |
||
Tamiru A., Bruce T.J., Woodcock C.M., et al., 2011. Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol. Lett, 14, 1075-1083.
DOI:10.1111/j.1461-0248.2011.01674.x |
||
Tamiru A., Bruce T.J.A., Richter A., et al., 2017. A maize landrace that emits defense volatiles in response toherbivore eggs possesses a strongly inducible terpene synthase gene. Ecol Evol, 7, 2835-2845.
DOI:10.1002/ece3.2017.7.issue-8 |
||
Tong X.H., Qi J.F., Zhu X.D., et al., 2012. The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway. Plant J, 71, 763-775.
DOI:10.1111/tpj.2012.71.issue-5 |
||
Veyrat N., Robert C.A.M., Turlings T.C.J., et al., 2016. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J. Ecol, 104, 591-600.
DOI:10.1111/1365-2745.12526 |
||
Walling L.L., 2000. The myriad plant responses to herbivores. J. Plant Growth Regul, 19, 195-216.
|
||
Wang L., Wu J.Q., 2013. The essential role of jasmonic acid in plant-herbivore interactions-using the wild tobacco Nicotiana attenuata as a model. J. Genet. Genom, 40, 597-606.
DOI:10.1016/j.jgg.2013.10.001 |
||
Wang X., Hu L.C., Zhou G.X., et al., 2011. Salicylic acid and ethylene signaling pathways are involved in production of rice trypsin proteinase inhibitors induced by the leaf folder Cnaphalocrocis medinalis (Guen, e). Chin. Sci. Bull, 56, 2351-2358.
DOI:10.1007/s11434-011-4568-y |
||
Wang Q., Li J., Hu L., et al., 2013. OsMPK3 positively regulates the JA signaling pathway and plant resistance to a chewing herbivore in rice. Plant Cell Rep, 32, 1075-1084.
DOI:10.1007/s00299-013-1389-2 |
||
Wouters F.C., Blanchette B., Gershenzon J., et al., 2016. Plant defense and herbivore counter-defense:benzoxazinoids and insect herbivores. Phytochem. Rev, 15, 1127-1151.
DOI:10.1007/s11101-016-9481-1 |
||
Wu J.Q., Baldwin I.T., 2009. Herbivory-induced signalling in plants:perception and action. Plant Cell Environ, 32, 1161-1174.
DOI:10.1111/pce.2009.32.issue-9 |
||
Wu J.Q., Baldwin I.T., 2010. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet, 44, 1-24.
DOI:10.1146/annurev-genet-102209-163500 |
||
Wu J., Hettenhausen C., Meldau S., et al., 2007. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell, 19, 1096-1122.
DOI:10.1105/tpc.106.049353 |
||
Xiao Y., Wang Q., Erb M., et al., 2012. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett, 15, 1130-1139.
DOI:10.1111/j.1461-0248.2012.01835.x |
||
Yan F., Liang X., Zhu X., 1999. The role of DIMBOA on the feeding of Asian corn borer Ostrinia furnacalis (Guenee) (Lep., Pyralidae). J. Appl. Entomol, 123, 49-53.
DOI:10.1046/j.1439-0418.1999.00304.x |
||
Yan Y.X., Christensen S., Isakeit T., et al., 2012. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell, 24, 1420-1436.
DOI:10.1105/tpc.111.094151 |
||
Yang J.O., Nakayama N., Toda K., et al., 2013. Elicitor(s) in Sogatella furcifera(Horvath) causing the japanese rice plant (Oryza sativa l.) to induce the ovicidal substance, benzyl benzoate. Biosci Biotechnol Biochem, 77, 1258-1261.
DOI:10.1271/bbb.130055 |
||
Yang C., Lu X., Ma B., et al., 2015. Ethylene signaling in rice and Arabidopsis:conserved and diverged aspects. Mol. Plant, 8, 495-505.
DOI:10.1016/j.molp.2015.01.003 |
||
Yang Y., Li L., Qu L.J., 2016. Plant mediator complex and its critical functions in transcription regulation. J. Integr. Plant Biol, 58, 106-118.
DOI:10.1111/jipb.v58.2 |
||
Yuan Y.X., Zhong S.H., Li Q., et al., 2007. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J, 5, 313-324.
DOI:10.1111/pbi.2007.5.issue-2 |
||
Zhang Y.T., Zhang Y.L., Chen S.X., et al., 2015. Proteomics of methyl jasmonate induced defense response in maize leaves against Asian corn borer. BMC Genom, 16, 224.
DOI:10.1186/s12864-015-1363-1 |
||
Zhang J., Luo T., Wang W.W., et al., 2017a. Silencing OsSLR1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens. Plant Cell Environ, 40, 2147-2159.
DOI:10.1111/pce.v40.10 |
||
Zhang L., Zhang F., Melotto M., et al., 2017b. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot, 68, 1371-1385.
|
||
Zhao Y., Huang J., Wang Z.Z., et al., 2016. Allelic diversity in an NLR genes BPH9 enables rice to combat planthopper variation. Proc. Natl. Acad. Sci. U. S. A, 113, 12850-12855.
DOI:10.1073/pnas.1614862113 |
||
Zhou G.X., Qi J.F., Ren N., et al., 2009. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J, 60, 638-648.
DOI:10.1111/tpj.2009.60.issue-4 |



