b. Kunming College of Life Sciences, University of Chinese Academy of Sciences, Kunming, Yunnan 650201, China;
c. School of Life Sciences, Yunnan University, Kunming, Yunnan 650091, China
蜜蜂两股大如茧, 应是前山花已开.
I saw honeybees carrying pollen loads large as silkworm cocoons on their legs. This suggests that the plants in the forest are in bloom now.
Jie Rao (1065e1129)
Pollination is simply the movement of pollen from the male organ of flowers (anther) to the receptive female parts of flowers (stigma) of the same or different plants. In most, terrestrial ecosystems pollination remains one of the most crucial and critical ecological services (sensu Gurevitch et al., 2006) as it sustains wildlife and human civilizations. Animal pollen vectors are needed for the reproduction of nearly 90% of angiosperm species (Ollerton et al., 2011). Furthermore, one in every three bites of food that humans consume on a daily basis is derived from animal-pollinated species (Holden, 2006).
Global change has affected and will continue to affect all ecosystems. Habitat loss and homogenization, coupled with pesticides, parasites/pathogens and invasive species have been identified as past and current threats to pollinators and plants at a global scale. Research from Europe, North America, and other countries have provided evidence of a widespread decline in pollinator populations and diversity (Thomann et al., 2013). This pollination crisis has continued to cause international concern in agro-ecosystems and in plant conservation programs (Klein et al., 2007; Potts et al., 2010; Burkle et al., 2013).
Experiments designed to test evolutionary hypotheses have shown that interactions between plants and prospective pollinators have played a major role in the diversification of angiosperms (Grant and Grant, 1965; Stebbins, 1970). Based on a complete genetic analysis of bees, including representatives from every extant family, Cardinal and Danforth (2013) concluded that bees originated in the Cretaceous approximately 123 Ma (million years ago; 113e132 Ma), concurrent with early diversification of the eudicots, a group comprising 75% of angiosperm species. The Grant-Stebbins model of plant diversification suggests that much of angiosperm speciation is based ultimately on "pollinator shifts leading to mechanical and/or ethological modes of floral isolation" (Grant and Grant, 1965; Stebbins, 1970; Grant, 1994). These shifts in pollination systems among related species in the same lineage are the key to understanding the diversification of certain plant groups. Taken together, these theories and hypotheses have provided a basis for examining evolutionary and ecological questions raised by pollination ecology, and understanding the implications of pollination for ecological services and conservation.
China, one of the largest countries in the world, has highly diverse landscapes and a long geological history. China harbors nearly 10% of angiosperm species worldwide and has long been considered as both "a museum and a cradle" for plant species (Wu et al., 2013; Lu et al., 2018). The "museum hypothesis" contends that diversity is based primarily on the survival of relict taxa representing ancient lineages due to the repeated establishment of refugia during the Tertiary (Wu et al., 2013). In contrast, the "cradle hypothesis" argues that novel and speciose lineages diversified following climatic shifts that resulted from recent changes in topography. This includes the formation of the Qinghai-Tibetan Plateau, which led to the development of a monsoon season in Southwest China; together, this change in topography and climate provided new habitats and promoted remarkable adaptive radiations (Lu et al., 2018). In particular, the United Nations regards the "Golden Arc" of southwestern China (Himalayas and Tibetan Plateau) as a biodiversity hot spot of plant endemism and their edible and ornamental cultivars (Ren et al., 2014a; b). By extension, China should also have a high diversity of antiphilous insect species. In fact, half of the world's known honeybee (Apis) are distributed through China (sensu Michener, 2000). Almost half of the world's bumblebee (Bombus) species are found here as well with their highest diversity in the mountains of southwestern China (Williams et al., 2009). Incomplete records kept by Fauna Sinica also suggest that c. 20% of all hawkmoth species (Sphingidae) occur in China (Zhu and Wang, 1997).
The practical importance of pollination in western Europe was recognized sporadically and incompletely towards the end of the 18th century. In Europe, the science of pollination biology emerged as a discrete and synthetic discipline within botanical research by the second half of the 19th century (Proctor et al., 1996). This occurred, in part because Charles Darwin, and his correspondents in Germany and North America, realized it was intrinsic to the study of natural selection (Allan, 1977). Outside of China, research on pollination ecology continues to focus on many issues, including global climate change. In contrast, China has a long history of applied ethnobotanical research (Hu, 2005) based on sustainable agriculture and traditional apiculture. The Chinese have a long tradition in attempting to understand the foraging behavior of honeybees as in the poem quoted as an epigraph for this review. However, pollination ecology must be regarded as a far younger sub-discipline. The earliest pollination references in China go back only as far as the 1970s, which means that programs of long-term field studies, intrinsic to pollination ecology, are little more than 40 years old (Huang, 2012).
More rapid development of this sub-discipline has occurred only within the past two decades. Three special issues (2007, 2012 and 2018) published in Biodiversity Sciences, and one special issue (2015) in Journal of Plant Ecology highlighted the recent development of pollination ecology in China (Huang, 2012; Barrett, 2015). The number of publications on pollination from China increases annually. In this review, we located all publications relevant to pollination ecology to build and maintain a large permanent database. Using this database, we identified and analyzed gaps and limitations in research on pollination ecology in China. This analysis will identify priorities for future studies by answering the following questions: 1) How much do we know about pollination systems for Chinese flora? 2) Compared with the development of research in pollination ecology worldwide, what research areas should we pursue in the future? 3) What can pollination ecologists do to understand and contribute to biodiversity and conservation in China? The critical issue for this review is to expose the important studies and future research topics that can only be done in China.
2. Material and methodsWe used an EndNote database of pollination biology publications (D. C. Inouye, personal communication) as a baseline for collection references. We searched all the publications in Dr. Inouye's database, recorded all the studies conducted in China, provided that plant species were native or naturalized components of the Chinese flora. A second series of scientific articles was collected by using key word searches (Pollinat* and China) found in the major scientific depositories including ISI Web of Science, Google Scholar, Researchgate and other online resources. All replicated records were deleted. Other pollination related studies on floral trait evolution using multiple species were also included (e.g. Eaton et al., 2012).
Articles in Chinese were searched in a similar way using the depository of China Knowledge Resource Integrated Database (www.cnki.net) and other Chinese online academic resources. As studies in floral biology are of uneven quality in Chinese journals we only recorded publications appearing in the journals listed in the China Science Citation Index (CSCI), such as "Biodiversity Sciences." All searches covered publications from the 1970s-2017 in mainland China and Taiwan. Publications from neighboring countries (Vietnam, Laos etc.) sharing the same plant species with China were also included. In our database, we recorded 548 publications in English, and 160 publications in Chinese.
To identify the largest plant families and genera in China which has been studied on their pollination, we did an analysis of publications on individual species. The taxonomic treatment of families was based on the APG system. Data on sexual reproduction in individual species, within the same lineage, was often incomplete with information limited to floral attractants (e.g., pigmentation patterns), or modes of intraspecific isolation (e.g., dicliny), and/or pollen-pistil interactions (e.g., self-incompatibility). To avoid replication, we only recorded a species within a lineage once even if there was more than one publication on this species.
To identify the important sub-divisions within pollination ecology studied in China and to suggest future directions for ongoing information we subdivided the analyses into ten categories understanding that such categories are synthetic. These categories included the following keywords and phrases. (1) Agriculture: pollination service, crop pollination, crop, medicinal/edible plants; (2) Approaches: methodology, techniques; (3) Floral presentation: phenology, floral life spans (anther dehiscence and stigmatic activity), nocturnal and diurnal flowers; (4) Attractants: color and scent biochemistry, color change/spectral analysis recognition and pollinator behavior; (5) Rewards: nectar, sugar concentration/volume, amino acid, pollen nutrition; (6) Breeding systems: Intra-/ inter-specific barriers, self-incompatibility, heterostyly, floral unisexuality, mating system, sex strategy; (7) Conservation: pollinator rarity, endangered species, global change, global warming, pollution, invasive species, pollinator decline, Anthropocene; (8) Functional ecology: floral longevity, metabolism and movement, perception of color and scent, cognition/behavior; (9) Ecology: pollination limitation, floral mimicry, competition/facilitation, networks, pollinator behavior (ethology), pollination efficiency, flower constancy, foraging strategies; (10) Evolutionary trends: hybridization/introgression, floral trait selection, floral trait evolution, pollinator mediated evolution, adaptive radiation, character displacement, co-evolution. Usually, each publication could be subdivided into categories as each publication recorded more than one of these words or phrases.
To further understand the coverage of pollination topics in these publications, we refined our database to make a sub-database including only publications in top journals (e.g., those with an impact factor ranked within the top 15%) from 2012 to 2016. We didn't count publications from 2017 as some full texts were unavailable. We do not suggest that papers not included in this subdatabase are unimportant. There were 45 papers in our database. All keywords or informative words in the title were extracted. The frequencies of each keywords were analyzed. In total, we extracted 347 keywords, re-categorizing them as 29 keywords.
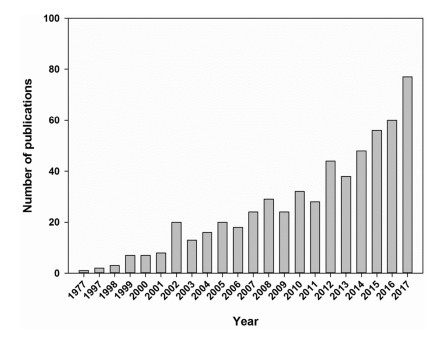
3. ResultsWe identified the first publication of pollination ecology in China as Wu (1977) based on its description of bee species dependent on domesticated Camellia oleifera by Wu (1977). Seeds of C. oleifera remain an important oil crop in southern China but bees that pollinate these flowers do not belong to the genus Apis (Xie et al., 2013) as its flowers secrete nectar toxic to Apis cerana (native) and commercial Apis mellifera (introduced). Hive populations of Apis species actually decline when placed in, or near, these plantations. Nectar analyses test positively for caffeine, raffinose, stachyose (Su et al., 2012). The legitimate pollinators of C. oleifera are Andrena camellia (Andrenidae) and Colletes gigas (Colletidae; Wu, 1977). Unfortunately, Chinese publications featuring field studies of pollination did not continue after this important finding until the 1990s. After 1998, the number of publication continued to increase annually reaching their highest numbers (77) in 2017 (Fig. 1).
|
| Fig. 1 The number of English publications on pollination biology conducted in China over the past 40 years (1977-2017). |
Pollination studies of species distributed in China cover more than 105 angiosperm families. Since 1990, however, 87 families have been represented by fewer than 10 species. For the remaining ten families, researchers have examined 16e100 species (Table 1). Publications were dominated by taxa representing the herbaceous and speciose genus Pedicularis (> 600 species worldwide, Orobanchaceaess; Robart et al., 2015) with about 300 species in China and the pollination of 100 species studied since Wang and Li (1998). The Pedicularis lineage forms extensive species flocks in sub-alpine to alpine meadows in the Himalayas. It appears that this genus became a priority of Chinese pollination biologists following a longterm interest in pollination mechanisms and interspecific isolation by the American botanist, Walter Macior (1926-2007), who studied North American assemblages and completed an initial study in China. As the majority of Pedicularis species are pollinated by Bombus species (Apidae), Macior's publications were used as models of interspecific isolation based on floral architecture/mechanics by the influential, American, evolutionary botanist, Verne Grant (1917-2007; Grant, 1994). With Chinese species vastly outnumbering the 28 species native North America the genus Pedicularis represents a model lineage for long-term projects on floral evolution and speciation.
| Families | No. species studied | Notes |
| Orobanchaceae | 100 | Most research was conducted in Pedicularis, the largest genus in this family. Following the model of the Macior, Pedicularis type of mechanical isolation was detailed on the mountains of Himalayan-Hengduan region |
| Orchidaceae | 58 | A review is provided by Tang et al. (2014), most studies focused on terrestrial orchids with deceptive (food mimic) pollination systems. |
| Asteraceae | 26 | Rapid evolution of floral traits on alpine Asteraceae by artificial warming and domestication of western honeybees was reported by Mu et al.(2014, 2015) |
| Ericaceae | 24 | Most studies focused on the largest genus, Rhododendron; bird pollination was reported for some alpine species (Georgian et al., 2015; Huang et al., 2017). |
| Leguminosae | 22 | |
| Zingiberaceae | 20 | The discovery of flexible style system is one of the most important contributions Chinese pollination ecologists have made to the world (Li et al., 2001). This is an economically and medicinally important taxon dominated by insect-pollinated species. |
| Labiatae | 19 | |
| Phyllanthaceae | 17 | |
| Liliaceae | 16 | |
| Ranunculaceae | 15 |
Publications on Pedicularis are followed by publications on 58 species in the Orchidaceae, representing taxa in four out of the five subfamilies (Table 1). In this case, a universal, long-term and scientific interest in the pollination of this family dates to the seminal work of Charles Darwin and, most likely, to the traditional Chinese affection for Cymbidium as one of the four gentlemen of flowers (Goody, 1993). Considering Chinese interests in food and medicinal species it is surprising that research on native members of the Fabaceae, Zingiberaceae and Lamiaceae lag so far behind (Table 1).
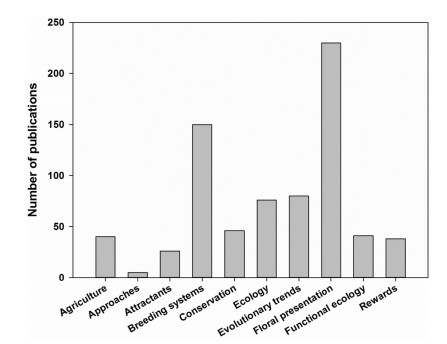
Publications covered all categories listed above, but papers on floral presentation and breeding systems (Fig. 2) dominated, accounting for 31.42% and 20.49% of the total publications respectively. We note, though, the low number of publications using new techniques and methods, such as floral scent headspace dynamic collection and GS-MS analysis, ecology network analysis, and other lab-based behavior experiments. Such approaches accounted for less than 1% of all publications. For example, functional aspects of floral rewards/signals (5.60%) and pollination networks accounted for less than 10 publications and appear restricted to field studies in sub-alpine and alpine meadows in the Himalayas. Only a small number of papers addressed research that used information on reproductive ecology to improve conservation programs under cycles of global climate change. Surprisingly for a food-conscious country, the role of pollination services in crops accounted for only 5.46% of publications.
|
| Fig. 2 The frequency distribution of the number of publications on different categories of pollination ecology in China. |
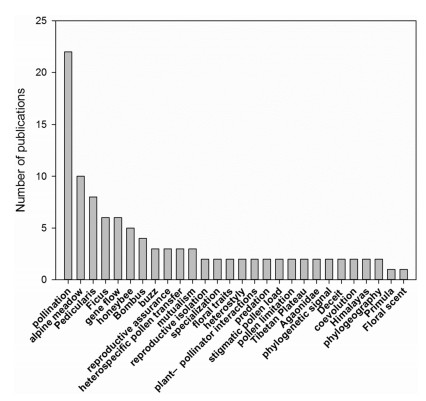
Similar to the results of the analysis of categories, we found that keywords were strongly biased in favor of studies on dominant plant families. Pollination as a keyword appeared 22 times, followed by alpine meadow (10 times), Ficus (six times), Pedicularis (six times) and gene flow (six times; Fig. 3). Honeybee and Bombus were listed as keywords five and four times respectively. Other keywords and/or phrases included buzz pollination, reproductive assurance, heterospecific pollen transfer, mutualism, reproductive isolation, specialization, floral traits, heterostyly, plant-pollinator interactions, predation, stigmatic pollen load and Tibetan Plateau.
|
| Fig. 3 Keywords frequency analysis of 45 selected publications on pollination ecology from 2012 to 2016. |
Pollination ecology has developed rapidly in China the last two decades. It appears that research on pollination in China does not match the worldwide concerns regarding the ongoing pollination crisis (Klein et al., 2007; Potts et al., 2010; Burkle et al., 2013). In China, the number of pollination publications has increased as the number of researchers and labs, as has been shown over the past success of seven national meetings on reproductive ecology. While research topics have also diversified, covering most of the subdivisions within pollination ecology, we continue to concentrate on the collection of very basic information on the life history of each plant species. More recently, Chinese researchers appear to be producing more hypothesis-driven papers that combine multiple approaches, many of which are already basic components of research in the West, e.g., functional tests of floral scent and color. Certain families are preferred and a limited number of families with herbaceous species may be preferred for field and lab research, especially those herbaceous species that occur with or have radiated from the mountains of Southwest China. Even though China clearly has many entomophilous trees, research on pollination of woody species in China has lagged behind.
To demonstrate trends in Chinese pollination ecology, we here highlight a few studies available only in China.
(1) High biodiversity combined with diverse landscapes along environmental gradients. The flora of China is extremely diverse with more than 30, 000 species of angiosperms described to date (Wu et al., 2013). The mountains of Southwest China are regarded as global hotspots of biodiversity with the highest endemic diversity of all extant but temperate floras (Myers et al., 2000). In this region, some speciose genera show their most extensive assemblages where habitats intergrade (e.g., sub-alpine and alpine meadows as well as sub-alpine forests) but do not show large scale range divergence (Fig. 4A, B). Pedicularis in China, as discussed above, is represented by more than 300 species at a 75% rate of endemism with a high degree of sympatry through the Himalayas. These species often have overlapping flowering seasons and appear to share the same bumblebee pollinators (see below). Other speciose genera through the Chinese Himalayas include Primula, Gentiana, and Rhododendron, and all appear to be dependent, to some extent, on bumblebee and A. cerana (see below) as primary or secondary pollinators (Ren et al., 2014a, b; Zhao et al., 2016).

|
| Fig. 4 Diverse landscapes, floras, and fauna in China. A. A typical alpine meadow with mass flowering at mid-summer on the mountains of Southwest China. B. A typical subalpine forest with multiple species of Rhododendron spp. co-occurring and co-blooming in early spring in the mountain region. C. A family-based agriculture system in China with vegetable gardens and orchards located around farmers' houses. D. Beekeeper's comb frame for the native, Apis cerana, one of the most important pollinators of flora in China, covering all ecosystems from lowlands to high elevations. |
Such extensive assemblages have provided us great long-term opportunities to test questions about how sympatric taxa share pollinators and how species pairs coexist at the community level (Huang and Shi, 2013; Armbruster et al., 2014; Corbet and Huang, 2014). These works have confirmed "Pedicularis type" isolation, a form of sympatric speciation in which Pedicularis species place pollen on different parts of the same bumblebee's body, reducing interspecific pollen transfer. However, based on a quantification of multiple preand post-pollination isolation barriers, Liang et al. (2018) also showed the importance of a suite of mechanical and post-pollination barriers.
As one of the world's biodiversity hotspots, the mountains of Southwest China, which harbor high plant diversity, are often characterized by a mosaic of microhabitats based on geology and elevation gradients. In these biomes, pollinators should also be distributed within similar geographical mosaics. The diverse landscapes and environmental gradients are "natural laboratories" for speciation. As elevation increases, we expect a decline in both flower and pollinator diversity, and at higher elevations we expect that bumblebees and certain flies will be the dominant pollen vectors (see reviews in Orford et al., 2015; Kerr et al., 2015). This is confirmed in a comparison network study by Zhao et al. (2016), in which they found that most species of pollinating insects declined in diversity and density as elevation increased but flowering herb diversity increased with elevation.
(2) Pollination systems shaped by super generalist foragers. Of the extant 10e12 honeybee species (sensu Michener, 2000) on earth, Chinese agriculture employs only the semidomesticated A. cerana and the commercial A. mellifera (Fig. 4D). The latter taxon is derived from a species complex native to Europe, Africa and the Middle East. Both are regarded as the most important pollinators of crop plants in China, but A. cerana is indigenous and probably evolved with the Sino-flora through the mid-Tertiary (Smith et al., 2014; You et al., 2005). This native honey bee is also important for the pollination of many wild species. However it is semimigratory through parts of its range with colonies, flying to higher elevations during the summer then retreating in autumn.
Bumblebees are one of most important pollinator groups globally. China harbors half of all known bumblebee species in the world with the highest diversity in the mountains of southwestern China (Williams et al., 2009). Bumblebees have physiological and behavioral adaptations that allow them to maintain their body temperatures as ambient temperatures decline with elevation and most species remove pollen from porose-porate anthers via sonication (buzz pollination). Bumblebee-plant interactions remain essential to ecosystems at higher elevations and Chinese studies of bumblebee pollination may be compared to literature on comparable, long-term projects in the Rockies of North America over the past 50 years. Paying closer attention to the research on bumblebee pollination in montane North America is important as China shares a number of bumblebee pollinated genera including Anenome, Cypripedium, Fritillaria, Lilium, Pedicularis, etc. Chinese-based research should test older North American-based hypotheses to see if pollination results are universal in montane regions. We suspect there will be some overlap, but in China the elevation gradients are much higher and bee and plant species diversity are much greater.
(3) Family based small-holder farming system and recent/ ongoing domestication of wild medicinal/edible plant. Western methods of agriculture are characterized by large field sizes devoted to monocultures of a limited number of crops. This type of agriculture employs a large number of hives of A. mellifera for pollination. In contrast, Chinese agroecosystems remain largely family-based with relatively small fields supporting a high diversity of crops and cultivars (Fig. 4C). These small-holder fields are often surrounded by forest in the montane areas, or small strips of native and/or disturbed habitats in southern China. Many are interrupted by spiny flower strips (Kremen et al., 2004). These agrolandscapes continue to provide nesting habitats and floral resources for wild pollinators supporting pollination services for insect-pollinated crops (Zou et al., 2017).
China is a center of origin and/or variation of many crop species and retains regions containing wild species and/or landraces (Ren et al., 2014a; b) as well as species still employed as traditional medicines. The country is also one vast gene bank for the descendants of ornamental primroses, gentians, peonies, rhododendrons, Meconopsis poppies etc. As argued in Ren et al.(2014a, b), ongoing domestication of wild plants provides opportunities for studying shifts in pollination/breeding systems. China could become a center for the study of the reproductive biology of cultivated species in early or transitional periods of domestication.
(4) Large geographical or inter-continental comparisons of pollination/breeding systems between China and other countries. Many angiosperm lineages show continuous or disjunctive distributions through China, other Eurasian regions and on other continents. We need to continue to compare the breeding, pollination and biomechanics of Chinese species with congeneric taxa outside our political borders to enrich our understanding of intraand interspecific evolution. For example, Epipactis veratrifolia, is distributed from east Asia through Europe. Populations of this species in Israel and India attract female, aphidophagous hoverflies as pollinators (Ivri and Dafni, 1977). Stökl et al. (2011) further indicated that E. veratrifolia mimics aphid alarm pheromones to attract hoverflies as pollinators, by producing α and β-pinene, β-myrcene, and β-phellandrene. These scent molecules attract and induce oviposition behaviors in female hoverflies. Jin et al. (2014) studied multiple populations of this species along the Nujiang river, Yunnan. They found that some inflorescences of E. veratrifolia were infested with aphids while they were still in bud and some hoverfly maggots developed to the third instar while flower buds opened. This indicates that adult female hoverflies are partly rewarded for oviposition. Although these flowers failed to secrete nectar, they mimicked both alarm pheromones and aphid colors to attract female hoverflies as their exclusive pollinators. This solved the puzzle of the origin of oviposition (brood site/aphid) mimicry suggesting that this deceptive pollination system evolved directly from earlier (partly) mutualistic systems. The fidelity of the original pollinator(s) has been maintained even though rewards (nectar/ brood site) were lost.
4.2. Where are the big gaps in Chinese pollination biology?However, compared with the ongoing research of our international colleagues, and the urgent need for pollination systems to solve big problems in Chinese conservation and sustainable agriculture, we still have a long way to go. First, research that examines multiple populations using multiple approaches is still scarce. To understand species-level traits on pollination, it is necessary to survey multiple populations within their distribution range, because pollinator faunas across time and space often vary at the population-level. Based on a large database on the outcrossing rates for 741 populations from 105 species, Whitehead et al. (2018) found substantial and prevalent among-population variation in their mating systems. Estimates of outcrossing rates from a single population were often highly unreliable indicators of the mating system of an entire species. Single populations with one or a few approaches are commonly found in Chinese-language publications, more than 30% only studied floral biology but without statistical analyses. Most publications lack floral signal analyses. One reason for this absence is the shortage of equipment to analyze floral pigmentation patterns and scents. Novel techniques and methods were limited in all the publications (but see Fang and Huang, 2013; Tang et al., 2015). We know there are bird-pollinated species in China, but the research is limited and under tested (Georgian et al., 2015; Huang et al., 2017).
Second, Chinese research has been more focused on wild species in montane regions of southwestern China. When we analyzed what families and keywords were most prevalent in papers published on pollination ecology in China, we found that most studies focused on mountain regions and wild plants. In fact, understanding reproductive adaptations to extremely high elevation is one of the most important achievements in this field, see below. In China, a broader array of ecosystems and biomes require investigation.
Third, taxonomy of certain insect groups is poorly understood in China that it is not possible to do in depth studies of plants pollinated by flies and bees belonging to families with the exception of eusocial taxa in the Apidae. Both pollinator identification and pollinator behavior studies are under developed. The first publication identified in this review studied and described the behavior of specialized bees pollinating Camellia olifera by Prof. Y. R. Wu. Her pioneering works on bee pollinators was one of most important contributions to Chinese agriculture. However, this tradition did not progress further. In fact, most pollination ecologists in China were trained as botanists. Only a few entomologists worked on insect-flower interactions. Consequently, well-trained entomologists are difficult to find for the identification of pollinators. In addition, we noticed that many pollination papers do not indicate who identified insects and where their vouchers are located. Bumblebees are the most well studied insect group in China due to the work of Paul Williams with Chinese colleagues (Williams et al., 2009). Based on our pollination network study on Yulong Mountain, Lijiang, northwest Yunnan, we systematically collected bumblebees visiting flowers in more than 10 meadows. In total, there were about 20 species of bumblebees identified by Paul Williams (Liang, unpublished data). This suggests how little we know about pollinator diversity. Other insect groups pollinating plants are also under studied e.g., moths (Lepidoptera) are a major pollinator group worldwide and are regarded as the dominant pollen vectors of flowers with crepuscular-nocturnal anthesis (Macgregor et al., 2014; Hahn and Brühl, 2016). However, currently, there are only four publications on hawkmoth pollination in China (Tao et al., 2018).
Fourth, functional aspects have seldom been tested. Plantpollinator interaction studies that examine UV floral patterns and use UV photography and insect visual models have been underexplored in China. Verhoeven et al. (2018) demonstrated the diversity of floral colors on the high elevation mountains in Lijiang, eastern Himalayas. Unusual flower color patterns were detected in the bumblebee buzz-pollinated genus Pedicularis and indicated a highly diverse signal system in this genus. As we mentioned above, floral mechanisms that ensure pollen deposits and pickups on different parts of the pollinator's body ("Pedicularis type" of mechanical isolation) were well characterized in a few co-existing species in China (Tong and Huang, 2016; Liang et al., 2018). Unfortunately, the visual and olfactory signal aspects of this model system has yet to be explored (Lunau et al., 2017; Verhoeven et al., 2018).
Fifth, the implications of pollination researches need to be emphasized. Most studies of pollination ecology in China today focus on wild species and, in only a few cases, on crops (Fang et al., 2012). Pollination ecologists and entomologists in China should pay more attention to both ethnic and industrial agro-ecosystems (Mitchell et al., 2009). The ethnic and rural systems are of particular importance due to the sheer diversity of edible, fiber and medicinal species under cultivation (López-Pujol et al., 2006; Li and Pritchard, 2009; Huang, 2011). The ecology and life-history of a number of threatened and endangered herbs and shrubs of the Chinese Himalayas remains understudied. Furthermore, conservation efforts look grim, at least for some wild and domesticated taxa, due to a combination of reduced populations and poaching (Ren et al., 2014a, b; Bernhardt et al., 2017).
4.3. What aspects of pollination research should be emphasized in China?First, China is the point of origin and variation for numerous crops and semi-domesticated species; therefore, Chinese researchers need to provide missing information of these species for the rest of the world. Second, diversification in China shows huge species flocks and assemblages especially through the Himalayas. These sites should be treated as living laboratories for studies on interspecific isolation, sympatry, and pollination networks. China is a center of diversity for the genera Apis and Bombus and it's time to concentrate efforts on the roles these insects play in reproductive success and diversification in angiosperms.
Pollinator-driven diversification has been generally considered important in such a scenario, that pollinator shifts play a major role in diversification (the Grant-Stebbins model of pollinator driven diversification of angiosperms), increasing biogeographical diversity through adaptive radiation (e.g., occupying empty pollinator-plant niches). This hypothesis should become our foundation for interpreting species richness and the high levels of endemism in southwestern China, which has the greatest number of temperate zone plant species on earth. Heterogeneous, and often highly specialized, pollination systems are found in this region but it is not understood how they precipitate speciation under adaptive radiation. The admirable progress of plant systematics in China (Lu et al., 2018) has provided robust phylogenetic trees for major plant groups. It is time to use these trees to test hypotheses about the relationship between species richness and the diversity of pollination systems within large clades of plant angiosperm. Specialized plant-pollinator interactions are less common in this region (Ren et al., 2011), as we showed using network studies, generalization structures were often detected in these super generalists (honeybee and bumblebee) dominated networks. Identifying phylogeographic patterns of members of the same plant lineage by comparing and contrasting adaptations for pollination in different microhabitats along the same ecological gradients (e.g., elevation, precipitation) should be insightful.
Pollination on the community level has rarely been studied in China. Currently, the network approach, as an investigative tool, is integrated into standard ecological research (Poisot et al., 2016 and the papers in the special issue of "Advances and Challenges in the Study of Ecological Networks" in Functional Ecology). Networks visualize and describe complex ecosystems contributing to our understanding of core questions in ecology including why and how so many species co-exist? However, a major challenge remains in our understanding of the role of ecological interactions in shaping trait evolution in a community context with multiple species interactions. The integration of phenotypic/functional traits such as volatile molecules (floral scents) and genomic data, into a plant community context should be a fruitful area in the near future (Kantsa et al., 2017, 2018).
Pollination networks are usually constructed and assessed by direct field observations, which commonly assumes that all flower visitors are true and equal pollinators. This assumption is often invalid and the use of such data, based on mere visitors to flowers, may lead to a misunderstanding of intrinsic pollination networks. A network approach with DNA barcoding for both plants and their pollinating insects will provide necessary information to understand how plant-pollinator interactions are structured at the community level and how these closely related pollinators (e.g., bumblebees) and plant species (e.g., Pedicularis) co-exist. Network studies at different levels of biological hierarchy (individual, species and community) will provide answers to species coexistence mechanisms, especially when plant species pairs share the same primary pollinator. Additionally, "minor" shifts of plant species to different pollinators, such as shifts from large bees (apids) to small bees (halictids or andrenids) may be just as significant as "major" shifts for diversification (Tao et al., 2018). The spatial-temporal divergence based on the individual insects' foraging pattern (DNA barcoding of pollen loads on insect bodies will provide such a database) may have also played role in structuring these communities.
4.4. Pollination implications and conservation of native species and cropsConserving any plant species means conserving a life history dependent on other organisms, including symbiotic fungi, sympatric and co-blooming flora, and pollinator guilds. For each species, we still need to ask the following basic questions: 1) What animals pollinate a particular population of plant species? 2) How does its pollination mechanisms (biomechanics) work? 3) Will a plant species self-pollinate or is an animal vector required?
At the 19th International Botanical Congress in Shenzhen, China in 2017, Drs. Peter Bernhardt and Zong-Xin Ren co-organized a symposium on "Pollination and breeding systems of endangered plant species". As we discussed with international scientists and the audience at our symposium, we still must encourage future research to answer the following questions: Does successful, sexual recombination in plant species always decline as their populations become smaller and fragmented due to environmental and/or anthropogenic stress? Do pollinators of endangered species always decline or become extinct as these plant populations decline? Are small populations that persist by changing their breeding systems (e.g., from self-incompatible to self-compatible) favored by directional selection? How can institutions manage and increase reproductive success of endangered species in remaining habitats based on current ecological and genetic information?
Pollinators, despite their ecological and agricultural importance, are under threat at a global scale. Future sustainability of pollinators and the service they provide requires anticipation of potential threats and opportunities before they occur, enabling timely implementation of policy and practice to prevent, rather than mitigate, further pollinator declines (Brown et al., 2016). To better understand limitations in our knowledge of pollination in agriculture systems in China, we must continue to address the following questions as proposed by Ren et al.(2014a, b). 1) Do these domesticated plants in China depend on animal pollen vectors to reproduce? 2) What are the most important pollinators of these crops and/or their wild relatives? 3) Are wild pollinator species more important for maximizing yield in some traditional Chinese crops than the introduced, domesticated and almost pandemic A. mellifera?
As a typical example highly cited by the international media, farmers in several counties in Sichuan Province pollinate apple and pear flowers manually using "pollination sticks" that are nothing more than wooden brushes tipped with chicken feathers or cigarette filters (Tang et al., 2003; Partap and Tang, 2012). Convincing as it may sound, this story has other explanations. Two major factors may drive this hand pollination practice in China. The heavy local use of insecticides may lead to the decline of wild pollinators and/or orchards are too contaminated with pesticides for beekeepers to risk renting their hives to the local producers. Second, some orchards were planted with self-incompatible cultivars or clonal grafts (as in commercial apples) requiring the recurrent use of inter-compatible pollen collected from neighboring or more distant orchards (Vereecken and Ren, in progress).
AcknowledgementsWe thank Prof. David C. Inouye of University of Maryland, USA for providing EndNote database of pollination biology publications. We also thank the following international colleagues for their valuable comments, suggestions and educations for promoting pollination research in China: Prof. Amots Dafni of Haifa University, Israel; Prof. Peter Bernhardt of Saint Louis University, USA; Prof. Spencer Barrett of University of Toronto, Canada; Prof. Klaus Lunau of Heinrich-Heine-Universität Düsseldorf, Germany; Dr. Nicolas J. Vereecken of Universite'Libre de Bruxelles, Belgium. Prof. Peter Bernhardt provided valuable comments, and edited the English text of an early draft of this manuscript. We thank all pollination ecologists, botanists and entomologists who contributed their efforts to understand pollination of Chinese plants. The EndNote database of pollination publication of China are available for sharing by contacting with correspondence author of this article. This project was supported by the National Natural Science Foundation of China (No. 31300199 and 41561014) and the Youth Innovation Promotion Association, Chinese Academy of Sciences (2014355).
Appendix A. Supplementary dataSupplementary data related to this article can be found at https://doi.org/10.1016/j.pld.2018.07.007.
Allan M., 1977. Darwin and His Flowers:the Key to Natural Selection. Taplinger Publishing Company, New York. |
||
Armbruster W.S., Shi X.Q., Huang S.Q., 2014. Do specialized flowers promote reproductive isolation? Realized pollination accuracy of three sympatric Pedicularis species. Ann. Bot, 113, 331-340.
DOI:10.1093/aob/mct187 |
||
Barrett S.C.H., 2015. The evolution of plant reproductive ecology in China. J. Plant Ecol, 8, 101-108.
DOI:10.1093/jpe/rtv028 |
||
Bernhardt P., Edens-Meier R., Grimm W., Ren Z.X., Towle B., 2017. Global collaborative research on the pollination biology of rare and threatened orchid species (Orchidaceae). Ann. Mo. Bot. Gard, 102(2), 364-376.
DOI:10.3417/D-16-00005A |
||
Brown M.J.F., Dicks L.V., Paxton R.J., Baldock K.C.R., Barron A.B., Chauzat M.-P., Freitas B.M., Goulson D., Jepsen S., Kremen C., Li J., Neumann P., Pattemore D.E., Potts S.G., Schweiger O., Seymour C.L., Stout J.C., 2016. A horizon scan of future threats and opportunities for pollinators and pollination. PeerJ, 4, e2249.
DOI:10.7717/peerj.2249 |
||
Burkle L.A., Marlin J.C., Knight T.M., 2013. Plant-pollinator interactions over 120 years:loss of species, co-occurrence and function. Science, 339, 1611-1615.
DOI:10.1126/science.1232728 |
||
Cardinal S., Danforth B.N., 2013. Bees diversified in the age of eudicots. Proc. Roy. Soc. Biol. Sci, 280, 20122686.
DOI:10.1098/rspb.2012.2686 |
||
Corbet S.A., Huang S.Q., 2014. Buzz pollination in eight bumblebee-pollinated Pedicularis species:does it involve vibration-induced triboelectric charging of pollen grains?. Ann. Bot, 114, 1665-1674.
DOI:10.1093/aob/mcu195 |
||
Eaton D.A.R., Fenster C.B., Hereford J., Huang S.Q., Ree R.H., 2012. Floral diversity and community structure in Pedicularis (Orobanchaceae). Ecology, 93(8), S182-S194.
|
||
Fang Q., Chen Y.-Z., Huang S.Q., 2012. Generalist passerine pollination of a winterflowering fruit tree in central China. Ann. Bot, 109(2), 379-384.
DOI:10.1093/aob/mcr293 |
||
Fang Q., Huang S.Q., 2013. A directed network analysis of heterospecific pollen transfer in a biodiverse community. Ecology, 94(5), 1176-1185.
DOI:10.1890/12-1634.1 |
||
Georgian E., Fang Z., Emshwiller E., Pidgeon A., 2015. The pollination ecology of Rhododendron floccigerum Franchet (Ericaceae) in Weixi, Yunnan Province, China. J. Pollinat. Ecol, 16(11), 72-81.
|
||
Goody J., 1993. The Culture of Flowers. University of Cambridge, Woolmough Bookbinding Ltd. Northampton, England. |
||
Grant V., 1994. Modes and origins of mechanical and ethological isolation in angiosperms. Proc. Natl. Acad. Sci. U. S. A, 91, 3-10.
DOI:10.1073/pnas.91.1.3 |
||
Grant V., Grant K.A., 1965. Flower Pollination in the Phlox Family. Columbia University Press, New York. |
||
Gurevitch J., Scheiner S.M., Fox G.A., 2006. The Ecology of Plants second ed. Sinauer Associates Inc., Massachusetts USA. |
||
Hahn M., Brühl C.A., 2016. The secret pollinators:an overview of moth pollination with a focus on Europe and North America. Arthropod Plant Interact, 10(1), 21-28.
DOI:10.1007/s11829-016-9414-3 |
||
Holden C., 2006. Report warns of looming pollination crisis in North America. Science, 314(5798), 397-397.
DOI:10.1126/science.314.5798.397 |
||
Hu S.Y., 2005. Food plants of China. Chinese University Press, Hong Kong. |
||
Huang H.W., 2011. Plant diversity and conservation in China:planning a strategic bioresource for a sustainable future. Bot. J. Linn. Soc, 166(3), 282-300.
DOI:10.1111/boj.2011.166.issue-3 |
||
Huang S.Q., 2012. Pollination biology in China in the 21st century:getting a good start. Biodivers. Sci, 20, 239-240.
|
||
Huang S.Q., Shi X.Q., 2013. Floral isolation in Pedicularis:how do congeners with shared pollinators minimize reproductive interference?. New Phytol, 199, 858-865.
DOI:10.1111/nph.12327 |
||
Huang Z.-H., Song Y.-P., Huang S.-Q., 2017. Evidence for passerine bird pollination in Rhododendron species. Aob Plants, 9, plx062.
|
||
Ivri Y., Dafni A., 1977. Pollination ecology of Epipactis consimilis Don. (Orchidaceae) in Israel. New Phytol, 79(1), 173-177.
DOI:10.1111/nph.1977.79.issue-1 |
||
Jin X.H., Ren Z.X., Xu S.Z., Wang H., Li D.Z., Li Z.Y., 2014. The evolution of floral deception in Epipactis veratrifolia (Orchidaceae):from indirect defense to pollination. BMC Plant Biol, 14(1), 63.
DOI:10.1186/1471-2229-14-63 |
||
Kantsa A., Raguso R.A., Dyer A.G., Sgardelis S.P., Olesen J.M., Petanidou T., 2017. Community-wide integration of floral colour and scent in a Mediterranean scrubland. Nat. Ecol. Evol, 1(10), 1502-1510.
DOI:10.1038/s41559-017-0298-0 |
||
Kantsa A., Raguso R.A., Dyer A.G., Olesen J.M., Tscheulin T., Petanidou T., 2018. Disentangling the role of floral sensory stimuli in pollination networks. Nat. Commun, 9(1), 1041.
DOI:10.1038/s41467-018-03448-w |
||
Kerr J.T., Pindar A., Galpern P., Packer L., Potts S.G., Roberts S.M., Rasmont P., Schweiger O., Colla S.R., Richardson L.L., Wagner D.L., Gall L.F., Sikes D.S., Pantoja A., 2015. Climate change impacts on bumblebees converge across continents. Science, 349(6244), 177-180.
DOI:10.1126/science.aaa7031 |
||
Klein A.M., Vaissiere B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C., Tscharntke T., 2007. Importance of pollinators in changing landscapes for world crops. Proc. Roy. Soc. Biol. Sci, 274(1608), 303-313.
DOI:10.1098/rspb.2006.3721 |
||
Kremen C., Williams N.M., Bugg R.L., Fay J.P., Thorp R.W., 2004. The area requirements of an ecosystem service:crop pollination by native bee communities in California. Ecol. Lett, 7(11), 1109-1119.
DOI:10.1111/ele.2004.7.issue-11 |
||
Li D.Z., Pritchard H.W., 2009. The science and economics of ex situ plant conservation. Trends Plant Sci, 14(11), 614-621.
DOI:10.1016/j.tplants.2009.09.005 |
||
Li Q.J., Xu Z.F., Kress W.J., Xia Y.M., Zhang L., Deng X.B., Gao J.Y., Bai Z.L., 2001. Flexible style that encourages outcrossing. Nature, 410(6827), 432-432.
DOI:10.1038/35068635 |
||
Liang H., Ren Z.X., Tao Z.B., Zhao Y.H., Bernhardt P., Wang H., 2018. Impact of preand post-pollination barriers on pollen transfer and reproductive isolation among three sympatric Pedicularis species. Plant Biol, 20, 662-673.
DOI:10.1111/plb.2018.20.issue-4 |
||
López-Pujol J., Zhang F.M., Ge S., 2006. Plant biodiversity in China:richly varied, endangered, and in need of conservation. Biodivers. Conserv, 15(12), 3983-4026.
DOI:10.1007/s10531-005-3015-2 |
||
Lunau K., Konzmann S., Winter L., Kamphausen V., Ren Z.-X., 2017. Pollen and stamen mimicry:the alpine flora as a case study. Arthropod Plant Interact, 11(3), 427-447.
DOI:10.1007/s11829-017-9525-5 |
||
Lu L.-M., Mao L.-F., Yang T., Ye J.-F., Liu B., Li H.-L., Sun M., Miller J.T., Mathews S., Hu H.-H., Niu Y.-T., Peng D.-X., Chen Y.-H., Smith S.A., Chen M., Xiang K.-L., Le C.-T., Dang V.-C., Lu A.-M., Soltis P.S., Soltis D.E., Li J.-H., Chen Z.-D., 2018. Evolutionary history of the angiosperm flora of China. Nature, 554, 234.
DOI:10.1038/nature25485 |
||
Macgregor C.J., Pocock M.J.O., Fox R., Evans D.M., 2014. Pollination by nocturnal Lepidoptera, and the effects of light pollution:a review. Ecol. Entomol, 40(3), 187-198.
|
||
Michener C.D., 2000. The Bees of the World. Johns Hopkins University Press, Baltimore, Maryland. |
||
Mitchell R.J., Irwin R.E., Flanagan R.J., Karron J.D., 2009. Ecology and evolution of plantepollinator interactions. Ann. Bot, 103, 1355-1363.
DOI:10.1093/aob/mcp122 |
||
Mu J., Griffin J.N., Niklas K.J., Sun S.C., 2014. Domesticated honey bees evolutionarily reduce flower nectar volume in a Tibetan lotus. Ecology, 95, 3161-3172.
DOI:10.1890/13-2055.1 |
||
Mu J., Peng Y., Xi X., Wu X., Li G., Niklas K.J., Sun S.C., 2015. Artificial asymmetric warming reduces nectar yield in a Tibetan alpine species of Asteraceae. Ann. Bot, 116, 899-906.
DOI:10.1093/aob/mcv042 |
||
Myers N., Mittermeier R.A., Mittermeier C.G., da Fonseca G.A.B., Kent J., 2000. Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853-858.
DOI:10.1038/35002501 |
||
Ollerton J., Winfree R., Tarrant S., 2011. How many flowering plants are pollinated by animals?. Oikos, 120(3), 321-326.
DOI:10.1111/more.2010.120.issue-3 |
||
Orford K.A., Vaughan I.P., Memmott J., 2015. The forgotten flies:the importance of non-syrphid Diptera as pollinators. Proc. Roy. Soc. Biol. Sci, 282, 20142934.
DOI:10.1098/rspb.2014.2934 |
||
Partap U., Tang Y., 2012. The human pollinators of fruit crops in Maoxian County, Sichuan, China. Mt. Res. Dev, 32(2), 176-186.
DOI:10.1659/MRD-JOURNAL-D-11-00108.1 |
||
Poisot T., Stouffer D.B., Kéfi S., 2016. Describe, understand and predict:why do we need networks in ecology?. Funct. Ecol, 30(12), 1878-1882.
DOI:10.1111/fec.2016.30.issue-12 |
||
Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O., Kunin W.E., 2010. Global pollinator declines:trends, impacts and drivers. Trends Ecol. Evol, 25(6), 345-353.
DOI:10.1016/j.tree.2010.01.007 |
||
Proctor M., Yeo P., Lack A., 1996. The Natural History of Pollination. Timber Press, Portland, Oregon. |
||
Ren Z.X., Li D.Z., Bernhardt P., Wang H., 2011. Flowers of Cypripedium fargesii(Orchidaceae) fool flat-footed flies (Platypezidae) by faking fungus-infected foliage. Proc. Natl. Acad. Sci. U. S. A, 108(18), 7478-7480.
DOI:10.1073/pnas.1103384108 |
||
Ren Z.X., Wang H., Bernhardt P., Camilo G., Li D.Z., 2014a. Which food-mimic floral traits and environmental factors influence fecundity in a rare orchid, Calanthe yaoshanensis?. Bot. J. Linn. Soc, 176(3), 421-433.
DOI:10.1111/boj.2014.176.issue-3 |
||
Ren Z.X., Wang H., Bernhardt P., Li D.Z., 2014b. Insect pollination and selfincompatibility in edible and/or medicinal crops in southwestern China, a global hotspot of biodiversity. Am. J. Bot, 101, 1700-1710.
DOI:10.3732/ajb.1400075 |
||
Robart B.W., Gladys C., Frank T., Kilpatrick S., 2015. Phylogeny and biogeography of North American and Asian Pedicularis (Orobanchaceae). Syst. Bot, 40, 229-258.
DOI:10.1600/036364415X686549 |
||
Smith K., Loh E., Rostal M., Zambrana-Torrelio C., Mendiola L., Daszak P., 2014. Pathogens, Pests, and Economics:Drivers of Honey Bee Colony Declines and Losses. EcoHealth, pp :1-12.
|
||
Stökl J., Brodmann J., Dafni A., Ayasse M., Hansson B.S., 2011. Smells like aphids: orchid flowers mimic aphid alarm pheromones to attract hoverflies for pollination. Proc. Roy. Soc. Biol. Sci, 278(1709), 1216-1222.
DOI:10.1098/rspb.2010.1770 |
||
Stebbins G.L., 1970. Adaptive radiation of reproductive characteristics in angiosperms. Ⅰ. Pollination mechanisms. Annu. Rev. Ecol. Systemat, 1, 307-326.
DOI:10.1146/annurev.es.01.110170.001515 |
||
Su R., Dong Y., Dong K., He S.Y., 2012. The toxic honey plant Camellia oleifera. J. Apicult. Res, 51(3), 277-279.
DOI:10.3896/IBRA.1.51.3.09 |
||
Tang G.D., Ou J.H., Luo Y.B., Zhuang X.Y., Liu Z.J., 2014. A review of orchid pollination studies in China. J. Systemat. Evol, 52(4), 411-422.
DOI:10.1111/jse.v52.4 |
||
Tang M., Hardman C.J., Ji Y., Meng G., Liu S., Tan M., Yang S., Moss E.D., Wang J., Yang C., Bruce C., Nevard T., Potts S.G., Zhou X., Yu D.W., Gilbert M., 2015. High-throughput monitoring of wild bee diversity and abundance via mitogenomics. Methods Ecol. Evol, 6(9), 1034-1043.
DOI:10.1111/2041-210X.12416 |
||
Tang, Y., Xie, J. S., Chen, K. M., 2003. Hand Pollination of Pears and its Implications for Biodiversity Conservation and Environmental Protection: a Case Study from Hanyuan County, Sichuan Province, China. Unpublished report submitted to the International Centre for Integrated Mountain Development (ICIMOD). http://www.internationalpollinatorsinitiative.org/jsp/studies/studies.jsp. (Accessed 1 February 2014).
|
||
Tao Z.B., Ren Z.X., Wang W.J., Bernhardt P., Li H.D., Wang H., 2018. Nocturnal hawkmoth and moth pollination of Habenaria limprichtii Schltr. (Orchidaceae) in sub-alpine meadows of the Yulong Snow Mountain (Yunnan, China). Bot. J. Linn. Soc, 187(3), 483-498.
DOI:10.1093/botlinnean/boy023 |
||
Thomann M., Imbert E., Devaux C., Cheptou P.O., 2013. Flowering plants under global pollinator decline. Trends Plant Sci, 18, 353-359.
|
||
Tong Z.Y., Huang S.Q., 2016. Pre-and post-pollination interaction between six coflowering Pedicularis species via heterospecific pollen transfer. New Phytol, 211(4), 1452-1461.
DOI:10.1111/nph.14005 |
||
Verhoeven C., Ren Z.X., Lunau K., 2018. False-colour photography:a novel digital approach to visualize the bee view of flowers. J. Pollinat. Ecol, 23(12), 102-118.
|
||
Whitehead M.R., Lanfear R., Mitchell R.J., Karron J.D., 2018. Plant mating systems often vary widely among populations. Front. Ecol. Evol, 6, 38.
DOI:10.3389/fevo.2018.00038 |
||
Wang H., Li D.Z., 1998. A preliminary study of pollination biology of Pedicularis(Scrophulariaceae) in Northwest Yunnan, China. Acta Bot. Sin, 40, 204-210.
|
||
Williams P., Tang Y., Yao J., Cameron S., 2009. The bumblebees of Sichuan (Hymenoptera:Apidae, Bombini). Syst. Biodivers, 7(2), 101-189.
DOI:10.1017/S1477200008002843 |
||
Wu Y.R., 1977. The pollinating bees on Camellia olifera with descriptions of 4 new species of the genus Andrena. Acta Entomol. Sin, 2, 199-204.
|
||
Wu, Z. Y., Raven, P. H., Hong, D. Y., 2013. Flora of China. In: Introduction, vol. 1. Science Press, Beijing, China and Missouri Botanical Garden Press, St. Louis, USA.
|
||
Xie Z.H., Chen X.M., Qiu J.S., 2013. Reproductive failure of Camellia oleifera in the plateau region of China due to a shortage of legitimate pollinators. Int. J. Agric. Biol, 15, 458-464.
|
||
You M.S., Xu D.M., Cai H.J., Vasseur L., 2005. Practical importance for conservation of insect diversity in China. Biodivers. Conserv, 14(3), 723-737.
DOI:10.1007/s10531-004-3922-7 |
||
Zhao Y.H., Ren Z.X., Lazaro A., Wang H., Bernhardt P., Li H.D., Li D.Z., 2016. Floral traits influence pollen vectors' choices in higher elevation communities in the Himalaya-Hengduan Mountains. BMC Ecol, 16, 26.
DOI:10.1186/s12898-016-0080-1 |
||
Zhu, H. F., Wang, L. Y., 1997. Lepidoptera: Sphingidae. In: Sinica, C. o. F. (Ed. ), Fauna Sinica: Insecta. Science Press, Beijing.
|
||
Zou Y., Bianchi F.J.J.A., Jauker F., Xiao H.J., Chen J.H., Cresswell J., Luo S.D., Huang J.K., Deng X.Z., Hou L.L., van der Werf W., 2017. Landscape effects on pollinator communities and pollination services in small-holder agroecosystems. Agric. Ecosyst. Environ, 246, 109-116.
DOI:10.1016/j.agee.2017.05.035 |



