Yunnan Province, located in southwestern China, covers a total area of 394, 000 square kilometers, about 84% of which is mountainous. The highest point is located in the north with an elevation of 6740 m, while the lowest point is in the south with an elevation of only 76.4 m above sea level. The average altitude of the province, similar to central Yunnan, is around 2000 m (http://www.wcb.yn.gov.cn/arti?id=2).
The complex topography and geography, highly variable climate, luxuriant vegetation, warm and wet currents from the Indian and Pacific Oceans in summer, and other abiotic and biotic factors, provided ample refuges during the Quaternary glaciations (Sun et al., 2017), and make Yunnan a favorable region for the growth and reproduction of higher fungi. The extremely high fungal diversity in this area has attracted much attention from mycologists both at home and abroad.
Here we review studies of this area from the late 1880s to the present, based on which an up-to-date view is provided on the patterns of diversity of higher fungi in Yunnan and the mechanisms that drive that diversity, with a special emphasis on ecological, geographical and geological factors that may have influenced the diversity of local higher fungi.
2. Brief history of fungal diversity studiesAs early as the Ming Dynasty, several ancient Chinese scholars, such as Lan Mao (1397-1476) and Li Shi-zhen (1518-1593), paid close attention to fungi for medical purposes. Records show that modern mycological research in China has a history of over 100 years (Zang and Li, 2008). Studies on higher fungi in Yunnan started at the end of the 19th century. Several western missionaries and explorers collected fungal specimens, together with plant and animal samples, from Yunnan for scientific studies. For example, J. M. Delavay made fungal collections from northwestern Yunnan in the late 1880s, which were then studied by a French mycologist, N. Patouillard(1886, 1890). Bovistella yunnanensis Pat. (type, FH 1343) and Hemiglossum yunnanense Pat. (type, FH 4884) are probably the earliest novel species of higher fungi described from Yunnan (Patouillard, 1890). However, only a limited number of works were published thereafter. It was H. Handel-Mazzetti who collected a sizeable number of fungal specimens from Yunnan during the 1910s and deposited them at the Herbarium of the Institute of Botany, University of Vienna. His collections were examined by R. Singer (Singer, 1935) and by K. Keissler and H. Lohwag (Keissler and Lohwag, 1937).
In the 1940s, Prof. F. L. Tai from National Tsing Hua University, together with his colleagues and students, carried out important studies on fungi in Yunnan (Chiu, 1945, 1948a, b; Tai, 1944; Tai and Hung, 1948). Approximately 100 new species of Amanitaceae, Boletales, Geoglossaceae, Nidulariales and Russulaceae were discovered and described in Yunnan from 1944 to 1948. Reports by Teng (1963) and Tai (1979) published the most inclusive lists for higher fungi in China at the time and have been recognized as fundamental works for mycological research in China.
Over the past 45 years, significant progress, especially by field investigators, has been made on studies of the higher fungi of southwestern China. Supported by two projects, the Comprehensive Scientific Expeditions in the Qinghai-Tibet Plateau (including the Hengduan Mountains region, 1973-1983) and the Bioresources Survey and Evaluation (1988-1990), researchers at the Chinese Academy of Sciences surveyed higher fungi in the region. During this same period, more and more fungal species were reported from southwestern China (Ying et al., 1994; Zang et al., 1996), which significantly advanced our knowledge of higher fungi in the region.
From the 1880s to the beginning of the 21st century, species of higher fungi in China were recognized based mainly on morphological characters and partially on geographical distribution (Bandoni and Zang, 1990; Dai, 1999; Horak, 1987; Maekawa and Zang, 1995; Petersen and Zang, 1990; Yang, 1997; Ying et al., 1994; Zang, 2006, 2013; Zang et al., 1996; Zhuang et al., 2001). Over the past 20 or so years, molecular phylogenetic techniques have advanced rapidly and been used to study higher fungi in Yunnan. This has helped us better understand species diversity and systematics of several fungal groups, such as Agaricales, Boletales, Helotiales, Hymenochaetales, Pezizales, Polyporales, and Russulales (Chen et al., 2011; Chen et al., 2016; Cui et al., 2016; Dai, 2010; Ge and Yang, 2017; Ge et al., 2018; Gelardi et al., 2014; Han et al., 2016; Li et al., 2009, 2014a, 2014b; Popa et al., 2014; Song et al., 2014; Vincenot et al., 2017; Wang, 2017; Wang et al., 2009, 2015; Weiß et al., 1998; Wu et al., 2016a, 2016b; Zeng et al., 2013; Zhang and Dai, 2005; Zhang et al., 2004; Zhao et al., 2013a; Zhuang et al., 2016, 2017).
3. General features of higher fungi diversity in Yunnan 3.1. Two prominent regions of diversity in YunnanThere are two regions rich in fungal diversity in Yunnan. One is the Eastern Himalayas and Hengduan Mountains located in northwestern Yunnan; the other is the tropical region of southern and southwestern Yunnan.
The Eastern Himalayas and Hengduan Mountains region is one of the world's 35 biodiversity hotspots (Mittermeier et al., 2000, 2005; Myers et al., 2000). The southern boundary of this region reaches down to Yunnan. Over 4000 fungal species, representing about 40% of known fungal taxa in China, have been reported in this area (Yang, 2005). About 500 type specimens of higher fungi were collected from this region and deposited in the Mycological Herbarium of the Institute of Microbiology (HMAS), the Cryptogamic Herbarium of Kunming Institute of Botany of the Chinese Academy of Sciences (HKAS), and the herbarium of Beijing Forestry University (BJFC).
The Indo-Burma biodiversity hotspot partially overlaps the southern and southwestern parts of Yunnan, areas with typical tropical climate and vegetation (Myers et al., 2000; Yang, 2005). Over the last forty years, many studies on higher fungi in tropical Yunnan have been carried out (Yang and Zang, 2003; Zang, 1980, 1987; 2006, 2013; Zang et al., 1996; Zeng et al., 2013; Zhuang, 2001). In total, 5056 species of 1192 genera of higher fungi (including lichens) from the whole of tropical China were recorded in a monograph edited by Zhuang (2001). Some of these fungi are present only in tropical Yunnan (Li et al., 2015; Yang, 2000, 2015; Qin et al., 2014; Yang and Zang, 2003). Recently, new taxa of Agaricaceae and Cantharellaceae were described from tropical Yunnan (Ge and Yang, 2017; Shao et al., 2016).
3.2. Improved understanding of species diversity in higher fungi from YunnanTo date, 17, 427 species of vascular plants are known from Yunnan, including numerous endemic species (Zhang and Lu, 2017; Chen et al., 2017). Given that the numbers of fungi (including lichens) occurring in a given area are estimated to be about six times that of vascular plants (Hawksworth, 1991, 2001), about 104, 000 fungal species may exist in Yunnan. Currently, about 6000 fungal species, including around 3000 species of higher fungi, have been reported from Yunnan (Yang, 2005; Yang et al., 2017b), suggesting that 95% of fungal species in Yunnan are likely undocumented.
Here we present studies on boletes (especially on the family Boletaceae) as an example to show that species diversity of higher fungi in Yunnan has been significantly underestimated. During the 1940s, Prof. W. F. Chiu carried out an excellent taxonomic study on Boletales in Yunnan and published one monograph on this order, recording over 54 species, including 46 species (and 18 novel species from Yunnan) of Boletaceae (Chiu, 1948a). From 1978 to 2006, Prof. M. Zang from the Kunming Institute of Botany, Chinese Academy of Sciences and Prof. J. Z. Ying from the Institute of Microbiology, Chinese Academy of Sciences, together with their colleagues, described at least 27 novel species of Boletaceae from Yunnan (for references, see Fig. 1). With advances in molecular phylogenetics over the last ten years, the number of new species within Boletaceae in Yunnan has exploded. Since 2011, 87 new species have been added to this family (Cui et al., 2016; Li et al., 2011; Wu et al., 2016a, 2016b; Zeng et al., 2017; Zhu et al., 2014), 56 of which were described based on type specimens collected from Yunnan (Fig. 1). At the same time, many species found in Yunnan, traditionally proposed to be conspecific with those reported from Europe and North America, were clarified as endemic to this area, China or East Asia (Cui et al., 2016; Feng et al., 2012). For example, a total of 24 porcini species were reported from China, most from Yunnan, based mainly on morphological features (Chiu, 1948a; Zang, 2006). However, most species reported in Chiu's and Zang's work were named according to descriptions of species found from Europe, North America or tropical Asia. Only two species, Boletus violaceofuscus W.F. Chiu and B. reticuloceps (M. Zang et al.) Q.B. Wang & Y.J. Yao, were originally described from Yunnan and Sichuan, respectively (Chiu, 1948a; Zang et al., 1993). Recent research (Cui et al., 2016; Feng et al., 2012; Zeng et al., 2014), which integrated molecular phylogenetic analyses and more detailed morphological observations that specifically emphasized the structure of pileipellis, revealed that the Chinese porcini have diverged greatly from their European and North American relatives. Fifteen species, 12 new to science, were subsequently documented (Cui et al., 2016; Dentinger, 2013; Feng et al., 2012; Zeng et al., 2014). Three of the 12 new species, namely Boletus bainiugan Dentinger, B. shiyong Dentinger and B. sinoedulis B. Feng et al., are traditionally collected and consumed by local people, widely traded in free markets, and exported to Europe. However, they were named as B. edulis, a species originally described from Europe, for a very long time (Chiu, 1948a; Zang, 2006). Similarly, one famous delicacy called Dahongjun, or "big red mushroom, " which is consumed and traded in southern Yunnan and adjacent areas, was previously considered a European species, Russula vinosa Lindblad (Wang et al., 2009). However, it has been shown that this delicacy belongs to a species complex of at least three cryptic species (Li et al., 2010), and the species with a distribution in southern Yunnan was formally described as R. griseocarnosa X.H. Wang et al. (Wang et al., 2009). To correctly identify higher fungi in Yunnan remains a huge challenge, especially for species not commonly consumed by humans.
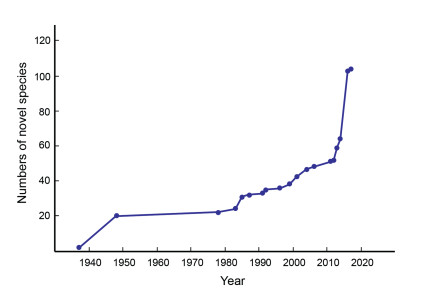
|
| Fig. 1 Numbers of novel species within Boleteceae described from Yunnan since 1937. Data were retrieved from Chiu (1948), Keissler and Lohwag (1937), Li et al. (2011), Li et al. (2014b), Wen and Ying (2001), Wu et al.(2016a, 2016b), Ying (1987), Ying and Ma (1985), Zang (1983), Zang (1985), Zang (1991), Zang (1992), Zang (1996), Zang and Petersen (2004), Zang and Zeng (1978), Zang and Zhang (2004), Zang et al. (1999), Zang et al. (2001), Zang et al. (2006), Zeng et al. (2017), Zeng et al. (2013), Zhao et al. (2014) and Zhu et al. (2014). |
Changes were also made in the systematics of some groups of higher fungi. For example, Wu et al. (2014) constructed a comprehensive molecular phylogenetic framework of the family Boletaceae by using sequences of four gene fragments, along with which four new subfamilies were described. Close to the same time, over 15 new genera were proposed (Hosen et al., 2013; Li et al., 2011, 2014b; Wu et al., 2016a, 2016b), seven of which were with type locations in Yunnan. This research has largely improved our knowledge of the classification of Boletaceae. For instance, 18 species described by Prof. W. F. Chiu (Chiu, 1948a) have recently been transferred to ten genera, nine of which were proposed after 2011 (Li et al., 2011; Wu et al., 2016a; Zhang et al., 2017; Zhu et al., 2015).
4. Floristic relationships of higher fungi in YunnanThe diversity of higher fungi in Yunnan may be the result of many factors, including but not limited to (1) geological events, (2) geographical factors, (3) diverse environments, and (4) coevolution with host plants (Yang, 2005). The floristic relationships of higher fungi in Yunnan to other parts of the world were elucidated mainly based on morphological features in the past (Yang and Zang, 2003; Zang, 1980, 1987). However, with the inclusion of more and more molecular data in phylogenetic analyses since the late 1990s, our understanding of the evolution of higher fungi in Yunnan, as well as those in China, East Asia and other continents of the world, has advanced significantly. Now, it is possible to construct more comprehensive molecular phylogenetic frameworks and illustrate geographical distribution patterns for certain fungal groups, which should help reveal evolutionary connections among fungal taxa from different continents, and address how such connections might have been formed (Cai et al., 2014; Du et al., 2012; Feng et al., 2012; Han et al., 2018).
4.1. Impacts of geological eventsThe mycobiota in China might have been influenced by orogeny, continental drifts, and climate changes in geological time. Since the collision of the Indian continent with Asia approximately 55 million years ago, tectonic activity in southwestern China has resulted in a series of dramatic landscape changes (Tapponnier et al., 2001) typified by the current topography of mountains and valleys nestled against the highest plateau on Earth. Due to the large-scale geological uplifts of the Qinghai-Tibet Plateau during the late Pliocene and Pleistocene, the arid areas in western and northwestern China spread further north and cover a much wider area (Zhang and Fang, 2012). Consequently, the fungal dispersals between East Asia and Europe virtually disappeared (Yang, 2005; Zhao et al., 2016a, b). Populations of the same species in East Asia and Europe, which were once continuously distributed across the Eurasian continent, may evolve independently and allopatric speciation may occur. This hypothesis has been supported by observations on the evolution of several fungal groups, such as Morchella, Boletus, Amanita sect. Phalloideae, and Laccaria (Du et al., 2012; Cai et al., 2014; Feng et al., 2012; Vincenot et al., 2017). Some closely related species, like Boletus reticulatus Schaeff. and B. bainiugan, can be found in Europe and southwestern China (Feng et al., 2012; Cui et al., 2016). In contrast, several species of Hydnum were shown to be shared by Europe and southwestern China, including Yunnan (Feng et al., 2016a). A possible explanation is that the Tethys region would have acted as a corridor for the dispersal of higher fungi between southwestern China and Europe. This hypothesis has been used to explain the discontinuous distribution of several plants in Europe and southwestern China (Sun, 2001).
Disjunctive distributions between East Asia and North America have been observed in several fungal groups, such as Boletus, Morchella, Hydnum and Strobilomyces, indicating possible historical mycobiota exchanges between these two regions (Du et al., 2012; Feng et al., 2012, 2016a; Han et al., 2018). Such historical exchanges would have been mediated by the Beringia route, which has repeatedly appeared and disappeared during different geological times, owing to historical climate fluctuations. Similarly, mainland East Asia was historically connected to Japan until the Last Glacial Maximum, which may have facilitated dispersals of fungal species between China and Japan. This has been supported by a recent study on Strobilomyces, in which 11 of 13 species were found to be shared by Japan and China (especially Yunnan) (Han et al., 2018). Other higher fungi shared by Japan and southwestern China include the species complex of Tuber indicum Cooke & Massee and Singerocybe alboinfundibuliformis (S.J. Seok et al.) Zhu L. Yang, J. Qin & Har. Takah (Kinoshita et al., 2011, 2018; Qin et al., 2014).
Historical fungal exchanges might have also happened between Southeast Asia and Yunnan. Han et al. (2018) indicated that after the African fungus Strobilomyces reached Southeast Asia through the boreotropics, it continued to migrate southward to Australia using continental fragments as "stepping-stones" between Australia and Asia, and then moved northward to East Asia via Yunnan. Thus, Southeast Asia might serve as a bridge for bidirectional migrations of fungi between Laurasia and Australia. The sharing of fungal species between Yunnan and Southeast Asia has also been observed in Hydnum and Cyptotrama (Feng et al., 2016a; Qin and Yang, 2016).
Southwestern China is adjacent to South Asia. The Himalayas, formed by the uplifting of the Qinghai-Tibet Plateau, may act as a barrier for the biota exchanges between China and countries located to the west of the Himalayas. However, some fungal species in subalpine forests or alpine meadows, like Boletus reticuloceps and Ophiocordyceps sinensis (Berk.) G.H. Sung et al., co-occur both east and west of the Himalayas (Thapa et al., 2014; unpublished data). As taxonomic studies on higher fungi in the western Himalayas are quickly being accumulated, such distribution patterns are expected for more fungi in this region. Furthermore, evolutionary questions about how such distribution patterns have formed and the role the Himalayas plays in the microevolutionary processes of these fungi are worthy of further exploration.
4.2. Geographical factorsGeographical isolation, followed by allopatric speciation, would also have contributed to the endemic fungal diversity in southwestern China, as suggested by studies on several ectomycorrhizal and saprophytic fungal groups, such as Chroogomphus, Phylloporus and Singerocybe (Li et al., 2009; Qin et al., 2014; Zeng et al., 2013). For example, C. tomentosus (Murrill) O.K. Mill. was once assumed to be a putative disjunct species between western North America and East Asia (e.g., Imai, 1938; Redhead, 1989), but later studies revealed that the East Asian populations represent a distinct species related to C. tomentosus (Miller, 2003; Miller and Aime, 2001). Recently, geographical divergences of Chroogomphus in the Northern Hemisphere were further evidenced. Li et al. (2009) indicated that although several vicariously paired or closely related species of Chroogomphus were present in East Asia and North America, most species had relatively narrow distribution ranges in Far East Asia and central Europe.
4.3. Diverse environmentsClimate, especially temperature and precipitation, affects the growth and distribution of fungi. The climate in Yunnan is significantly affected by monsoons. Monsoons bring abundant rainfall, and, as a result, humid tropical evergreen rainforests cover the southern part of the province. Central Yunnan generally has a subtropical climate, while the northern part shows a typical alpine climate. The diverse climate types and environments allow different fungi to specialize and thrive. For instance, Imleria parva Xue T. Zhu & Zhu L. Yang grows exclusively under subtropical mixed forests of Pinus and Castanopsis in southern Yunnan; I. obscurebrunnea (Hongo) Xue T. Zhu & Zhu L. Yang is distributed in forests dominated by Fagaceae in southern Yunnan; whereas I. subalpina Xue T. Zhu & Zhu L. Yang can only be found from subalpine forests with Picea and Abies as structural species (Zhu et al., 2014). Similarly, we observed that Tricholoma highlandense Zhu L. Yang et al. occurs on acid red soil in forests dominated by Pinus yunnanensis in the Yunnan Plateau between elevations of 2400 and 2800 m, while T. sinopardinum Zhu L. Yang et al. grows on calcareous soil in forests dominated by Picea, Betula, Populus, or Quercus in subalpine areas between elevations of 3300 and 4100 m (Yang et al., 2017a).
4.4. Co-evolution between fungi and host plants and fungal specificity to substratesPlants of Pinaceae, Fagaceae, Betulaceae, Salicaceae, and Dipterocarpaceae are abundantly distributed in Yunnan, providing ideal and diverse niches for various ectomycorrhizal higher fungi (Halling, 2001). Previous research has suggested that ecological speciation might have contributed to the high diversity of fungi (Li et al., 2009). For example, during the co-evolution of Chroogomphus and its hosts, host specificity generally increased, and host specificity would have driven some sympatric speciation events. For instance, C. rutilus (Schaeff.) O.K. Mill. had a sympatric distribution pattern with C. purpurascens (Lj.N. Vassiljeva) M.M. Nazarova, but with plant hosts in different subgenera of Pinus (Li et al., 2009).
Substrate specificity may drive speciation and evolution of saprotrophic fungi (Qin et al., 2018). For example, most species of the genus Strobilurus grow on decaying cones of various conifers. It was recently shown that some sympatric species of this genus are specific to certain substrates (Qin et al., 2018). For instance, S. orientalis Zhu L. Yang & J. Qin and S. pachycystidiatus Zhu L. Yang & J. Qin are restricted to dead cones of Pinus armandii, a five-needle pine, while S. luchuensis Har. Takah. & Taneyama only occurs on dead cones of three-needle pines, such as P. yunnanensis, and P. luchuensis. These findings suggest that substrate specificity might have contributed to the evolution of Strobilurus, in addition to geographical migration and vicariance events (Qin et al., 2018).
5. Microevolution of higher fungi in Yunnan and adjacent regionsThree of the world's 35 biodiversity hotspots, Mountains of Southwest China, Eastern Himalaya-Nepal-India and Indo-BurmaIndia-Myanmar, intersect in Yunnan (Mittermeier et al., 2005; Myers et al., 2000). This makes mountainous Yunnan and its adjacent regions ideal for studying the evolution of plants, animals, and higher fungi. Numerous phylogeographical studies have been carried out, mainly on plants and animals, to understand their evolutionary histories and the factors that drive their evolution. Two important hypotheses, "isolation of rivers" (Zhang et al., 2010, 2011) and "isolation of sky islands" (He and Jiang, 2014; Luo et al., 2015), have been proposed. These two hypotheses identify large rivers and high mountains as gene flow barriers for river-associated species and subalpine/alpine organisms respectively. Recent phylogeographic studies on the Tuber indicum species complex and the Alpine Porcini, Boletus reticuloceps, indicated that multiple factors, in addition to big rivers and high mountains, might have shaped the evolution of higher fungi in Yunnan and adjacent regions (Feng et al., 2016b, 2017; Fig. 2).
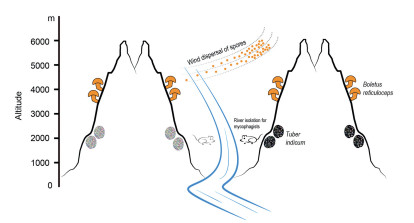
|
| Fig. 2 A diagram showing how large rivers and high mountains may influence gene flow of hypogenous and above-ground mushrooms. Rivers may act as barriers to migration for mycophagists like mice and thus block gene flow of hypogenous mushrooms (like Tuber indicum). In contrast, above-ground mushrooms (like Boletus reticuloceps) can disperse their spores via winds and thus high mountains cannot block gene flow of such fungi. These conclusions are summarized from Feng et al. (2016b) and Feng et al. (2017). |
The species complex of Tuber indicum, known as the Chinese black truffle, is distributed in southwestern China, Taiwan island of China, and Japan (Chen et al., 2011; Kinoshita et al., 2011, 2018; Qiao et al., 2018; Zhang et al., 2005). In southwestern China, its distribution is restricted to Yunnan and Sichuan, where three large rivers, Nujiang, Lancangjiang and Jinshajiang Rivers, run through. The species complex in this region contains two cryptic species, namely T. indicum Cooke & Massee and T. himalayense B.C. Zhang & Minter, although the nomenclature of these two species remains unresolved (Chen et al., 2011; Qiao et al., 2018; Zhang et al., 2005). By analyzing sequences from four gene fragments for over 300 individuals of T. indicum collected in its known distribution range, Feng et al. (2016b) revealed that large rivers in Yunnan and Sichuan could act as barriers to gene flow among different populations of T. indicum. Three major geographic groups, namely W, C and N, can be found in areas to the west of the paleo-Red River, between east of the paleo-Red River and south of the Jinshajiang River, and to the north of Jinshajiang River. As a hypogeous fungus that produces belowground ascocarps, the dispersal of spores of T. indicum is mainly dependent on the help of mycophagists, like mice and other small rodents (Maser et al., 2008). The existence of large rivers may form barriers to migration for such mycophagists, indirectly blocking gene flow of T. indicum (Feng et al., 2016b).
Boletus reticuloceps is an ectomycorrhizal fungus exclusively distributed under subalpine forests dominated by Picea and Abies. Its distribution pattern is similar to plants and animals that show an "isolated by sky islands" genetic structure. Surprisingly, Feng et al. (2017) suggested that gene flow of B. reticuloceps was not limited by high mountains in certain ranges, which means that "isolation of sky islands" may not efficiently influence the evolution of this fungus. One explanation may be that the on-ground fruiting bodies of B. reticuloceps bear spores that can be spread by wind over certain distances (less than 300 km, unpublished data). Despite this, three genetic groups, distributed in the East Himalayas (EH), northern Hengduan Mountains (NHM), and southern Hengduan Mountains (SHM), respectively, were identified in B. reticuloceps. Further analyses showed that ecological heterogeneity (especially precipitations and host plants) could have contributed to the divergence between the SHM and the NHM-EH groups, while physical barriers (the Mekong-Salween Divide) could have led to the divergence of the NHM and the EH groups (Feng et al., 2017).
6. Concluding remarksThis review and discussion has focused on studies using morphological characters, along with molecular evidence available since the beginning of the 21st century. As indicated by Wu et al. (2000), studies based on morphological characters alone can hardly provide a comprehensive understanding of fungal diversity and evolution. In order to further understand the diversity of higher fungi in Yunnan, special emphasis should be placed on (1) more international collaboration on fungal diversity, (2) reconstruction of molecular phylogenetic frameworks for fungal groups in higher ranks (such as order) and research on the evolution and ecological adaptations of higher fungi using genome-scale data, and (3) integration of multi-disciplinary approaches to investigate associations among higher fungi and other organisms, like host plants, and between fungi and environments.
6.1. Understanding fungal diversity and relationships from a global perspectiveAs summarized above, higher fungi in Yunnan show a great deal of endemism but also have evolutionary connections with species from other regions of the world. However, to date only limited studies have considered fungal groups worldwide (Han et al., 2018; Hosaka et al., 2008; Matheny et al., 2009; Skrede et al., 2011). Thus, additional international collaborations are urgently needed to improve our understanding of fungal diversity and evolution from a global perspective.
6.2. Applying genome-wide analysis to study systematics, ecology and adaptations of higher fungiFrom a single gene to multiple genes, the use of DNA sequences in phylogenetic analyses has revolutionized the study of fungal systematics, and this revolution is still being accelerated with the rapid development of sequencing techniques. Pioneering studies have exhibited the usefulness of genome-scale data and even genome sequences for the study of fungal systematics (Binder et al., 2013; Sato et al., 2017). A set of 80-gene data has helped better resolve relationships among different species of Strobilomyces (Sato et al., 2017). The small size of fungal genomes (about tens to hundreds Mb) makes genome sequencing for fungi much cheaper than that of most animals and plants. In addition, the costs for genome sequencing are gradually decreasing. Therefore, the application of genome sequences in the systematics of more and more fungal groups, especially at higher hierarchical ranks, like families and orders, is expected.
The use of genome sequences in studies of higher fungi is not restricted to systematics. There are many higher fungi that form ectomycorrhizal symbioses with several plant families, like Pinaceae, Fagaceae and Betulaceae. Several famous edible mushrooms in Yunnan, like boletes, truffles and T. matsutake (S. Ito & S. Imai) Singer, are ectomycorrhizal species. By using genome information, we can better understand how these commercial fungi interact with their host plants and subsequently provide guidelines for the artificial cultivation of these delicacies. Recently, the mystery of this mutualism has been partially uncovered by using genome and transcriptome information, involving a reduced complement of genes encoding plant cell wall-degrading enzymes (PCWDEs) and the addition of several mycorrhiza-induced small secreted proteins (MiSSPs) (Kohler et al., 2015; Martin et al., 2016). Interestingly, most of such MiSSPs are lineage-specific (Martin et al., 2016).
Ecological adaptation is a hot topic in evolutionary studies. In Yunnan and adjacent regions, several studies have been conducted to understand how organisms (especially animals) adapt to high elevations (Qiu et al., 2012; Yu et al., 2016; Zhao et al., 2013b; Zhou et al., 2014). There are numerous higher fungi distributed at high elevations, and how they adapt to such harsh environments remains unknown. Our current phylogeographic study on Boletus reticuloceps has suggested that ecological heterogeneity might have driven divergences between southern and northern populations in the Hengduan Mountains (Feng et al., 2017). Further study employing genome/transcriptome sequencing may help reveal the mechanism that drives the ecological adaptation of higher fungi like B. reticuloceps.
6.3. Interpreting interactions between higher fungi and environmentsHigher fungi are important components of certain ecosystems, actively interacting with their living environments. The existence of certain ectomycorrhizal fungal group may influence the distribution of their host plants, and vice versa. Understanding such interactions between higher fungi and their living environments is a key goal in ecological studies and has attracted scientists' attention for a long time. Some attempts to elucidate these interactions in southern China have been made (Gao et al., 2013, 2015). Yunnan harbors a great deal of diverse vegetation. In certain places, subtropical, temperate and subalpine forests are distributed vertically within distances as small as only tens of kilometers, making it ideal to address how higher fungi interact with different environments to form their current distributions. Such study urgently needs the integration of mycological and ecological approaches.
AcknowledgementsThe authors are very grateful to Dr. Hong Luo for improving the English. The two anonymous reviewers are acknowledged for their insightful comments on the manuscript. This work was financed by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000), the National Natural Science Foundation of China (31770030) and Yunnan Applied Basic Research Project (2017FB022)
Bandoni R.J., Zang M., 1990. On an undescribed Tremella from China. Mycologia, 82, 270-273.
DOI:10.1080/00275514.1990.12025876 |
||
Binder M., Justo A., Riley R., et al., 2013. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia, 105, 1350-1373.
DOI:10.3852/13-003 |
||
Cai Q., Tulloss R.E., Tang L.P., et al., 2014. Multi-locus phylogeny of lethal amanitas:implications for species diversity and historical biogeography. BMC Evol. Biol, 14, 143.
DOI:10.1186/1471-2148-14-143 |
||
Chen J., Guo S.-X., Liu P.-G., 2011. Species recognition and cryptic species in the Tuber indicum complex. PLoS One, 6, e14625.
DOI:10.1371/journal.pone.0014625 |
||
Chen, J. H., Deng, T., Zhang, D. G., et al., 2017. Spermatophyta. In: Gao, Z. W., Sun, H. (Eds. ), Checklist of Living Species in Yunnan. Yunnan Publishing Group, Yunnan Science and Technology Press, Kunming, pp. 177-510.
|
||
Chen J.J., Cui B.K., Dai Y.C., 2016. Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia, 37, 21-36.
DOI:10.3767/003158516X689666 |
||
Chiu W.F., 1945. The Russulaceae of Yunnan. Lloydia, 8, 31-59.
|
||
Chiu W.F., 1948a. The boletes of Yunnan. Mycologia, 40, 199-231.
DOI:10.2307/3755085 |
||
Chiu W.F., 1948b. The Amanitaceae of Yunnan. Sci. Rept. Natl. Tsing Hua Univ. Ser. B, Biol. Psychol. Sci, 3(3), 165-178.
|
||
Cui Y.Y., Feng B., Wu G., et al., 2016. Porcini mushrooms (Boletus sect. Boletus) from China. Fungal Divers, 81, 189-212.
DOI:10.1007/s13225-015-0336-7 |
||
Dai Y.C., 1999. Phellinus sensu lato (Aphyllophorales, Hymenochaetales) in East Asia. Acta Bot. Fenn, 166, 1-115.
|
||
Dai Y.C., 2010. Hymenochaetaceae (Basidiomycota) in China. Fungal Divers, 45, 131-343.
DOI:10.1007/s13225-010-0066-9 |
||
Dentinger B.T., 2013. Nomenclatural novelties. Index Fungorum, 29, 1.
|
||
Du X.H., Zhao Q., O'Donnell K., et al., 2012. Multigene molecular phylogenetics reveals true morels (Morchella) are especially species-rich in China. Fungal Genet. Biol, 49, 455-469.
DOI:10.1016/j.fgb.2012.03.006 |
||
Feng B., Liu J.W., Xu J., et al., 2017. Ecological and physical barriers shape genetic structure of the Alpine Porcini (Boletus reticuloceps). Mycorrhiza, 27, 261-271.
DOI:10.1007/s00572-016-0751-y |
||
Feng B., Wang X.H., Ratkowsky D., et al., 2016a. Multilocus phylogenetic analyses reveal unexpected abundant diversity and significant disjunct distribution pattern of the Hedgehog Mushrooms (Hydnum L.). Sci. Rep, 6, 25586.
DOI:10.1038/srep25586 |
||
Feng B., Xu J., Wu G., et al., 2012. DNA sequence analyses reveal abundant diversity, endemism and evidence for Asian origin of the porcini mushrooms. PLoS One, 7, e37567.
DOI:10.1371/journal.pone.0037567 |
||
Feng B., Zhao Q., Xu J., et al., 2016b. Drainage isolation and climate change-driven population expansion shape the genetic structures of Tuber indicum complex in the Hengduan Mountains region. Sci. Rep, 6, 21811.
DOI:10.1038/srep21811 |
||
Gao C., Shi N.-N., Liu Y.-X., et al., 2013. Host plant genus-level diversity is the best predictor of ectomycorrhizal fungal diversity in a Chinese subtropical forest. Mol. Ecol, 22, 3403-3414.
DOI:10.1111/mec.12297 |
||
Gao C., Zhang Y., Shi N.-N., et al., 2015. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. New Phytol, 205, 771-785.
DOI:10.1111/nph.13068 |
||
Ge Z.-W., Jacobs A., Vellinga E.C., et al., 2018. A multi-gene phylogeny of Chlorophyllum (Agaricaceae, Basidiomycota):new species, new combination and infrageneric classification. MycoKeys, 32, 65-90.
DOI:10.3897/mycokeys.32.23831 |
||
Ge Z.-W., Yang Z.-L., 2017. Pseudolepiota zangmui gen. et sp nov. (Agaricaceae, Basidiomycota), a new white-spored mushroom from China. Phytotaxa, 312, 247-255.
DOI:10.11646/phytotaxa.312.2 |
||
Gelardi M., Vizzini A., Horak E., et al., 2014. Paxillus orientalis sp. nov. (Paxillaceae, Boletales) from south-western China based on morphological and molecular data and proposal of the new subgenus Alnopaxillus. Mycol. Prog, 13, 333-342.
DOI:10.1007/s11557-013-0919-1 |
||
Halling R.E., 2001. Ectomycorrhizae:Co-evolution, significance, and biogeography. Ann. Mo. Bot. Gard, 88, 5-13.
DOI:10.2307/2666128 |
||
Han L.H., Feng B., Wu G., et al., 2018. African origin and global distribution patterns:evidence inferred from phylogenetic and biogeographical analyses of ectomycorrhizal fungal genus Strobilomyces. J. Biogeogr, 45, 201-212.
DOI:10.1111/jbi.13094 |
||
Han M.L., Chen Y.Y., Shen L.L., et al., 2016. Taxonomy and phylogeny of the brownrot fungi:Fomitopsis and its related genera. Fungal Divers, 80, 343-373.
DOI:10.1007/s13225-016-0364-y |
||
Hawksworth D.L., 1991. The fungal dimension of biodiversity:magnitude, significance, and conservation. Mycol. Res, 95, 641-655.
DOI:10.1016/S0953-7562(09)80810-1 |
||
Hawksworth D.L., 2001. The magnitude of fungal diversity:the 1.5 million species estimate revisited. Mycol. Res, 105, 1422-1432.
DOI:10.1017/S0953756201004725 |
||
He K., Jiang X., 2014. Sky islands of Southwest China. Ⅰ:an overview of phylogeographic patterns. Chin. Sci. Bull, 59, 585-597.
DOI:10.1007/s11434-013-0089-1 |
||
Horak E., 1987. Agaricales from Yunnan, China Ⅰ. Trans. Mycol. Soc. Jap, 28, 171-188.
|
||
Hosaka K., Castellano M.A., Spatafora J.W., 2008. Biogeography of Hysterangiales(Phallomycetidae, Basidiomycota). Mycol. Res, 112, 448-462.
DOI:10.1016/j.mycres.2007.06.004 |
||
Hosen M.I., Feng B., Wu G., et al., 2013. Borofutus, a new genus of Boletaceae from tropical Asia:phylogeny, morphology and taxonomy. Fungal Divers, 58, 215-226.
DOI:10.1007/s13225-012-0211-8 |
||
Imai S., 1938. Studies Agaricaceae of Hokkaido. Ⅱ. Journ. Facul. Agr. Hokk.Imper. Univ, 43, 179-378.
|
||
Keissler, K., Lohwag, H., 1937. Fungi. In: Handel-Mazzetti H (Hrg. ). Symbolae Sinicae, Band 2. Julius Springer, Wien.
|
||
Kinoshita A., Nara K., Sasaki H., et al., 2018. Using mating-type loci to improve taxonomy of the Tuber indicum complex, and discovery of a new species, T. longispinosum. PLoS One, 13, e0193745.
DOI:10.1371/journal.pone.0193745 |
||
Kinoshita A., Sasaki H., Nara K., 2011. Phylogeny and diversity of Japanese truffles(Tuber spp.) inferred from sequences of four nuclear loci. Mycologia, 103, 779-794.
DOI:10.3852/10-138 |
||
Kohler A., Kuo A., Nagy L.G., et al., 2015. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet, 47, 410-415.
DOI:10.1038/ng.3223 |
||
Li H.J., Cui B.K., Dai Y.C., 2014a. Taxonomy and multi-gene phylogeny of Datronia(Polyporales, Basidiomycota). Persoonia, 32, 170-182.
DOI:10.3767/003158514X681828 |
||
Li M.C., Liang J., Li Y., et al., 2010. Genetic diversity of Dahongjun, the commercially important "Big Red Mushroom" from southern China. PLoS One, 5, e10684.
DOI:10.1371/journal.pone.0010684 |
||
Li Y., Li T.H., Yang Z.L., et al., 2015. Atlas of Chinese Macrofungal Resources. Central China Farmers' Publishing House, Zhengzhou. |
||
Li Y.C., Feng B., Yang Z.L., 2011. Zangia, a new genus of Boletaceae supported by molecular and morphological evidence. Fungal Divers, 49, 125-143.
DOI:10.1007/s13225-011-0096-y |
||
Li Y.-C., Ortiz-Santana B., Zeng N.-K., et al., 2014b. Molecular phylogeny and taxonomy of the genus Veloporphyrellus. Mycologia, 106, 291-306.
DOI:10.3852/106.2.291 |
||
Li Y.C., Yang Z.L., Tolgor B., 2009. Phylogenetic and biogeographic relationships of Chroogomphus species as inferred from molecular and morphological data. Fungal Divers, 38, 85-104.
|
||
Luo D., Yue J.P., Sun W.G., et al., 2015. Evolutionary history of the subnival flora of the Himalaya-Hengduan Mountains:first insights from comparative phylogeography of four perennial herbs. J. Biogeogr, 43, 31-43.
|
||
Maekawa N., Zang M., 1995. Corticiaceous fungi (Aphyllophorales, Basidiomycotina) collected in Yunnan, China. Bull. Nat. Sci. Mus. (Tokyo) Ser. B, 21, 87-94.
|
||
Martin F., Kohler A., Murat C., et al., 2016. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol, 14, 760-773.
DOI:10.1038/nrmicro.2016.149 |
||
Matheny P.B., Aime M.C., Bougher N.L., et al., 2009. Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. J. Biogeogr, 36, 577-592.
DOI:10.1111/jbi.2009.36.issue-4 |
||
Maser C., Claridge A.W., Trappe J.M., 2008. Trees, Truffles, and Beasts:How Forests Function. Rutgers University Press, New Jersey. |
||
Miller O.K., 2003. The Gomphidiaceae revised:a worldwide perspective. Mycologia, 95, 176-183.
DOI:10.1080/15572536.2004.11833147 |
||
Miller, O. K., Aime, M. C., 2001. Systematics, ecology and world distribution in the genus Chroogomphus (Gomphidiaceae). In: Misra, J. K., Horn, B. W. (Eds. ), Trichomycetes and Other Fungal Groups. Sceince Publishers Inc., Enfield, pp. 314-333.
|
||
Mittermeier, R. A., Gil, P. R., Hoffman, M., et al., 2005. Hotspots Revisited: Earth's Biologically Richest and Most Endangered Terrestrial Ecoregions. Conservation International and Agrupacion Sierra Madre, Monterrey.
|
||
Mittermeier, R. A., Myers, N., Mittermeier, C. G., 2000. Hotspots: Earth's Biologically Richest and Most Endangered Terrestrial Ecoregions. CEMEX, Mexico City.
|
||
Myers N., Mittermeier R.A., Mittermeier C.G., et al., 2000. Biodiversity hotspots for conservation priorities. Nature, 403, 853-858.
DOI:10.1038/35002501 |
||
Patouillard N., 1886. Quelques champignons de la Chine récolté par M. l'abbé Delavay dans la province du Yunnan. Rev. Mycol. Toulouse, 8, 179-182.
|
||
Patouillard N., 1890. Quelques champignons de la Chine récolté par M. l'abbé Delavay. Rev. Mycol. Toulouse, 12, 133-136.
|
||
Petersen R.H., Zang M., 1990. Ramaria subgenera Ramaria and Laeticora in Yunnan. Acta Bot. Yunnanica, 11, 363-396.
|
||
Popa F., Rexer K.-H., Donges K., et al., 2014. Three new Laccaria species from Southwest China (Yunnan). Mycol. Prog, 13, 1105-1117.
|
||
Qiao P., Tian W., Liu P., et al., 2018. Phylogeography and population genetic analyses reveal the speciation of the Tuber indicum complex. Fungal Genet. Biol, 113, 14-23.
DOI:10.1016/j.fgb.2018.02.001 |
||
Qin J., Feng B., Yang Z.L., et al., 2014. The taxonomic foundation, species circumscription and continental endemisms of Singerocybe:evidence from morphological and molecular data. Mycologia, 106, 1015-1026.
DOI:10.3852/13-338 |
||
Qin, J., Horak, E., Popa, F., et al., 2018. Species diversity, distribution patterns and substrate specificity of Strobilurus. Mycologia. https://doi.org/10.1080/00275514.2018.1463064.
|
||
Qin J., Yang Z.L., 2016. Cyptotrama (Physalacriaceae, Agaricales) from Asia. Fung.Biol, 120, 513-529.
DOI:10.1016/j.funbio.2016.01.009 |
||
Qiu Q., Zhang G., Ma T., et al., 2012. The yak genome and adaptation to life at high altitude. Nat. Genet, 44, 946-949.
DOI:10.1038/ng.2343 |
||
Redhead S.A., 1989. A biogeographical overview of the Canadian mushroom flora. Can. J. Bot, 67, 3003-3062.
DOI:10.1139/b89-384 |
||
Sato H., Tanabe A.S., Toju H., 2017. Host shifts enhance diversification of ectomycorrhizal fungi:diversification rate analysis of the ectomycorrhizal fungal genera Strobilomyces and Afroboletus with an 80-gene phylogeny. New Phytol, 214, 443-454.
DOI:10.1111/nph.14368 |
||
Shao S.-C., Buyck B., Tian X.-F., et al., 2016. Cantharellus phloginus, a new pinkcolored species from southwestern China. Mycoscience, 57, 144-149.
DOI:10.1016/j.myc.2015.12.004 |
||
Singer R., 1935. Supplemente zu meiner Monographie der Gattung Russula. Ann. Mycol, 33, 297-352.
|
||
Skrede I., Engh I.B., Binder M., et al., 2011. Evolutionary history of Serpulaceae(Basidiomycota):molecular phylogeny, historical biogeography and evidence for a single transition of nutritional mode. BMC Evol. Biol, 11, 230.
DOI:10.1186/1471-2148-11-230 |
||
Song J., Chen Y.Y., Cui B.K., et al., 2014. Morphological and molecular evidence for two new species of Laetiporus (Basidiomycota, Polyporales) from southwestern China. Mycologia, 106, 1039-1050.
DOI:10.3852/13-402 |
||
Sun H., 2001. Tethys retreat and Himalayas-Hengduanshan Mountains uplift and their significance on the origin and development of the Sino-Himalayan elements and alpine flora (in Chinese). Acta Bot. Yunnanica, 24, 273-288.
|
||
Sun H., Zhang J.W., Deng T., et al., 2017. Origins and evolution of plants diversity in the Hengduan Mountains, China. Plant Divers, 39, 161-166.
DOI:10.1016/j.pld.2017.09.004 |
||
Tai F.L., 1944. Studies in the Geoglossaceae of Yunnan. Lloydia, 7, 146-162.
|
||
Tai F.L., 1979. Sylloge Fungorum Sinicorum. Science Press, Beijing. |
||
Tai F.L., Hung C.S., 1948. Nidulariales of Yunnan. Sci. Rep. Nat. Tsing Hua Univ, 3, 30-40.
|
||
Tapponnier P., Xu Z.Q., Roger F., et al., 2001. Oblique stepwise rise and growth of the Tibet Plateau. Science, 294, 1671-1677.
DOI:10.1126/science.105978 |
||
Teng S.C., 1963. Fungi of China. Science Press, Beijing. |
||
Thapa D.D., Panthi S., Rai R.K., et al., 2014. An assessment of Yarsagumba(Ophiocordyceps sinensis) collection in Dhorpatan Hunting Reserve. Nepal. J. Mt. Sci, 11, 555-562.
DOI:10.1007/s11629-013-2692-7 |
||
Vincenot L., Popa F., Laso F., et al., 2017. Out of Asia:biogeography of fungal populations reveals Asian origin of diversification of the Laccaria amethystina complex, and two new species of violet Laccaria. Fungal Biol, 121, 939-955.
DOI:10.1016/j.funbio.2017.08.001 |
||
Wang X.H., 2017. Seven new species of Lactarius subg. Lactarius (Russulaceae) from southwestern China. Mycosystema, 36, 1463-1482.
|
||
Wang X.H., Buyck B., Verbeken A., et al., 2015. Revisiting the morphology and phylogeny of Lactifluus with three new lineages from southern China. Mycologia, 107, 941-958.
DOI:10.3852/13-393 |
||
Wang X.H., Yang Z.L., Li Y.C., et al., 2009. Russula griseocarnosa sp. nov. (Russulaceae, Russulales), a commercially important edible mushroom in tropical China:mycorrhiza, phylogenetic position, and taxonomy. Nova Hedwigia, 88, 269-282.
DOI:10.1127/0029-5035/2009/0088-0269 |
||
Weiß M., Yang Z.L., Oberwinkler F., 1998. Molecular phylogenetic studies in the genus Amanita. Can. J. Bot, 76, 1170-1179.
|
||
Wen H.-A., Ying J.-Z., 2001. Supplementary notes on the genus Strobilomyces from China â…¡. Mycosystema, 20, 297-300.
|
||
Wu G., Feng B., Xu J., et al., 2014. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers, 69, 93-115.
DOI:10.1007/s13225-014-0283-8 |
||
Wu G., Li Y.C., Zhu X.T., et al., 2016a. One hundred noteworthy boletes from China. Fungal Divers, 81, 25-188.
DOI:10.1007/s13225-016-0375-8 |
||
Wu G., Zhao K., Li Y.C., et al., 2016b. Four new genera of the fungal family Boletaceae. Fungal Divers, 81, 1-24.
DOI:10.1007/s13225-015-0322-0 |
||
Wu Q.X., Mueller G.M., Lutzoni F.M., et al., 2000. Phylogenetic and biogeographic relationships of eastern Asian and eastern North American disjunct Suillus species (Fungi) as inferred from nuclear ribosomal RNA ITS sequences. Mol.Phylog. Evol, 17, 37-47.
DOI:10.1006/mpev.2000.0812 |
||
Yang Z.L., 1997. Die Amanita-Arten von Südwestchina. Bibl. Mycol, 170, 1-240.
|
||
Yang Z.L., 2000. Notes on five common but little known higher Basidiomycetes from tropical Yunnan, China. Mycotaxon, 74, 45-56.
|
||
Yang, Z. L., 2005. Diversity and biogeography of higher fungi in China. In: Xu, J. (Ed. ), Evolutionary Genetics of Fungi. Horizon Bioscience, Norfolk, pp. 35-62.
|
||
Yang Z.L., 2015. Atlas of the Chinese Species of Amanitaceae. Science Press, Beijing. |
||
Yang Z.L., Ding X.X., Kost G., et al., 2017a. New species in the Tricholoma pardinum complex from Eastern Himalaya. Phytotaxa, 305, 1-10.
DOI:10.11646/phytotaxa.305.1 |
||
Yang, Z. L., Ge, Z. W., Li, Y. C., et al., 2017b. Macrofungi. In: Gao, Z. W., Sun, H. (Eds. ), Checklist of Living Species in Yunnan. Yunnan Publishing Group. Yunnan Science and Technology Press, Kunming, pp. 3-63.
|
||
Yang Z.L., Zang M., 2003. Tropical affinities of higher fungi in southern China. Acta Bot. Yunnanica, 25, 129-144.
|
||
Ying J.Z., 1987. Supplement notes on genus Strobilomyces from China. Acta Mycol. Sinica(Suppl. Ⅰ ), 305-308.
|
||
Ying J.-Z., Ma Q.-M., 1985. New taxa and records of the genus Strobilomyces in China. Acta Mycol. Sinica, 4, 95-120.
|
||
Ying J.Z., Zang M., Zong Y.C., et al., 1994. Economic Macrofungi from Southwestern China. Science Press, Beijing. |
||
Yu L., Wang G.-D., Ruan J., et al., 2016. Genomic analysis of snub-nosed monkeys(Rhinopithecus) identifies genes and processes related to high-altitude adaptation. Nat. Genet, 48, 947-952.
DOI:10.1038/ng.3615 |
||
Zang M., 1980. The phytogeographical distribution of higher fungi and their evaluation of natural resources, Yunnan and Xizang (Tibet). Acta Bot. Yunnanica, 2, 152-187.
|
||
Zang M., 1983. A tentative subdivision and two new species of Boletus from Yunnan, China. Acta Mycol. Sinica, 2, 12-17.
|
||
Zang M., 1985. Notes on the Boletales from eastern Himalayas and adjacent of China. Acta Bot. Yunnanica, 7, 383-401.
|
||
Zang M., 1987. The Mycogeography of tropical fungi from Yunnan, Tibet. Acta Mycol. Sinica(Suppl. Ⅰ), 407-418.
|
||
Zang M., 1991. Boletus brevitubusda new taxon of genus Boletus from Yunnan. Acta Mycol. Sinica, 10, 117-119.
|
||
Zang M., 1992. Sinoboletus, a new genus of Boletaceae from China. Mycotaxon, 45, 223-227.
|
||
Zang M., 1996. A contribution to the taxonomy and distribution of the genus Xerocomus from China. Fungal Sci, 11, 1-15.
|
||
Zang M., 2006. Flora Fungorum Sinicorum, Vol., 22, Boletaceae Ⅰ. Science Press, Beijing. |
||
Zang M., 2013. Flora Fungorum Sinicorum, Vol., 44, Boletaceae Ⅱ. Science Press, Beijing. |
||
Zang M., Chen C.M., Sittigul C., 1999. Some new and interesting taxa of Boletales from tropical Asia. Fungal Sci, 18, 19-25.
|
||
Zang M., Li B., Xi J.X., 1996. Fungi of the Hengduan Mountains. Science Press, Beijing. |
||
Zang M., Li T.-H., Petersen R.H., 2001. Five new species of Boletaceae from China. Mycotaxon, 80, 48-487.
|
||
Zang M., Li X.J., 2008. Studies on fungi and bryophytes from Yunnan in the last one hundred years-review and prospect. Acta Bot. Yunnanica, 30, 382-386.
|
||
Zang M., Petersen R.H., 2004. Notes on tropical boletes from Asia. Acta Bot. Acta Bot. Yunnanica, 22, 619-627.
|
||
Zang M., Yang Z.-L., Zhang Y., 2006. On an undescribed Sinoboletus from China. Mycosystema, 25, 366-367.
|
||
Zang M., Yuan M.S., Gong M.Q., 1993. Notes on and additions to Chinese members of the Boletales. Acta Mycol. Sinica, 12, 275-282.
|
||
Zang M., Zeng X.L., 1978. A preliminary study on the family Paxillaceae of Yunnan and Tibet, China. Acta Microbiol. Sin, 18, 279-286.
|
||
Zang M., Zhang D.-Z., 2004. Two new species of Basidiomycota. Acta Bot. Yunnanica, 26, 633-636.
|
||
Zeng N.K., Liang Z.Q., Tang L.P., Li Y.C., Yang Z.L., 2017. The genus Pulveroboletus(Boletaceae, Boletales) in China. Mycologia, 109, 422-442.
DOI:10.1080/00275514.2017.1331689 |
||
Zeng N.K., Liang Z.Q., Yang Z.L., 2014. Boletus orientialbus, a new species with white basidioma from subtropical China. Mycoscience, 55, 159-163.
DOI:10.1016/j.myc.2013.07.004 |
||
Zeng N.K., Tang L.P., Li Y.C., et al., 2013. The genus Phylloporus (Boletaceae, Boletales) from China:morphological and multilocus DNA sequence analyses. Fungal Divers, 58, 73-101.
DOI:10.1007/s13225-012-0184-7 |
||
Zhang D.-R., Chen M.-Y., Murphy R.W., et al., 2010. Genealogy and palaeodrainage basins in Yunnan Province:phylogeography of the Yunnan spiny frog, Nanorana yunnanensis (Dicroglossidae). Mol. Ecol, 19, 3406-3420.
DOI:10.1111/j.1365-294X.2010.04747.x |
||
Zhang, G. F., Lu, S. G., 2017. Pteridophyte. In: Gao, Z. W., Sun, H. (Eds. ), Checklist of Living Species in Yunnan. Yunnan Publishing Group, Yunnan Science and Technology Press, Kunming, pp. 143-176.
|
||
Zhang L.F., Yang J.B., Yang Z.L., 2004. Molecular phylogeny of eastern Asian species of Amanita (Agaricales, Basidiomycota):taxonomic and biogeographic implications. Fungal Divers, 17, 219-238.
|
||
Zhang L.F., Yang Z.L., Song D.S., 2005. A phylogenetic study of commercial Chinese truffles and their allies:taxonomic implications. FEMS Microbiol. Lett, 245, 85-92.
DOI:10.1016/j.femsle.2005.02.028 |
||
Zhang L.S., Fang X.Q., 2012. Palaeogeography of China:the Formation of China's Natural Environment. Science Press, Beijing.. Science Press, Beijing. |
||
Zhang M., Li T.H., Gelardi M., et al., 2017. A new species and a new combination of Caloboletus from China. Phytotaxa, 309, 118-126.
DOI:10.11646/phytotaxa.309.2 |
||
Zhang T.-C., Comes H.P., Sun H., 2011. Chloroplast phylogeography of Terminalia franchetii (Combretaceae) from the eastern Sino-Himalayan region and its correlation with historical river capture events. Mol. Phylogenet. Evol, 60, 1-12.
DOI:10.1016/j.ympev.2011.04.009 |
||
Zhang X.Q., Dai Y.C., 2005. Flora Fungorum Sinicorum. Vol, 29, Hymenochaetaceae. Science Press, Beijing. |
||
Zhao C.L., Cui B.K., Dai Y.C., 2013a. New species and phylogeny of Perenniporia based on morphological and molecular characters. Fungal Divers, 58, 47-60.
DOI:10.1007/s13225-012-0177-6 |
||
Zhao K., Wu G., Feng B., et al., 2014. Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycol. Prog, 13, 1127-1136.
|
||
Zhao M.R., Huang C.Y., Wu X.L., et al., 2016a. Genetic variation and population structure of the mushroom Pleurotus ferulae in China inferred from nuclear DNA analysis. J. Integr. Agric, 15, 2237-2246.
DOI:10.1016/S2095-3119(16)61383-7 |
||
Zhao M.R., Zhang J.X., Chen Q., et al., 2016b. The famous cultivated mushroom Bailinggu is a separate species of the Pleurotus eryngii species complex. Sci. Rep, 6, 33066.
DOI:10.1038/srep33066 |
||
Zhao S., Zheng P., Dong S., et al., 2013b. Whole-genome sequencing of giant pandas provides insights into demographic history and local adaptation. Nat. Genet, 45, 67-71.
DOI:10.1038/ng.2494 |
||
Zhou X., Wang B., Pan Q., et al., 2014. Whole-genome sequencing of the snubnosed monkey provides insights into folivory and evolutionary history. Nat. Genet, 46, 1303-1310.
DOI:10.1038/ng.3137 |
||
Zhu X.-T., Li Y.-C., Wu G., et al., 2014. The genus Imleria (Boletaceae) in East Asia. Phytotaxa, 191, 81-98.
DOI:10.11646/phytotaxa.191.1 |
||
Zhu X.-T., Wu G., Zhao K., et al., 2015. Hourangia, a new genus of Boletaceae to accommodate Xerocomus cheoi and its allied species. Mycol. Prog, 14, 37.
DOI:10.1007/s11557-015-1060-0 |
||
Zhuang, W. Y. (Ed. ), 2001. Higher Fungi of Tropical China. Mycotaxon LTD, Ithaca.
|
||
Zhuang W.Y., Zeng Z.Q., Liu X.X., 2016. Taxonomic revision of the genus Dicephalospora (Helotiales) in China. Mycosystema, 35, 791-801.
|
||
Zhuang W.Y., Zheng H.D., Ren F., 2017. Taxonomy of the genus Bisporella (Helotiales) in China with seven new species and four new records. Mycosystema, 36, 401-420.
|



