b. Department of Economic Plants and Biotechnology, Yunnan Key Laboratory for Wild Plant Resources, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China;
c. Key Laboratory of Biogeography and Bioresource in Arid Land, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Ürümqi 830011, Xinjiang, China
Arbuscular mycorrhizal fungi (AMF) are ubiquitous components of the soil microbiome that form nutritional associations with a great majority of plants in various ecosystems (Smith and Read, 2008). AMF belong to the phylum Glomeromycota and generally form mutualistic symbioses with their host plants, providing mineral nutrients to plants in exchange for photosynthates (Johnson, 2010). They play an important role in regulating plant productivity and maintaining plant diversity (Vogelsang et al., 2006; Wagg et al., 2011). Consequently, changes in AMF communities may strongly affect ecosystems. Thus, in order to optimize ecosystem management, it is important to improve our understanding of the factors that influence AMF communities.
Fertilizers have been commonly used to increase plant productivity. However, improper application of chemical fertilizers may change plant community structure and reduce species diversity (Bret-Harte et al., 2001; Clark and Tilman, 2008; Madan et al., 2007). Moreover, fertilization considerably decreases AMF diversity and abundance (Treseder and Allen, 2002). High levels of homogenous P supply have been shown to strongly suppress AMF colonization (Olsson et al., 1997), abundance, and diversity (Camenzind et al., 2014; Chen et al., 2014; Lin et al., 2012). In contrast, the effects of N fertilization on AMF are controversial. Some investigations have suggested that high levels of N fertilization have suppressive effects on AMF (Albizua et al., 2015; Verbruggen et al., 2013), whereas others have found no significant impact (Tian et al., 2013; Williams et al., 2013). This lack of consistency suggests that the effects of fertilization on AMF may be context dependent. Apart from fertilization, many other factors may play a role in shaping AMF communities in an ecosystem, including N and P availability ratios (Cheng et al., 2013; Williams et al., 2017), soil pH (Rousk et al., 2010), AMF species identity (Wang et al., 1993), and plant community composition (Smith and Read, 2008). How other factors interact with fertilization in shaping AMF community is not fully understood.
Root hemiparasitic plants are green plants that retain the ability to photosynthesize, but rely on host plants for optimal performance, extracting nutrients and other resources from host roots via specialized root structures called haustoria (Irving and Cameron, 2009). Root hemiparasitic plants often suppress host growth and productivity (Press and Phoenix, 2005). Furthermore, root hemiparasitic plants can alter plant community structure and diversity by changing competitive relationships between host and non-host plant species (Bao et al., 2015; Borowicz and Armstrong, 2012; Hedberg et al., 2005). Like AMF, root hemiparasitic plants form strong and direct nutritional associations with host plants and are ubiquitous components of many ecosystems (Press and Phoenix, 2005; Irving and Cameron, 2009). However, these two groups of organisms clearly have contrasting effects on host plants and shape plant community structure by different mechanisms. An interesting question is whether the presence of root hemiparasites alters how fertilization affects AMF communities.
Despite extensive research on how fertilizers alter plant community composition, AMF abundance and diversity, and root hemiparasitic plant performance (Borowicz and Armstrong, 2012; Gibson and Watkinson, 1991; Davies and Graves, 2000; Liu et al., 2017), few studies have examined how fertilizers affect soil AMF abundance and community composition in soils where root hemiparasitic plants occur. Because AMF and root hemiparasites affect host plant nutrition differently, we hypothesized that root hemiparasite infestation, which substantially deprives the host of nutrients, may offset the suppressive effects of fertilization, which are the result of high nutrient levels.
Pedicularis kansuensis Maxim. (Orobanchaceae) is a root hemiparasitic species widely distributed and rapidly spreading in the subalpine zone of western China. With an estimated spreading rate of 3.3 × 103 ha year-1, this species has become a severe impediment to forage grass production for the local livestock industry (Liu et al., 2008; Sui et al., 2015). At present, no effective practice has been found to control this root hemiparasite. Our previous field experiments showed that N and P fertilization significantly reduce P. kansuensis biomass and change plant community structure (Liu et al., 2017). However, it remains unclear whether fertilization regimes affect AMF abundance and community structure.
In this study, our overall aim was to test whether fertilization suppresses AMF when plant communities are heavily infested by root hemiparasitic plants. Specifically, we asked three questions: (1) Does fertilization have a suppressive effect on AMF spore abundance, hyphae length density or root colonization in a plant community heavily infested by P. kansuensis? (2) Does fertilization affect the diversity or community composition of AMF in a plant community heavily infested by P. kansuensis? (3) Do other factors such as soil depth, soil pH or plant biomass affect AMF abundance and community composition under these fertilization regimes? To answer these questions, we collected plant and soil samples from field experimental plots that had been fertilized with N or P for three continuous years to control against heavy infestation by P. kansuensis. Answering these questions will not only help identify the factors that influence AMF communities, but also contribute to an improved understanding of how these biotic and abiotic factors interact to shape AMF communities.
2. Materials and methods 2.1. Study site and experimental designSampling was carried out in the research field at Bayanbulak subalpine grassland (42°53.1'N, 83°42.5'E, 2500 m), where N and P fertilizers had been applied for three continuous years to test their control effect against P. kansuensis. Bayanbulak Grassland is located in the southern Tianshan Mountains of the Xinjiang Uygur autonomous region of China (Gong et al., 2010). It is a typical subalpine meadow with Stipa purpurea and Festuca ovina as dominant plant species. The annual temperature is -4.8 ℃, with the lowest monthly mean occurring in January (-27.4 ℃) and the highest in July (11.2 ℃) (Li et al., 2012). The mean annual precipitation is 276.2 mm.
Fertilization treatments have been conducted since 2014 (Liu et al., 2017). In 2013, one 100 m × 100 m study site was chosen based on two criteria: heavy P. kansuensis infestation and similarity to the surrounding plant community. Sixteen blocks (4 m × 3 m) were randomly assigned for fertilization tests. A-2 m buffer zone was maintained between blocks to avoid nutrient diffusion into adjacent blocks. Four fertilization treatments were included: (1) non-fertilized control (CK), (2) N applied at 3 g N m-2 yr-1 as urea (N3), (4) N applied at 9 g N m-2 yr-1 as urea (N9), and (4) P applied at 10 g Ca(H2PO4)2·H2O m-2 yr-1. Each treatment had four replicates in a completely randomized design. Fertilizers were divided into two equal parts to increase fertilization efficiency while reducing ammonia volatilization from urea. The treatments were applied twice a year, mid-June and mid-July in 2014, 2015, and 2016, respectively.
2.2. Plant and soil samplingPlant and soil samples were collected in September 2016 in a similar manner as described by Liu et al. (2017), except that a circle quadrat 30 cm in diameter was used instead of a 1 m × 1 m square quadrat to facilitate the assessment of above-ground biomass per unit area for each plant functional group (grasses, forbs, legumes, and P. kansuensis). Briefly, a portable circle quadrat frame was randomly landed on plots that had not been previously sampled in each block. For each fertilization regime, four replicate quadrats were sampled, resulting in 16 sampling quadrats in total. Aboveground plant tissues within the circle quadrat were clipped at soil surface, sorted into different functional groups, and weighed separately for dry shoot biomass after drying at 80 ℃ for 48 h. Soil cores were taken using a soil auger (8 cm in diameter and 10 cm in depth; Eijkelkamp Agrisearch Equipment, the Netherlands) at three depths (0-10 cm, 10-20 cm, and 20-30 cm) from the center of each circle quadrat. Soil cores were stored in plastic bags and transported back to the lab immediately after collection. After extraction from soil, roots were carefully brushed, and washed with water. Roots from the same soil core were pooled together as it was not possible to separate them into different functional groups. Subsamples were taken for AMF colonization assessment and other analyses. The remainder was oven-dried at 80 ℃ for 48 h and weighed. Dry weight (DW) of the subsamples used for checking AMF colonization and other analyses were calculated from the ratio between fresh weight, dry weight of the remainder and fresh weight of the subsamples. Total root DW for each soil core was presented as a sum of dry weight of all extracted roots. Soil was sieved through a 1-mm mesh to remove root fragments and other debris. Soil subsamples for molecular analyses were stored at -80 ℃ before DNA extraction. A small amount of soil subsamples was taken from the remainder for water content measurement before air-drying at room temperature for soil nutrient analysis, extraction of AMF spores and extraradical hyphae.
2.3. Measurement of AMF colonization, spore abundance and extraradical hyphae length densityTo assess AMF colonization in each treatment, a weighed subsample of root material (diameter < 1 mm, about 100 root segments) was taken, cleared in 10% KOH and put in a 5% lactic acid solution, then stained with 0.05% Trypan blue in lactic acid (v/v), according to Phillips and Hayman (1970). The percent of root length hyphae, arbuscules/coils and vesicles were quantified by a gridline intersect method (Giovannetti and Mosse, 1980). Spores of AMF in soil samples were extracted by a wet sieving and decanting method (Brundrett et al., 1994). Briefly, 10 g of dry soil sample was suspended in water and filtered through a set of sieves with pore sizes ranging from 0.15 to 0.038 mm, then centrifuged in 50% sucrose solutions at 2000 rpm for 2 min to separate the spores from the soil particles. Extraradical hyphae length density was determined as described by Jakobsen et al. (1992). Twenty-five fields of view were examined under a microscope at 200× for AMF hyphae determination as described by Miller et al. (1995).
2.4. Soil chemical analysisThe soil moisture was measured by oven-drying the weighed samples at 105 ℃ for 24 h and calculating water content with a fresh weigh and a corresponding dry weigh. Soil pH was determined by a soil-to-water ratio of 1:2 using a pH meter (Mettler-Toledo 320, Mettler Toledo Instruments Co. Ltd., Greifensee, Switzerland). Soil organic matter was determined using a modified Walkley-Black chromic acid wet oxidation method (Wang et al., 2012). Total N concentrations were analyzed with the elemental analyzer (Vario EL Ⅲ Element Analyzer, Elementar, Hanau, Germany) following the burning method at 850 ℃ and 1180 ℃, respectively. Soil ammonium (NH4+-N) and nitrate (NO3--N) were measured with a Lachat Flow Injection Analyzer Quikchem 8500 S2 (Lachat instruments, Hach company, United States). Soil total P concentrations were determined with the HCl-extractable fraction method (Rodrigues et al., 2016). Soil available P was measured using the molybdate-blue colorimetric method (Mehlich, 1984).
2.5. Soil DNA extractionSoil DNA was extracted from 0.25 g soil sample using PowerSoilTM DNA Isolation Kit (MOBIO Laboratories, Carlsbad, CA, USA) following the manufacturer's protocol. Total DNA was extracted from 48 soil samples (3 samples from different soil depths for each of the 16 sampling quadrats from the four fertilization regimes). The concentration and purity of extracted DNA was checked on NanoDrop 2000. All extracted DNA was stored at -80 ℃ until further use.
2.6. PCR amplificationDNA samples were diluted to 10 ng μL-1 in DEPC water before PCR amplification. Partial sequences of AMF 18S rRNA genes were amplified from the extracted soil DNA via a nested PCR method, with GeoA2-AML2 as the first primer pair and NS31-AMDG the second. First round PCR was carried out at a final volume of 25 μL which contained 2 μL diluted DNA, 1 μL concentration of primers, 8.5 μL double-distilled H2O and 12.5 μL premix (Tsingke, Beijing) with the following cycling conditions: 95 ℃ for 3 min, 30 × (94 ℃ for 1 min, 58 ℃ for 50 s and 72 ℃ for 1 min) and 72 ℃ for 10 min. The PCR product was diluted with double-distilled H2O (1:50) and 2 μL of the diluted DNA was used as template for second round PCR amplification. Conditions for second round PCR were the same as first round PCR. All PCR products were examined on 1% (w/v) agarose gels with ethidium bromide staining to confirm the product integrity. Second round PCR products were purified with the DNA Gel Extraction Kit (Tsingke, Beijing) to obtain the predicted DNA fragments. The purified PCR products from all samples were mixed and further sequenced by the Second-generation sequencerIllumina Miseq sequencer at Chengdu Institute of Biology, Chinese Academy of Science.
Sequence trimming and quality control were conducted using QIIME V1.9.0 (http://qiime.org/tutorials/tutorial.html) following the suggested pipeline (Caporaso et al., 2010). Sequences < 200 bp or with average quality scores < 30 were removed (Edgar et al., 2011), and instances of exact barcode matching, two-nucleotide mismatch during primer matching, and reads containing ambiguous characters were removed. Usearch V8.0 was used to detect and remove chimeric sequences. A total of 813, 301 high-quality and chimera-free reads with an average length of 300 ± 50 bp were collected by sequencing, and there were 9857 sequences for each sample, which were sufficient to capture the majority of AMF information, as shown by the plateaus observed in the curves. Operational taxonomic units (OTU) were defined using a 97% similarity cutoff. The raw sequence data have been deposited in the Sequence Read Archive of the National Center for Biotechnology Information, USA (NCBI; Accession no. SRP136946).
2.7. Statistical data analysisOne-way analysis of variance (ANOVA) and Least Significant Difference (LSD) multiple range test were used for analyzing the effects of fertilization or soil depth on plant biomass, total root colonization and vesicle colonization of AMF, soil AMF spore density and extraradical hyphae length density. Data for percentage root length colonized by vesicles were transformed by natural logarithm (ln) before the analyses in order to improve normality and homogeneity of residuals. Spearman rank correlations were used to calculate the relationships between environmental variables (soil properties and plant biomass) and AMF community structure at genera levels. Statistical analysis of ANOVA and correlations were carried out using the Statistical Product and Service Solution (SPSS) software (version 19.0, IBM Corporation, Armonk, New York, USA).
AMF α-diversity can be represented by evenness, richness and Shannon diversity. According to Bonfim et al. (2016), Shannon index: H' = -Σ(Pilog[Pi]), where Pi = ni/N, ni = number of individuals of the species i, and N = total number of individuals of all species; Pielou's evenness index: J = H'/Log(S), in which H' is value obtained by the Shannon index and S is the total number of species (da Silva et al., 2015); richness was determined by the total number of species identified in fertilizer treatment, whereas mean species richness was obtained by the mean of the number of species obtained per sample (Melo et al., 2018).
To explore the relationship between AMF OTU compositions and treatments, and the environmental variables (including soil depth and fertilizer treatment) were fitted as vectors onto the nonmetric multidimensional scaling (NMDS) plot using function "envfit" in the R version (R Development Core Team, 2011). Redundancy analysis (RDA, length of gradient < 4) was applied with 9999 permutations and combinations of different soil depths and plants to infer relationships between AMF community composition in soil and soil chemical parameters using CANOCO 4.5 (http://www.canoco5.com/).
3. Results 3.1. Soil propertiesSoil properties varied between different soil depths. Soil available N (sum of nitrate and ammonium N), available P, and organic matter content were all significantly higher at 0-10 cm (P < 0.01) than at other depths. The soil pH at 0-10 cm was reduced significantly more in P-fertilized soil (P < 0.01) than in the non-fertilized control. Soil pH was generally lower at 0-10 cm (P < 0.05), whereas it was higher at 20-30 cm (P < 0.05) (Table 1).
| Soil depths | CK | N3 | N9 | P | |
| Total N (%) | 0-10 cm | 0.3 ± 0.01ABa | 0.4 ± 0.02Aa | 0.3 ± 0.03Ba | 0.3 ± 0.03Ba |
| 10-20 cm | 0.3 ± 0.04Aa | 0.3 ± 0.01Ab | 0.3 ± 0.02Aa | 0.3 ± 0.03Aa | |
| 20-30 cm | 0.3 ± 0.05Aa | 0.3 ± 0.02Ab | 0.3 ± 0.01Aa | 0.2 ± 0.04Aa | |
| Total P (g/kg) | 0-10 cm | 2.3 ± 0.11Aa | 2.5 ± 0.09Aa | 2.5 ± 0.07Aa | 2.7 ± 0.20Aa |
| 10-20 cm | 2.2 ± 0.08Aa | 2.3 ± 0.05Aab | 2.4 ± 0.10Aa | 2.2 ± 0.16Aa | |
| 20-30 cm | 2.2 ± 1.12Aa | 2.2 ± 0.09Ab | 2.0 ± 0.07Ac | 2.3 ± 0.13Aa | |
| Organic matter contents (%) | 0-10 cm | 8.0 ± 0.25ABa | 7.6 ± 0.45ABa | 6.7 ± 0.47Ba | 8.1 ± 0.50Aa |
| 10-20 cm | 5.7 ± 0.44Ab | 5.7 ± 0.08Ab | 5.6 ± 0.32Ab | 5.5 ± 0.17Ab | |
| 20-30 cm | 3.7 ± 0.38Ac | 3.8 ± 0.28Ac | 3.8 ± 0.14Ac | 3.9 ± 0.19Ac | |
| Available N (mg kg-1) | 0-10 cm | 65.2 ± 4.00Aa | 65.6 ± 3.23Aa | 79.2 ± 9.30Aa | 66.9 ± 2.30Aa |
| 10-20 cm | 44.6 ± 4.38Ab | 38.9 ± 1.80Ab | 37.6 ± 1.13Ab | 43.3 ± 2.41Ab | |
| 20-30 cm | 20.1 ± 3.95Ac | 24.1 ± 4.37Ac | 22.3 ± 3.30Ab | 27.1 ± 3.95Ac | |
| Available P (mg kg-1) | 0-10 cm | 38.6 ± 9.12Ba | 16.6 ± 6.12Ba | 28.7 ± 7.59Ba | 175.5 ± 6.75Aa |
| 10-20 cm | 21.4 ± 3.77Bab | 17.4 ± 2.43Ba | 17.9 ± 2.10Ba | 42.9 ± 6.86Ab | |
| 20-30 cm | 14.1 ± 2.75Ab | 18.4 ± 6.46Aa | 15.0 ± 5.18Aa | 24.8 ± 9.17Ab | |
| Moisture (%) | 0-10 cm | 35.4 ± 6.09Aa | 35.7 ± 8.02Aa | 35.4 ± 4.41Aa | 50.9 ± 5.24Aa. |
| 10-20 cm | 38.2 ± 3.65Aa | 33.6 ± 3.87Aa | 35.6 ± 3.18Aa | 43.1 ± 3.31Aab | |
| 20-30 cm | 31.5 ± 0.66Aa | 32.7 ± 4.29Aa | 38.6 ± 3.12Aa | 34.2 ± 2.90Ab | |
| pH | 0-10 cm | 7.7 ± 0.06Ab | 7.8 ± 0.08Ab | 7.8 ± 0.11Ab | 7.5 ± 0.06Bb |
| 10-20 cm | 8.1 ± 0.11Aa | 8.1 ± 0.08Aa | 8.1 ± 0.12Aab | 7.9 ± 0.07Ba | |
| 20-30 cm | 8.1 ± 0.10Aa | 8.2 ± 0.09Aa | 8.2 ± 0.10Aa | 8.1 ± 0.04Aa | |
| Data are means ± SE (n= 4). CK, non-fertilized control; N3, fertilized with low N level at a rate of 3 g N m-2 yr-1 as urea; N9, fertilized with high N level at a rate of 9 g N m-2 yr-1 as urea; P, fertilized with 10 g Ca (H2PO4)2·H2O m-2 yr-1. Different uppercase letters indicate difference between different treatments within the same soil layer; different lowercase letters indicate difference between different soil layers of the same treatment. Significant differences across treatments within each variable were determined using Fisher's least significant difference (LSD) test (P < 0.05) after one-way ANOVA and are indicated by dissimilar letters. | |||||
Shoot biomass of grasses and forbs increased slightly (not statistically significant) after N and P fertilization, whereas P. kansuensis biomass decreased significantly after high N and P fertilization (by 95.61% and 52.63%, respectively). After three years of fertilization, legume biomass was high in P-fertilized quadrats, whereas it was negligible in other quadrats (Table 2). Roots were mainly distributed in the 0-10-cm soil layer regardless of fertilization regime (P < 0.01). P fertilization significantly increased root biomass at 0-10 cm (P = 0.011) (Table 2).
| Shoot Biomass (g/m2) | Root Biomass (g/m2) | |||||||
| Grasses | Forbs | P. kansuensis | Legumes | 0-10 cm | 10-20 cm | 20-30 cm | ||
| CK | 275.3 ± 40.81A | 68.3 ± 18.57A | 11.4 ± 2.47A | - | 121.1 ± 29.49Ba | 29.7 ± 10.81Ab | 14.7 ± 2.30Ab | |
| N3 | 339.7 ± 51.15A | 91.0 ± 11.23A | 7.7 ± 1.63AB | - | 159.3 ± 20.79Ba | 50.7 ± 17.86Ab | 11.6 ± 1.64Ab | |
| N9 | 324.5 ± 91.01A | 83.5 ± 32.55A | 0.5 ± 0.00C | - | 228.2 ± 55.62ABa | 46.2 ± 8.52Ab | 23.3 ± 6.96Ab | |
| P | 285.6 ± 46.35A | 58.9 ± 10.83A | 5.4 ± 1.58BC | 34.1 ± 0.01 | 272.2 ± 26.32Aa | 33.6 ± 10.9Ab | 26.4 ± 9.61Ab | |
| Data are means ± SE (n =4). CK, non-fertilized control; N3, fertilized with low N level at a rate of 3 g N m-2 yr-1 as urea; N9, fertilized with high N level at a rate of 9 g N m-2 yr-1 as urea; P, fertilized with 10 g Ca (H2PO4)2·H2O m-2 yr-1. For shoot biomass, different uppercase letters indicate difference between different treatments within the same kind of plant. For root biomass, different uppercase letters indicate difference between different treatments within the same soil layer; different lowercase letters indicate difference between different soil layers of the same treatment. Significant differences across treatments within each variable were determined using Fisher's least significant difference (LSD) test (P < 0.05) after one-way ANOVA and are indicated by dissimilar letters. | ||||||||
Hyphae and vesicles were the most commonly observed AMF structures in sampled roots.However, arbuscules were verypatchy in all samples (lessthan1%).The total and vesicle colonization of N9 and P fertilization groups at 0-10 cm were significantly higher (P < 0.01) than at other soil layers. Vesicle and total colonization levels for fertilized groups at 10-20 cm were lower than for the non-fertilized control at the same soil depth (Fig. 1A and B). Soil spore abundance of AMF increased gradually under the fertilization gradient (Fig. 1C), especially in the P-fertilized group (P < 0.01). After P fertilization, soil spore numbers increased by 67.24%, 66.86% and 74.82% in three soil layers respectively. Hyphae length density increased by 59.74% at 0-10 cm in the P-fertilized group (P < 0.05) (Fig. 1D).
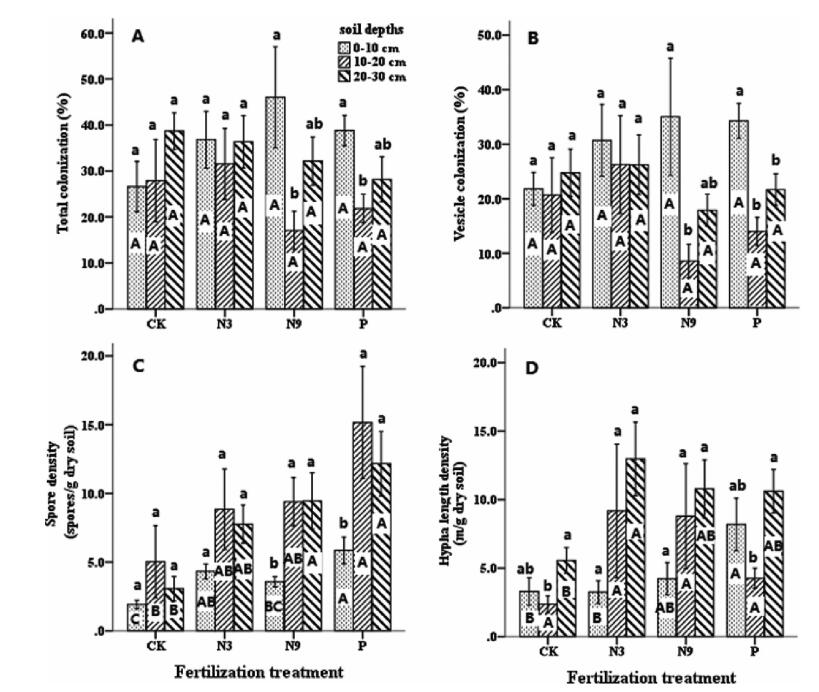
|
| Fig. 1 Total colonization (A) and vesicle colonization (B) by AMF in roots, AMF spore density (C) and extraradical hyphae length density (D) varied across different fertilization regimes. Data are means ± SE (n = 4). CK, non-fertilized control; N3, fertilized with low N level at a rate of 3 g N m-2 yr-1 as urea; N9, fertilized with high N level at a rate of 9 g N m-2 yr-1 as urea; P, fertilized with 10 g Ca (H2PO4)2·H2O m-2 yr-1. Different uppercase letters indicate difference between different treatments within the same soil layers; different lowercase letters indicate difference between different soil layers of the same treatment. Significant differences across treatments within each variable were determined using Fisher's least significant difference (LSD) test (P < 0.05) after one-way ANOVA and are indicated by dissimilar letters |
In total, 43 AMF OTUs were detected, belonging to 7 genera within 6 families: Ambisporaceae (3 OTUs within Ambispora), Archaeosporaceae (2 OTUs within Archaeospora), Diversisporaceae (3 OTUs within Diversispora), Gigasporaceae (2 OTUs within Gigaspora), Glomeraceae (24 OTUs within Funneliformis, Glomus, Rhizophagus and three new genus-like clades), Paraglomeraceae (1 OTU within new genus-like clade). Eight OTUs were only named to class. The most abundant genus was Glomus, accounting for 57.66% of all AMF genera, followed by Diversispora (33.19%), Gigaspora (2.57%), Archaeospora (1.3%). The relative abundance of Ambispora, Funneliformis and Rhizophagus was much lower. Relative abundances of individual genera varied greatly at different soil depths and were Significant correlated to fertilization regimes (Fig. 2A and B).
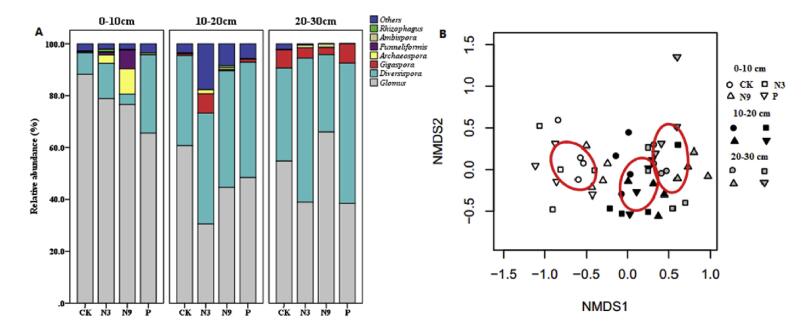
|
| Fig. 2 Relative abundance of AMF taxa (A) and non-metric multidimensional scaling (NMDS) ordination of AMF OTU composition in different soil layers (B). Notes: AMF taxa not named to any genera were presented as others. CK, non-fertilized control; N3, fertilized with low N level at a rate of 3 g N m-2 yr-1 as urea; N9, fertilized with high N level at a rate of 9 g N m-2 yr-1 as urea; P, fertilized with 10 g Ca (H2PO4)2·H2O m-2 yr-1 |
Fertilization did not significantly affect AMF α-diversity, as indicated by similarities in Shannon diversity index, richness of OTUs, and Pielou's evenness in the same soil layers under different fertilization regimes (Fig. 3). However, soil depths showed a significant impact on Shannon index and particularly richness of AMF. In all treatments, AMF richness at 0-10 cm was higher (P < 0.05) than that at deep layers. When more N fertilizer was applied, the Shannon index of AMF was higher at 0-10 cm than at 20-30 cm (P = 0.046).
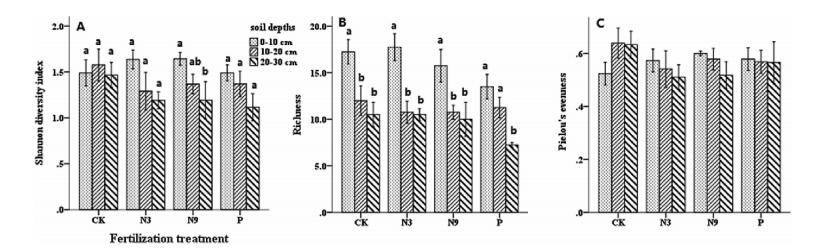
|
| Fig. 3 Mean Shannon diversity index (A), Richness of AMF OTUs (B), Pielou's evenness (C). Data are means ± SE (n = 4). CK, non-fertilized control; N3, fertilized with low N level at a rate of 3 g N m-2 yr-1 as urea; N9, fertilized with high N level at a rate of 9 g N m-2 yr-1 as urea; P, fertilized with 10 g Ca (H2PO4)2·H2O m-2·yr-1. Different lowercase letters indicate difference between different soil layers of the same treatment. Significant differences across treatments within each variable were determined using Fisher's least significant difference (LSD) test (P < 0.05) after one-way ANOVA and are indicated by dissimilar letters |
The effect that changes in soil properties had on relative abundance varied depending on both AMF genus and fertilization regime. The relative abundance of Glomus, Archaespora and Rhizophagus showed strong negative correlations with soil depth and soil pH (P < 0.05), but showed positive correlations with soil available N, organic content and root biomass (P < 0.01). In contrast, Diversispora and Gigaspora showed the opposite pattern. For these genera, soil depth and soil pH were positively correlated (P < 0.001), whereas soil available N, organic content, and root biomass were negatively correlated (P < 0.001). The relative abundance of Glomus and Rhizophagus increased with total N (P < 0.05), but Diversispora and Gigaspora decreased as soil total N increased. The relative abundance of Archaeospora was positively correlated with soil total P (P < 0.05), whereas the relative abundance of Gigaspora decreased with increasing total P and available P (P < 0.05). Relatively more Funneliformis was detected in the topsoil layer than in deeper layers. Interestingly, a positive correlation between biomass of P. kansuensis and the relative abundance of Diversispora was observed (P < 0.05) (Table 3).
| Soil AMF genus | Correlation to soil variables and plant biomass | |||||||||||||
| Layers | pH | TN | TP | AN | AP | Organic content |
Water content |
Root biomass |
Shoot biomass |
Grasses biomass |
Forbs biomass |
P. kansuensis biomass |
Legume biomass |
|
| Glomus | -0.477*** | -0.527*** | 0.434** | 0.119 | 0.478*** | 0.142 | 0.412** | 0.254 | 0.439** | 0.055 | 0.101 | 0.088 | -0.245 | -0.0404 |
| Archaeospora | -0.657*** | -0.418** | 0.099 | 0.317* | 0.582*** | 0.226 | 0.558*** | 0.046 | 0.586*** | 0.071 | 0.026 | 0.064 | -0.130 | 0.0109 |
| Diversispora | 0.494*** | 0.505*** | -0.368** | -0.143 | -0.500*** | -0.040 | -0.446** | -0.152 | -0.518*** | -0.114 | -0.150 | -0.167 | 0.321* | 0.0652 |
| Gigaspora | 0.772*** | 0.494*** | -0.319* | -0.300* | -0.701*** | -0.358* | -0.675*** | -0.279 | -0.579*** | 0.105 | 0.117 | 0.095 | -0.052 | 0.1021 |
| Ambispora | 0.024 | 0.235 | 0.054 | 0.103 | -0.047 | 0.044 | 0.087 | -0.084 | -0.040 | 0.040 | 0.068 | 0.171 | -0.029 | -0.1232 |
| Funneliformis | -0.336* | -0.242 | 0.194 | 0.103 | 0.263 | -0.037 | 0.254 | 0.100 | 0.268 | -0.148 | -0.120 | -0.054 | -0.020 | -0.1779 |
| Rhizophagus | -0.398** | -0.292* | 0.285* | 0.200 | 0.312* | -0.080 | 0.378** | 0.059 | 0.362* | -0.198 | -0.278 | 0.094 | 0.007 | -0.1548 |
| *P < 0.05, **P < 0.01, ***P < 0.001; mean AMF genera are in bold; negative correlations are in italic, positive correlations are in bold. | ||||||||||||||
Based on RDA analyses, different environmental variables had differential effects on soil AMF community (P = 0.001) (Fig. 4). The main factors that influenced AMF composition, regardless of soil depth, were soil pH, organic content, available nitrogen and root biomass (Fig. 4A). At 0-10 cm, no significant influence was detected by any of the tested factors on AMF community composition (Fig. 4B). For soil at 10-20 cm, the main factors that shaped AMF communities were soil pH and soil water content (Fig. 4C). For soil at 20-30 cm, AMF community composition was significantly influenced by soil total N, biomass, and the relative abundance of P. kansuensis (P < 0.05), which, combined, explain 64% of the variation observed (Fig. 4D).

|
| Fig. 4 Redundancy analysis (RDA) relating AMF community structure to soil properties and plants biomass at different soil depths. AMF community structure in all depths (A) and three soil depths separately (B-D) under different treatments. The percentages in the axis labels indicate the variance explained by that axis |
Fertilization did not affect AMF colonization significantly (Fig. 1A and B). In this study, fertilization was applied at a rate of 3 or 9 g m-2 N as urea (corresponding to 1.4 or 4.25 g m-2 N), or 10 g m-2 P as Ca (H2PO4)2·H2O (corresponding 4.6 g m-2 P). Similar fertilization rates have been reported to cause significant suppression of AMF colonization in croplands (Liu et al., 2016; Bakhshandeh et al., 2017), but not in an alpine meadow ecosystem (Liu et al., 2012). However, when we examined the soil available P and N of our experimental plots, we found that due to relatively high background soil nutrient levels, the moderate fertilization in this study resulted in soil N and P levels comparable to those of heavily fertilized treatments described by Liu et al. (2012), where AMF colonization was strongly suppressed. The root hemiparasite P. kansuensis may have offset, or at least delayed the suppressive effects of fertilization on AMF colonization by causing host nutrient deprivation. In a previous study on the same experimental plots, we observed that the response of plant biomass to fertilization was delayed (Liu et al., 2017). It would be interesting to test whether a suppressive effect of fertilization on AMF colonization can be detected as we continue the fertilization experiments for another couple of years. We observed that sampled roots had very patchy arbuscules. A previous field experiment that investigated the effects of long-term N and P fertilization (8 years) on AMF in an alpine meadow ecosystem also observed very few arbuscular structures. For these studies, AP was high. According to Kobae et al. (2017), this may inhibit arbuscular formation.
AMF colonization levels varied among different soil depths after high N and P fertilization treatments; specifically, AMF colonization was higher in topsoil than in soil at 10-20 cm (Fig. 1A and B). Previous research found that when phosphorus concentration exceeded 7.5 g m-2, AMF colonization of maize roots was lower in the topsoil (0-20 cm) than in the subsoil (20-40 cm) (Wang et al., 2017). But Kabir et al. (1998) found that AMF colonization was highest at a soil depth of 0-15 cm. The inconsistency of these results may be related to the heterogeneity of P distribution in the soil or to plant root distribution. In this study, when soil was fertilized with more N or P, plant roots grew well and were mainly distributed at 0-10 cm. This may have significantly increased AMF colonization at this soil depth.
Fertilization affected soil spore abundance and hyphae length density. Spore abundance and hyphae length density were higher in soil fertilized with P (4.6 g P m-2 yr-1) than in the non-fertilized control. This was inconsistent with other studies. Liu et al. (2012) found that when soil was fertilized with 30 g m-2 yr-1 of (NH4)2HPO4 (corresponding to 6.4 g N and 7 g P m-2 yr-1), soil spore abundance reached its peak, but hyphae length density of AMF decreased along a fertilization gradient. Johnson et al. (2003) indicated that in N-enriched (more than 10 g m-2) plots, spore abundance and hyphae length density were lower compared to that in ambient plots, but when N and P were applied together, spore abundance was significantly high. Sheng et al. (2013) found that with 3.5 g m-2 P, spore abundance increased, but the hyphae length density decreased. In our study, soil spore abundance and hyphae length density increased after fertilizer was added, especially under P fertilization conditions. Because AMF form mutualistic associations with plants and obtain C resources from host plants in exchange for mineral elements, under fertilization conditions, plants may be less dependent on AMF for nutrients. In this case, C allocation to AMF may decrease, causing the abundance of AMF to decrease. In our research, the presence of root hemiparasitic plants led to nutrient stress for host plants. As a result, in nutrient-poor soil, the growth of host plants and the abundance of AMF were suppressed. However, when fertilizer was applied, this situation improved. The reasons are as follows. On the one hand, root hemiparasitic plants consume lower levels of nutrients from host plants; on the other hand, the competition for nutrients between AMF and root hemiparasites is alleviated. When this occurs, there will be no surplus of nutrients even under the fertilization condition. This may explain why fertilization did not inhibit, but, on the contrary, appeared to promote AMF abundance. Strigolactones, plant hormones that enhance the germination of AMF spores and promote AMF hyphae branching (Mori et al., 2016), have been shown to increase in response to P deficiency (Al-Babili and Bouwmeester, 2015; Andreo-Jimenez et al., 2015). In our study, the presence of root hemiparasitic plants, which often cause nutrient (including P) deprivation in their hosts, may have increased excretion of strigolactones in host plants and thereby promoted AMF propagation.
Forty-three OTUs were detected in this subalpine grassland ecosystem, suggesting relatively high AMF biodiversity (Chen et al., 2014; Santos et al., 2006; Vandenkoornhuyse et al., 2002). Glomus was a dominant genus under all fertilization regimes. This was consistent with previous reports on the dominance of this genus in many ecosystems (Lin et al., 2012; Oehl et al., 2004; Santos et al., 2006). It has been suggested that Glomus spp. are more capable of colonizing via fragments of mycelium when compared with other AMF species (Biermann and Linderman, 1983). Furthermore, Giovannetti et al. (1999) suggested that Glomus spp. can easily form anastomoses among mycelia and therefore have the ability to reestablish an interconnected network after mechanical disruption. Interestingly, the distribution of Glomus and Archaeospora differed from that of Diversispora and Gigaspora. The former were mainly distributed in topsoil, especially in N-fertilized quadrats, whereas the latter mainly occurred in deeper soil, where available N was low. These results suggest that 3-year N fertilizer practice could change soil AMF community.
AMF diversity was not affected by fertilization. This does not agree with previous studies that showed that soil AMF diversity decreases after fertilization (de Pontes et al., 2017; Tian et al., 2013; Wang et al., 2011). The influence of soil fertilizer on the diversity of AMF remains controversial, and the inconsistency of these results may be attributed to the application rate of fertilization (Mathimaran et al., 2005), soil properties (Verbruggen et al., 2012) and host plant species (Gosling et al., 2013). In our study, nitrogen and phosphate fertilizer application did not exert an overall significant effect on AMF diversity. One possible explanation is the existence of root hemiparasitic plants. Fertilization allows host plants to absorb essential nutrients from soil, thereby lowering the nutrient stress caused by hemiparasitic plants. However, root hemiparasitic plants continue to absorb nutrients from host plants. AMF and hemiparasitic plants compete for nutrients and host plants still need AMF to provide the mineral nutrients for their growth. Hence, fertilization does not significantly influence AMF diversity. AMF species richness was higher in topsoil than in deep soil (Fig. 3B). Hence, shallow and deep soil layers are both necessary to fully evaluate AMF diversity. This phenomenon corresponds to a study by Taniguchi et al. (2012), in which AMF richness decreased at lower soil depths, even while more than five phylotypes were observed at depths up to 100 cm. The soil surface and seasonal experience swings, result in different soil temperature and moisture at depth (Brady and Weil, 2002). Both soil moisture (Schimel et al., 1999) and soil temperature (Zogg et al., 1997) have been found to influence soil AMF richness (Chaparro et al., 2012).
5. ConclusionsThis is the first study to investigate the influence of fertilization on AMF communities under field conditions where root hemiparasitic plants exist. In contrast to suppressive effects reported by many previous studies, fertilization showed no significant effects on AMF root colonization or AMF species diversity. Instead, we observed that soil spore abundance and extraradical hyphae length density increased markedly. Further investigations are required to unravel the underlying mechanisms of this phenomenon.
AcknowledgmentsWe thank the two anonymous reviewers for their comments that greatly improved the manuscript. The research was financially supported by the Natural Science Foundation of China (U1303201, No. 31400440 and No. 31370512), China Agriculture Research System (CARS-34), Natural Science Foundation of Yunnan Province (2016FB059), Natural Science Foundation of Yunnan Province (2016FB059), and funding for Airong Li from The Youth Innovation Promotion Association of Chinese Academy of Sciences and the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2014HB047).
Appendix A. Supplementary dataSupplementary data related to this article can be found at https://doi.org/10.1016/j.pld.2018.05.001.
Al-Babili S., Bouwmeester H.J., 2015. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol, 161-186.
|
||
Albizua A., Williams A., Hedlund K., et al., 2015. Crop rotations including ley and manure can promote ecosystem services in conventional farming systems. Appl. Soil Ecol, 95, 54-61.
DOI:10.1016/j.apsoil.2015.06.003 |
||
Andreo-Jimenez B., Ruyter-Spira C., Bouwmeester H.J., Lopez-Raez J.A., 2015. Ecological relevance of strigolactones in nutrient uptake and other abiotic stresses, and in plant-microbe interactions below-ground. Plant Soil, 394, 1-19.
DOI:10.1007/s11104-015-2544-z |
||
Bakhshandeh S., Corneo P.E., Mariotte P., et al., 2017. Effect of crop rotation on mycorrhizal colonization and wheat yield under different fertilizer treatments. Agric. Ecosyst. Environ, 247, 130-136.
DOI:10.1016/j.agee.2017.06.027 |
||
Bao G., Suetsugu K., Wang H., et al., 2015. Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol. Res, 30, 507-515.
DOI:10.1007/s11284-015-1248-4 |
||
Biermann B., Linderman R.G., 1983. Use of vesicular mycorrhizal roots, intraradical vesicles and extraradical vesicles as inoculum. New Phytol, 95, 97-105.
DOI:10.1111/nph.1983.95.issue-1 |
||
Bonfim J.A., Vasconcellos R.L.F., Gumiere T., et al., 2016. Diversity of arbuscular mycorrhizal fungi in a Brazilian Atlantic forest toposequence. Microb. Ecol, 71, 164-177.
DOI:10.1007/s00248-015-0661-0 |
||
Borowicz V.A., Armstrong J.E., 2012. Resource limitation and the role of a hemiparasite on a restored prairie. Oecologia, 169, 783-792.
DOI:10.1007/s00442-011-2222-7 |
||
Brady, N. C., Weil, R. R., 2002. The Nature and Properties of Soils. Pearson Education, Upper Saddle River.
|
||
Bret-Harte M.S., Shaver G.R., Zoerner J.P., et al., 2001. Developmental plasticity allows Betula nana to dominate tundra subjected to an altered environment. Ecology, 82, 18-32.
DOI:10.1890/0012-9658(2001)082[0018:DPABNT]2.0.CO;2 |
||
Brundrett, M., Melville, L., Peterson, L., 1994. Practical Methods in Mycorrhiza Research: Based on a Workshop Organized in Conjunction with the Ninth North American Conference on Mycorrhizae. University of Guelph, Ontario, Canada.
|
||
Camenzind T., Hempel S., Homeier J., et al., 2014. Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Global Change Biol, 20, 3646-3659.
DOI:10.1111/gcb.12618 |
||
Caporaso J.G., Kuczynski J., Stombaugh J., et al., 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods, 7, 335-336.
DOI:10.1038/nmeth.f.303 |
||
Chaparro J.M., Sheflin A.M., Manter D.K., et al., 2012. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils, 48, 489-499.
DOI:10.1007/s00374-012-0691-4 |
||
Chen Y.-L., Zhang X., Ye J.-S., et al., 2014. Six-year fertilization modifies the biodiversity of arbuscular mycorrhizal fungi in a temperate steppe in Inner Mongolia. Soil Biol. Biochem, 69, 371-381.
DOI:10.1016/j.soilbio.2013.11.020 |
||
Cheng Y., Ishimoto K., Kuriyama Y., et al., 2013. Ninety-year, but not single, application of phosphorus fertilizer has a major impact on arbuscular mycorrhizal fungal communities. Plant Soil, 365, 397-407.
DOI:10.1007/s11104-012-1398-x |
||
Clark C.M., Tilman D., 2008. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature, 451, 712-715.
DOI:10.1038/nature06503 |
||
Davies D.M., Graves J.D., 2000. The impact of phosphorus on interactions of the hemiparasitic angiosperm Rhinanthus minor and its host Lolium perenne. Oecologia, 124, 100-106.
DOI:10.1007/s004420050029 |
||
Edgar R.C., Haas B.J., Clemente J.C., et al., 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27, 2194-2200.
DOI:10.1093/bioinformatics/btr381 |
||
Gibson C.C., Watkinson A.R., 1991. Host selectivity and the mediation of competition by the root hemiparasite Rhinanthus-Minor. Oecologia, 86, 81-87.
DOI:10.1007/BF00317393 |
||
Giovannetti M., Azzolini D., Citernesi A.S., 1999. Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl. Environ. Microbiol, 65, 5571-5575.
|
||
Giovannetti M., Mosse B., 1980. Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol, 84, 489-500.
DOI:10.1111/j.1469-8137.1980.tb04556.x |
||
Gong Y., Hu Y., Adeli M., et al., 2010. Alpine grassland community characteristics at the different stages of degenerating succession in Bayanbulak. J. Arid Land Resour. Environ, 24, 149-152.
|
||
Gosling P., Mead A., Proctor M., et al., 2013. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol, 198, 546-556.
DOI:10.1111/nph.12169 |
||
Hedberg A.M., Borowicz V.A., Armstrong J.E., 2005. Interactions between a hemiparasitic plant, Pedicularis canadensis L. (Orobanchaceae), and members of a tallgrass prairie community. J. Torrey Bot. Soc, 132, 401-410.
DOI:10.3159/1095-5674(2005)132[401:IBAHPP]2.0.CO;2 |
||
Irving, L. J., Cameron, D. D., 2009. You are what you eat: interactions between root parasitic plants and their hosts. In: Kader, J. C., Delseny, M. (Eds. ), Advances in Botanical Research, vol. 50.
|
||
Jakobsen I., Abbott L.K., Robson A.D., 1992. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium -subterr aneum L. 1.Spread of hyphae and phosphorus inflow into roots. New Phytol, 120, 371-380.
DOI:10.1111/nph.1992.120.issue-3 |
||
Johnson N.C., 2010. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol, 185, 631-647.
DOI:10.1111/j.1469-8137.2009.03110.x |
||
Johnson N.C., Rowland D.L., Corkidi L., et al., 2003. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology, 84, 1895-1908.
DOI:10.1890/0012-9658(2003)084[1895:NEAMAA]2.0.CO;2 |
||
Kabir Z., O'Halloran I.P., Widden P., et al., 1998. Vertical distribution of arbuscular mycorrhizal fungi under corn (Zea mays L.) in no-till and conventional tillage systems. Mycorrhiza, 8, 53-55.
DOI:10.1007/s005720050211 |
||
Kobae Y., Sisaphaithong T., Hanai S., Tomioka R., Tanaka A., Yano K., et al., 2017. Varietal differences in the growth responses of rice to an arbuscular mycorrhizal fungus under natural upland conditions. Plant Signal. Behav, 12.
|
||
Li K., Gong Y., Song W., et al., 2012. No significant nitrous oxide emissions during spring thaw under grazing and nitrogen addition in an alpine grassland. Global Change Biol, 18, 2546-2554.
DOI:10.1111/j.1365-2486.2012.02704.x |
||
Lin X., Feng Y., Zhang H., et al., 2012. Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in North China revealed by 454 pyrosequencing. Environ. Sci. Technol, 46, 5764-5771.
DOI:10.1021/es3001695 |
||
Liu W., Zhang Y., Jiang S., et al., 2016. Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Sci. Rep, 6.
|
||
Liu Y., Hu Y., Yu J., et al., 2008. Study on harmfulness of Pedicularis myriophylla and its control measures. Arid Zone Res, 25, 778-782.
|
||
Liu Y., Shi G., Mao L., et al., 2012. Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol, 194, 523-535.
DOI:10.1111/j.1469-8137.2012.04050.x |
||
Liu Y., Taxipulati T., Gong Y., et al., 2017. N-P Fertilization Inhibits growth of root hemiparasite Pedicularis kansuensis in natural grassland. Front. Plant Sci, 8.
|
||
Madan N.J., Deacon L.J., Robinson C.H., 2007. Greater nitrogen and/or phosphorus availability increase plant species cover and diversity at a High Arctic polar semidesert. Polar Biol, 30, 559-570.
DOI:10.1007/s00300-006-0213-7 |
||
Mathimaran N., Ruh R., Vullioud P., et al., 2005. Glomus intraradices dominates arbuscular mycorrhizal communities in a heavy textured agricultural soil. Mycorrhiza, 16, 61-66.
DOI:10.1007/s00572-005-0014-9 |
||
Mehlich A., 1984. Mehlich-3 soil test extractant-a modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal, 15, 1409-1416.
DOI:10.1080/00103628409367568 |
||
Melo C.D., Luna S., Kruger C., et al., 2018. Communities of arbuscular mycorrhizal fungi under Picconia azorica in native forests of Azores. Symbiosis, 74, 43-54.
DOI:10.1007/s13199-017-0487-2 |
||
Miller R.M., Reinhardt D.R., Jastrow J.D., 1995. External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia, 103, 17-23.
DOI:10.1007/BF00328420 |
||
Mori N., Nishiuma K., Sugiyama T., Hayashi H., Akiyama K., 2016. Carlactone-type strigolactones and their synthetic analogues as inducers of hyphal branching in arbuscular mycorrhizal fungi. Phytochemistry, 130, 90-98.
DOI:10.1016/j.phytochem.2016.05.012 |
||
Oehl F., Sieverding E., Mader P., et al., 2004. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia, 138, 571-583.
|
||
Olsson P.A., Baath E., Jakobsen I., 1997. Phosphorus effects on the mycelium and storage structures of an arbuscular mycorrhizal fungus as studied in the soil and roots by analysis of fatty acid signatures. Appl. Environ. Microbiol, 63, 3531-3538.
|
||
Phillips J.M., Hayman D.S., 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc, 55, 158.
|
||
de Pontes J.S., Oehl F., Pereira C.D., et al., 2017. Diversity of arbuscular mycorrhizal fungi in the Brazilian's Cerrado and in soybean under conservation and conventional tillage. Appl. Soil Ecol, 117, 178-189.
|
||
Press M.C., Phoenix G.K., 2005. Impacts of parasitic plants on natural communities. New Phytol, 166, 737-751.
DOI:10.1111/j.1469-8137.2005.01358.x |
||
Rodrigues M., Pavinato P.S., Withers P.J.A., et al., 2016. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ, 542, 1050-1061.
DOI:10.1016/j.scitotenv.2015.08.118 |
||
Rousk J., Baath E., Brookes P.C., et al., 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J, 4, 1340-1351.
DOI:10.1038/ismej.2010.58 |
||
Santos J.C., Finlay R.D., Tehler A., 2006. Molecular analysis of arbuscular mycorrhizal fungi colonising a semi-natural grassland along a fertilisation gradient. New Phytol, 172, 159-168.
DOI:10.1111/nph.2006.172.issue-1 |
||
Schimel J.P., Gulledge J.M., Clein-Curley J.S., et al., 1999. Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga. Soil Biol. Biochem, 31, 831-838.
DOI:10.1016/S0038-0717(98)00182-5 |
||
Sheng M., Lalande R., Hamel C., et al., 2013. Effect of long-term tillage and mineral phosphorus fertilization on arbuscular mycorrhizal fungi in a humid continental zone of Eastern Canada. Plant Soil, 369, 599-613.
DOI:10.1007/s11104-013-1585-4 |
||
da Silva D.K.A., de Souza R.G., Velez B.A.D., et al., 2015. Communities of arbuscular mycorrhizal fungi on a vegetation gradient in tropical coastal dunes. Appl. Soil Ecol, 96, 7-17.
DOI:10.1016/j.apsoil.2015.06.009 |
||
Smith S.E., Read D.J., 2008. Mycorrhizal Symbiosis. Academic Press, New York. |
||
Sui X.-L., Huang W., Li Y.-J., et al., 2015. Host shoot clipping depresses the growth of weedy hemiparasitic Pedicularis kansuensis. J. Plant Res, 128, 563-572.
DOI:10.1007/s10265-015-0727-6 |
||
Taniguchi T., Usuki H., Kikuchi J., et al., 2012. Colonization and community structure of root-associated microorganisms of Sabina vulgaris with soil depth in a semiarid desert ecosystem with shallow groundwater. Mycorrhiza, 22, 419-428.
DOI:10.1007/s00572-011-0417-8 |
||
Tian H., Drijber R.A., Zhang J.L., et al., 2013. Impact of long-term nitrogen fertilization and rotation with soybean on the diversity and phosphorus metabolism of indigenous arbuscular mycorrhizal fungi within the roots of maize (Zea mays L.). Agric. Ecosyst. Environ, 164, 53-61.
DOI:10.1016/j.agee.2012.09.007 |
||
Treseder K.K., Allen M.F., 2002. Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi:a model and field test. New Phytol, 155, 507-515.
DOI:10.1046/j.1469-8137.2002.00470.x |
||
Vandenkoornhuyse P., Husband R., Daniell T.J., et al., 2002. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol. Ecol, 11, 1555-1564.
DOI:10.1046/j.1365-294X.2002.01538.x |
||
Verbruggen E., van der Heijden M.G.A., Rillig M.C., et al., 2013. Mycorrhizal fungal establishment in agricultural soils:factors determining inoculation success. New Phytol, 197, 1104-1109.
DOI:10.1111/j.1469-8137.2012.04348.x |
||
Verbruggen E., Van Der Heijden M.G.A., Weedon J.T., et al., 2012. Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils. Mol. Ecol, 21, 2341-2353.
DOI:10.1111/j.1365-294X.2012.05534.x |
||
Vogelsang K.M., Reynolds H.L., Bever J.D., 2006. Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol, 172, 554-562.
DOI:10.1111/nph.2006.172.issue-3 |
||
Wagg C., Jansa J., Schmid B., et al., 2011. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett, 14, 1001-1009.
DOI:10.1111/ele.2011.14.issue-10 |
||
Wang C., White P.J., Li C., 2017. Colonization and community structure of arbuscular mycorrhizal fungi in maize roots at different depths in the soil profile respond differently to phosphorus inputs on a long-term experimental site. Mycorrhiza, 27, 369-381.
DOI:10.1007/s00572-016-0757-5 |
||
Wang F.Y., Hu J.L., Lin X.G., et al., 2011. Arbuscular mycorrhizal fungal community structure and diversity in response to long-term fertilization:a field case from China. World J. Microbiol. Biotechnol, 27, 67-74.
DOI:10.1007/s11274-010-0427-2 |
||
Wang G.M., Stribley D.P., Tinker P.B., et al., 1993. Effects of pH on arbuscular mycorrhiza. 1. Field observations on the long-term liming experiments at Rothamsted and Woburn. New Phytol, 124, 465-472.
DOI:10.1111/nph.1993.124.issue-3 |
||
Wang X.J., Wang J.P., Zhang J., 2012. Comparisons of three methods for organic and inorganic carbon in calcareous soils of Northwestern China. PLoS One, 7.
|
||
Williams A., Borjesson G., Hedlund K., 2013. The effects of 55 years of different inorganic fertiliser regimes on soil properties and microbial community composition. Soil Biol. Biochem, 67, 41-46.
DOI:10.1016/j.soilbio.2013.08.008 |
||
Williams A., Manoharan L., Rosenstock N.P., et al., 2017. Long-term agricultural fertilization alters arbuscular mycorrhizal fungal community composition and barley (Hordeum vulgare) mycorrhizal carbon and phosphorus exchange. New Phytol, 213, 874-885.
DOI:10.1111/nph.14196 |
||
Zogg G.P., Zak D.R., Ringelberg D.B., et al., 1997. Compositional and functional shifts in microbial communities due to soil warming. Soil Sci. Soc. Am. J, 61, 475-481.
DOI:10.2136/sssaj1997.03615995006100020015x |



