-ecoregional basis for conservation planning and implementation;
-a unified scoring system that is used in regional systematic planning for reserve design, monitoring and assessment of efficiency of a reserve network, and creation of seed banks and living collections;
-a focus on population demography and the presence of naturally occurring regeneration as the key criteria for defining the conservation status of a species and the appropriate major focus of the species recovery plan;
-creation of multi-species living collections that preserve species genetic variation and provide material for in situ actions;
-experimental translocation of threatened species into multiple locations within and outside their known range.
Adopting and implementing these strategies successfully and more fully in China requires that the country changes PA legislation and improves PA management, the National Science Foundation of China (NSFC) re-prioritizes the type of research that receives research funds, and local scientists improve their approach toward information sharing.
As one of 17 megadiverse countries (Noss, 1990), China is a hotspot extremely rich in biodiversity and endemism, and harboring more relic lineages of plant taxa than any other country in the world. The Chinese flora has as many as 34, 984 native higher plant species (The Ministry of Environmental Protection of China, 2011). Anthropogenic disturbance, a result of rapid economic development and population growth, severely threatens Chinese plant species, which require wide-scale and immediate conservation action. This makes China one of the highest priorities for global biodiversity conservation and poses a huge challenge for Chinese conservation biologists. An estimated 15%-20% of wild higher plants in China are currently endangered (The Ministry of Environmental Protection of China, 2011), with many more projected to reach this status in the following decades. The country Red List for higher plants based on the IUCN Red List Categories and Criteria (Version 3.1) includes 27 globally extinct, 10 extinct in the wild, 15 regionally extinct, 583 critically endangered, 1297 endangered, 1887 vulnerable, 2723 near-threatened, 24, 296 least concern, and 3612 data deficient species (Chinese Academy of Sciences and Ministry of Environmental Protection of the People's Republic of China, 2013).
Volis (2016c) briefly described the problems of plant conservation in China focusing on endangered species with extremely small population sizes. In this paper, I would like to present a more general concept relevant to all threatened Chinese plant species. This concept is founded on several cornerstones. One is that the current focus of plant conservation on passive protection of fragmented natural habitats appears inadequate in light of only the accelerating loss of species and natural habitats that we are witnessing globally (Heywood, 2016, 2017). In the Anthropocene, the future of conservation lies in habitat restoration and wide-scale plant introductions, not only within, but also outside the known historical range of the species (Volis, 2016b, 2017b). Second is that ex situ and in situ conservation must be tightly linked and be integral parts of systematic conservation planning (Volis, 2016d, 2017a).
In addition, widespread local adaptation resulting from spatially varying environmental conditions, and therefore an ecoregional approach in conservation planning, appear especially relevant for plant conservation in China. There are at least 20 centers of endemism, all located in central and southern China and coinciding with the main mountain ranges in the area (Lopez-Pujol et al., 2011; Zhang et al., 2017b). Such a localized distribution of many taxa suggests that the formation of the local flora in different mountain regions took place under conditions of no gene flow between the mountain ranges due to their physical isolation or environmental differences. This observation has important conservation implications. Systematic conservation planning (Margules and Pressey, 2000) for plants in China must utilize regional planning based on recognizing the importance of ecologically significant variation in adaptive traits (Volis, 2016d). Some of the differences between species and species assemblages in different mountain ranges may have resulted from specific environmental conditions to which the local species adapted. It must not, therefore, be assumed a priori that populations of the same species in different mountain ranges will be genetically similar or face similar challenges to survival. Given these factors, let us consider the steps required to efficiently conserve China's unique flora.
Since its introduction (Margules and Pressey, 2000), systematic conservation planning has become a widely accepted operational approach covering both planning and implementation of conservation. This approach, in its latest modifications (Pressey and Bottrill, 2009; Sarkar and Illoldi-Rangel, 2010), necessarily includes the following stages:
● delimitation of the planning area,
● collection of data,
● setting conservation goals,
● evaluation of the existing protected area (PA) network,
● design of expansions, implementation of conservation actions, and
● long-term maintenance of biodiversity in the network.
In this review, I will discuss how these steps can be modified for plant species, and the specific conditions in China, while providing additional insights where pertinent. Adopting systematic conservation planning has been accepted by the Ministry of Environmental Protection of China (2011), and I hope that this paper will contribute to its successful implementation.
Delimitation and mapping of ecoregionsThe first step of systematic conservation planning is delimiting the planning area with a spatial framework of units based on ecological or political criteria (Pressey and Bottrill, 2009; Sarkar and Illoldi-Rangel, 2010). Global assessments of biodiversity are important for prioritizing and focusing attention on broad regions of the highest conservation concern. However, most decisions about protective management are made at finer spatial scales; more detailed assessment within each of these regions for the purposes of area prioritization is called regional conservation planning. Designing networks of conservation areas is most efficient if conducted within the context of biologically defined units, as the distribution of species and communities rarely coincides with administrative units (Olson et al., 2001). To illustrate, a province such as Yunnan includes several very different climatic zones, while distinct geomorphologic formations such as mountain ranges often occupy parts of more than one province.
Thus, the first step of systematic conservation planning in China should be ecological land classification; i.e., dividing the Chinese territory into regional conservation units based on geomorphology, climate and vegetation types (Fig. 1). Defined on varying spatial scales from climate, landform, hydrology, vegetation, and soil data, these units (ecoregions) provide a consistent spatial framework for ecological modeling, biodiversity conservation policies and management, and systematic conservation planning at national and sub-national levels (Cleland et al., 1997; Bryce et al., 1999; Dinerstein, 2000; Groves et al., 2000, 2002; Bottrill et al., 2012). The Convention on Biological Diversity (http://www.cbd.int/sp/targets/rationale/target-11/) and the Global and European Strategies for Plant Conservation (http://www.plants2020.net/implementing-the-gspc-targets/) specifically target the effective conservation of ecoregions.
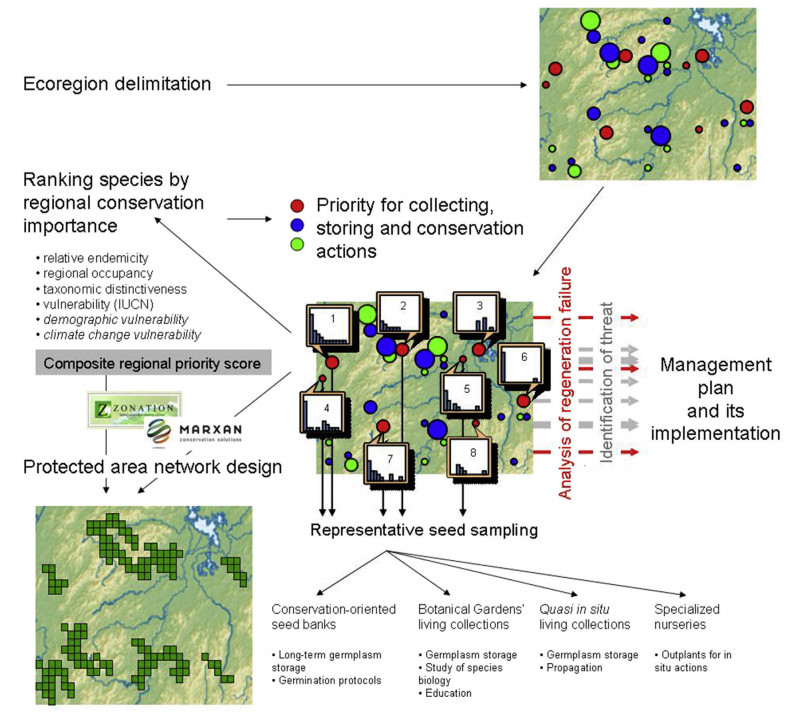
|
| Fig. 1 A scheme of the proposed regional conservation planning and implementation applied to a hypothetical ecoregion of China. Each colored circle denotes a population of one of three species with the circle size and color corresponding to a population size and species identity, respectively. All populations of one species (in red) are provided with size class distributions. In size class distribution histograms the x and y axes are size classes and plant density per unit area, respectively. The populations 3, 6 and 8 have easily identifiable regeneration problems. |
Defining and mapping ecoregions is a well-established process for countries in North, Central and South America (Wiken et al., 1989; Gallant et al., 1995; Josse et al., 2003; Sayre et al., 2008; Omernik and Griffith, 2014), Australia (Commonwealth of Australia, 2012), New Zealand (Leathwick et al., 2003), and many European countries (Klijn et al., 1995; Ioja et al., 2010; Tuvi et al., 2011; Blasi et al., 2014). Recently, the ecoregion approach has been extended globally (Potter and Hargrove, 2012). Many land use planning agencies and conservation organizations base their plans on ecoregional delimitation (e.g., Cowling et al., 2003; Thorne et al., 2006). In ecological restorations, setting boundaries for seed transfer zones also has an ecoregional base (Miller et al., 2011; Bower et al., 2014), as will be discussed below.
The Ministry of Environmental Protection of China (2011) recognized eight ecoregions and 35 priority areas for conservation, but the former are too broad to be useful for conservation planning, while the latter were identified from parameters not directly useful for defining ecoregions (e.g., species richness and endemism, regional representativeness, economic and scientific values of species). Higher-resolution and more practical delineations of China's territory into biologically defined regions include priority natural ecosystems (Li et al., 2003) (129 natural ecosystems of forests, grasslands, meadows, deserts and wetlands) and natural vegetation types (559 natural vegetation formations) (Zhang, 2007b). The latter two delineations can be readily converted into formally defined ecoregions at different resolution scales, as already done in several studies (Wu et al., 2006, 2011; Xu et al., 2006).
It must be noted, that in China, ecoregions in most cases will include territories from more than one province (as for example the case with Wuling, Dalou, Daba and many other mountain ranges) and their conservation planning and management requires close coordination between the relevant state and provincial governmental bodies.
Species and population inventorySpatially explicit data on biodiversity is a prerequisite for conservation decision-making and is therefore fundamental to systematic conservation planning. Thus, conservation within an ecoregion begins with species and population inventory (Fig. 1). The resulting regional species occurrence database should include all threatened species found in the area, including GPS coordinates and the approximate size for every population. The need for the collecting, summarizing and disseminating this information has not been recognized as a matter of priority by relevant Chinese administrative bodies. Moreover, many Chinese conservation biologists are reluctant to deposit their data on species occurrence not only in the Global Biodiversity Information Facility (GBIF, http://www.gbif.org/), but elsewhere. As a result, information on species occurrence is very difficult to obtain. As I noted earlier (Volis, 2016c), for the efficient conservation of Chinese flora, a searchable database and repository for conservation-related information is of fundamental importance. This should above of all include datasets on species occurrence, geo-referenced databases of nature reserves (NRs) and other types of protected areas, and information on active or completed conservation programs. For efficient overall coordination, scientists need to make their data publicly available as a matter of standard procedure.
Demographic surveyingWithin each delimited region, every population of a threatened species must be visited for at least a preliminary survey of the population demographic structure (Fig. 1). In small populations, all reproducing adults must be mapped. Currently, even for critically endangered Chinese species, we have only information about the population locations and rough estimates of population sizes but very seldom their demographic structure, not to mention projected population viability. The preliminary demographic survey should ideally be followed by properly organized long-term observations on population demography and reproductive phenology. Such monitoring is seldom carried out on a sufficient scale, in China or elsewhere, and in its absence, it is not possible to accurately identify the threats to species persistence, develop a management plan or make a seed collection calendar. One reason why so many species in the IUCN Red List data appear as "data deficient" is because of the lack of properly organized demographic surveys as a standard procedure of species assessment. Given the heavy reliance of many organizations and policymakers on this list to guide conservation practices, it is vital to have accurate, demography-based and up-to-date species assessments. A demographic survey should be accompanied by a study of species biology, viz. of the mode of pollination and major pollinators, seed dispersal, breeding structure, presence of seed dormancy, age at maturity and pattern of fruit/seed production. This is, of course, an ideal situation, but should be approached for all species of conservation concern as directly and efficiently as possibly.
Demographic surveying must focus on the ability of species to regenerate. Given that formerly existing species assemblages and biotic interactions in a vast majority of Chinese natural habitats, as in other parts of the world, have been altered, many extant populations of threatened plant species, even in the PAs, do not regenerate naturally. The regeneration niche is destroyed if either the reproductive, dispersal or recruitment niches become unsuitable, e.g., by altering the soil or light conditions so that germination becomes impossible, or by extirpating pollinators/seed dispersers and thus hindering seed production/dispersal. Many species of high-conservation priority (e.g. those in the PSESP species list; State Forestry Administration of China, 2012) are long-living plants with very localized distributions and usually represented by small, isolated populations with limited or absent recruitment. The probable lack of recruitment in isolated populations of these species has thus far received very limited attention. Even if problems with natural regeneration are reported, no recommendations about what to do beyond "better protection" are usually recorded.
A demographic survey will identify those populations that have problems with recruitment (Fig. 1). For these, observations on flowering and fruiting and introduction experiments manipulating germination and growth conditions can be used to reveal why population regeneration fails. Depending on the cause, the possible solutions may include thinning competing vegetation, introducing nurse plants, planting more individuals of the target species to increase pollinator visitation rate, and so forth.
Very few studies of threatened plant species in China have so far focused on population demography and regeneration. In this respect, the studies of Yongchuan Yang with colleagues must be acknowledged as standing out in their scope and methodology. Their analyses of population demography, community composition and abiotic environment provided invaluable insights on the status of natural populations of such emblematic Chinese endangered relicts as Ginkgo biloba (Tang et al., 2012), Metasequoia glyptostroboides (Tang et al., 2011), Cathaya argyrophylla (Qian et al., 2016), Davidia involucrata (Qian et al., 2017) and others.
Ranking threatened species by conservation valueWhile the ecoregion inventories of threatened species will result in lists of locally occurring species with each species assigned to one of three IUCN categories (CR, EN or VU), this information about species vulnerability may be insufficient for setting conservation priorities. Species assigned to the same IUCN category can differ dramatically in many important attributes. For example, one of two endangered species can represent a regional endemic, while another may have its major distribution outside the region. Or one species can represent a monotypic genus and the second a genus with several hundred species that closely resemble each other. Thus, regional conservation planning must have a more nuanced but straightforward and easy-to-use procedure for ranking species by their conservation priority. One approach, as opposed to using a single qualitative variable (IUCN category), is to employ a composite variable that combines several quantitative estimates of species rarity and vulnerability. Freitag with colleagues proposed to complement quantitative analogs of the IUCN categories (RV) by three additional estimates: relative endemicity (the proportion of a species' range found in a region) (RE), regional occupancy (RO) and taxonomic distinctiveness (RTD) (Freitag and Van Jaarsveld, 1997; Freitag et al., 1997). These estimates can be calculated in different ways depending on spatial scale and quality of the species occurrence data using either spatial units, area occupied or number of populations.
For each species, total geographic range or "extent of occurrence" should be estimated as the general distribution of the species throughout China, i.e., as total distribution area (km2). The distribution of each species in the region is calculated in terms of relative endemicity and area of occupancy scores.
Relative endemicity score (RE) is a proportion of the species' total distribution range in China falling within the ecoregion. For example:
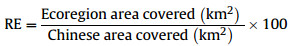
|
According to this equation, species that are increasingly restricted to the ecoregion receive increasingly larger RE scores. Species with RE scores of one have distributions entirely restricted to the region (true regional endemicity).
Regional occupancy score (RO) is a more refined estimate of regional extent of occurrence. Higher scores get regionally less common species (those with smaller areas of occupancy and smaller number of populations). For example:
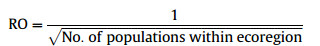
|
Relative taxonomic distinctiveness score (RTD) estimates the taxonomic distinctiveness of a species. Taxonomically more distinct taxa receive higher scores because they contribute proportionately more to regional biodiversity (Vane-Wright et al., 1991, 1994). An equation that can be applied to all hierarchical classifications was proposed by Freitag and Van Jaarsveld (1997):
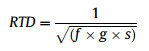
|
where f is the number of families in the order to which the taxon belongs, g is the number of genera in the family and s is the number of species in the genus to which a particular species belongs. Only those species, genera and families occurring in China are included in the calculation procedure.
The above three estimates complement the quantitative analog of the IUCN categories, Relative vulnerability scores (RV). The RV scores are based on Red Data Book categories, i.e. "critically endangered", "endangered", "vulnerable" and "near threatened" weighted as 1.0, 0.75, 0.5 and 0.25, respectively. Species not recorded in the Red Data Book are given a score of zero.
I propose two novel criteria, demographic vulnerability and climate change vulnerability.
Demographic vulnerability score (DV) is the proportion of the populations having regeneration problems such as lack of seedlings, young plants or reproducing adults:
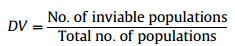
|
According to this equation, species with regeneration problems receive increasingly larger scores.
Climate change vulnerability score (CV) is a proportion of the habitat currently suitable for the species within the ecoregion that will remain suitable despite ongoing climate change:
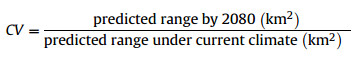
|
The predicted ranges may be obtained from species occurrence data through ecological niche modeling.
Following Freitag and Van Jaarsveld (1997) for simplicity, these six scores can be given equal weights, summed and averaged to obtain a total composite score for each regionally occurring species, referred to as regional priority score (RPS):
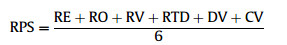
|
Regional systematic planning utilizing a unified scoring system as described above will give the highest priority to the most endangered and phylogenetically distinct local endemics with regeneration problems. As discussed below, this should help conservationists design reserves, monitor and assess the efficiency of a reserve network, as well as plan and create seed banks and botanic garden living collections.
Prioritizing conservation areas and designing PA networksBecause in any given region the total amount of land available for conservation is limited, the purpose of systematic conservation planning is to establish an efficient system of conservation areas for the long-term conservation of biodiversity (Margules and Pressey, 2000). Therefore, after identifying areas of conservation value, these areas should be prioritized for protection and the protected area network designed. Traditionally, such decision-making has been based on expert opinion, but nowadays prioritizing areas, as well as designing protected area networks, are customarily aided by computer-based decision-support tools. The latter utilize datadriven, inherently quantitative approaches based on spatial data on biodiversity and other environmental and socioeconomic factors (Pressey et al., 1993; Margules and Pressey, 2000).
Reserve selection can be viewed as an optimization problem - a search for a compromise between resource allocation (land area) and conservation efficiency. The ultimate goal of this optimization is, whilst minimizing cost, to create protected area networks that will protect all conservation targets (species or habitats), include as many elements of biodiversity as possible, and be sufficiently large and well-connected. Even if the creation of a regional conservation plan starts from the identification of core areas that contain regionally important habitats or charismatic species (cf. Noss et al., 1999; Shriner et al., 2006), it should proceed to formal computerbased reserve selection, because umbrella and flagship species are usually poor surrogates for regional biodiversity (Andelman and Fagan, 2000).
Despite the proliferation of decision support software tools for use in systematic conservation planning (e.g. ConsNet, Ciarleglio et al., 2009; C-Plan, Pressey et al., 2009; LQGraph, Fuller and Sarkar, 2006; Marxan, Ball et al., 2009; Zonation, Moilanen et al., 2009), these techniques share several common basic principles. Firstly, the regional area is divided into a set of spatial units either based on existing boundaries (e.g., administrative, ecological, watershed or land ownership boundaries) or grid cells of a required resolution level. Each of the discrete spatial units ("planning units") is assigned a conservation value which is estimated depending on the focus - which can be on an individual species, habitat or ecosystem. Quantification of a conservation value can be based on the species occupancy (presence or absence), species abundance, the area of a species habitat within each planning unit, habitat conditions, and so forth. The problem formulation can be as simple as a binary decision of whether to include a planning unit in the selected set, or more complicated, such as selecting which actions to implement within a planning unit. In either case, the value of each planning unit for inclusion or with respect to an action must be quantified, along with some measure of the cost of implementing the action. The targets for achieving conservation goals can be set for each species of conservation concern but the common target in the reserve design is a habitat area to be protected. The outcome of the area selection procedure depends on the data input, the initial and subsequent selection rules and the sequence in which selection rules are used in an algorithm.
Selecting sites by species richness is inappropriate because a proper solution is not a set of richest sites, but a set of sites whose species assemblages complement each other and together embrace the largest species pool (Pressey et al., 1993; Williams et al., 1996). Therefore, most of the existing selection algorithms are based on the concept of complementarity, i.e., a measure of the degree to which a site contributes to the representation of biodiversity features that are not adequately represented in the existing set (Pressey et al., 1993). In addition to complementarity, two other key concepts have guided the reserve selection algorithms: irreplaceability and vulnerability.
Irreplaceability is a measure of how any particular selected site is essential for achieving targets. Sites with lower irreplaceability can be replaced by other, unselected sites, if for some reasons the former cannot be included in a conservation area network, whereas sites with high irreplaceability can not be replaced and therefore should not in principle be open to negotiation (Pressey et al., 1994). Technically, irreplaceability value is the summed number of times each planning unit was chosen in a set of runs of the algorithm. Complementarity is implicit in irreplaceability. Units with a high value include high numbers of species with low species overlap compared with other units, while those that have a low value do overlap in species composition with many other units, although they can still have high biodiversity.
Vulnerability is about persistence of biodiversity in the planning unit, i.e., a probability of biodiversity loss to current or imminent threatening processes (Pressey et al., 1996; Wilson et al., 2005), which amongst others include urbanization, infrastructure development, mining, agriculture, logging, grazing and the spread of invasive plants and animals. A comprehensive assessment of vulnerability should consider the threat effects as well as the dynamic responses of threats to conservation actions. The unit vulnerability score for multiple threats can be calculated by differentially weighting threats to reflect their relative impacts.
All the currently utilized reserve selection algorithms produce either a set of sites that represent the maximum number of species in a given number of sites, or all species in the minimum number of sites (known as maximum coverage and minimum set problems), referred to as a "solution." A solution for the former is found via simulated annealing (e.g., as implemented in Marxan, Ball et al., 2009) and for the latter via reverse stepwise search (e.g., as implemented in Zonation, Moilanen et al., 2009). Weighting species by their conservation values has mostly been overlooked by these methods. Several authors have discussed the usefulness of weighting species by their taxonomic or genetic distinctiveness, rarity, endemicity or economic value in reserve selection (e.g., Freitag et al., 1997; Arthur et al., 2002; Onal, 2004), but very few have actually used species weighting in reserve selection algorithms (Freitag et al., 1997; Arponen et al., 2005; Fiorella et al., 2010).
Most existing algorithms for reserve selection give equal weights to the species, but there are exceptions. For example, in the Zonation program (Moilanen et al., 2009) weighting species by their conservation values is possible. This software optimizes a solution by capitalizing on the natural patterns of species cooccurrences in the landscape to include the maximum possible proportion of each species distribution within a solution. Utilization of the software option to weight species by their conservation value will change a focus in reserve network design, as was shown by Fiorella et al. (2010), from maximizing overall species protection to maximizing protection of the most threatened species. Species can be prioritized not only by their IUCN Red List Categories (Fiorella et al., 2010), but also by a combination of criteria (Freitag et al., 1997; Arponen et al., 2005). The regional priority scores described above can be particularly useful for this purpose (Fig. 1).
The search for an efficient reserve design must account for the predicted effects of climate change (Araujo et al., 2004; Hannah et al., 2007). To enable species to adjust to their ranges in response to changing climatic conditions, it is often recommended to increase ecological connectivity - the flow of propagules, organisms and ecological processes across landscapes, by either increasing suitable habitat at range margins or establishing corridors to make species movement possible (Heller and Zavaleta, 2009; Krosby et al., 2010). The creation of corridors was the most commonly recommended management response in early climate change literature (e.g., Peters and Darling, 1985), but there is little guidance in the literature for corridor implementation beyond common-sense reasoning (Heller and Zavaleta, 2009). In many cases, because of technical challenges of establishing corridors, the connectivity can be co-incidentally improved by a mere increase in habitat area and habitat quality (Hodgson et al., 2009).
Another commonly suggested management response to the effects of climate change is a poleward expansion of reserve boundaries or buffer zones (Noss, 2001; Hodgson et al., 2009). But one should keep in mind that a species can track a 3 ℃ increase in temperature by moving less than 500 m upward in elevation, while tracing an analogous latitudinal shift would require poleward movement of almost 400 km (Krosby et al., 2010). Thus, in situations when an environment sharply changes in elevation over short geographic distances an increase in the reserve size can provide enough opportunities for a species to find a more favorable habitat where it can survive. However, it is more likely that a change in reserve size or configuration will not significantly change the extent of variation in habitat conditions. For many species the distance required to adjust their ranges (the temperature isoclines are expected to shift more than 1 km/year in many systems; Loarie et al., 2009) will be too great to be accommodated by simply expanding reserve boundaries. For these species, the creation of steppingstone reserves, even of small size, along climatic gradients may be a better means to enable range shifts. Analysis of a relationship between the species' current distribution and climate through SDM, especially if the relevant paleobotanic and paleoclimatic data are available, may make it possible to suggest optimal reserve locations and sizes, as well as to identify areas that will have suitable conditions in the future and therefore a high probability of protecting the target species.
Ex situ: seed collectionsIn parallel with demographic/ecogeographic surveying, conservationists should plan and initiate seed collecting in all reproducing populations of threatened species (Fig. 1). Volis(2016c, d) provides detailed guidelines for collecting seeds of threatened species. It is probably important to stress once again that ⅰ) sampling should be done repeatedly over several years so as not to harm the population's rate of reproduction in any one year or simply because seed production is too low to take an adequate sample all at once; ⅱ) collecting seeds from individual plants and keeping their seeds separately as maternal lines (families) should always be preferred over bulk collections; and ⅲ) it is desirable to distinguish long-term and short-term seed collections, the first being a "strategic" source of germplasm to be used for studying species biology, propagation and renewal, but not for in situ actions, whereas the second to be used exclusively in in situ actions either directly or after propagation.
The ecoregion concept central to the proposed integrated conservation strategy, should be used for more than species and population inventory, making priority lists, and enabling reserve design. This concept should also provide a basis for seed collecting and movement of the seeds across the species range in in situ actions, through delineation of the seed zones. As not all populations of a species will necessarily be adapted to other parts of the species' range, seed sources must be matched to geographic locations for which they are suited. This idea was coined in the concept of seed transfer zones in forestry as early as in the 1950s, with the latter zones defined as geographically bounded and ecologically similar regions where seeds can be collected and used with a higher rate of success. As seed zone delineation on a species-by-species basis is costly and time-consuming, a solution is to use provisional seed transfer zones defined by relevant climate variables (e.g., winter minimum temperature, maximum mean monthly temperature and annual precipitation) based on maps that are overlaid with defined ecoregions at the appropriate scale (Bower et al., 2014).
In a study by Bower et al. (2014), in which Omernik's level Ⅲ ecoregions (Omernik, 1987) were superimposed over 64 provisional seed zones representing areas of high climatic similarity, the combined model produced more variation partitioned among zones than either system alone (Bower et al., 2014). The areas distinguished by a combination of the two approaches were climatically similar yet ecologically different, and therefore account for adaptation not only to climate but also to the other environmental features that vary spatially. However, despite the higher proportion of variation explained, the finer subdivision of provisional seed zones (i.e., usage of level Ⅳ ecoregions) should be discouraged because they produce too many zones to be practical from a land management perspective (Bower et al., 2014).
The above knowledge can be adjusted on the basis of available species-specific information about its biology (life history, mating system, seed dispersal distance), spatial pattern of genetic variation, and the range of microsites where it grows. For example, outcrossing species with long-distance seed dispersal are likely to have larger seed transfer zones than those that are highly selfing with spatially-localized seed dispersal. At present, there are three national gene banks in China and a large network of botanical gardens holding their own seed banks of various degrees of sophistication. However, as noted earlier (Volis, 2016c), the efficiency of this impressive infrastructure for conservation of threatened species in China, like in most other countries, is low. Firstly, collecting seeds of common and widespread species is often emphasized in seed collecting programs simply because they are more easily found. To obtain samples of many threatened species, which often exist as small populations sparsely distributed in remote places, special efforts must be made to obtain seed samples, especially considering that it is highly desirable to bank seeds from more than one population. Seed collections of threatened species, if they exist, represent an unknown or low number of populations and accessions with information on stored accessions very difficult or impossible to access. In the majority of situations, the germplasm stored in seed banks is inaccessible for any in situ actions. Living collections in Botanical Gardens, potentially useful as seed sources, in many cases lack information on place of origin which strongly limits their utility for in situ usage.
To the extent possible and appropriate, I suggest the following amendments to the seed bank concept and management. To make seed banks useful for conservation, and encourage seed bank managers to invest in collecting seeds of species which pose a collecting challenge, it is necessary to convert at least some existing seed banks into seed banks that specialize in conservation and restoration, while at the same time making the collected seeds available for in situ actions. These 'conservation seed banks' should be designed to store, handle and use large number of seeds per species rather than just large number of species, and must have an emphasis on collecting and storage of as much as possible of the species genetic variation. Although it is desirable to preserve genetically adequate seed samples of all species, the rarest species must be the first priority for collection and storage.
The idea of collecting and storing only species to be used in in situ actions is utilized in 'restoration seed banks' (Merritt and Dixon, 2011). Although these facilities are created to provide the large quantities of seed needed in ecological restoration projects, experience of existing restoration seed banks should be learned and adopted for the specific needs of conservation seed banks.
Ex situ: living collectionsTraditionally, the major depositories for living plants, especially rare and threatened species, are botanical gardens. In China, the role of botanical gardens in plant conservation has been recognized by the government and the Chinese Academy of Sciences (Huang et al., 2002; Huang, 2011). The conservation of threatened plant species is included in the mission statements of many Chinese botanic gardens, and living collections of threatened plant species exist in three large (Xishuangbanna Tropical Botanical Garden, South China Botanical Garden and Kunming Botanical Garden), and at least 13 smaller, Chinese gardens. However, very few of these collections have significant in situ conservation value for reasons discussed below.
Although botanical gardens perform important for conservation botanical and ecological research, their role in conservation should not be overestimated. Botanical gardens have numerous limitations in their utility for maintaining living collections, with the major weakness being their capacity. The number of genetically distinct individuals is automatically limited by the space available in which to grow them. Traditional guidelines for ex situ conservation (e.g., 10-50 individual plants from five natural populations) are impossible to meet for the majority of botanical gardens (Chen et al., 2009), where display is an important criterion too. In most cases, only a few living individuals can be preserved in botanical gardens. In some cases, these may represent precious samples of taxa now extinct in nature and should be cherished as such. For example, the only ex situ collection of Picea neoveitchii (a narrow endemic species of Qinling Mountains) consists of just two individuals cultivated at the Xi'an Botanical Garden (Zhang, 2007a). However, in the overwhelming majority of cases, the living collections of botanical gardens represent a negligible fraction of the existing species genetic diversity.
To play a more useful role in plant conservation, botanical gardens should adopt the creation of living collections of threatened species as equally important to public service, botanical research and education, and share responsibilities with one another. Especially if they could assume regional roles, these gardens could begin to play a much more active and effective role in saving plants from extinction. Botanical gardens worldwide are working in various degrees toward these objectives and networking such efforts in China would be especially important in achieving national goals. An appropriate unified management strategy of botanical garden collections is described in Volis (2017a). It includes setting regional conservation priorities (in association with the conservation agencies at a national/regional level) for the species to be conserved, creation of genetically representative collections for high priority species and usage of these collections in in situ actions. For conservation, the value of existing and future living collections will be a function of the species conservation status and how well the collection represents its natural genetic variation (Volis, 2017a). In all cases, however, living collections should be valued for what they are.
Integration of ex situ and in situHaving space and logistic limitations for preserving species genetic diversity and providing material for in situ actions ex situ seed and living collections can be enhanced through the creating living collections of needed capacity under natural, semi-natural or artificial conditions. Two such concepts are "forest gene banks" (Uma Shaanker and Ganeshaiah, 1997; Uma Shaanker et al., 2001, 2002) and "quasi in situ" (Volis and Blecher, 2010; Volis, 2015). The main idea of the forest gene bank is to use a particular existing population as an in situ sink into which genetic material from several source sites is introduced and maintained. The genetically diverse sink population serves as a repository of the species gene pool and a natural breeding habitat. This approach can be useful in cases when introduction of non-locals will not lead to outbreeding depression (i.e., in situations when plants originate from the same climatic zone and similar biotic environments) and can i) greatly improve the local population genetic variation and eliminate inbreeding depression; and 2) provide a large quantity of vigorous (due to heterosis) seedlings for creating new and reinforcing existing populations. The quasi in situ concept proposes creation of new instead of using existing populations as depositories of genetically variable source material. The quasi in situ site maintains a living collection of individuals from populations sharing the same climatic zone and biotic/abiotic environment, has natural or seminatural conditions and would be legally protected. The detailed guidelines for choosing material, planting, and management of the quasi in situ living collections are provided in Volis and Blecher (2010) and Volis (2016d).
The quasi in situ concept seems to have been adopted in China through so-called 'near situ'1 conservation. In Chinese plant conservation literature published after 2014, when the first paper using this term appeared (Xu and Guo, 2014) the term "near situ" rapidly gained popularity and became widespread. Conservation through "near situ" is often referred to or recommended without any explanation what is meant, as something widely accepted and understood by everyone. This, however, is actually not the case, as the term has never been clearly defined. From the papers using this term we learn that this term embraces any study in which outplants are introduced near the site from which they came. This, apart from rather technical questions about the source material, planting design, maintenance of outplants etc., raises an immediate basic question - for what purpose are they planted? Planting seedlings or saplings outside their original locations and not into a location where this species still grows can have two purposes. One is to S. Volis / Plant Diversity 40 (2018) 91e105 97 re-create a once-existing ecosystem using threatened and/or least concern plant species. Another is to create a living collection that preserves as much as possible the existing species genetic diversity while avoiding the severe maintenance costs and space limitations of ex situ living collections in botanical gardens and arboreta. There are two approaches that specifically pursue the above two conservation goals: inter situs2 - outplanting for restoring a degraded environment (Burney and Burney, 2007); and quasi in situ - outplanting for preserving species genetic diversity ( and Blecher, 2010). I perceive the term "near situ" as either duplicating one of the above, or mixing them, depending on the situation. In any case, this does not justify using this term in the literature.
1 The term 'near situ' presumably means near site and in Latin would be correctly rendered prope situm.
2 Originally and ungrammatically termed 'inter situ'.
Quasi in situ living collections can be useful for more than just germplasm preservation. Space limitations of the traditional seed banks, problems with storing non-orthodox seeds, and the negative impacts of seed harvesting on local population dynamics (Broadhurst et al., 2008) call for an intermediate stage of seed propagation. Quasi in situ living collections can be part of a solution. The plants maintained in these collections can be a reliable source of seeds to be used in situ (i.e. in reinforcement or translocation). Recent technological developments that overcome dormancy, promote germination, and reduce pathogen attack will greatly increase the number of seedlings obtained from these seeds (Turner et al., 2013; Madsen et al., 2012, 2014).
Seedlings are preferred over direct seeding for in situ conservation actions because of frequently high mortality and low establishment of the latter (Guerrant and Kaye, 2007; Menges 2008; Godefroid et al., 2011). Raising a large number of seedlings or plantlets from seeds in botanical gardens can be difficult because of space requirements. Specialized nurseries are the best option for producing a large number of seedlings (Fig. 1). However, in some cases one of two other approaches can be used. In "in situ seedling banks" (Pritchard et al. 2014), seeds are sown and seedlings are maintained in the forest understory. This method is easy to apply, because the seedling banks can be established in a wide range of forests: natural, degraded or planted forests, including monoculture tree plantations. But it can only be used for species that have high seed viability and germination rates, in addition to few specialized requirements for successful germination. Another approach is to use wildlings. Old individuals of many threatened plant species can be found in villages or farmland where they are maintained by farmers and are used (fruits, medicine, shade) or retained for cultural, religious or esthetic reasons. These trees often produce a large number of seeds that become seedlings having no future in the agricultural landscape but that can be used for creation of new populations in a suitable natural location..
In situ: introduction and habitat restorationChina's Strategy for Plant Conservation (CSPC) (China's Strategy for Plant Conservation Editorial Committe, 2008) declared the nation's commitment to protect ~90% of China's national key protected plants through in situ efforts (Target 7), and to reintroduce 10% of China's threatened plant species to their natural habitats and establish monitoring programs to track management success (Target 8). However, to achieve these goals several methodological principles would need to be adopted.
One principle is that the steady decline in population size of many species will not necessarily be halted by passive conservation via protection of a particular area; in fact, preventing these populations from extinction requires their active management.
Only a small number of nature reserves protect primeval and not secondary forests. Virtually all ecosystems protected in nature reserves have undergone some degree of human-induced change in the past that disrupted species interactions and ecological processes that once existed. In addition, exploitation, fragmentation and environmental degradation has reduced the population sizes of many species below the viability threshold. The existing individuals indicate that the survival of the species at a specific location is possible in principle, but only if the factors contributing to their population decline are identified and eliminated. The identification of these factors requires the proper understanding of ecological processes within an ecosystem as well as the population dynamics of endangered species. Therefore, if CSPC Target 8 defines "management success" as the survival of reintroduced plants, it should be made clear that even high rates of survival for a time will not guarantee that their population decline will stop. Ultimately, the success of the reintroduction project must be assessed by the successful creation of the new generations from the introduced plants. And the latter in many cases will prove impossible without active interventions (e.g., introduction of nurse plants, thinning of competing species or rewilding). Analysis and restoration (if needed) of the regeneration niche must be a precondition for any reintroduction. For example, an analysis of regeneration in M. glyptostroboides revealed that over the last 50 years activities of the local human residents, such as selective cutting, harvesting of firewood, and especially cultivation of cash plants in the understory, have made natural recruitment of M. glyptostroboides (Tang et al., 2011) impossible. Neither strict protection nor reinforcement actions will rescue this population unless the conditions under which natural germination of M. glyptostroboides was occurring normally (as evidenced by Chu and Cooper, 1950) are restored.
The second principle is the recognition of the importance of carrying out wide-scale plant introductions, not only within but also outside the known species historical range. Traditionally, introduction outside the historic range has been considered one of the last resort conservation actions that can be taken to prevent species extinction (Hoegh-Guldberg et al., 2008). There is now a growing consensus that this view needs to be reconsidered given the current rates of disappearance and alteration of natural habitats, and climate change effects on the species ranges (Millar and Brubaker, 2006; Vitt et al., 2010, 2016; Thomas, 2011; Butterfield et al., 2016; Volis, 2016b). In fact, for many endangered species, undisturbed reference habitats do not exist nowadays, as in the case of M. glyptostroboides described above. Insuring the future for these species will likely be possible over the long run only through introduction into multiple suitable habitats both inside and outside their known range, accompanied by additional restoration interventions applied to the habitats (Volis, 2016b). Introduction of multiple threatened species into multiple locations, accompanied by such interventions as thinning of competing vegetation or liberation cutting has been described as a coherent strategy for the conservation-oriented restoration concept (Volis, 2016b). This concept provides a set of recommendations for the identification of suitable multi-species assemblages prior to introduction, planting design and addressing inter-specific interactions (Volis, 2016a).
Following Frankham et al. (2014), the introduced population should ideally be at least 100 families and around 10, 000 individuals. For species that are on the brink of extinction this number of families will be impossible to achieve because of the small number of adults in extant populations that can act as the mother plants. On the other hand, the required number of individuals (5000e10, 000) derived from all available mother plants will often be achievable after properly managed propagation. For a 2 Originally and ungrammatically termed 'inter situ'. species introduced into multiple sites within potential seed and pollen flow distance from one other, a mosaic of these populations can be considered a single meta-population in which the recommended number of introduced individuals is present.
Active management of a threatened species necessarily involves preparing an Action Plan specifying the interventions needed for species recovery. The level of management intervention will depend on the nature and severity of the threat to the populations/ habitats. Possible interventions include weeding, eradication/control of exotic species, assisted pollination to increase seed set, liberation cutting to improve seedling and sapling survival, predator and pest control, introduction of nurse plants, creation of deadwood, augmenting dispersers, pollinators and ecological engineers (Heywood, 2016; Volis, 2016a). If plant translocation is performed, it is essential that the outplanting protocol includes planting maps, GPS locations, and unique identification tags with sequential numbers for the introduced plants. The chances of any Action Plan succeeding will increase with better site evaluation and preparation prior to planting, monitoring and continuous site care, the use of larger numbers and more genetically variable seed source, and the use of larger and more mature individuals for planting (Guerrant and Kaye, 2007; Godefroid et al., 2011; Dalrymple et al., 2011, 2012; Volis, 2016a; Maschinski and Albrecht, 2017).
Analysis of population demography that starts from a preliminary survey of population demographic structure followed by tracing plant fates through time must be a necessary part of every in situ management plan. First, this is needed to access the species conservation status. Second, this is the only way to identify the threats and devise appropriate management interventions. And third, long-term monitoring is essential to assess the project success and make adjustments to the Action Plan when needed. For a project involving plant translocation, the monitoring of introduced and comparison with the natural populations will help to manage the introduced populations optimally. For example, demographic comparisons of the introduced and natural populations of Centaurea corymbosa revealed that the former had higher survival but lower fecundity than the latter, with a management recommendation to increase plant density to improve mate availability for self-incompatible flowering individuals (Colas et al., 2008).
The introduction of potentially maladapted genotypes is a major concern in translocations and habitat restoration. Genotypes poorly adapted to local conditions have a low chance of establishment and could even negatively affect the characteristics of adjacent native populations adapted to local environmental conditions through gene flow (McKay et al., 2005). This means that plant germplasm must be adapted to the local environment, yet also possess sufficient diversity to cope with changing biotic and abiotic conditions. A solution is to use ecoregion-based seed transfer zones and quasi in situ living collections.
In situ: PA policiesChina has an impressive network of 2856 PAs, including 428 national NRs, covering an area of 137 million hectares (Miller-Rushing et al., 2017). However, as previously noted (Volis, 2016c), the efficiency of this network must be strongly reinforced if much of the rapidly vanishing biodiversity is to be rescued, especially given the deterioration of ecosystems under the pressures of an unprecedentedly fast-developing economy.
The most serious problems that compromise the effectiveness of many PAs in China are institutional conflicts in planning and management, improper area categorization and zoning, and a conflict between the objectives of generating revenue and conservation. These problems have been recognized and intensively discussed in the last two decades (e.g., Li and Han, 2001; Xu and Melick, 2007; Ma et al., 2009; Tang et al., 2009; Xu et al., 2012; Wang et al., 2012; Zinda, 2014; Cao et al., 2015; Buckley et al., 2016; Miller-Rushing et al., 2017; Zhang et al., 2017a). Before going over these issues and discussing the required amendments, however, I would like to present another problem that is less commonly considered by Chinese conservation biologists (but see Tang et al., 2011; Qian et al., 2017). The problem is in a strict prohibition of any interventions in PAs paradoxically combined with their pursuit of economic benefits and very liberal policy toward visitors and local residents. To exemplify the latter, 7% of national parks permit the sale of goods made from endangered species (Zhong et al., 2015). But even under the very liberal policy, illegal tree cutting is reported from 7% of PAs nationwide; wildlife poaching in 5%; collection of herbs in 11%; illegal grazing in 12%; and damage to native trees in 15% (Zhong et al., 2015).
The Chinese Nature Reserves policy explicitly states that the reserve authorities need, and have the right, to develop these same lands they protect, for the economic benefits of the reserve and its staff (China's National Committee on Man and Biosphere, 2000). It appears to be unthinkable and logically impossible to simultaneously protect the land in its natural state (which is coined in the IUCN definition of a NR), and develop and manage it for maximum profit. Predictably, such policy will stimulate the reserve authorities to prioritize profits at the expense of land protection, resulting in the loss of ecosystem integrity and naturalness (e.g., Ma et al., 2009).
Formally, NRs in China are managed based on the three-zone (core, buffer, and experimental zones) biosphere reserve model of UNESCO (Batisse, 1982): the core zone, where visitation and any activities are prohibited, is surrounded by a buffer zone; scientific research and ecological monitoring is allowed in this buffer zone, which is itself surrounded by an experimental zone; the experimental zone not only permits but supports economic development that benefits local residents while simultaneously maintaining a healthy environment". However, in China, applying these regulations has turned out to be unrealistic, and in fact almost no NR conforms to them. According to the Protected Area Task Force report (PATF, 2004) mapped zones are rarely marked in the field and often completely ignored in practice, with hardly any PA in China free of human settlements, farming, and/or widespread unsustainable harvesting of resources. Not surprisingly, many reserve managers are unsure about even the exact area and extent of their reserves, with many parks existing only on paper (Sang et al., 2011). The core zones of some NRs fail to cover the main conservation target, but include villages and even towns (Zhang et al., 2017a). Infrastructure developments in the experimental zone are controlled by the Nature Reserve Review Committees, but the latter are often under strong pressure from local governments and in many cases can not stop detrimental infrastructure developments without interventions from higher-ranked supervising agencies. Zhang et al. (2017a) reports that the State Forestry Administration (SFA) has refused to approve any applications for infrastructure development in 30 National NRs since February 2016, because the planned activities, such as mining, hydro-development and tourism, were in direct conflict with the primary objectives of conservation. In total, the core and buffer zones on the average account for 56% of the area of Chinese NRs (L. Zhang et al., 2017a). Even assuming that both core and buffer zones in Chinese NRs control human impacts in accord with category Ia (which unfortunately is not the case), the previously mentioned number still is much lower than what is recommended by IUCN. IUCN recommends that the PA category based on management objectives applies to more than 75% of its area (Dudley 2013). The Chinese NRs with de facto human habitation in buffer zones cannot be considered as category Ⅰ or even Ⅱ, but should instead be classified as category Ⅳ only (Buckley et al., 2016). Available data on the number of residents in NRs, including core areas, are unthinkable. According to Li et al. (2009), in 2008 there were c. 10 million residents in c. 2000 NRs. Xu et al. (2012) reported that 1085 villagers were resettled from the core areas of the Wuyanling National NR, Zhejiang Province; and that over 250, 000 people reside in the Sanjiangyuan National NR, Qinghai Province. The population density of many parks and reserves exceeds 60 persons km-2 (Wang et al., 2012). In many NRs, rapidly developing tourism is practiced de facto in the buffer and even core zones, even though PA regulations restrict such activities to the experimental zone only (Tang et al., 2009).
In summary, Chinese PAs, including NRs, hardly correspond to IUCN categories Ⅰ-Ⅳ, as has already been recognized by the World Database on Protected Areas (WDPA) (WDPA, 2010). Nevertheless, allowing infrastructure works within even core zones, and largely ignoring damage from local residents, PAs have in place contradictory policies prohibiting conservation interventions necessary for maintaining viable ecosystems or rescuing endangered species. For example, the management of populations of most relict tree species - e.g., C. argyrophylla (Qian et al., 2016), Thuja sutchuenensis (Tang et al., 2015), Tetracentron sinense (Tang et al., 2013), Taiwania cryptomerioides (He et al., 2015); D. involucrata, Cercidiphyllum japonicum var. sinense and Euptelea pleiospermum (Tang and Ohsawa, 2002) - requires the control of competing broad-leaved tree species by thinning and liberation cutting. But the latter activities are usually not allowed by NR policies. This dilemma will need to be resolved if the target species are to be conserved effectively. For NRs to function properly in conservation, a number of locally applied conservation actions must be allowed. The incomplete list of the latter includes eradication of exotic species, assisted pollination to increase seed set, removing obstacles to seedling recruitment, augmenting dispersers, reinforcement of existing and creation of new populations by artificially or naturally propagated material (Volis, 2016a). In 2017, China has revised 'The Nature Reserve Regulation' and made some previously prohibited management actions (e.g. controlled burning to facilitate species regeneration) possible for NRs, but this is not enough.
At the same time a policy of "blind eyes" toward local residents should be halted. Local residents in many PAs are not even aware that their activities within the PA, such as logging, coppicing, planting profitable plants in the understory, and collecting seeds and seedlings are prohibited by law. Human population in these areas only grows as a result of increasing visitation by tourists, and this leads to further deterioration and fragmentation of the natural habitat. The government must stimulate, e.g., by providing the fellowships, the younger generations to leave instead of stay in the national parks and especially NRs, with strong educational companies explaining the esthetic and cultural values of nature beyond practical needs. The abovementioned activities of local residents must be prohibited (not just on paper) and strictly controlled by rangers. The following example provided by Lamb and Gilmour (2003) is highly relevant for China. India's Buxa Tiger Reserve, on the borders of Nepal and Bhutan, contained 25, 000 people living in 37 forest villages in 1987, when the NR was established and a logging ban was imposed. The ban removed a major source of income for the local villages but did not provide them with an alternative. As a result, they have continued illegal felling, which increased the overall rate of habitat degradation despite its protection status. There are hundreds if not thousands of similar situations in China, including the iconic Wolong NR, where the growing population of local residents caused higher loss of high-quality habitat than before the reserve was created and even higher than outside the reserve (Liu et al., 2001).
Despite claims that China is a unique place where "many landscapes have been maintained and/or shaped by generations of human activity" (Xu and Melick, 2007), numerous studies have clearly demonstrated that permanent human residence within protected areas is incompatible with long-term preservation of these ecosystems (Liu et al., 2001; Piao et al., 2011; Tang et al., 2011; Qian et al., 2017). These studies show that traditional and apparently long-practiced human activities are a direct threat to critically endangered species and entire ecosystems. We can only guess how many species have disappeared locally and globally as a result of traditional (i.e. continued during centuries) human impacts in China.
In situ: PA governance and planningIn China, PAs are managed through a hierarchical system which is aligned with the country's administrative divisions. Major policies and regulations affecting PAs are set by the central government, but implemented at the provincial and county level through a complex bureaucracy. This management system has been criticized as inefficient due to inconsistent regulatory standards across the various departments involved, their conflicts of interest, and a lack of effective inter-departmental communication and information sharing. In China, the State Environmental Protection Administration (SEPA) is formally responsible for the overall integrated management of conservation zones, but actually 10 different ministries or administrations manage protected areas. For example, 80% of NRs in China are managed by the State Forestry Administration (SFA), while the other 20% are administered by the Ministry of Environmental Protection (MEP), the State Oceanic Administration (SOA), the Ministry of Agriculture (MOA), Chinese Academy of Sciences (CAS), the Ministry of Water Resources (MWR) or the Ministry of Land and Resources (MLR) (Zhang et al., 2017a). The responsibilities of these governmental units often overlap, which can lead to conflicts in decision-making because of different values and priorities they have.
Over 100 national-level PAs, which are considered the best designed and managed, are simultaneously administered by at least two government agencies (Zhang et al., 2017a). This is in part because PAs tend to declare multiple management objectives to qualify for financial support from several government agencies. However, when agencies share management responsibilities for PAs, they often compete for prestige and control; because the process for reconciling administrative conflicts is not straightforward and lacks transparency, powerful agencies can take priority over less powerful agencies, which may negatively affect PAs with impunity (PATF, 2004; Zinda, 2014; Zhang et al., 2017a). Moreover, protected-area designations are susceptible to fiscal and political agendas, which allows not only upgrading areas to higher level, but also downgrading areas to lower levels (Jim and Xu, 2004). Because different PA types focus on different management objectives, it is not surprising that in areas with multiple-agency administration, tourism is over-developed at the expanse of conservation. For example, Mt. Lushan in central China and Five Connected Lakes in northeast China, each designated by four national-level "titles" (National Nature Reserve, National Forest Park, National Geopark and National Scenic Spot), both suffer from over-developed tourism (Zhang et al., 2017a).
Tourism is a new economic growth priority enthusiastically pursued by local governments, because, in contrast to mining and hydropower development, which are subject to requisitions from higher levels of government, tourism as a rule is entirely within local control. Unfortunately, local governments pursue this revenue by making the smallest investments and without allocating new lands for protection, i.e., often by just converting existing NRs to National Parks. Thus, instead of creating new protected territories with protected categories lower than NRs, the existing NRs become crowded with visitors, packed with hotels, restaurants and shops, and intersected by roads. Often, sightseeing trails and commercial tourist facilities are built through the core zones. Clearly, these developments can only increase threats to biodiversity in the area. Although many local governments proclaim protecting nature as an important goal of their regional development, and directly control the practical management of PAs, actions on behalf of this goal do not support active conservation or ecological monitoring. Instead, funds are allocated to building high-profile tourist attractions and supporting them infrastructure (Wang et al., 2012; Zinda, 2014). Actually, because of their stricter regulations, NRs are considered by local governments as obstacles to economic development and seen as targets for re-designation as parks or scenic interest areas. The Regulations on Scenic Interest Areas (2006) do not even mention biodiversity conservation among their objectives; the latter are natural resource use and ecotourism, followed by deriving economic benefits (DWCNRM, 2007; Xu et al., 2012). Not surprisingly, the redesigned NRs rapidly become pure commercial enterprises which are intended to protect a scenic landscape, but not biodiversity.
It remains unclear what is better for China - a streamlined, centralized management system or a system allowing local governments to manage all types of PAs (Zhang 2006; Wang et al., 2012). What is clear, however, is that the new organizational arrangements should allow inter-governmental agency or agencies across national-, provincial-, and local-scales to efficiently coordinate decision-making. A new system is required, with clear laws and procedures that make it much more difficult to downgrade NRs to lower protection categories, comply with the IUCN rules in designation of PA categories and their zonation, and have financial structures allocating sufficient budgets for operational conservation costs such as ecosystem monitoring, patrolling by rangers and, when needed, active interventions.
It must be noted that China does make significant efforts to improve current conditions, and has just drafted the State institutional reform plan (http://www.chinadaily.com.cn/a/201803/14/WS5aa8a001a3106e7dcc141987.html). According to this plan, China will establish a 'Ministry of Ecological Environment' and a 'Ministry of Natural Resources' to better reconcile the demands of economic development and environmental protection. This institutional restructuring is supposed to enforce overall governance, and to be more efficient than the existing system of separated governance. However, we will have to wait for the reform to take effect to judge its success.
There is a need to adopt systematic conservation planning for both establishing and managing NRs. Every protected area must have a comprehensive monitoring program to trace the ecosystem changes over time. All the issues described above, and the fact that tourist development and management in many NRs is done by commercial companies having purely economic interests, makes such monitoring particularly important. As has been noted by several authors, the magnitude of the investments in infrastructure in Chinese NRs has a strong potential to inadvertently and irreparably damage their ecosystems (Li and Han, 2001; Ma et al., 2009; Zinda, 2014; Miller-Rushing et al., 2017).
The effectiveness of NRs cannot be evaluated in terms of time and money spent on monitoring and patrolling, but only by the conservation outcomes evident in such parameters as changes in population sizes of the target species (which usually are the flagship, or, most threatened species), and in the area of the target habitat and degree to which it is intact.
In situ: gaps in the existing PA networkLike elsewhere in the world, the majority of NRs in China have been established opportunistically, piecemeal and not through systematic conservation planning, and usually in areas less suitable for agriculture and extractive development (Wu et al., 2011; Zhang et al., 2017c). Therefore, despite the impressive number of PAs in China, there are many imbalances and gaps in the current PA network.
PA distribution is strongly biased by the long history of human habitation in China, with eastern regions historically having a higher population density and stronger socioeconomic development than other parts of the country. Areas of high conservation value in these regions are few, small and highly scattered among farmlands and human residential areas (Wu et al., 2011, 2014). Protection of the last remaining patches of natural landscapes in these highly developed and populated regions through different forms of PAs must be strongly reinforced, and accompanied by developing restoration plans for those transformed landscapes that can be reverted to an appropriate previous state (e.g., forest plantations and abandoned farmlands) and located strategically so as to connect PAs and form corridors and a mosaic that facilitates seed and pollen flow among them. Among the existing forms of PAs, plant micro-reserves are especially suited to the highly fragmented natural landscapes of Eastern China (Volis, 2016c; Fos et al., 2017).
Other adjustments to the PA network should be made strategically by focusing on biodiversity hotspots and those with the greatest urgency for conservation actions. Zhang et al. (2017a) provided a list of the first priority ecoregions in China to be focused on. The range and categorization of the highest priority PAs in these ecoregions also need adjustments to ensure their strict protection. Several assessments of the Chinese network of PAs, based mostly on the World Database on Protected Areas (WDPA), revealed that all but one of the ecoregions located entirely within China's borders have no coverage by PAs in IUCN Categories Ⅰ-Ⅳ (Soutullo et al., 2008; Jenkins and Joppa, 2009; Wu et al., 2011). Only two of China's PAs included in the WDPA are assigned to Category Ⅳ, with the remainder assigned to Categories Ⅴ-Ⅵ (sustainable use) or having not been categorized at all (WDPA, 2010). Based on these results, Wu et al. (2011) concluded that China has the world's largest deficit in strict protection, and that almost all of China's NRs are designated for sustainable use rather than for nature conservation. Although, according to Chinese law, the primary purpose of NRs in China is to protect endangered species and natural ecosystems (Xu and Melick, 2007; et al. 2007), the reality is different.
Budgetary considerationsThere is a strong imbalance in funding provided by the Chinese Academy of Sciences for research in conservation. Ecological experimental research, which has explicit practical conservation implications, did not exist until recently in China, and only in the last decade has some progress been in this field made (e.g. Ren et al., 2010, 2012; 2016; Zhang et al., 2013; Bruelheide, 2014; Wang et al., 2014). In the current Chinese plant conservation literature, the number of articles that report experimental conservation field work (e.g., experimentally manipulated germination or seedling growth conditions, reintroduction/translocation experiments) amount to only a handful, while many more deal with listing of endangered species and the selection of priority areas for protection.
Among papers devoted to a particular threatened plant species there is disproportionate amount which describe the development of microsatellite markers, patterns of genetic variation, or phylogeographic structure compared to papers presenting field work. Although studying genetic relationships among plant populations and species within China is important, and strong progress continues to be made in this research area, far less attention is being given to field studies which examine the ways that populations function, information that is of critical importance for conservation. Experimental conservation field work takes more time than genetic analyses, which are therefore more often selected for dissertation work or individual papers. The predominance of proposals on genetic variation of threatened species results in a disproportional allocation of money for conservation-related genetic studies than for studies of ecological niche or population demography of threatened species.
This situation has to be radically changed. If the overall aim is to conserve Chinese plants, plant conservation proposals submitted to the National Science Foundation of China (NSFC) that focus on field observations and experiments should be selectively funded.
Information sharingInteractive learning is a prerequisite for efficient conservation. Therefore, availability of the information on active or completed conservation programs is of critical importance both for improving the basis of conservation efforts and coordinating them. Conservation cannot be as effective without adequate systems of obtaining and disseminating the pertinent information as it is gained (Meek et al., 2015).
Some conservation biologists doubt the effectiveness of major scientific journals in promoting conservation (Shanley and Lopez, 2009; McKinley et al., 2012; Gossa et al., 2015). That is clearly an untenable generality, but project reports and other 'grey literature' produced by field practitioners and conservationists, may seldom get communicated to a wide audience (Milner-Gulland et al., 2010; Amend et al., 2014). Highly focused and pertinent articles in the "gray literature" contribute little to academic success in China or elsewhere, which means that there is little attraction for Chinese scientists to prepare them. Instead, scientists tend to concentrate on broad, often theoretical articles that they are able to publish in major conservation or other biological journals with high impact factors. As a result, many Chinese conservation projects are either published in Chinese in local journals or remain unpublished, are inadequately described and analyzed, and poorly publicized. The same tends to be true in other parts of the world. Those papers that do appear in major journals are often poorly connected to practice, and emphasize theory or planning rather than implementation. Reviews analyzing conservation project outcomes, summarizing new conservation tools and presenting detailed methodologies that can be useful for protected area managers or other practitioners are extremely rare.
To change this situation, a system must be developed for conservation biologists to publish their results in appropriate journals, but still have them contribute to their academic advancement. Several established and new journals do publish research, monitoring results and case studies on the effects of conservation interventions, a topic that is underrepresented in major conservation journals (Conservation Evidence, Oryx, New Forests, PARKS, Plant Diversity).
ConclusionsThe crucial components of the proposed integral conservation strategy follow:
- conservation planning and implementation has an ecoregional basis;
- regional systematic planning can best utilize a unified scoring system that gives the highest priority to the most endangered local endemics with regeneration problems; this system is used in reserve design, monitoring and assessment of efficiency of a reserve network, and creation of seed banks and living collections;
- population demography and the presence of naturally occurring regeneration are the key criteria for defining the conservation status of a species and the appropriate major focus of the species recovery plan;
- analysis and understanding of abiotic and biotic niche of species are crucial not only for restoring the population habitat but also for choosing sites for reintroduction and translocation, with the latter done experimentally in multiple locations;
- seeds collected in natural populations may be used for creation of multi-species living collections in semi-natural or natural environments (e.g., NR buffer zones)
- living collections can provide material for in situ actions such as reinforcement, reintroduction and translocation.
Adopting and implementing these strategies successfully and more fully in China requires that the country changes PA legislation and improves PA management, the NSFC funds ecological field research, and local scientists improve their attitudes toward information sharing.
AcknowledgmentsThis work was supported by the CAS/SAFEA International Partnership Program for Creative Research Teams. I would like to thank Peter Raven, Vernon Heywood and two anonymous reviewers for valuable comments on an earlier version of the manuscript.
Amend, T., et al., 2014. Publishing for the Protected Area Community: a Vision for PARKS from its Editorial Board, 20. 2.
|
||
Andelman S.J., Fagan W.F., 2000. Umbrellas and flagships:efficient conservation surrogates or expensive mistakes? Proc. Natl. Acad. Sci. U. S. A, 97, 5954-5959.
DOI:10.1073/pnas.100126797 |
||
Araujo M.B., et al., 2004. Would climate change drive species out of reserves? An assessment of existing reserve-selection methods. Global Change Biol, 10, 1618-1626.
DOI:10.1111/gcb.2004.10.issue-9 |
||
Arponen A., et al., 2005. The value of biodiversity in reserve selection:representation, species weighting, and benefit functions. Conserv. Biol, 19, 2009-2014.
DOI:10.1111/cbi.2005.19.issue-6 |
||
Arthur J.L., et al., 2002. Analysis of the threshold and expected coverage approaches to the probabilistic reserve site selection problem. Environ. Model. Assess, 7, 81-89.
DOI:10.1023/A:1015693531132 |
||
Ball, I. R., et al., 2009. Marxan and relatives: software for spatial conservation prioritisation. In: Moilanen, A., et al. (Eds. ), Spatial Conservation Prioritisation: Quantitative Methods and Computational Tools. Oxford University Press, Oxford, UK, pp. 185-195.
|
||
Batisse M., 1982. The biosphere reserve:a tool for environment conservation and management. Environ. Conserv, 9, 101-111.
DOI:10.1017/S0376892900019937 |
||
Blasi C., et al., 2014. Classification and mapping of the ecoregions of Italy. Plant Biosyst, 148, 1255-1345.
DOI:10.1080/11263504.2014.985756 |
||
Bottrill M.C., et al., 2012. Evaluating perceived benefits of ecoregional assessments. Conserv. Biol, 26, 851-861.
DOI:10.1111/cobi.2012.26.issue-5 |
||
Bower A.D., et al., 2014. Generalized provisional seed zones for native plants. Ecol. Appl, 24, 913-919.
DOI:10.1890/13-0285.1 |
||
Broadhurst L.M., et al., 2008. Seed supply for broadscale restoration:maximizing evolutionary potential. Evol. Appl, 1, 587-597.
|
||
Bruelheide H., et al., 2014. Designing forest biodiversity experiments:general considerations illustrated by a new large experiment in subtropical China. Meth. Ecol. Evol, 5, 74-89.
DOI:10.1111/2041-210X.12126 |
||
Bryce S.A., et al., 1999. Ecoregions -a geographic framework to guide risk characterization and ecosystem management. Environ. Pract, 1, 141-155.
DOI:10.1017/S1466046600000582 |
||
Buckley R., et al., 2016. How pristine are China's parks? Front. Ecol. Evol, 4, 136.
|
||
Burney D.A., Burney L.P., 2007. Paleoecology and "inter-situ" restoration on Kauai, Hawaii. Front. Ecol. Environ, 5, 483-490.
DOI:10.1890/070051 |
||
Butterfield B.J., et al., 2016. Prestoration:using species in restoration that will persist now and into the future. Restor. Ecol, 25, S155-S163.
|
||
Cao M., et al., 2015. Analysis of the network of protected areas in China based on a geographic perspective:current status, issues and integration. Sustainability, 7, 15617-15631.
DOI:10.3390/su71115617 |
||
Chen J., et al., 2009. Tropical botanical gardens:at the in situ ecosystem management frontier. Trends Plant Sci, 14, 584-589.
DOI:10.1016/j.tplants.2009.08.010 |
||
China's National Committee on Man and Biosphere, 2000. Study of Sustainable Management Policies for China's Nature Reserves. Scientific and Technical Documents Publishing House.
|
||
China's Strategy for Plant Conservation Editorial Committe, 2008. China's Strategy for Plant Conservation. Guangdong Science & Technology Press, Guangzhou.
|
||
Chinese Academy of Sciences and Ministry of Environmental Protection of the People's Republic of China, 2013. Red List of Biodiversity in China-Higher Plants (in Chinese). http://www.mep.gov.cn/gkml/hbb/bgg/201309/W020130912562095920726.pdf.
|
||
Chu K.-L., Cooper W.S., 1950. An ecological reconnaissance in the native home of Metasequoia glyptostroboides. Ecology, 31, 260-278.
DOI:10.2307/1932391 |
||
Ciarleglio M., et al., 2009. ConsNet:new software for the selection of conservation area networks with spatial and multi-criteria analyses. Ecography, 32, 205-209.
DOI:10.1111/eco.2009.32.issue-2 |
||
Cleland, D. T., et al., 1997. National hierarchical framework of ecological units. In: Boyce, M. S., Haney, A. (Eds. ), Ecosystem Management Applications for Sustainable Forest and Wildlife Resources. Yale University Press, New Haven, CT, pp. 181-200.
|
||
Colas B., et al., 2008. Restoration demography:a 10-year demographic comparison between introduced and natural populations of endemic Centaurea corymbosa(Asteraceae). J. Appl. Ecol, 45, 1468-1476.
DOI:10.1111/jpe.2008.45.issue-5 |
||
Commonwealth of Australia, 2012. Interim Biogeographic Regionalisation for Australia, Version 7.
|
||
Cowling R.M., et al., 2003. A conservation plan for a global biodiversity hotspot -the Cape Floristic Region, South Africa. Biol. Conserv, 112, 191-216.
DOI:10.1016/S0006-3207(02)00425-1 |
||
Dalrymple, S. E., et al., 2012. A meta-analysis of threatened plant reintroductions from across the globe. In: Maschinski, J., Haskins, E. H. (Eds. ), Plant Reintroduction in a Changing Climate: Promises and Perils. Island Press, Washington, D. C, pp. 31-50.
|
||
Dalrymple, S. E., et al., 2011. Are Re-introductions an Effective Way of Mitigating Against Plant Extinctions?, CEE Review 07-008 (SR32). Collaboration for Environmental Evidence. www.environmentalevidence.org/SR32.html.
|
||
Dinerstein, E., 2000. A Workbook for Conducting Biological Assessments and Developing Biodiversity Visions for Ecoregion-based Conservation. World Wildlife Fund, Washington, DC.
|
||
Dudley, N., 2013. Guidelines for Applying Protected Area Management Categories. IUCN, Gland, Switzerland.
|
||
DWCNRM, Department of Wildlife Conservation and Nature Reserve Management of the State Forestry Administration, 2007. Researches on China's Nature Reserve legislation. Chinese Forestry Publishing House, Beijing, China (In Chinese).
|
||
Fiorella K., et al., 2010. Methodological considerations in reserve system selection:a case study of Malagasy lemurs. Biol. Conserv, 143, 963-973.
DOI:10.1016/j.biocon.2010.01.005 |
||
Fos S., et al., 2017. Plant micro-reserves in Valencia (E. Spain):a model to preserve threatened flora in China?. Plant Divers, 39, 383-389.
DOI:10.1016/j.pld.2017.10.002 |
||
Frankham R., et al., 2014. Genetics in conservation management:revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol. Conserv, 170, 56-63.
DOI:10.1016/j.biocon.2013.12.036 |
||
Freitag S., Van Jaarsveld A.S., 1997. Relative occupancy, endemism, taxonomic distinctiveness and vulnerability:prioritizing regional conservation actions. Biodivers. Conserv, 6, 211-232.
DOI:10.1023/A:1018392019594 |
||
Freitag S., et al., 1997. Ranking priority biodiversity areas:an iterative conservation value-based approach. Biol. Conserv, 82, 263-272.
DOI:10.1016/S0006-3207(97)00040-2 |
||
Fuller T., Sarkar S., 2006. LQGraph:a software package for optimizing connectivity in conservation planning. Environ. Model. Software, 21, 750-755.
DOI:10.1016/j.envsoft.2006.01.005 |
||
Gallant, A. L., et al., 1995. Ecoregions of Alaska. US Government Printing Office, Washington (DC). US Geological Survey Professional Paper 1567.
|
||
Godefroid S., et al., 2011. How successful are plant species reintroductions? Biol. Conserv, 144, 672-682.
DOI:10.1016/j.biocon.2010.10.003 |
||
Gossa C., et al., 2015. The research-implementation gap:how practitioners and researchers from developing countries perceive the role of peer-reviewed literature in conservation science. Oryx, 49, 80-87.
DOI:10.1017/S0030605313001634 |
||
Groves, C., et al., 2000. Designing a Geography of Hope: a Practitioner's Handbook for Ecoregional Conservation Planning. The Nature Conservancy, Arlington (VA).
|
||
Groves C.R., et al., 2002. Planning for biodiversity conservation:putting conservation science into practice. Bioscience, 52, 499-512.
DOI:10.1641/0006-3568(2002)052[0499:PFBCPC]2.0.CO;2 |
||
Guerrant E.O.J., Kaye T.N., 2007. Reintroduction of rare and endangered plants:common factors, questions and approaches. Aust. J. Bot, 55, 362-370.
DOI:10.1071/BT06033 |
||
Hannah L., et al., 2007. Protected area needs in a changing climate. Front. Ecol. Environ, 5, 131-138.
DOI:10.1890/1540-9295(2007)5[131:PANIAC]2.0.CO;2 |
||
He L.-Y., et al., 2015. Forest structure and regeneration of the tertiary relict Taiwania cryptomerioides in the Gaoligong mountains, Yunnan, southwestern China. Phytocoenologia, 45, 135-155.
DOI:10.1127/phyto/2015/0038 |
||
Heller N.E., Zavaleta E.S., 2009. Biodiversity management in the face of climate change:a review of 22 years of recommendations. Biol. Conserv, 142, 14-32.
DOI:10.1016/j.biocon.2008.10.006 |
||
Heywood V.H., 2016. In situ conservation of plant species -an unattainable goal?. Isr. J. Plant Sci, 63, 211-231.
|
||
Heywood V.H., 2017. Plant conservation in the Anthropocene -challenges and future prospects. Plant Divers, 39, 314-330.
DOI:10.1016/j.pld.2017.10.004 |
||
Hodgson J.A., et al., 2009. Climate change, connectivity and conservation decision making:back to basics. J. Appl. Ecol, 46, 964-969.
DOI:10.1111/jpe.2009.46.issue-5 |
||
Hoegh-Guldberg O., et al., 2008. Assisted colonization and rapid climate change. Science, 321, 345-346.
DOI:10.1126/science.1157897 |
||
Huang H., 2011. Plant diversity and conservation in China:planning a strategic bioresource for a sustainable future. Bot. J. Linn. Soc, 166, 282-300.
DOI:10.1111/boj.2011.166.issue-3 |
||
Huang H., et al., 2002. Conserving native plants in China. Science, 297, 935-936.
|
||
Ioja C.I., et al., 2010. The efficacy of Romania's protected areas network in conserving biodiversity. Biol. Conserv, 143, 2468-2476.
DOI:10.1016/j.biocon.2010.06.013 |
||
Jenkins C.N., Joppa L., 2009. Expansion of the global terrestrial protected area system. Biol. Conserv, 142, 2166-2174.
DOI:10.1016/j.biocon.2009.04.016 |
||
Jim C.Y., Xu S.S.W., 2004. Recent protected-area designation in China:an evaluation of administrative and statutory procedures. Geogr. J, 170, 39-50.
DOI:10.1111/geoj.2004.170.issue-1 |
||
Josse, C., et al., 2003. Ecological Systems of Latin America and the Carribbean: a Working Classification of Terrestrial Systems. Nature-Serve, Arlington.
|
||
Klijn F., et al., 1995. Ecoregions and ecodistricts:ecological regionalizations for The Netherlands' environmental policy. Environ. Manag, 19, 797-813.
DOI:10.1007/BF02471933 |
||
Krosby M., et al., 2010. Ecological connectivity for a changing climate. Conserv. Biol, 24, 1686-1689.
DOI:10.1111/cbi.2010.24.issue-6 |
||
Lamb, D., Gilmour, D., 2003. Rehabilitation and Restoration of Degraded Forests. IUCN, Gland, Switzerland and Cambridge, UK in collaboration with WWF, Gland, Switzerland.
|
||
Leathwick J.R., et al., 2003. An environmental domain classification of New Zealand and its use as a tool for biodiversity management. Conserv. Biol, 17, 1612-1623.
DOI:10.1111/cbi.2003.17.issue-6 |
||
Li D.Q., et al., 2003. Research on the National Forestry Nature Reserve System Plan.. China Land Press, Beijing. |
||
Li J.Q., et al., 2009. Problems and countermeasures on collective forest tenure reform in the nature reserves in China. For. Resour. Manag, 12, 1-8.
|
||
Li W., Han N., 2001. Ecotourism management in China's nature reserves. Ambio, 30, 62-63.
DOI:10.1579/0044-7447-30.1.62 |
||
Liu J.G., et al., 2001. Ecological degradation in protected areas:the case of Wolong Nature Reserve for giant pandas. Science, 29, 98-101.
|
||
Loarie S.R., et al., 2009. The velocity of climate change. Nature, 462, 1052-U1111.
DOI:10.1038/nature08649 |
||
Lopez-Pujol J., et al., 2011. Centres of plant endemism in China:places for survival or for speciation?. J. Biogeogr, 38, 1267-1280.
DOI:10.1111/jbi.2011.38.issue-7 |
||
Ma Z., et al., 2009. Conflicts between biodiversity conservation and development in a biosphere reserve. J. Appl. Ecol, 46, 527-535.
DOI:10.1111/jpe.2009.46.issue-3 |
||
Madsen M.D., et al., 2014. Improving restoration of exotic annual grass-invaded rangelands through activated carbon seed enhancement technologies. Rangel. Ecol. Manag, 67, 61-67.
DOI:10.2111/REM-D-13-00050.1 |
||
Madsen M.D., et al., 2012. Agglomerating seeds to enhance native seedling emergence and growth. J. Appl. Ecol, 49, 431-438.
DOI:10.1111/j.1365-2664.2012.02118.x |
||
Margules C.R., Pressey R.L., 2000. Systematic conservation planning. Nature, 405, 243-253.
DOI:10.1038/35012251 |
||
Maschinski, J., Albrecht, M. A., 2017. Center for plant conservation's best practice guidelines for the reintroduction of rare plants. Plant Divers.
|
||
McKay J.K., et al., 2005. "How local is local?" A review of practical and conceptual issues in the genetics of restoration. Restor. Ecol, 13, 432-440.
DOI:10.1111/rec.2005.13.issue-3 |
||
McKinley D.C., et al., 2012. When peer-reviewed publications are not enough! Delivering science for natural resource management. For. Pol. Econ, 21, 1-11.
DOI:10.1016/j.forpol.2012.03.007 |
||
Meek M.H., et al., 2015. Fear of failure in conservation:the problem and potential solutions to aid conservation of extremely small populations. Biol. Conserv, 184, 209-217.
DOI:10.1016/j.biocon.2015.01.025 |
||
Menges E.S., 2008. Restoration demography and genetics of plants:when is a translocation successful? Aust. J. Bot, 56, 187-196.
|
||
Merritt D.J., Dixon K.W., 2011. Restoration seed banks-a matter of scale. Science, 332, 424-425.
DOI:10.1126/science.1203083 |
||
Millar, C. I., Brubaker, L. B., 2006. Climate change and paleoecology: new contexts for restoration ecology. In: Falk, D. A., et al. (Eds. ), Foundations of Restoration Ecology. Society for Ecological, Restoration, International. Island Press, Washington, D. C, pp. 315-340.
|
||
Miller-Rushing A.J., et al., 2017. A Chinese approach to protected areas:a case study comparison with the United States. Biol. Conserv, 210, 101-112.
DOI:10.1016/j.biocon.2016.05.022 |
||
Miller S.A., et al., 2011. Can an ecoregion serve as a seed transfer zone? Evidence from a common garden study with five native species. Restor. Ecol, 19, 268-276.
DOI:10.1111/j.1526-100X.2010.00702.x |
||
Milner-Gulland E.J., et al., 2010. Do we need to develop a more relevant conservation literature?. Oryx, 44, 1-2.
DOI:10.1017/S0030605309991001 |
||
Ministry of Environmental Protection of China (EMP), 2011. National Biodiversity Conservation Strategy and Action Plan (2011-2030). China Environmental Science Press, Beijing, China (In Chinese).
|
||
Moilanen, A., et al., 2009. The Zonation framework and software for conservation prioritization. In: Moilanen, A., et al. (Eds. ), Spatial Conservation Prioritization: Quantitative Methods and Computational Tools. Oxford University Press, Oxford, UK, pp. 196-210.
|
||
Noss R.F., 1990. Indicators for monitoring biodiversity:a hierarchical approach. Conserv. Biol, 4, 355-364.
DOI:10.1111/cbi.1990.4.issue-4 |
||
Noss R.F., 2001. Beyond Kyoto:forest management in a time of rapid climate change. Conserv. Biol, 15, 578-590.
DOI:10.1046/j.1523-1739.2001.015003578.x |
||
Noss, R. F., et al., 1999. Core areas: where nature reigns. In: Soulé, M. E., Terborgh, J. (Eds. ), Continental Conservation: Scientific Foundations of Regional Reserve Networks. Island Press, Washington, DC, pp. 99-128.
|
||
Olson D.M., et al., 2001. Terrestrial ecoregions of the worlds:a new map of life on Earth. Bioscience, 51, 933-938.
DOI:10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2 |
||
Omernik J.M., 1987. Ecoregions of the conterminous United States. Ann. Assoc. Am. Geogr, 77, 118-125.
DOI:10.1111/j.1467-8306.1987.tb00149.x |
||
Omernik J.M., Griffith G.E., 2014. Ecoregions of the conterminous United States:evolution of a hierarchical spatial framework. Environ. Manag, 54, 1249-1266.
DOI:10.1007/s00267-014-0364-1 |
||
Onal H., 2004. First-best, second-best, and heuristic solutions in conservation reserve site selection. Biol. Conserv, 115, 55-62.
DOI:10.1016/S0006-3207(03)00093-4 |
||
PATF, 2004. Protected Area Task Force Report to the China Council for International Cooperation on Environment and Development (CCICED). Evaluation on and Policy Recommendations to the Protected Area System of China. State Environmental Protection Administration, Beijing.
|
||
Peters R.L., Darling J.D.S., 1985. The greenhouse effect and nature reserves. Bioscience, 35, 707-717.
DOI:10.2307/1310052 |
||
Piao Z., et al., 2011. Human-wildlife competition for Korean pine seeds:vertebrate responses and implications for mixed forests on Changbai Mountain, China. Ann. For. Sci, 68, 911-919.
DOI:10.1007/s13595-011-0097-8 |
||
Potter K.M., Hargrove W.W., 2012. Determining suitable locations for seed transfer under climate change:a global quantitative method. N. For, 43, 581-599.
|
||
Pressey R.L., Bottrill M.C., 2009. Approaches to landscape-and seascape-scale conservation planning:convergence, contrasts and challenges. Oryx, 43, 464-475.
DOI:10.1017/S0030605309990500 |
||
Pressey R.L., et al., 1996. How well protected are the forests of north-eastern New South Wales?-Analyses of forest environments in relation to formal protection measures, land tenure, and vulnerability to clearing. For. Ecol. Manag, 85, 311-333.
DOI:10.1016/S0378-1127(96)03766-8 |
||
Pressey R.L., et al., 1993. Beyond opportunism:key principles for systematic reserve selection. Trends Ecol. Evol, 8, 124-128.
DOI:10.1016/0169-5347(93)90023-I |
||
Pressey R.L., et al., 1994. Shades of irreplaceability:towards a measure of the contribution of sites to a reservation goal. Biodivers. Conserv, 3, 242-262.
DOI:10.1007/BF00055941 |
||
Pressey, R. L., et al., 2009. The C-Plan conservation planning system: origins, applications, and possible futures. In: Moilanen, A., et al. (Eds. ), Spatial Conservation Prioritization. Oxford University Press, Oxford, UK, pp. 211-234.
|
||
Pritchard H.W., Moat J.F., Ferraz J.B.S., et al., 2014. Innovative approaches to the preservation of forest trees. For. Ecol. Manag, 333, 88-98.
DOI:10.1016/j.foreco.2014.08.012 |
||
Qian, S., et al., 2017. Conservation and development in conflict: regeneration of wild Davidia involucrata (Nyssaceae) communities weakened by bamboo management in south-central China. Oryx 1-10.
|
||
Qian S., et al., 2016. Effective conservation measures are needed for wild Cathaya argyrophylla populations in China:insights from the population structure and regeneration characteristics. For. Ecol. Manag, 361, 358-367.
DOI:10.1016/j.foreco.2015.11.041 |
||
Regulations on Scenic Interest Areas, 2006. State Council of China.
|
||
Ren H., et al., 2016. The use of grafted seedlings increases the success of conservation translocations of Manglietia longipedunculata (Magnoliaceae), a Critically Endangered tree. Oryx, 50, 437-445.
DOI:10.1017/S0030605315000423 |
||
Ren H., et al., 2010. Moss is a key nurse plant for reintroduction of the endangered herb, Primulina tabacum Hance. Plant Ecol, 209, 313-320.
DOI:10.1007/s11258-010-9754-5 |
||
Ren H., et al., 2012. Reintroduction of Tigridiopalma magnifica, a rare and critically endangered herb endemic to China. Oryx, 46, 391-398.
DOI:10.1017/S0030605311000615 |
||
Sang W., et al., 2011. Securing a future for China's wild plant resources. Bioscience, 61, 720-725.
DOI:10.1525/bio.2011.61.9.11 |
||
Sarkar S., Illoldi-Rangel P., 2010. Systematic conservation planning:an updated protocol. Nat. Conserv, 8, 19-26.
DOI:10.4322/natcon.00801003 |
||
Sayre, R., et al., 2008. Terrestrial ecosystems of south America. In: Campbell, J. C., et al. (Eds. ), North America Land Cover Summit. North America Land Cover Summit, Washington, DC, pp. 131-152.
|
||
Shanley P., Lopez C., 2009. Out of the loop:why research rarely reaches policy makers and the public and what can be done. Biotropica, 41, 535-544.
DOI:10.1111/j.1744-7429.2009.00561.x |
||
Shriner S.A., et al., 2006. Reserve networks based on richness hotspots and representation vary with scale. Ecol. Appl, 16, 1660-1673.
DOI:10.1890/1051-0761(2006)016[1660:RNBORH]2.0.CO;2 |
||
Soutullo A., et al., 2008. Linking political and scientifically derived targets for global biodiversity conservation:implications for the expansion of the global network of protected areas. Divers. Distrib, 14, 604-613.
|
||
State Forestry Administration of China, 2012. The Saving and Conservation Program on Extremely Small Populations in China. State Forestry Administration of China.
|
||
Tang C.Q., Ohsawa M., 2002. Tertiary relic deciduous forests on a humid subtropical mountain, Mt. Emei, Sichuan, China. Folia Geobot, 37, 93-106.
DOI:10.1007/BF02803193 |
||
Tang C.Q., et al., 2013. Population persistence of a tertiary relict tree Tetracentron sinense on the Ailao mountains, Yunnan, China. J. Plant Res, 126, 651-659.
DOI:10.1007/s10265-013-0559-1 |
||
Tang C.Q., et al., 2011. Population structure of relict Metasequoia glyptostroboides and its habitat fragmentation and degradation in south-central China. Biol. Conserv, 144, 279-289.
DOI:10.1016/j.biocon.2010.09.003 |
||
Tang, C. Q., et al., 2015. Community structure and survival of tertiary relict Thuja sutchuenensis (Cupressaceae) in the subtropical Daba mountains, southwestern. China. PLoS One 10.
|
||
Tang. C.Q., et al., 2012. Evidence for the persistence of wild Ginkgo biloba (Ginkgoaceae) populations in the Dalou Mountains, southwestern China. Am. J. Bot, 99, 1408-1414.
DOI:10.3732/ajb.1200168 |
||
Tang X.P., et al., 2009. Policy Study on Ecotourism in Nature Reserves in China.. Beijing Press, Beijing, China. |
||
Thomas C.D., 2011. Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol. Evol, 26, 216-221.
DOI:10.1016/j.tree.2011.02.006 |
||
Thorne J.H., et al., 2006. A conservation design for the central coast of California and the evaluation of mountain lion as an umbrella species. Nat. Area J, 26, 137-148.
DOI:10.3375/0885-8608(2006)26[137:ACDFTC]2.0.CO;2 |
||
Turner S.R., et al., 2013. Seed treatment optimizes benefits of seed bank storage for restoration-ready seeds:the feasibility of prestorage dormancy alleviation for mine-site revegetation. Restor. Ecol, 21, 186-192.
DOI:10.1111/rec.2013.21.issue-2 |
||
Tuvi E.-L., et al., 2011. Establishment of protected areas in different ecoregions, ecosystems, and diversity hotspots under successive political systems. Biol. Conserv, 144, 1726-1732.
DOI:10.1016/j.biocon.2011.03.008 |
||
Uma Shaanker R., Ganeshaiah K.N., 1997. Mapping genetic diversity of Phyllanthus emblica:forest gene banks as a new approach for in situ conservation of genetic resources. Curr. Sci, 73, 163-168.
|
||
Uma Shaanker, R., et al., 2001. A new approach to conservation of genetic resources of forest trees: promise and processes. In: Uma Shaanker, R., et al. (Eds. ), Forest Genetic Resources: Status, Threats and Conservation Strategies. Oxford and IBH Publishing Co, pp. 263-271.
|
||
Uma Shaanker, R., et al., 2002. Forest gene banks -a new integrated approach for the conservation of forest tree genetic resources. In: Engels, J. M. M., et al. (Eds. ), Managing Plant Genetic Resources. CABI Publishing, Nosworthy. UK, pp. 229-235.
|
||
Vane-Wright R.I., Humphries C.J., Williams P.H., 1991. What to protect?-Systematics and the agony of choice. Biol. Conserv, 55, 235-254.
DOI:10.1016/0006-3207(91)90030-D |
||
Vane-Wright, R. I., Smith, C. R., Kitching, I. J., 1994. Systematic assessment of taxic diversity by summation. In: Forey, P. L., Humphries, C. J., Vane-Wright, R. I. (Eds. ), Systematics and Conservation Evaluation. Systematics Association Special vol. 50. Clarendon Press, Oxford, pp. 309-326.
|
||
Vitt P., et al., 2016. Assisted migration as a climate change adaptation strategy:lessons from restoration and plant reintroductions. Isr. J. Plant Sci, 63, 250-261.
|
||
Vitt P., et al., 2010. Assisted migration of plants:changes in latitudes, changes in attitudes. Biol. Conserv, 143, 18-27.
DOI:10.1016/j.biocon.2009.08.015 |
||
Volis S., 2016a. Conservation-oriented restoration -how to make it a success?. Isr. J. Plant Sci, 63, 276-296.
|
||
Volis S., 2016b. Conservation meets restoration -rescuing threatened plant species by restoring their environments and restoring environments using threatened plant species. Isr. J. Plant Sci, 63, 262-275.
|
||
Volis S., 2016c. How to conserve threatened Chinese species with extremely small populations?. Plant Divers, 38, 53-62.
DOI:10.1016/j.pld.2016.05.004 |
||
Volis S., 2016d. Species-targeted plant conservation:time for conceptual integration. Isr. J. Plant Sci, 63, 232-249.
|
||
Volis S., 2017a. Conservation utility of botanic garden living collections:setting a strategy and appropriate methodology. Plant Divers, 39, 365-372.
DOI:10.1016/j.pld.2017.11.006 |
||
Volis, S., 2017b. Plant Conservation in the Anthropocene: definitely Not Win-win but Maybe Not Lose-lose?, Reference Module in Earth Systems and Environmental Sciences. Elsevier.
|
||
Volis S., Blecher M., 2010. Quasi in situ -a bridge between ex situ and in situ conservation of plants. Biodivers. Conserv, 19, 2441-2454.
DOI:10.1007/s10531-010-9849-2 |
||
Wang G., et al., 2012. National park development in China:conservation or commercialization?. Ambio, 41, 247-261.
DOI:10.1007/s13280-011-0194-9 |
||
Wang Z., et al., 2014. Effects of stocking rate on the variability of peak standing crop in a desert steppe of Eurasia grassland. Environ. Manag, 53, 266-273.
DOI:10.1007/s00267-013-0186-6 |
||
WDPA, 2010. World Database on Protected Areas Annual Release 2010. www. wdpa. org.
|
||
Wiken, E. B., et al., 1989. Terrestrial Ecoregions of Canada. Provisional Map 1: 7, 500, 000. Sustainable Development Branch, Environment Canada, Ottawa(Canada).
|
||
Williams P., et al., 1996. A comparison of richness hotspots, rarity hotspots, and complementary areas for conserving diversity of British birds. Conserv. Biol, 10, 155-174.
DOI:10.1046/j.1523-1739.1996.10010155.x |
||
Wilson K., et al., 2005. Measuring and incorporating vulnerability into conservation planning. Environ. Manag, 35, 527-543.
DOI:10.1007/s00267-004-0095-9 |
||
Wu B., et al., 2006. Setting biodiversity conservation priorities in the Forests of the Upper Yangtze Ecoregion based on ecoregion conservation methodology. Chin. Biodivers, 14, 87-97.
|
||
Wu R., et al., 2014. Optimized spatial priorities for biodiversity conservation in China:a systematic conservation planning perspective. PLoS One, 9, e103783.
DOI:10.1371/journal.pone.0103783 |
||
Wu R., et al., 2011. Effectiveness of China's nature reserves in representing ecological diversity. Front. Ecol. Environ, 9, 383-389.
DOI:10.1890/100093 |
||
Xu Z., Guo H., 2014. Near situ conservation for wild plant species with extremely small populations. Plant Divers. Resour, 36, 533-536.
|
||
Xu J., Melick D.R., 2007. Rethinking the effectiveness of public protected areas in southwestern China. Conserv. Biol, 21, 318-328.
DOI:10.1111/cbi.2007.21.issue-2 |
||
Xu J., et al., 2012. A review and assessment of nature reserve policy in China:advances, challenges and opportunities. Oryx, 46, 554-562.
DOI:10.1017/S0030605311000810 |
||
Xu W., et al., 2006. Priority analysis on conserving China's terrestrial ecosystems. Acta Ecol. Sin, 26, 271-280.
|
||
Zhang C.Z., 2006. The debate and its essential on managing system of the World Heritage Site. Commer. Res, 340, 175-179.
|
||
Zhang, D., 2007a. Plant Germplasm Resources and Conservation. Shanghai Scientific and Technical Publishers, Shanghai, China.
|
||
Zhang H., et al., 2013. Grazer exclusion alters plant spatial organization at multiple scales, increasing diversity. Ecol. Evol, 3, 3604-3612.
|
||
Zhang L., et al., 2017a. Biodiversity conservation status in China's growing protected areas. Biol. Conserv, 210, 89-100.
|
||
Zhang M.-G., et al., 2017b. Priority areas for the conservation of perennial plants in China. Biol. Conserv, 210, 56-63.
|
||
Zhang, X. S., 2007b. Vegetation Map of People's Republic of China (1: 1, 000, 000). Geology Press, Beijing.
|
||
Zhang Y., et al., 2017c. Integrated maps of biodiversity in the Qinling Mountains of China for expanding protected areas. Biol. Conserv, 210, 64-71.
DOI:10.1016/j.biocon.2016.04.022 |
||
Zhong L., et al., 2015. Environmental and visitor management in a thousand protected areas in China. Biol. Conserv, 181, 219-225.
DOI:10.1016/j.biocon.2014.11.007 |
||
Zinda, J. A., 2014. Making national parks in Yunnan: shifts and struggles within the ecological state. In: Yeh, E., Coggins, C. (Eds. ), Mapping Shangrila: Nature, Personhood, and Sovereignty in the Sino-Tibetan Borderlands. University of Washington Press, Seattle, pp. 105-128.
|
||
Zong C., Ma J.Z., He L., 2007. Achievements of the nature reserve construction in the past fifty years in China. Forest Resour. Manag, 2, 2-6.
|



