b. Shanghai Key Laboratory of Plant Functional Genomics and Resources, Shanghai 201602, China;
c. Lushan Forestry Bureau, Ya'an 625600, Sichuan, China
Consisting of over 1800 accepted species names, Begonia L. (Begoniaceae) is one of the most diverse plant taxa and the fifth or sixth largest angiosperm genus (Phutthai and Hughes, 2016; Tian et al., 2017). The number of species in this genus has increased sharply over the past 20 years, and based on literature, field surveys and communication with begonia researchers across the world, we predict that the number of species may actually in total between 2000 and 2500. Begonia is mainly native to moist tropical and subtropical climates, and is mostly distributed in Asia, America and Africa. It is one of the most attractive ornamentals with both beautiful foliage and flowers. In "Year of the" crops program hosted by the National Garden Bureau of USA, begonia was voted the annual of 2016 (Hilinske, 2016). Some begonias species are also used as medicines or vegetable (Guan et al., 2007).
Although global begonia diversity is huge, most species are narrowly distributed, with a few exceptions, such as Begonia palmata D. Don, Begonia longifolia Blume and Begonia handelii Irmsch., which are native to several Asian countries including China, India, Vietnam, Myanmar, and Laos. The wild populations of many species face increasing risk of extinction due to their unique and isolated habitats, which are subject to the negative effects of climate change and agricultural activity. In addition, the illegal collection of wild plants with ornamental or medicinal value has increased as improved transportation systems, online commerce and rapid shipping have made it easier to access and obtain plant materials. Therefore, more species will likely be categorized into rare and endangered class and need immediate protection. However, to accurately understand the diversity of begonias, field surveys of germplasm must be carried out and taxonomical issues must be resolved. Unfortunately, evaluation of the habitat and resources of wild begonias, and their conservation status, has lagged behind policymaking demands. This may expedite the extinction of currently endangered species. The status of Chinese begonias mirrors that of global begonia conservation. Developing a sustainable conservation strategy based on a deep understanding of germplasm diversity, taxonomical status, and current conservation status of species and related ecosystems is therefore urgent.
1. Diversity of Chinese begoniasCompared to other countries, China has the largest or second largest number of Begonia species in the world. Chinese begonias have amazingly diverse distribution ranges, climate adaptations, habitats, habits, phenology, and morphology (e.g., plant size and characteristics of foliage, flower and fruit).
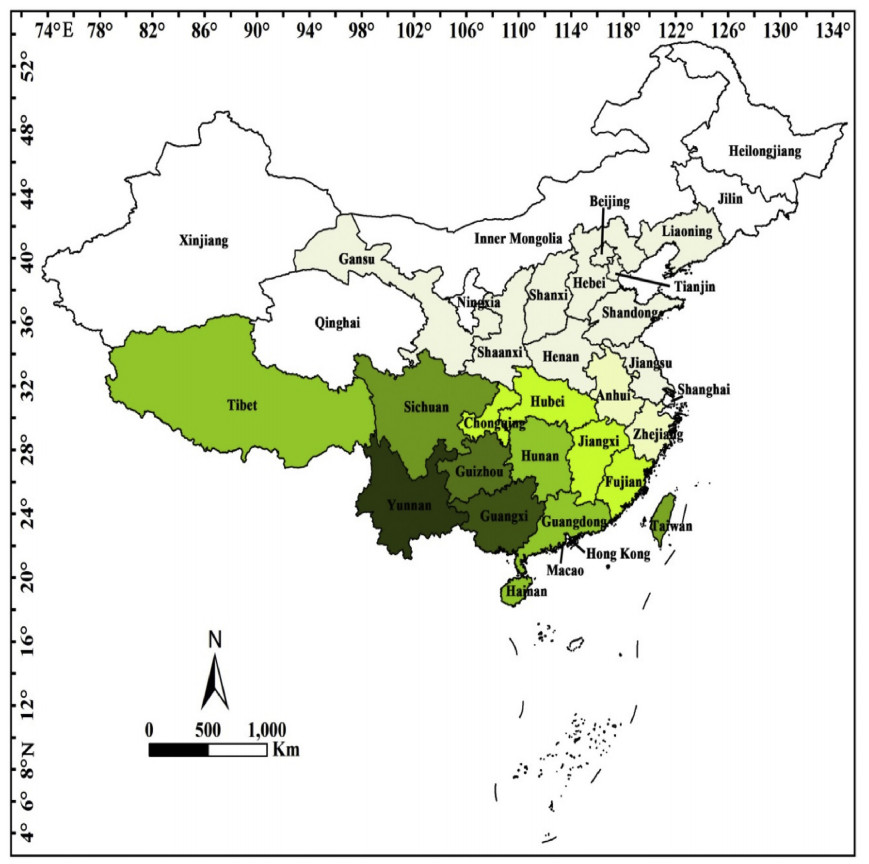
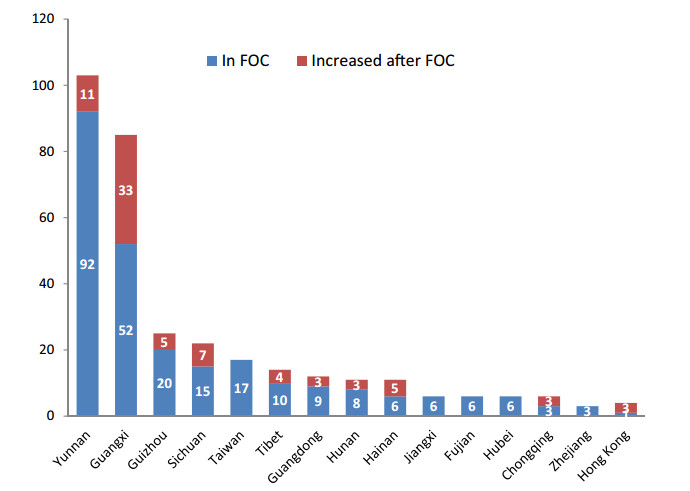
1.1. Distribution, climate conditions and habitatWild begonias are widely distributed in China, from the south tropical, subtropical to the north temperate regions (Fig. 1). The southwestern regions (Yunnan, Guizhou, Sichuan, and Tibet) have the largest number of taxa, followed by some of the southern regions (Guangxi, Guangdong, Hainan) and Taiwan. No species occurs in Macao, Heilongjiang, Jilin, Inner Mongolia, Qinghai, Shanghai or Xinjiang (Fig. 1). Based on plant surveys, Lingyuan county, Liaoning province is the northern boundary of Chinese begonia distribution, and Hainan province is the southern boundary. The distribution range extends east to Taiwan and west to Tibet. Taking into account only the currently accepted species of Chinese begonias, the largest number of taxa are located in Yunnan (103), followed by Guangxi (85), where, since the publication of Flora of China in 2007, the number of begonia species has increased most. There is only one species (Begonia grandis Dryand.) that is distributed in the northern provinces or municipalities (Anhui, Beijing, Gansu, Hebei, Henan, Liaoning, Shandong, Shanxi, Tianjin) above Hubei province (Fig. 2). Begonia grandis, followed by B. palmata and then B. longifolia, has the largest distribution range and may be found in most parts of China, except for Xinjiang, Qinghai, Heilongjiang, Jilin, Inner Mongolia, Hainan, Shanghai, Hong Kong and Macao (Li et al., 2014).
|
| Fig. 1 Regional distribution map for wild begonia species in China. Data are based on Flora of China (Gu et al., 2007) and species described before 2017. Darker colors indicate more begonia species distributed in this region, although the same color does not mean an even distribution across the region. Grey represents 1 species; grey-yellow, 2-4 species; yellow-green, 5-10 species; pale green, 11-20 species; green, 21-30 species; deep green, 80-100 species; and dark green, 101-120 species. |
|
| Fig. 2 Number of Begonia species distributed in the provincial and municipal administrative regions of China. The total number of species = the number of species recorded in FOC (blue) þ the newly recorded and discovered species after FOC (red); provinces with less than two species were not listed; FOC: Flora of China (Gu et al., 2007). |
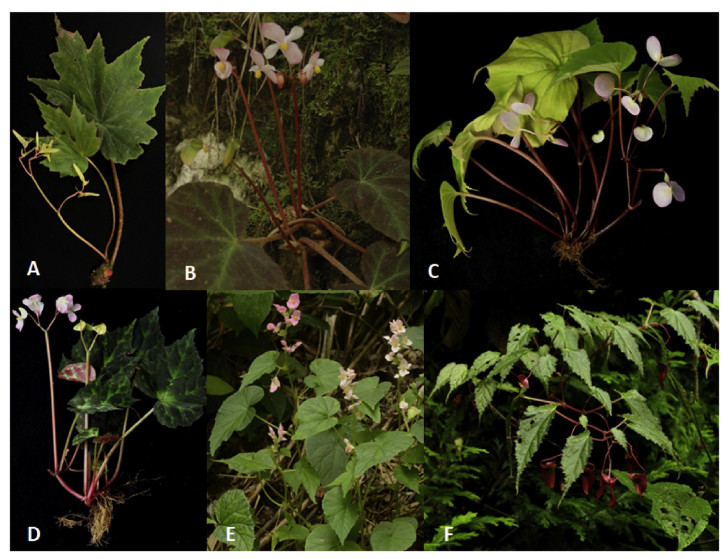
Chinese begonias grow at elevations from 50 to 3500 m alongside streams or waterfalls, on cliffs, steep slopes under forests, rock surfaces, at the entrances and inside caves even, and at least two species are epiphytes (Fig. 3). Some species require higher humidity for survival, such as Begonia alveolata Yu, Begonia hainanensis Chun & F. Chun, Begonia versicolor Irmsch. and Begonia wenshanensis C. H. Hu ex C. Y. Wu & T. C. Ku, whereas other species like Begonia fimbristipula Hance, Begonia henryi Hemsl., and Begonia peltatifolia H. L. Li. can adapt to seasonally dry conditions and are only found in the rocky mountain areas (Fig. 3F-J). A few species need special microenvironments to survive; for instance, Begonia coptidifolia H. G. Ye, F. G. Wang, Y. S. Ye & C. I Peng only grows on rock surfaces in the middle or along the sides of little streams under the forest in a nature reserve in Guangdong province (Fig. 3E); Begonia arboreta Y. M. Shui only grows on the branches and trunks of trees near the top of a mountain in South Yunnan (Fig. 3K). More than twenty species occur in karst caves, particularly in Guangxi and Yunnan, and some of these are cave-endemic. B. peltatifolia has the thickest leaf of all Chinese begonias and its similarity to succulent plants allows it to tolerate extremely dry conditions (Fig. 3G). B. grandis is considered a hardy species which can tolerate temperatures below -20 ℃ (Li et al., 2014).

|
| Fig. 3 Habitats of the wild begonias in China. A Evergreen forest of southwestern Yunnan is a common habitat for many species; B Rock surfaces on riverside cliffs of a valley for Begonia cavaleriei H. Lév., Begonia edulis H. Lév., B. kaiyangensis sp. nov., etc.; C Little valley for B. grandis; D The area near a waterfall for B. cavaleirei, B. grandis, Begonia labordei H. Lév.; E Little streams for B. coptidifolia; F Lime stone mountain for Begonia rubropunctata S. H. Huang & Y. M. Shui; G Surface of limestone for B. pelatifolia; H Surface of Danxia cliff for B. henryi; I, J Rock caves for B. fimbristipula Hance (I), B. labordei and B. grandis (J); K Tree trunk and branches with moss for B. arboreta. Arrows indicate where begonias grow. |
The habit of Chinese begonias is rich in diversity, containing nearly all growth forms of this genus: tuberous, semi-tuberous, moniliform, rhizomatous, trailing (scandent), and shrub-like (Fig. 4). The majority of species (i.e., over 150 species) are rhizomatous (Fig. 4E), including Begonia algaia L. B. Sm. & Wassh., Begonia circumlobata Hance, B. edulis, B. handelii, Begonia hemsleyana Hook. f., Begonia limprichtii Irmsch., B. palmata, Begonia pedatifida H. Lév. There are over 40 tuberous species (Fig. 4C), including B. fimbristipula, B. grandis, B. henryi, B. labordei, and Begonia taliensis Gagnep. The trailing-scandent type has at least four species: Begonia ruboides C. M. Hu ex C. Y. Wu & T. C. Ku, Begonia auritistipula Y. M. Shui & W. H. Chen, Begonia rhynchocarpa Y. M. Shui & W. H. Chen and one newly discovered species (Fig. 4F). There are three subshrub species (Begonia acetosella Craib, Begonia coptidimontana C. Y. Wu, B. longifolia) (Fig. 4G), three monliform species (Begonia coelocentroides Y. M. Shui & Z. D. Wei, Begonia wilsonii Gagnep. and Begonia xishuiensis T. C. Ku) (Fig. 4D), and two semi-tuberous species (Begonia augustinei Hemsl. and Begonia wuzhishanensis C. I Peng, X. H. Jin & S. M. Ku) (Fig. 4A and B).

|
| Fig. 4 Diverse habits of Chinese begonias. A Semi-tuberous type: Begonia augustinei, deciduous species with short rhizome without clear internodes; B Pseudo-tuberous: B. wuzhishanensis, deciduous with orchid-root-like rhizome; C Tuberous: B. fimbristipula, deciduous with single ball-shaped tuber; D Moniliform: B. wilsonii, deciduous with the tubers connected as a chain; E Rhizomatous: B. pedatifida, evergreen with clear nodes and internodes; F Trailing: B. sp. nov., evergreen with very long stem and internodes, the leaves remain on most of the nodes; G Shrub-like: B. acetosella, evergreen shrub-like with fibrous roots only. |
An important trait for identifying Begonia species is the occurrence or absence of aerial (erect) stems. According to this trait, Chinese begonias can generally be divided into three types (Fig. 5): type Ⅰ, species without an aerial stem, including the majority of tuberous species such as Begonia guishanensis S. H. Huang & Y. M. Shui (Fig. 5A) and over half the number of rhizomatous species such as B. augustinei (Fig. 5B); type Ⅱ, species with short erect stems or flowering stems occurring only at anthesis, including a small number of tuberous species like Begonia ravenii C. I Peng & Y. K. Chen (Fig. 5C) and some rhizomatous species like Begonia pulchrifolia D. K. Tian & C. H. Li (Fig. 5D); and type Ⅲ, species of plants which always have aerial stems, including three species of tuberous begonias like Begonia cehengensi T. C. Ku, B. grandis, and Begonia modestiflora Kurz (Fig. 5E), a few rhizomatous species like B. palmata, and several shrub-like species such as B. acetosella, B. coptidimontana (Fig. 5F) and B. longifolia.
|
| Fig. 5 Chinese begonia species with and without aerial stem. A, B Species without aerial stems: A Begonia guishanensis (tuberous type), B B. augustinei (Rhizomatous or subtuberous type); C, D Species with short aerial stem only developed at anthesis: C B. ravenii (tuberous), D B. pulchrifolia (rhizomatous); E, F Species always with aerial stem: E B. modestiflora (tuberous, non-branched to few branched stems), F B. coptidimontana (shrub-like, branched). |
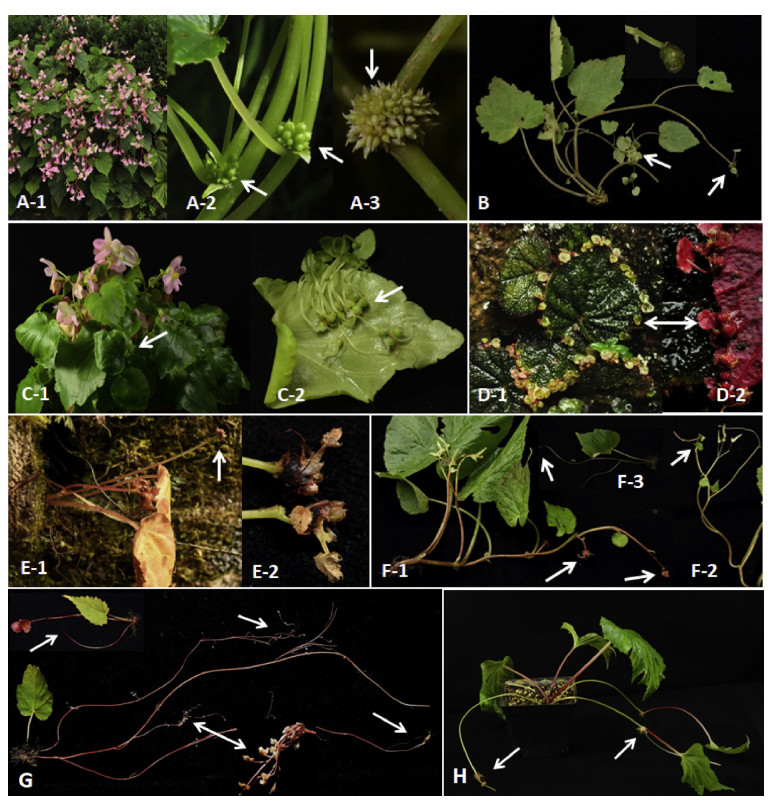
Besides natural reproduction by seeds, at least eight species of tuberous Chinese begonias may produce aerial tubers, stolons and stolon-like stems for asexual propagation (Fig. 6). Two species produce bulbils on the stem, namely B. grandis (several to numerous bulbils develop on the leaf axil) (Fig. 6A) and B. cehengensis (bulbils develop on the tip of the main and branched stems) (Fig. 6B); two species produce bulbils on the leaf, including Begonia parvula H. Lév. - & Vaniot (some populations in Guizhou province form bulbils on the veins of the leaf back) (Fig. 6C) and Begonia glechomifolia C. M. Hu ex C. Y. Wu & C. Z. Ku (bulbils form on leaf edge) (Fig. 6D). Begonia josephii A DC. produces bulbils usually on the rachis tip (Fig. 6E). Begonia flagellaris Hara, a newly recorded species in Tibet, is very special and produces stolons (from underground tubers) and stolon-like stems above the inflorescence, both producing bulbils (Fig. 6F). Interestingly, Begonia dioica Buch.-Ham. ex D. Don (also newly recorded Tibetan species) produces one to several long stolons only from the previous year's underground tuber, and numerous tiny bulbils develop on stolon tips (Fig. 6G). Similar to Saxifraga stolonifera Curtis and strawberries, B. ravenii produces a long stolon from the current year's underground tuber, and each node of the stolon forms a tuber and then develops into a plantlet (Fig. 6H).
|
| Fig. 6 Chinese begonia species with vegetative reproduction via tubers or stolons. A, B Aerial tubers producing on stem: A-1 to A-3 Bulbil clusters at leaf axial of Begonia grandis; B Bulbils on the tips of stem in B. cehengensis; C, D Aerial tubers producing on leaf: Bulbils on the veins of leaf back in B. parvula (C-1, C-2); Bulbils on leaf back edge of B. glechomifolia (D-1, D-2); E Aerial tubers producing on inflorescence: Bulbils on the rachis tips of B. josephii (E-1, E-2); F-H Aerial tubers producing on stolon-like stem and stolon: Bulbils on the stolen-like stems connected with (F-1, F-2) or without (F-3) inflorescence in B. flagellaris; G Tiny bulbils on tips of long stolons in B. dioica; H Bulbils and plantlets on stolon of B. ravenii. Arrows indicate where bulbils form. |
Most Chinese begonias are monecious, whereas only six species (all in Sect. Sphenanthera) are diecious, namely B. acetosella, B. handelii, Begonia lancangensis S. H. Huang, Begonia silletensis subsp. mengyangensis M. Tebbitt & K. Y. Guan, Begonia aborensis Dunn (new record in Tibet), and Begonia roxiburigii (Miq.) A. DC. (in southern Tibet).
Plant and leaf size vary greatly among species and populations, and even within a population (Fig. 7). In China, B. parvula and Begonia peii C. Y. Wu are the smallest and shortest begonias, the plants of which are only about 1e10 cm in height. The smallest leaf in these two species is only about 1 cm in diameter (Fig. 7A). B. acetosella, B. edulis, B. longifolia and B. palmata are among the tallest begonias, and the plants which in the wild may reach above 1.5 m in height (Fig. 7B). The largest leaf recorded was in B. edulis (above 50 cm in diameter) (Fig. 7D) and Begonia megalophyllaria C. Y. Wu (nearly 50 cm in diameter) (Fig. 7E), and the longest petiole of all species was observed in B. megalophyllaria (above 120 cm long) (Fig. 7F). In both B. labordei and B. fimbristipula (Fig. 7C), a huge variation of plant and leaf size is observed even in the same population, and the leaf size may range from 2 cm to 25 cm, with more than a 20-fold difference among adult plants.

|
| Fig. 7 Variation of plant and leaf size in Chinese begonias. A B. parvula (one of the smallest species in Begonia); B B. longifolia (above 150 cm tall); C B. fimbristipula (huge variation in leaf size between individuals); D B. edulis (the largest leaf greater than 50 cm in diameter); E, F B. megalophyllaria (leaf blade 45 cm thick, petiole over 120 cm long). |
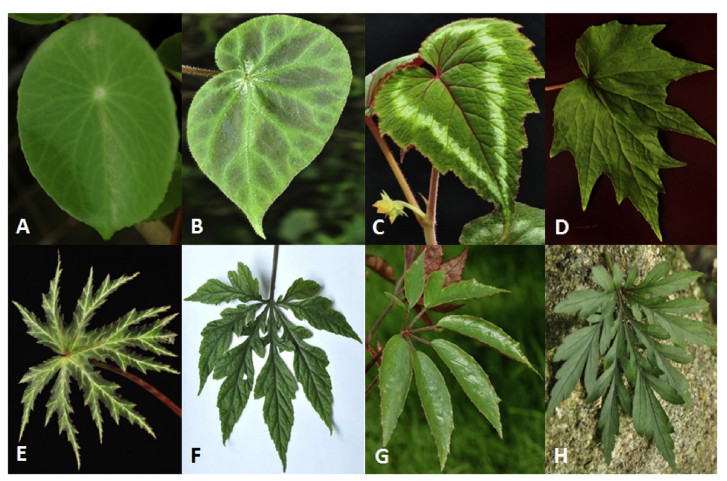
Chinese begonias also have amazingly diverse leaf types and colors. Simple, sub-compound, and compound leaves are all found in Chinese begonias (Fig. 8). Most species have simple leaves. Simple leaf blades are peltate and non-peltate, from entire to deeply lobed (Fig. 8A-E). The sub-compound leaf is only seen in Begonia jinyunensis C. I Peng, B. Ding & Q. Wang without real petiolules (Fig. 8F). Only five species have compound leaves, including B. coptidifolia (Fig. 8H), Begonia fangii Y. M. Shui & C. I Peng (Fig. 8G), B. hemsleyana, Begonia leipingensis D. K. Tian, L. H. Yang & C. Li and a new species from Guangxi. The largest variation in leaf margin is observed in B. palmata, B. circumlobata, and B. pedatifida.
|
| Fig. 8 Diversity in leaf morphology of Chinese begonias. A Peltate simple leaf: Begonia peltatifolia; B Entire simple leaf: B. luochengensis S. M. Ku, C. I Peng & Yan Liu; C Simple leaf with double teeth: B. cathayana Hemsl.; D Shallow lobed leaf: B. palmata; E Deep lobed leaf: B. rubropunctata; F Sub- or pseudo-compound leaf with underdeveloped petioles: B. jingxiensis D. Fang & Y. G Wei; G Compound leaf: B. fangii; H Compound leaf with secondary lobes: B. coptidifolia. |
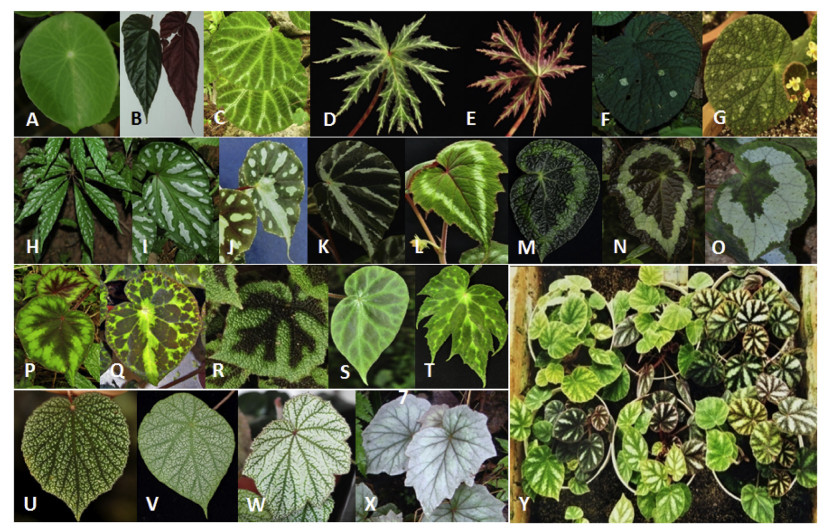
The most common color for Chinese begonia leaves is green. However, leaves with various other colors and diverse coloration patterns are also commonly seen in many species (Fig. 9). Previous research has shown that leaf variegation occurs in 84 of 203 Chinese begonia taxa (Cui and Guan, 2013). Variegated leaves can be mainly divided into vein-related and non-vein related types. Actually, based on our survey, most species of Chinese begonias have at least two leaf color types. The most extreme examples of color variation in begonia leaves include B. versicolor and Begonia ningmingensis D. Fang, Y. G. Wei & C. I Peng, in the former, the leaves can be divided into more than fifteen types based on differences in color and colorationpatterns (Fig. 9Y). Leaf variegation may provide a useful clue for classification of begonias, but also increases the difficulty in species identification, particularly for those sharing similar leaf morphological characteristics.
|
| Fig. 9 Diverse leaf color and variegation patterns in Chinese begonias. A B. peltatifolia with green leaf only; B B. longifolia (rarely seen dark-green leaf surface type); C B. ningmingensis (white-vein type); D, E B. rubropunctata (two sides); F Begonia gulinqingensis S. H. Huang & Y. M. Shui (white spotted type); G Begonia longistyla Y. M. Shui & W. H. Chen (scattered spotted type); H B. hemsleyana ('Silvery Pearl' selected from natural mutants); I B. handelii (variegated type); J Begonia pseudodryadis C. Y. Wu (white spots or bands on middle vein and between veins); K B. sp. nov.(variegated type with white color along veins); L B. cathayana (light green leaf variety with orange flower); M, N Begonia longiciliata C. Y. Wu (dark-leaf type), O Begonia aurantiflora C. I Peng, Yan Liu & S. M. Ku (white-ring-band leaf type); P Begonia polytricha C. Y. Wu (leaf with red-dark center, green middle and red-dark edge); Q B. henryi (variegated leaf type); R Begonia masoniana Irmsch. (dark bands along main veins); S B. luochengensis (dark-green between veins); T B. pulchrifolia (deeply lobed type); U B. frimbristipula (white spotted leaf type); V Begonia setulosopeltata C. Y. Wu (white spots evenly distributed between veins); W Begonia malipoensis S. H. Huang & Y. M. Shui (densely white spotted type); X B. palmata (nearly white leaf type); Y B. versicolor (seedlings, highest diversity in leaf color and coloration pattern of Begonia). |
The most commonly occurring flower colors in Chinese begonias are white, pink, and red (Fig. 10); however, a few species, such as Begonia aurantiflora, a variety of B. cathayana, Begonia crocea C. I Peng, Begonia flaviflora H. Hara, Begonia hekouensis S. H. Huang, Begonia xanthina Hook. and a new species recently found in Tibet, have orange flowers (Fig. 10I). A small number of species have light green-yellow flowers, such as Begonia filiformis Irmsch., Begonia liuyanii C. I Peng, S. M. Ku & W. C. Leong, and B. masoniana (Fig. 10G). The number of tepals ranges from 2 to 4 (rarely 5-6) in male flowers and 2 to 6 (rarely 7e8) in female flowers (Fig. 10). Double flowers are extremely rare in wild begonias. Except for red-purple stamens in Begonia purpureofolia S. H. Huang & Y. M. Shui, the stamens of all other species are yellow. The morphological characteristics of fruits and wings are very diverse in Chinese begonias (Fig. 11). The shape and length of wings may vary largely among species, and sometimes even in a species, or the same population, particularly in B. grandis (Li et al., 2014).

|
| Fig. 10 Diversity of flower tepal number and color in Chinese begonias. A-E Male flowers: A Begonia wangii Yu (tepal 2); B B. pelatifolia (tepal 3, more often 4); C Begonia chishuiensis T. C. Ku (petal 4); D, E B. longifolia (tepal normally 4, rarely 6); F-K Female flowers: F B. henryi (tepal 2); G Begonia filiformis Irmsch. (tepal 3); H Begonia silletensis subsp. mengyangensis M. Tebbitt et K. Y. Guan (tepal 4); I Begonia crocea C. I Peng (tepal 5); J-K B. longifolia (tepal normally 6, rarely 8). |

|
| Fig. 11 Morphological diversity in fruits and wings of Chinese begonias. A Begonia pulvinifera C. I Peng & Yan Liu; B B. henryi; C Begonia curvicarpa S. M. Ku, C. I Peng & Yan Liu; D B. chishuiensis; E B. coptidimontana; F B. palmata; G B. acetosella; H B. handelii; I B. longifolia; J Begonia leprosa Hance. |
The classification of the sections of Chinese Begonia has been under debate (Irmscher, 1939; Ku, 1999; Shui et al., 2002; Gu et al., 2007), although nine sections have been generally recognized. Defining a section of Begonia is largely based on characteristics of female flowers, particularly the ovary, one of the most important traits for species identity in this genus. Dozens of species have been placed in Begonia sect. Platycentrum (Klotzsch) A. DC., which is characterized by 2-locular ovary and bifid axile plancentae (Fig. 12A), sect. Diploclinium (Wight) A. DC., which is characterized by 3-locular ovary and bifid axile plancentae (Fig. 12B), sect. Coelocentrum Irmscher, which is characterized by 1-locular ovary and bifid parietal plancentae (Fig. 12C); a few species are included in sect. Reichenheimia (Klotzsch) A. DC., which is characterized by 1- locular ovary and undivided axile plancentae (Fig. 12D), sect. Leprosae (T.C. Ku) Y.M. Shui which is characterized by 3-locular ovary, bifid axile plancentae and clavate fruit (Fig. 12E), and sect. Sphenanthera (Hassk.) Warb. which is characterized by 3e4 (7) locular ovary and bifid to multifid axile plancentae (Fig. 12F-H); whereas each of other three sections, sect. Petermannia (Klotzsch) A. DC., sect. Alicida C. B. Clarke, and sect. Parvibegonia A. DC., contains one species only. As field survey continues, new sections of begonias will likely be discovered in China.

|
| Fig. 12 Transected ovary or fruit showing locule and placentae in different sections of Chinese Begonia. A Begonia sect. Platycentrum (B. pulchrifolia); B sect. Diploclinium (B. labordei); C sect. Coelocentrum (Begonia cirrossa L. B. Smith & Wasshausen); D sect. Reichenheimia (Begonia xingyiensis T. C. Ku); E sect. Leprosae (Begonia cylindrical D. R. Liang & X. X. Chen); F-H sect. Sphananthera (F: B. longifolia; G, H: Begonia aborensis Dunn). |
Natural hybridization occurs very often in Chinese Begonia particularly in those regions with numerous species. Based on our extensive field survey and experimental analysis on natural hybridization of begonias in China, about 50 populations of 30 natural hybrids have been discovered among 26 species, accounting for 14% of currently accepted species (Tian et al., 2017). Begonia hemsleyana and B. longifolia had the highest cross frequency in nature and each has crossed with seven other species. Begonia palmata contributed 17 hybrid populations, the largest number in all species (Fig. 13). Most hybrids were observed in South Yunnan, followed by Taiwan, Guangxi and Tibet. Hybridization between species is unidirectional in most cases, and the majority of hybrids are F1 individuals, which still rely on parents or hybridization zones to maintain hybrid population stability, and therefore, they have not been established as self-perpetuating populations. In Chinese Begonia, there are fewer and more sparsely distributed natural hybrids than parents, which limits backcrosses; hybrids are therefore not harmful to their parents, but increase plant diversity and the chance of obtaining a new ornamental cultivar by natural processes. For these reasons, we suggest that regions with natural hybrids and numerous species should be given priority when planning in situ conservation.

|
| Fig. 13 Natural hybridization between Begonia hemsleyana and B.. palmata. A Natural hybrid zone: a, variegated leaf type of B. hemsleyana as maternal parent; b, the hybrid with white spots on leaves; c, non-variegated type of hybrid; B B. palmata (with various leaf colors among individuals) as paternal parent. |
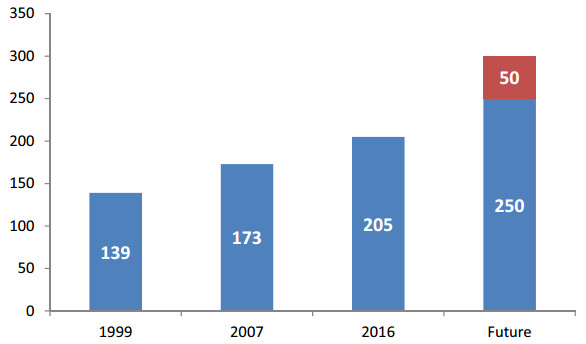
Begoina grandis, known for over a thousand years in ancient Chinese literature as qiu haitang, is probably the earliest documented species in Begonia (Li et al., 2014). It was scientifically named and described in 1791 (Dryander, 1791) and noted as the first species given a scientific name in the native Chinese begonias. In the 1990s, a large number of Chinese begonia species were discovered and described, 139 of which were documented in Flora Reipublicae Popularis Sinicae (Ku, 1999) (Fig. 14). More species have continued to be discovered and described. In 2007, the FOC was published, recording 173 species (Gu et al., 2007). In the past ten years, Chinese taxonomists have made great efforts to explore native begonias and have newly described over 30 species, most of them discovered in the karst regions of Guangxi (Li et al., 2016). At the end of 2016, the total number of accepted species of Chinese begonias had reached 205 (Fig. 14). However, based on our recent surveys, understanding the germplasm of Chinese begonias still requires extensive field exploration; furthermore, at least 40 new species currently await description. Accounting for synonyms of several species under our revision, we estimate that in the future the total number of begonia species in China will exceed 250 (250-300) (Fig. 14).
|
| Fig. 14 The number of Chinese begonia species is predicted to increase. Note: For 1999, the data are taken from the Flora Reipublicae Popularis Sinicae (Ku, 1999); for 2007 the data are taken from the Flora of China (Gu et al., 2007). Future data are based on our estimation according to field surveys and communication with begonia researchers and collectors. |
Huge diversity exists not only at the species level but also within populations of a species. Therefore, to better understand germplasm diversity in Chinese begonias, population studies based on morphological and genetic approaches are extremely necessary, particularly for highly diverse species with a wide distribution range and large variation in morphology, including B. circumlobata, B. edulis, B. fimbristipula, B. grandis, B. handelii, B. labordei, B. palmata, and B. pedatifida. In the past five years, we investigated population diversity and phylogeography in B. grandis (Li et al., 2014). Our results support the hypothesis that this species should be revised at the subspecies level. An investigation on population diversity of B. edulis is ongoing at Shenzhen Fairy Lake Botanical Garden of China. The remaining species need future study.
Taking into account the large number of species, wide distribution, huge diversity in morphology and habitat, high frequency of natural hybridization, as well as the limited number of taxonomists and funds available for research, the germplasm of Chinese begonias is still not well understood. To improve our understanding of the distribution, morphological characteristics, phenology and conservation status for Chinese begonias, future taxonomical work is required that explores new taxa, investigates population diversity, and revises previously described taxa.
3. Conservation of Chinese begonias 3.1. Evaluation of threatened begonia speciesBiodiversity, at all three level-sdgenes, species and ecosystems, is the basis for the sustainability, productivity and resilience of agricultural systems. It plays a key role in maintaining healthy ecosystems and thereby sustaining ecosystem services to the evergrowing human population (Santamaria, 2012). Understanding the diversity of Begonia and the associated ecosystem is the first step for sustainable conservation. However, the exact number of world begonias still remains unclear. Few studies have evaluated the conservation status of begonias worldwide, and so far, only 60 of the total 1800 species have been recorded in the IUCN red list database (IUCN, 2017). Of the listed species, only five are native to China, including B. cavaleriei, Begonia dentatobracteata C. Y. Wu, B. hainanensis, B. hemsleyana, and B. peltatifolia (Table 1). In China, most begonia species have been evaluated based on IUCN Categories and Criteria, 49 of which were designated as Near Threatened to Extinct taxa in the Red List of China's Biodiversity (RLCB) (The Ministry of Environmental Protection, Chinese Academy of Sciences, 2013); and 14 have been listed as protected species at the province level (e.g., in Hainan and Zhejiang); while, after having been described as new taxa, five species have been evaluated using the standard IUCN red list (Table 1). However, after carefully reviewing these taxa according to our field survey and specimen study, the listed risk categories for many species may not reflect their true status. For instance, in the IUCN database, B. cavaleriei was listed as vulnerable (VU). However, in fact, it is not only widely distributed in Guangxi, Yunnan, Guizhou and Chongqing, but also may adapt to dry environments; therefore, it, at most, may be evaluated as near threatened (NT) (Table 1). Similarly, B. hemsleyana, should be not considered as VU but NT due to a relatively wide distribution range in both Yunnan and Guangxi. Therefore, the threatened status for some evaluated species has been exaggerated and may consequently mislead government and researchers when developing policies for species conservation. Erroneous evaluations have also been made on the Red List of China's Biodiversity (RLCB): Higher Plants (2013) (Table 1) lists Begonia sublongipes Y. M. Shui as extinct (EX), but living plants of this species have recently been observed in several places in Hainan province; B. labordei, which is listed as near threatened (NT), is widely distributed in many places in Yunnan, Guangxi and Guizhou, where its populations are generally in good condition, and therefore should be designated least concern (LC) at most (Table 1). The reason for such erroneous conclusions is likely due to incomplete specimen review, inadequate field survey and the low number of begonia specialists working in China and elsewhere. Meanwhile, other begonia species with data deficiency need to be evaluated, some of which, based on our knowledge, should be included on the threatened list. It is otherwise impossible to develop a good strategy and practice for conservation of Chinese begonias. Currently, a four-year project is being conducted by Dr. Daike Tian's team at the Shanghai Chenshan Plant Science Research Center (SCPSRC), CAS and Shanghai Chenshan Botanical Garden (SHCBG) that aims to fully revise the taxonomy of Chinese begonias and evaluate their conservation status using both IUCN standards and Chinese criteria. A database for wild Chinese begonias will be available as soon as this project is completed. This work will definitely improve our knowledge on the germplasm of Chinese begonias and provide a useful reference for basic research, conservation and utilization of the natural resources of this genus.
| Species (including low taxa) | Extinction risk category | Endemic to China | ||
| IUCN | RLCB | Other source | Y (Yes)/N (No) | |
| Begonia acetosella var. acetosella | NT | N | ||
| B. algaia | NT | a | Y | |
| B. asteropyrifolia | EN | Y | ||
| B. aurantiflora | EN | Y | ||
| B. baviensis | NT | N | ||
| B. biflora | VU | Y | ||
| B. cavaleriei | VU | Y | ||
| B. cehengensis | VU | Y | ||
| B. coptidifolia | CR | Y | ||
| B. curvicarpa | NT | Y | ||
| B. daxinensis | NT | Y | ||
| B. debaoensis | VU | Y | ||
| B. dentatobracteata | VU | VU | Y | |
| B. dielsiana | NT | Y | ||
| B. digyna | a | Y | ||
| B. discreta | NT | N | ||
| B. filiformis | NT | Y | ||
| B. fimbristipula | a, b | Y | ||
| B. forrestii | NT | N | ||
| B. grandis | a | Y | ||
| B. grandis subsp. holostyla | NT | Y | ||
| B. grandis subsp. sinensis | a, c | Y | ||
| B. grandis var. unialata | NT | Y | ||
| B. guangxiensis | EN | Y | ||
| B. gulinqingensis | d (EN) | Y | ||
| B. hainanensis | EN | b | Y | |
| B. handelii | b | N | ||
| B. hemsleyana | VU | Y | ||
| B. howii | b | Y | ||
| B. jinyunensis | e (VU) | Y | ||
| B. labordei | NT | N | ||
| B. leipingensis | f (CR) | Y | ||
| B. liuyanii | VU | Y | ||
| B. longifolia | b | N | ||
| B. longistyla | NT | Y | ||
| B. luochengensis | NT | Y | ||
| B. manhaoensis | NT | Y | ||
| B. masoniana | VU | N | ||
| B. megalophyllaria | VU | Y | ||
| B. mengtzeana | VU | Y | ||
| B. morifolia | NT | Y | ||
| B. obliquifolia | VU | Y | ||
| B. palmata | b | N | ||
| B. palmata var. bowringiana | b | Y | ||
| B. peii | NT | Y | ||
| B. pellionioides | g (CR) | Y | ||
| B. peltatifolia | EN | b | Y | |
| B. pengii | EN | Y | ||
| B. pinglinensis | NT | Y | ||
| B. platycarpa | NT | Y | ||
| B. polytricha | NT | Y | ||
| B. pulchrifolia | h (EN) | Y | ||
| B. reflexisquamosa | NT | Y | ||
| B. retinervia | NT | Y | ||
| B. rhynchocarpa | NT | Y | ||
| B. rotundilimba | NT | Y | ||
| B. semiparietalis | VU | Y | ||
| B. setulosopeltata | EN | Y | ||
| B. subcoriacea | VU | Y | ||
| B. subhowii | NT | N | ||
| B. sublongipes | EX | b, g (CR) | Y | |
| B. tsoongii | EN | Y | ||
| B. umbraculifolia | VU | Y | ||
| B. variifolia | VU | Y | ||
| B. wuzhishanensis | i (EN) | Y | ||
| B. xingyiensis | NT | Y | ||
| B. xishuiensis | VU | Y | ||
| B. zhengyiana | NT | Y | ||
| Note: The table only includes the taxa with the risk category above Near Threatened. CR, Critically Endangered; EN, Endangered; EX, Extinct; NT, Near Threatened; VU, Vulnerable; IUCN, International Union for the Conservation of Nature (2017); RLCB, Red List of China's Biodiversity: Higher Plants (The Ministry of Environmental Protection, Chinese Academy of Sciences, 2013); a, In The List of Wild Plants under Protection of Zhejiang Province (The Forestry Department of Zhejiang Province, 2012); b, In The List of Wild Plants under Protection of Hainan Province (The People's Government of Hainan Province, 2006); c, In The List of Wild Plants under Priority Protection of Beijing (The People's Government of Beijing Municipality, 2008); d, EN in Ma et al. (2007); e, VU in Ding et al. (2014); f, CR in Li et al. (2016); g, CR Wang et al. (2015); h, EN in Tian et al. (2015); i, EN in Tian et al. (2014). | ||||
Early conservation work on wild begonias began with Dr. Kaiyun Guan's team at the Kunming Institute of Botany, Chinese Academy of Sciences. In 1995, native Chinese begonia species were collected mainly from Yunnan province for ex situ conservation. Since 1996, graduate students and PhD candidates from KIB have contributed greatly to conservation biology studies on begonias (Tian, 1999; Xiang, 2001; Li, 2006; Ma, 2008; Yang, 2010; Cui, 2012). Since then, the number of begonias collected has continued to increase and in 2017 stands at 410 taxa (Table 2). Over the past ten years, Dr. Cecelia Koo, Botanical Conservation Center (CKBCC), Fairy Lake Botanical Garden, Xiamen Botanical Garden, and Shanghai Chenshan Botanical Garden have made a great progress in the collection and conservation of begonias (Table 2). Specifically, the begonia collection at CKBCC in Taiwan has already reached 1238 taxa, including species collected in China and the rest of the world. Although several other Chinese botanical gardens have introduced begonias since the 1960s, the collection number has not increased much due to either poor management or the absence of a targeted research project or team.
| Institution | Location | Earliest time | Total taxa | Species | Cultivar | Native Sp. |
| Beijing Florascape Co, Ltd. | Beijing | 2013 | 120 | 80 | 40 | 60 |
| Dr. Cecelia Koo Botanic Conservation Center | Taiwan | 2007 | 1238 | 680 | 558 | 165 |
| Fairylake Botanical Garden, CAS | Shenzhen | 2008 | 390 | 270 | 120 | 140 |
| Guilin Botanical Garden, CAS | Guilin | 1991 | 150 | 150 | 30 | 90 |
| Kunming Institute of Botany, CAS | Kunming | 1974 | 410 | 210 | 200 | 150 |
| Shanghai Botanical Garden | Shanghai | 1974 | 250 | 100 | 150 | 50 |
| Shanghai Chenshan Botanical Garden, CAS | Shanghai | 2009 | 330 | 224 | 106 | 190 |
| South China Botanical Gardens, CAS | Guangzhou | 1963 | 90 | 60 | 30 | 40 |
| Xiamen Botanical Garden | Xiamen | 2010 | 360 | 210 | 150 | 110 |
| Earliest time, the earliest recorded time of introducing begonia for each institution in China; Total taxa, the taxa including all types of species, subspecies, variety, form and cultivar; Native Sp. includes species and lower taxa. Data were updated April, 2017. CAS, Chinese Academy of Sciences. | ||||||
Recently, in order to improve the survival rate and growth quality of begonias conserved ex situ, researchers and graduate students have paid more attention to disease, tissue culture, microleafcutting, seed dormancy, growth medium, light quality and intensity for plant growth (Tian et al., 2000a, 2000b; Ma et al., 2005; Yang et al., 2010; Zhao et al., 2016, 2017), and exploring new, efficient approaches for conserving difficult-to-grow species (Fig. 15). Through improved integrated approaches for propagation and cultivation management, extremely endangered species, such as B. coptidifolia, B. hainanensis and Begonia hongkongensis F. W. Xing, have been successfully conserved ex situ at SHCBG (Fig. 15). For in situ conservation of Chinese begonias, Dr. Daike Tian's team at SCPSRC and SHCBG is evaluating the status of wild species in the protected areas, including national and regional nature reserves and parks. However, excluding a trial for B. hongkongensis in 2014, little work has been done on reintroduction. Public education about begonias, on the other hand, has been improved by the increasing number of begonia exhibitions.

|
| Fig. 15 Integrative approaches for conserving begonias in Chenshan Botanical Garden, Shanghai. A-C Propagation of B. coptidifolia by micro-leafcutting; D-F Propagation of B. peltatifolia by tissue culture; G Propagation by stem/leaf cuttings or growing small sized species to maintain high moisture under transparent plastic cover; H-I Self-designed poly-boxes for growing begonias requiring relatively high moisture. |
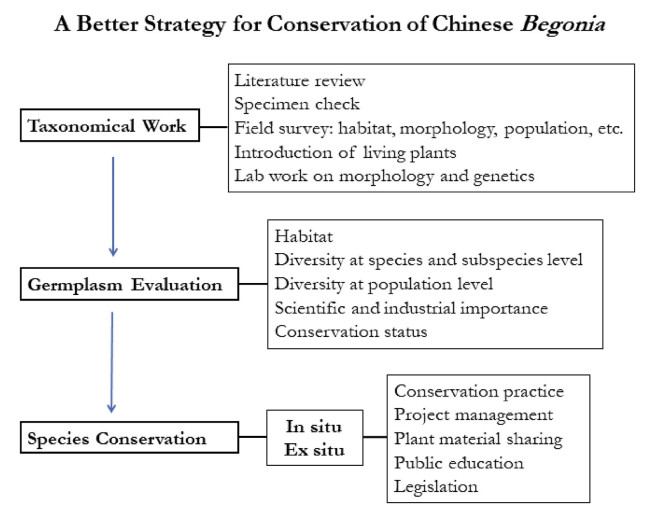
Most currently known species of wild Chinese begonias (~200) have been conserved ex situ in several major botanical gardens. However, the funds and labor invested on conservation work are still very low. Much work is needed for improving the quality of the living collection and establishing high conservation practice standards. In contrast, illegal collection and over-exploitation of wild begonia resources still persist in many places for ornamental and medicinal use (Fig. 16). Therefore, we propose a strategic plan to better understand and more effectively conserve germplasm of Chinese begonias (Fig. 17).

|
| Fig. 16 Overharvesting or illegal collection of wild Chinese begonias for medicinal and ornamental uses. A-C Medicinal begonias for sale at a local market in Jingxi County, Guangxi province, China (A Begonia wangii; B B. picturata Yan Liu, S. M. Ku & C. I Peng; C B. cathayana); D Illegal collection of the rhizomes of B. emeiensis C. M. Hu ex C. Y. Wu & T. C. Ku and the tubers of Asparagus cochinchinensis (Loureiro) Merrill from Mount Emei National Park, Sichuan province (Photos A t to C were provided by Mr. Chongjian Zhou). |
|
| Fig. 17 A strategy for improved conservation of Chinese Begonia. |
There are still many new begonia taxa that require investigation and description. Moreover, in some instances, previously described species require taxonomic revision. Taxonomic work is therefore critical, forming the basis for germplasm evaluation and conservation. Literature review and specimen study are both important, and further field surveys are needed. For the difficult-to-identify taxa, living plants should be introduced for further observation and study of morphological characteristics. To understand population diversity and interspecific differences in Begonia, taxonomic work must be supported by an integrated approach that includes morphology, cytology, molecular biology, and biochemistry. Germplasm evaluation should not be limited to habitat, diversity of all levels of species, subspecies and population, and importance in science and industry, but also to IUCN conservation status, taking into consideration distribution and habitat, population size, genetic structure and utilization.
Plant conservation generally includes both in situ and ex situ practices, and often requires a long-term project even only for a specific species. Besides a good living collection, ex situ conservation should also include seed banks and gene banks when possible. Establishing a satellite or species-targeted protected area is necessary for endangered species located outside of current nature reserves. Due to limited financial and human resources, priority should be given to the most medicinally, or ornamentally, important and endangered taxa.
Plant conservation is not a simple procedural job, and the success of a project largely relies on effective project management, the extent of sharing plant material, technique and experience, and long-term public education on the importance of biodiversity. Therefore, government, researchers, and local residents should all actively participate in the project. Finally, driven by the rapid development of transportation systems and online commerce, the illegal collection and exchange of wild begonia plants has increased sharply; consequently, some species face immediate risk of extinction. Legislation has therefore become extremely urgent for protecting the wild plants, particularly species with high ornamental and medicinal value.
AcknowledgementsThis work was supported by the funds from National Natural Science Foundation of China (31570199) and the project of Shanghai Landscaping Administration Bureau (F122416). We also gratefully thank the following persons for providing data on living begonia collection: Mr. Chunming Chen from Dr. Cecelia Koo Botanic Conservation Center of Taiwan, Mrs. Weihua Cui from Kunming Botanical Garden of Kunming Institute of Botany, Chinese Academy of Sciences (CAS); Mr. Wenguan Wang from Fairylake Botanical Garden of Shenzhen & CAS; Dr. Youfan Ding from Xiamen Botanical Garden; Dr. Wenxiu Tang from Guilin Botanical Garden of Guangxi & CAS, Mrs. Caixia Peng from South China Botanical Garden of CAS; Mr. Jiaqi Qin from Shanghai Botanical Garden; and Mr. Wenke Dong from Beijing Florascape Co., Ltd.
Appendix A. Supplementary dataSupplementary data related to this article can be found at https://doi.org/10.1016/j.pld.2018.06.002.
Cui, W. H., 2012. Diversity of Leaf Variegation in Chinese Begonias and its Relevance to Geographic Distribution and Other Factors. Kunming Institute of Botany, Chinese Academy of Sciences (Master thesis). (Chinese with English Abstract).
|
||
Cui W.H., Guan K.Y., 2013. Diversity of leaf variegation in Chinese begonias. Plant Divers. Resour, 35(2), 119-127.
|
||
Ding B., Nakamura K., Kono Y., Ho M.J., Peng C.I., 2014. Begonia jinyunensis(Begoniaceae, section Platycentrum), a new palmately compound leaved species from Chongqing. China. Bot. Stud, 55(1), 1-8.
DOI:10.1186/1999-3110-55-1 |
||
Dryander, J. C., 1791. Begonia grandis Dryand. Trans. Linn. Soc. London 1, 163.
|
||
Gu, C. Z., Peng, C. I., Turland, N. J., 2007. Begoniaceae. In: Wu, C. Y., Raven, P. H. (Eds. ), Flora of China. Vol. 13 (Clusiaceae through Araliaceae). Science Press & Missouri Botanical Garden, Beijing & St. Louis, Missouri.
|
||
Guan K.Y., Yamaguchi H., Li J.X., Li H.Z., Ma H., 2007. Traditional uses of begonias(Begoniaceae) in China. Acta Bot. Yunnanica, 29(1), 58-66.
|
||
Hilinske, J., 2016. The toot of it all: plant of the year 2016. In: The Journal Times. http://journaltimes.com/lifestyles/home-and-garden/the-root-of-it-all-plantof-the-year/article_1c6ce319-2ab6-5aa8-950f-9dc92cd71f92.html (Accessed 16 May 2017).
|
||
Irmscher E., 1939. Die Begoniaceen Chinas und ihre Bedeutung für die Frage der Formbildung in polymorphen Sippen. Mitteilungen aus dem Institut für Allgemeine Botanik in Hamburg, 10, 431-557.
|
||
IUCN, 2017. The IUCN Red List of Threatened Species. Version 2017-1. Downloaded on 16 May, 2017. www. iucnredlist. org.
|
||
Ku, T. C., 1999. Begoniaceae. In: Ku, T. C. (Ed. ), Flora Reipublicae Popularis Sinica, vol. 52(1). Science Press, Beijing, Addenda (Chinese).
|
||
Li C., Yang L.H., Tian D.K., Chen Y., Wu R.J., Fu N.F., 2016. Begonia leipingensis(Begoniaceae), a new compound-leaved species with unique petiolule pattern from Guangxi of China. Phytotaxa, 244(1), 045-056.
DOI:10.11646/phytotaxa.244.1 |
||
Li X.J., Tian D.K., Li C., Liu K.M., Li X.P., Nakata M., 2014. The history, culture, utilization, germplasm diversity and research advances of Begonia grandis Dry. Bot. Res, 3, 117-139.
|
||
Li, H. Z., 2006. Conservation Biology of Begonia Section Reidhenheimia (Klotzsch) a. DC. from China. Kunming Institute of Botany, Chinese Academy of Sciences(Master thesis). (Chinese with English Abstract).
|
||
Ma, H., 2008. The Phylogeny and Conservation Biology of the Begonia Sect. Diploclinium (Lindl. ) a. DC. from China. Kunming Institute of Botany, Graduate School of Chinese Academy of Sciences (Doctoral Dissertation). (Chinese with English Abstract).
|
||
Ma H., Li H.Z., Guan K.Y., 2005. Study on characters of seed germination of four species of Begonia from China. Seed, 24(11), 10-13.
|
||
Ma H., Li H.Z., Guan K.Y., Lu Y.X., 2007. Begonia gulinqingensis, a rare plant and its wild resource status and biological characters. J. Yunnan Agric. Univ. (Nat. Sci.), 22(3), 337-339.
|
||
Phutthai T., Hughes M., 2016. A new species and a new record in Begonia sect. Platycentrum (Begoniaceae) from Thailand. Gard. Bull. Singap, 68(1), 99-107.
DOI:10.3850/S2382581216000077 |
||
Santamaria L., 2012. Evolution and biodiversity:the evolutionary basis of biodiversity and its potential for adaptation to global change. Evol. Appl, 5(2), 103-106.
DOI:10.1111/j.1752-4571.2011.00232.x |
||
Shui Y.M., Peng C.I., Wu C.Y., 2002. Synopsis of the Chinese species of Begonia(Begoniaceae), with a reappraisal of sectional delimitation. Bot. Bull. Acad. Sin, 43, 313-327.
|
||
The Forestry Department of Zhejiang Province, 2012. List of the Wild Plants Under Protection of Zhejiang Province. http://www.zjly.gov.cn/art/2012/5/28/art_1275955_4716477.html (in Chinese) (Accessed May 2017).
|
||
The Ministry of Environmental Protection, Chinese Academy of Sciences, 2013. Red List of China's Biodiversity: Higher Plants. http://www.mep.gov.cn (in Chinese)(Accessed May 2017).
|
||
The People's Government of Beijing Municipality, 2008. The List of the Wild Plants Under Priority Protection of Beijing Municipality. http://www.gov.cn/gzdt/2008-03/18/content_923338.htm (in Chinese) (Accessed May 2017).
|
||
The People's Government of Hainan Province, 2006. The List of the Wild Plants Under Protection of Hainan Province. Decree No. 78. (in Chinese).
|
||
Tian, D. K., 1999. Horticulture Study on Begonia Versicolor (Master thesis). Kunming Institute of Botany, Chinese Academy of Sciences (Chinese with English abstract).
|
||
Tian D.K., Li C., Li C.H., Li X.J., 2015. Begonia pulchrifolia (sect. Platycentrum), a new species of Begoniaceae from Sichuan of China. Phytotaxa, 207(3), 242-252.
DOI:10.11646/phytotaxa.207.3 |
||
Tian D.K., Li C., Tong Y., Fu N.F., Wu R.J., 2017. Occurrence and characteristics of natural hybridization of Begonia in China. Biodivers. Sci, 25(6), 654-674.
DOI:10.17520/biods.2017050 |
||
Tian D.K., Li C., Yan Y.H., Li X.P., Meng J., 2014. Begonia intermedia, a new species of Begoniaceae from Hainan, China. Phytotaxa, 166(2), 114-122.
DOI:10.11646/phytotaxa.166.2 |
||
Tian D.K., Guan K.Y., Guo R.X., Li J.X., 2000a. Propagation and cultivation of Begonia versicolor. Guihaina, 21(4), 375-380.
|
||
Tian D.K., Li J.X., Guan K.Y., Li Z.J., 2000b. Prevention and cure of the powdery mildew disease on begonias. Plant Prot, 26(4), 33-34.
|
||
Wang J., Chen W.H., Mark H., Yang Z.Z., Shui Y.M., 2015. Additional notes on the Begonia Sect. Petermannia (Begoniaceae) from China. Plant Divers. Resour, 37(5), 563-568.
|
||
Xiang, J. Y., 2001. Taxonomy and Conservation Biology of Begonia Sect. Sphenanthera(Hassk. ) a. DC. from China. Kunming Institute of Botany, Chinese Academy of Sciences (Master thesis). (Chinese with English Abstract).
|
||
Yang, L. H., 2010. Morphology and Conservation Biology of Several Species of Begonia from China. Kunming Institute of Botany, Graduate School of Chinese Academy of Sciences (Master thesis). (Chinese with English Abstract).
|
||
Yang L.H., Hu X.J., Guan K.Y., 2010. Preliminary studies on characteristics of seed germination and storage of three begonia species. J. Yunnan Agric. Univ, 25(6), 844-849.
|
||
Zhao B., Fu N.F., Xiang Y.C., Tian D.K., 2017. Effects of light intensity and planting substrates on the growth of Begonia ningmingensis 'Ningming Silver', a new begonia cultivar. Guihaia, 37(9), 1153-1160.
|
||
Zhao B., Fu N.F., Xiang Y.C., Tian D.K., 2016. Effects of light intensity on the growth of Begonia cucullata and B. wallichinana. Acta Agric. Shangshai, 32(6), 128-133.
|



