b. College of Life Science, University of Yunnan, Kunming, 650091, China;
c. Yunnan Academy of Tobacco Agricultural Sciences, Kunming, 650021, China
The ATP-binding cassette (ABC) transporters belong to a large protein superfamily present in all living organisms, and are involved in the transportation of a wide range of molecules across membranes when energized by ATP hydrolysis (Lefevre et al., 2015; Nuruzzaman et al., 2014). The ABC transporter family has been divided according to structural features into full-size and half-size transporters. One of the well-known full-size subfamilies is the pleiotropic drug resistance (PDR) transporters.
In plants, PDR transporters constitute one of the biggest subfamilies of ABC transporters, and have recently been shown to perform critical physiological functions in plant response to biotic stresses (van den Brule et al., 2002; Lefevre et al., 2015; Nuruzzaman et al., 2014). PDR2 in Petunia hybrid and PDR5 in Nicotiana tabacum are involved in herbivore defense (Sasse et al., 2016; Bienert et al., 2012). In Nicotiana plumbaginifolia, NpPDR1 is induced by biotic stresses; and silencing NpPDR1 leads to plants highly susceptible to a group of fungal and oomycete pathogens which include Botrytis cinerea and Phytophthora nicotianae race 0 (Bultreys et al., 2009; Stukkens et al., 2005). PENETRATION (PEN) 3 (also called PDR8 or ABCG36) is required for Arabidopsis non-host resistance (Stein et al., 2006). Evidence also suggests that PDR transporters (e.g. AtPDR12 and NtPDR1) function in resistance against bacterial wilt disease in tobacco and Arabidopsis plants (Seo et al., 2012). However, it is not clear if PDR transporters play a role in plant defense in Nicotiana attenuata–Alternaria alternata (tobacco pathotype) interaction.
N. attenuata is an annual wild diploid tobacco native to western North America, and has been extensively used as a host for studying herbivore-elicited responses (Wu and Baldwin, 2010). Similar to cultivated tobacco, N. attenuata is attacked by A. alternata, a notorious necrotrophic fungus that causes brown spot disease in tobacco species in the field (Schuck et al., 2014). In response to this fungal infection, N. attenuata plants activate abscisic acid (ABA)-mediated stomatal defense (Sun et al., 2014a), as well as the jasmonate (JA) and ethylene signaling pathways (Sun et al., 2014b, 2017; Li and Wu, 2016).
In this study, two PDRs, which were up-regulated after A. alternata inoculation, were selected to test their roles in plant resistance via a virus-induced gene silencing technique, and the regulation of the transcriptional levels of these two genes by JA and ethylene was also investigated.
Materials and methods Plant and fungal materialSeeds of the 33rd generation of an inbred line of N. attenuata (Solanaceae) were defined as the wild type (WT) genotype in experiments. Stably transformed lines of irAOC (Kallenbach et al., 2012), irACO (von Dahl et al., 2007) and Ov-etr1 (von Dahl et al., 2007) were provided by Professor Ian T. Baldwin (Max-Planck Institute for Chemical Ecology, Jena, Germany). Seed germination and plant growth were conducted as described by Krugel et al. (2002).
A. alternata was grown and inoculated as described by Sun et al. (2014b).
Methyl jasmonate (MeJA) and Ethephon treatmentsMeJA treatment is the most commonly used means of eliciting JA-regulated responses in plants; when applied, the chemical is quickly de-methylated into jasmonic acid by plant MeJA-esterase (Wu et al., 2008). Ethephon is one of the most widely used synthetic plant growth regulators, and is converted into ethylene, thereby eliciting ethylene-induced responses in plants. 1 mM MeJA (www.sigmaaldrich.com) and 5 mM ethephon (www.sangon.com) were prepared by distilled water, and sprayed directly on the source–sink transition leaves (0 leaves) of 35-day-old rosette staged N. attenuata plants until run off with a fine mist of either compound at the indicated concentrations. The treated plants were immediately covered with a plastic bag, and harvested after 1, 3, 6, 12 h. Distilled water was sprayed and used as a control treatment.
Generation of VIGS plantsSpecific 137-bp PCR fragment used for silencing NaPDR1 (XM_019378156) was amplified by the primer pair XZ31 and XZ32 (all primers used in this study were included in Supplementary table). The 135-bp specific PCR fragment used for silencing NaPDR1-like (XM_019396612) was cloned by primer pair XZ35 and XZ36. In addition, a 188-bp PCR fragment used for silencing NaPDR1 and NaPDR1-like together was amplified by primers ABC01 and ABC02. All PCR products were cloned into pTV00 through BamH Ⅰ and Hind Ⅲ respectively. To generate NaPDR1- (VIGS PDR1 plant) and NaPDR1-like-silenced plants (VIGS PDR1-like plant), as well as NaPDR1 and NaPDR1-like-co-silenced plants (VIGS PDR1 and 1-like plant), Agrobacterium tumefaciens GV3101 cells carrying the above individual construct were combined with those cells having pBINTRA, and were inoculated into 25-day-old N. attenuata leaves through 1 mL syringe without a needle.
To monitor the progress of VIGS, we silenced phytoene desaturase (PDS), which causes plants to show visible bleaching of green tissues two to three weeks after transformation (Saedler and Baldwin, 2004; Wu et al., 2008; Sun et al., 2017). When the leaves of PDS-silenced plants began to bleach, the source–sink transition leaves of EV and VIGS plants were used for experiments. Around 20 plants were inoculated with each construct, and all VIGS experiments were repeated twice.
Real-time PCR assayTotal RNA was extracted from leaf lamina in an approximately 1.5 by 1.5 cm2 area which encompassed the inoculation site. The preparation of total RNA and cDNA was performed as described by Wu et al. (2013). Real-time PCR was conducted on a CFX Connect qPCR System (Bio-Rad) with iTaq Universal SYBR Green Supermix (Bio-Rad) and gene-specific primers according to the manufacturer's instructions. For each analysis, a linear standard curve obtained from threshold cycle number versus log DNA quantity was constructed by using a dilution series of a specific cDNA sample, and the levels of the transcript in all unknown samples were calculated according to the standard curve. Finally, relative transcript levels of target genes were obtained by dividing the extrapolated transcript levels of the target genes by the levels of a housekeeping gene Actin Ⅱ as an internal standard from the same sample. The expression level of housekeeping gene Actin Ⅱ was not altered in leaves either inoculated with A. alternata at 1 and 3 dpi, or treated with MeJA (or Ethephone) at 1 and 3 h (Supplementary data 1).
Statistical analysesAll samples represent biological replicates. Sample sizes are indicated in the figure legends. Unpaired two-tailed Student's t-tests and ANOVA were used to compare sets of data obtained from different treatments as indicated in figure legends. All experiments were repeated twice.
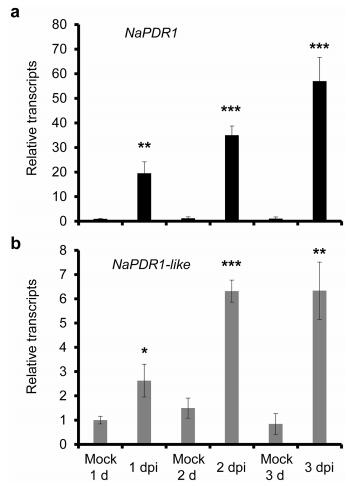
Results Elicitation of NaPDR1 and NaPDR1-like transcripts by A. alternataTranscriptome analysis revealed that after source–sink transition leaves (0 leaves) were inoculated with A. alternata for one day, two pleiotropic drug resistance transporter genes (NaPDR1 and NaPCR1-like) were up-regulated. To confirm these data, we quantified NaPDR1 (Gene bank accession number: XM_019378156) and NaPDR1-like gene (Gene bank accession number: XM_019396612) expression levels using real-time PCR analysis. Both NaPDR1 and NaPDR1-like expression was strongly elicited in 0 leaves at one day post inoculation (1 dpi), and reached its highest levels at 3 dpi (Fig. 1).
|
| Fig. 1 Elicitation of NaPDR1 and NaPDR1-like after A. alternata inoculation. NaPDR1 (a) or NaPDR1-like (b) transcripts were measured by real-time PCR in 0 leaves treated with mock or with A. alternata at 1, 2, and 3 days post inoculation (dpi). All transcriptional levels were normalized with a housekeeping gene Actin Ⅱ. Values are means ± SE for fives replicates. Asterisks indicate the level of significant difference between mock and infected leaves (Student's t-test: *, P < 0.05; **, P < 0.01; ***, P < 0.005). |
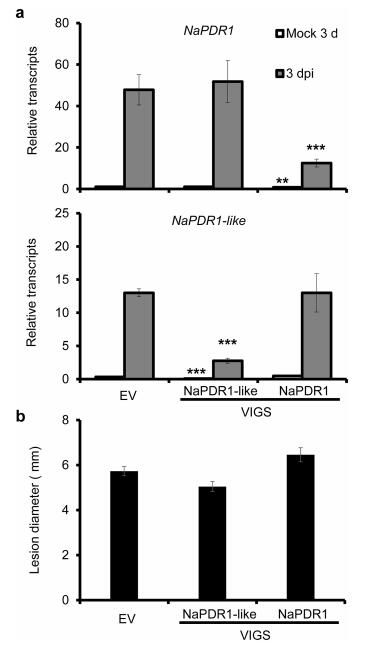
To study the role of NaPDR1 or NaPDR1-like in N. attenuata resistance to A. alternata, both genes were silenced individually by virus-induced gene silencing (VIGS). Three days after inoculation with A. alternata, plants transformed with NaPDR1 VIGS constructs (VIGS PDR1 plants) had only 26% of the NaPDR1 transcripts that plants transformed with empty vector (EV plant) or NaPDR1-like VIGS constructs (VIGS PDR1-like plant) had, suggesting NaPDR1 was successfully silenced in VIGS NaPDR1 plants without affecting the transcriptional level of NaPDR1-like (Fig. 2a). VIGS PDR1 plants inoculated with A. alternata developed necrotic lesions in 0 leaves of about the same size as EV plants (Fig. 2b). This result indicates that silencing NaPDR1 did not affect N. attenuata resistance to A. alternata. The transcriptional level of A. alternata-elicited NaPDR1-like in VIGS PDR1-like plants was silenced by 79% compared with EV or VIGS NaPDR1 plants (Fig. 2a). VIGS PDR1-like plants developed slightly smaller necrotic lesions in 0 leaves compared to EV or VIGS NaPDR1 plants, although the differences were not significant (Fig. 2b). All these data suggest that silencing NaPDR1 or NaPDR1-like individually does not affect N. attenuata resistance to A. alternata.
|
| Fig. 2 Silencing NaPDR1 or NaPDR1-like individually had no effect on N. attenuata resistance to A. alternata. a) Mean (±SE) NaPDR1 and NaPDR1-like transcripts were measured by real-time PCR in ten replicates of 0 leaves of EV, VIGS PDR1 and VIGS PDR1-like plants at 3 dpi. b) Mean (±SE) diameter of necrotic lesions of fifteen replicates of 0 leaves of EV, VIGS PDR1 and VIGS PDR1-like plants at 6 dpi. Asterisks indicate the level of significant difference between A. alternata-elicited EV and VIGS plants with the same treatments (Student's t-test: **, P < 0.01; ***P < 0.005). |
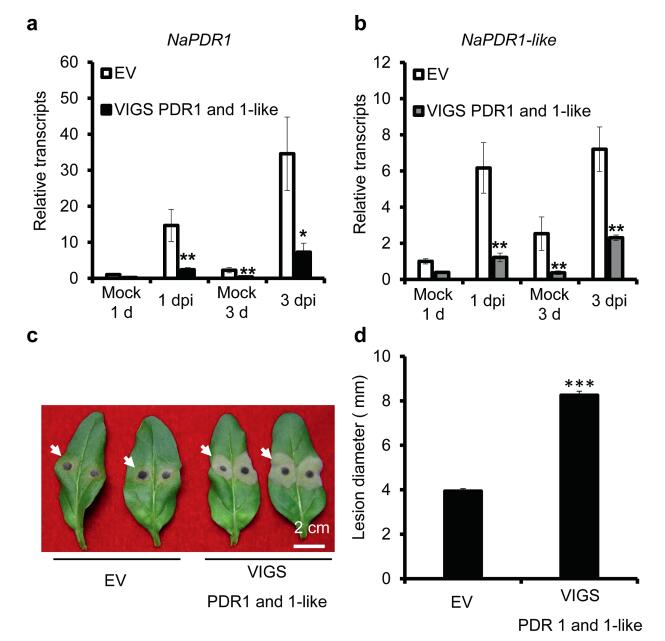
NaPDR1 and NaPDR1-like proteins share high sequence similarity (Supplementary data 1). To investigate whether they function redundantly in N. attenuata resistance to A. alternata, we co-silenced NaPDR1 and NaPDR1-like. When both NaPDR1 and NaPDR1-like genes were successfully silenced (Fig. 3a and b), necrotic lesion diameter at 6 dpi doubled (8.25 cm) in 0 leaves compared to those with the same leaf position in EV plants (4 cm) (Fig. 3c and d). This indicates that plants co-silenced for NaPDR1 and NaPDR1-like are more susceptible to A. alternata.
|
| Fig. 3 Co-silencing NaPDR1-like and NaPDR1 lead to plants highly susceptible to A. alternata. a) Mean (±SE) NaPDR1 transcripts were measured by real-time PCR in six replicates of 0 leaves of EV and VIGS PDR1 and 1-like plants at 1 dpi and 3 dpi. b) Mean (±SE) NaPDR1-like transcripts were measured by Real-time PCR in six replicates of 0 leaves of EV and VIGS PDR1 and 1-like plants at 1 dpi and 3 dpi. c) Photographs were taken of two 0 leaves of EV plants and two 0 leaves of VIGS PDR1 and 1-like plants at 6 dpi. White arrows indicate lesions. d) Mean (±SE) diameter of necrotic lesions of fifteen replicates of 0 leaves of EV, VIGS PDR1 and 1-like plants at 6 dpi. Asterisks indicate the level of significant differences between EV and VIGS PDR1 and 1-like plants (Student's t-test: *, P < 0.05; **, P < 0.01; ***, P < 0.005). |
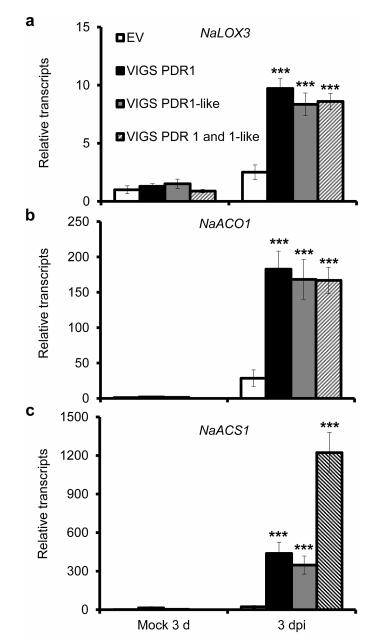
We have previously shown that both JA and ethylene signaling pathways are activated in N. attenuata plants after A. alternata inoculation. Furthermore, JA-deficient and ethylene-insensitive plants develop larger lesions than WT plants after inoculation with A. alternata (Sun et al., 2014b, 2017; Li and Wu, 2016). These findings indicate that JA and ethylene signaling pathways play important roles in host defense response. To investigate whether the high susceptibility of NaPDR1 and NaPDR1-like co-silenced plants to A. alternata was due to inhibition of the JA and ethylene signaling pathways, we examined the transcriptional levels of three JA and ethylene biosynthetic genes (NaLOX3, NaACS1, and NaACO1) (Halitschke and Baldwin, 2003; Sun et al., 2017) at 3 dpi, when PDRs were highly expressed. As expected, all three genes were up-regulated in WT plants at 3 dpi (Fig. 4). However, their transcripts were elicited to a higher level in VIGS PDR1, VIGS PDR1-like, and VIGS PDR1 and 1-like plants (Fig. 4). For NaACS1, even higher levels were elicited in leaves of VIGS PDR1 and 1-like plants (Fig. 4). These results suggest that the JA and ethylene signaling pathways were activated at higher levels in PDR-silenced plants.
|
| Fig. 4 Elicitation of NaLOX3, NaACS1, NaACO1 by A. alternata in PDR-silenced plants. Mean (±SE) NaLOX3 (a) NaACO1 (b) and NaACS1 (c) transcripts were measured by real-time PCR in five replicates of 0 leaves at 3 dpi. Asterisks indicate the level of significant difference between EV and VIGS plants at 3 dpi (Student's t-test: ***, P < 0.005). |
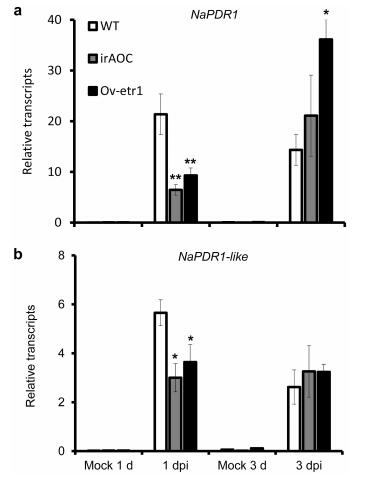
Neither NaPDR1 nor NaPDR1-like was significantly induced by exogenous methyl jasmonate (MeJA) (Fig. 5). Both genes were strongly elicited after plants were treated with ethephon, a synthetic compound which decomposes into ethylene upon metabolism by plants (Fig. 5). These results indicate that ethylene signaling might play a role in the regulation of NaPDR1 and NaPDR1-like transcripts. We thus tested whether A. alternata-elicited transcripts of both genes decreased in plants in which allene oxide cyclase was silenced (JA-deficient irAOC plants), and plants that over-expressed an Arabidopsis ethylene receptor mutant etr1-1 gene (Ov-etr1 plants are insensitive to exogenous ethylene) (Sun et al., 2014b, 2017; Kallenbach et al., 2012; von Dahl et al., 2007). As expected, both NaPDR1 and NaPDR1-like transcript levels decreased significantly in irAOC, irACO and Ov-etr1 plants at 1 dpi but not at 3 dpi (Fig. 6), indicating that both JA and ethylene signaling are only partially involved in the elicitation of NaPDR1 and NaPDR1-like transcripts by A. alternata.

|
| Fig. 5 Elicitation of NaPDR1-like and NaPDR1 transcripts by methyl jasmonate and ethephon. Mean (±SE) NaPDR1 (a) and NaPDR1-like (b) transcripts were measured by real-time PCR in five replicates of 0 leaves treated with water (as control), MeJA and Ethephon at 1, 3, 6, and 12 h. The different letters indicate the level of significant difference (Tukey's HSD test, P < 0.05). |
|
| Fig. 6 Reduction of A. alternata-induced NaPDR1-like and NaPDR1 transcripts at 1 dpi but not 3 dpi in plants impaired with JA or ethylene signaling pathway. Mean (±SE) NaPDR1 (a) and NaPDR1-like (b) transcripts were measured by real-time PCR in five replicates of 0 leaves treated with mock or A. alternata at 1 and 3 dpi. Asterisks indicate the level of significant difference between WT and transgenic plants (Student's t-test: *, P < 0.05; **, P < 0.01). |
An increasing number of reports have shown that PDRs are involved in plant resistance against pathogens (Bultreys et al., 2009; Stukkens et al., 2005; Stein et al., 2006; Seo et al., 2012; Shibata et al., 2016). Our results strongly support the idea that both NaPDR1 and NaPDR1-like play important roles in N. attenuata resistance to A. alternata. We found that NaPDR1 and NaPDR1-like were both strongly elicited by A. alternata inoculation. In addition, co-silencing NaPDR1 and NaPDR1-like increased the susceptibility of N. attenuata plants to A. alternata. However, silencing NaPDR1 and NaPDR1-like individually did not affect A. alternata-induced lesion size after inoculation, indicating that both genes function redundantly during plant resistance to the fungus.
The JA and ethylene pathways are two of the most important phytohormonal signaling pathways involved in plant resistance to necrotrophic pathogens (Glazebrook, 2005; Mengiste, 2012; Campos et al., 2014). Indeed, N. attenuata plants strongly impaired in JA/ethylene biosynthesis or perception have been shown to be highly susceptible to the necrotrophic fungal pathogen A. alternata (Sun et al., 2014b, 2017). In this study, fungal inoculation of NaPDR1 and NaPDR1-like co-silenced plants increased transcriptional levels of NaLOX3, NaACS1 and NaACO1, three key enzyme genes strongly responsive to exogenous JA or ethylene respectively. This indicates that both JA and ethylene signalling were strongly elicited in VIGS PDR1 and VIGS PDR1-like plants. Thus, the increased susceptibility of NaPDR1 and NaPDR1-like co-silenced plants to A. alternata was not because the JA or ethylene signaling pathways were blocked.
Many phytohormones and pathogens are involved in the regulation of PDR genes expression. This is the case for NtPDR5, which is induced by MeJA and P. nicotianae (Bienert et al., 2012). We found that NaPDR1 and NaPDR1-like transcripts were strongly induced after A. alternata inoculation. When plants were treated with ethephon, high levels of NaPDR1 and NaPDR1-like transcripts were elicited, whereas they were not significantly induced after MeJA treatments. These data suggest that the elicitation of NaPDR1 and NaPDR1-like transcripts by A. alternata may be regulated by the ethylene signaling pathway. The fact that the levels of A. alternata-elicited transcripts of NaPDR1 and NaPDR1-like were decreased in JA-deficient (irAOC) or ethylene-insensitive (Ov-etr1) plants at 1 dpi but not at 3 dpi indicates that the fungus-induced both PDR transcripts were only partially depended on the endogenous JA and ethylene signaling pathways.
It is not clear why NaPDR1 and NaPDR1-like co-silenced plants showed increased susceptibility to A. alternata. One possibility is that these plants may be unable to transport important 'chemical weapons' required for defense against the fungus, similar to how Nicotiana benthamiana plants inoculated with Phytophthora infestans are unable to transport capsidiol (Shibata et al., 2016). Alternatively, when inoculated with A. alternata plants co-silenced with NaPDR1 and NaPDR1-like may simply get more stressed because they are unable to transport important metabolites which are currently unknown, and those stresses lead to plants more vulnerable to A. alternata.
Taken together, our results indicate that both NaPDR1 and NaPDR1-like function redundantly, and play important roles in N. attenuata resistance to A. alternata. Both PDR transcripts were highly elicited after fungal inoculation, which appears to be partially dependent on JA and ethylene signaling pathways.
AcknowledgementsWe thank Biological Technology Open Platform of Kunming Institute of Botany (CAS) for greenhouse and instrument services. This project is supported by the NSFC (Grant No. 31670262), Key Project of Applied Basic Research Program of Yunnan (Grant No. 2014FA040), and 100-Oversea-Top-Talents Recruitment plan of Yunnan to Jinsong Wu, Project of Applied Basic Research Program of Yunnan (Grant No. 2017FB048) to Lan Ma, and founding (Grant No. 110201603008) to Dunhuang Fang.
Appendix A. Supplementary dataSupplementary data related to this article can be found at https://doi.org/10.1016/j.pld.2018.01.001.
Bienert M.D., Siegmund S.E., Drozak A., et al., 2012. A pleiotropic drug resistance transporter in Nicotiana tabacum is involved in defense against the herbivore Manduca sexta. Plant J, 72, 745-757.
DOI:10.1111/tpj.2012.72.issue-5 |
||
Bultreys A., Trombik T., Drozak A., et al., 2009. Nicotiana plumbaginifolia plants silenced for the ATP-binding cassette transporter gene NpPDR1 show increased susceptibility to a group of fungal and oomycete pathogens. Mol. Plant Pathol, 10, 651-663.
DOI:10.1111/mpp.2009.10.issue-5 |
||
Campos M.L., Kang J.H., Howe G.A., 2014. Jasmonate-triggered plant immunity. J. Chem. Ecol, 40, 657-675.
DOI:10.1007/s10886-014-0468-3 |
||
Glazebrook J., 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol, 43, 205-227.
DOI:10.1146/annurev.phyto.43.040204.135923 |
||
Halitschke R., Baldwin I.T., 2003. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J, 36, 794-807.
DOI:10.1046/j.1365-313X.2003.01921.x |
||
Kallenbach M., Bonaventure G., Gilardoni P.A., et al., 2012. Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proc. Natl. Acad. Sci. U. S. A, 109, E1548-E1557.
DOI:10.1073/pnas.1200363109 |
||
Krugel T., Lim M., Gase K., et al., 2002. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology, 12, 177-183.
DOI:10.1007/PL00012666 |
||
Lefevre F., Baijot A., Boutry M., 2015. Plant ABC transporters: time for biochemistry? Biochem. Soc. Trans, 43, 931-936.
|
||
Li J., Wu J., 2016. Scopolin, a glycoside form of the phytoalexin scopoletin, is likely involved in the resistance of Nicotiana attenuata against Alternaria alternata. J. Plant Pathol, 98, 641-644.
|
||
Mengiste T., 2012. Plant immunity to necrotrophs. Annu. Rev. Phytopathol, 50, 267-294.
DOI:10.1146/annurev-phyto-081211-172955 |
||
Nuruzzaman M., Zhang R., Cao H.Z., et al., 2014. Plant pleiotropic drug resistance transporters: transport mechanism, gene expression, and function. J. Integr.Plant Biol, 56, 729-740.
DOI:10.1111/jipb.v56.8 |
||
Saedler R., Baldwin I.T., 2004. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. J. Exp. Bot, 55, 151-157.
|
||
Sasse J., Schlegel M., Borghi L., et al., 2016. Petunia hybrida PDR2 is involved in herbivore defense by controlling steroidal contents in trichomes. Plant Cell Environ, 39, 2725-2739.
DOI:10.1111/pce.v39.12 |
||
Schuck S., Weinhold A., Luu V.T., et al., 2014. Isolating fungal pathogens from a dynamic disease outbreak in a native plant population to establish plantpathogen bioassays for the ecological model plant Nicotiana attenuata. PLoS One, 9, e102915.
DOI:10.1371/journal.pone.0102915 |
||
Seo S., Gomi K., Kaku H., et al., 2012. Identification of natural diterpenes that inhibit bacterial wilt disease in tobacco, tomato and Arabidopsis. Plant Cell Physiol, 53, 1432-1444.
DOI:10.1093/pcp/pcs085 |
||
Shibata Y., Ojika M., Sugiyama A., et al., 2016. The full-size ABCG transporters NbABCG1 and Nb-ABCG2 function in pre- and postinvasion defense against Phytophthora infestans in Nicotiana benthamiana. Plant Cell, 28, 1163-1181.
DOI:10.1105/tpc.15.00721 |
||
Stein M., Dittgen J., Sanchez-Rodriguez C., et al., 2006. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell, 18, 731-746.
DOI:10.1105/tpc.105.038372 |
||
Stukkens Y., Bultreys A., Grec S., et al., 2005. NpPDR1, a pleiotropic drug resistance-type ATP-binding cassette transporter from Nicotiana plumbaginifolia, plays a major role in plant pathogen defense. Plant Physiol, 139, 341-352.
DOI:10.1104/pp.105.062372 |
||
Sun H., Hu X., Ma J., et al., 2014a. Requirement of ABA signalling-mediated stomatal closure for resistance of wild tobacco to Alternaria alternata. Plant Pathol, 63, 1070-1077.
DOI:10.1111/ppa.2014.63.issue-5 |
||
Sun H., Song N., Ma L., et al., 2017. Ethylene signalling is essential for the resistance of Nicotiana attenuata against Alternaria alternata and phytoalexin scopoletin biosynthesis. Plant Pathol, 66, 277-284.
DOI:10.1111/ppa.2017.66.issue-2 |
||
Sun H., Wang L., Zhang B., et al., 2014b. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp.Bot, 65, 4305-4315.
DOI:10.1093/jxb/eru203 |
||
van den Brule S., Muller A., Fleming A.J., et al., 2002. The ABC transporter SpTUR2 confers resistance to the antifungal diterpene sclareol. Plant J, 30, 649-662.
DOI:10.1046/j.1365-313X.2002.01321.x |
||
von Dahl C.C., Winz R.A., Halitschke R., et al., 2007. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J, 51, 293-307.
DOI:10.1111/j.1365-313X.2007.03142.x |
||
Wu J., Baldwin I.T., 2010. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet, 44, 1-24.
DOI:10.1146/annurev-genet-102209-163500 |
||
Wu J., Wang L., Baldwin I.T., 2008. Methyl jasmonate-elicited herbivore resistance:does MeJA function as a signal without being hydrolyzed to JA?. Planta, 227, 1161-1168.
DOI:10.1007/s00425-008-0690-8 |
||
Wu J., Wang L., Wunsche H., et al., 2013. Narboh D, a respiratory burst oxidase homolog in Nicotiana attenuata, is required for late defense responses after herbivore attack. J. Integr. Plant Biol, 55, 187-198.
DOI:10.1111/jipb.2013.55.issue-2 |



