b. FASMAC Co., Ltd., 5-1-3 Midorigaoka, Atsugi, Kanagawa 243-0041, Japan
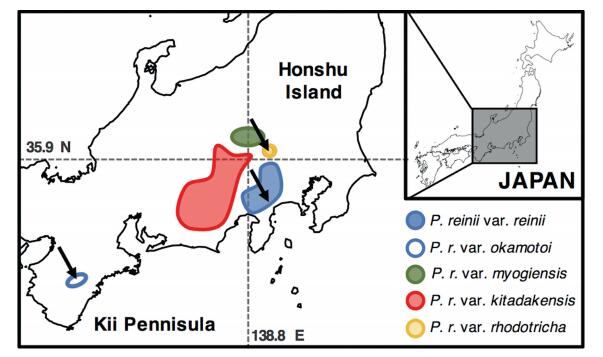
Primula reinii Franch. et Sav., a perennial herb belonging to the Primula section Reinii, occurs on wet shaded rocky cliffs in the mountains of Japan (Richards, 2003). The species comprises four narrow endemic varieties (Fig. 1, Yamazaki, 1993): P. reinii var. reinii, P. reinii var. myogiensis Hara, P. reinii var. kitadakensis (Hara) Ohwi, and P. reinii var. rhodotricha (Nakai et Maek.) Yamaz. In addition, P. reinii var. okamotoi (Koidz.) Murata., which is found on the Kii Peninsula, is a synonym of var. reinii (Fig. 1). However, molecular phylogenic analyses using both chloroplast and nuclear DNA have shown distinct sequence divergence between vars. reinii and okamotoi (Yamamoto et al., 2017b).
|
| Fig. 1 Presumed range of Primula sect. Reinii species. Black arrows indicate the populations sampled. |
P. reinii is the most attractive representative in sect. Reinii because these primrose plants have a small number of relatively large flowers just above their very dwarf emerging foliage (Richards, 2003). Furthermore, these plants, which are threatened species, are very localized and rare in the wild. Based on their rarity, and reductions in the numbers of individuals and populations, due to anthropogenic activities, all four varieties of P. reinii are listed on the latest Japanese Red List (Ministry of the Environment, 2017), and are assigned to the 'Critically Endangered' (vars. rhodotricha and myogiensis) or 'Vulnerable' (vars. reinii and kitadakensis) categories. Despite the need for conservation, little is known of the life history, reproductive system, or vegetative characteristics of these plants.
Recent ecological and genetic studies have examined P. reinii var. rhodotricha, a typical species in sect. Reinii that faces a risk of extinction (Yamamoto et al., 2013, 2017a). Yamamoto et al. (2017a) reported molecular evidence of population depletion of the critically endangered primrose using 11 microsatellite markers that were originally developed for Primula sieboldii E. Morren. Furthermore, they also revealed a relationship between genetic diversity and the population sizes of Reinii species, and suggested that a purge of recessive detrimental genes to increase homozygosity could prevent additional genetic degradation in their wild habitat (Yamamoto et al., 2017a). However, only six microsatellite loci were used in that study to assess the genetic diversity of these species. Therefore, additional highly polymorphic molecular markers are required to investigate genetic status more reliably and to conduct effective conservation activities for P. reinii. Even in var. rhodotricha, additional microsatellite markers are needed to measure the degree of inbreeding and inbreeding depression (e.g., pedigree analysis) to improve their low fertility (approximately 5% in fruition, Yamamoto et al., 2017a). In this study, we isolated and characterized 43 genomic microsatellite markers for P. reinii, which will be powerful tools aiding assessment of their genetic diversity.
2. Materials and methodsTo develop useful microsatellite markers for P. reinii, which comprises several narrow endemic taxa, genomic DNA from three varieties (vars. reinii, okamotoi, and rhodotricha) was extracted from leaf tissues collected from each population (Fig. 1) using a modified CTAB protocol (Doyle, 1990). Each genomic DNA sample was used for library preparation with the KAPA HyperPlus Kit (Kapa Biosystems, Wilmington, MA, USA). Sequencing analyses was performed on the MiSeq Benchtop Sequencer (Illumina, San Diego, CA, USA) using a 2 × 250-bp read length for each DNA sample. Raw reads of each sample were quality trimmed (Q > 20) using Sickle (https://github.com/najoshi/sickle). High-quality reads from the three samples, vars. reinii, okamotoi, and rhodotricha, were assembled, using Velvet (Zerbino and Birney, 2008), into 246, 887, 313, 719, and 285, 839 contigs, respectively. Potential microsatellite regions with at least five repeats were detected in each assembled draft genome sequence using QDD ver. 2.1 (Meglécz et al., 2010). QDD was the most versatile software for estimating microsatellites based on next generation sequencing datasets in our pipeline. In total, 505, 732, and 562 microsatellite markers were predicted for each taxon, of which 73, 70, and 65 markers were selected as candidate microsatellite markers for vars. reinii, okamotoi, and rhodotricha, respectively. Primers were designed automatically using the Primer3 algorithm (Rozen and Skaletsky, 2000) implemented in QDD. Due possibly to low coverage (attributed to the large genome size of these plants), as well as lineage divergence among taxa (Yamamoto et al., 2017b), common microsatellite regions were not found in this study.
To assess amplification and polymorphism at all 208 candidate microsatellite loci, additional leaf tissues from 32 individuals were collected from a natural population for each taxon. PCR amplifications were conducted in 10-μL reaction mixtures containing 1.0 μL of DNA solution (0.1 ng/μL), 0.20 μL of each primer (10 μmol/L), 2.0 μL of 5 × PCR buffer, 0.80 μL of dNTP mixture (each 2.5 mM), 0.20 μL of PrimeSTAR GXL polymerase (0.25 U; Takara Bio, Kusatsu, Shiga, Japan), and 5.6 μL distilled water. Each forward primer was labeled using FAM, VIC, NED, and/or PET. Amplifications consisted of an initial denaturation at 98 ℃ for 5 min, 39 amplification cycles using a touchdown protocol of 98 ℃ for 30 s, annealing for 30 s, 68 ℃ for 40 s, and a final extension at 68 ℃ for 2 min. The annealing temperatures were 63, 62, and 61 ℃ for 9 cycles and 59, 58, and 53 ℃ for 30 cycles. Fragment analysis was performed using the 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA). For each locus, the number of alleles (NA), expected heterozygosity (HE), inbreeding coefficient (FIS), and deviations from Hardy–Weinberg equilibrium were calculated using Arlequin 3.5 (Excoffier and Lischer, 2010).
3. Results and discussionOf 208 candidate microsatellite markers, 98 (47%), 71 (34%), and 39 (19%) were di-, tri-, and tetranucleotides, respectively. The most common di-and trinucleotide repeats were (AG)n(25%) and (TTA)n (13%), respectively. No common motif was found among the tetranucleotide repeats. The motifs (AG)n, (CT)n, (TC)n, and (AT)n accounted for 35% of the 208 candidate microsatellite markers.
Of the 208 candidate primer pairs tested, a total of 43 loci were amplified, displayed a clear polymorphism, and were in Hardy–Weinberg equilibrium (p > 0.05). All sequences were deposited in GenBank/DDBJ/EMBL (Table 1). The 19 loci developed for P. reinii var. reiniidisplayed relatively high polymorphism; the average values for NA, HE, and FIS were 4.16, 0.56, and 0.05, respectively. Meanwhile, the 10 loci for var. rhodotricha showed relatively low polymorphism, with values of 2.60, 0.39, and 0.08 for NA, HE, and FIS, respectively. Similarly, the 14 loci for var. okamotoi showed low polymorphism, with average values for NA, HE, and FIS of 2.14, 0.35, and 0.03, respectively.
| Locus | Primer sequence (5′→3′) | Repeat motif | Size range | NA | HE | FIS | Accession no. |
| For Primula reinii var. reinii | |||||||
| Pre_2 | F: TGGCAAATGGGAGCTTAGCA | (TA)9 | 228–236 | 5 | 0.756 | 0.198 | LC217340 |
| R: GAGGTTGTTTACGTGCCGTG | |||||||
| Pre_5 | F: ACACTGCTTTCTGCTAGCTCT | (CT)12 | 146–158 | 4 | 0.604 | 0.068 | LC217341 |
| R: CAGACAAATTATAATCAGCTCACCG | |||||||
| Pre_7 | F: TGACATTTGCATAATTGTTAATTTGGA | (TC)11 | 144–160 | 5 | 0.699 | 0.240 | LC217342 |
| R: TGTGGGTATGTAGTCCTGCA | |||||||
| Pre_9 | F: GGCAACCAAACAAACTCCTATAGT | (GA)11 | 202–212 | 3 | 0.693 | −0.173 | LC217343 |
| R: TCCTGAGCGTTTACCAAACTCA | |||||||
| Pre_10 | F: CAGTTGAGAAGATCGATCAGACT | (AG)11 | 149–165 | 5 | 0.696 | −0.033 | LC217344 |
| R: ATCATTTGGCTTTCTACAGCTTT | |||||||
| Pre_18 | F: TTGGACTTTGCGCTCATAAGC | (TC)10 | 200–210 | 6 | 0.825 | 0.129 | LC217345 |
| R: CTTGTTCTCTTCAACCCTTTGCT | |||||||
| Pre_28 | F: AGCCTTGCAGGAAGATCAAGAA | (AG)9 | 253–263 | 4 | 0.398 | −0.020 | LC217346 |
| R: ACTCTAGCACACGTAGAGCA | |||||||
| Pre_31 | F: ACGGCATGAATTTGAAGAATTGGA | (GA)9 | 277–290 | 3 | 0.305 | 0.179 | LC217347 |
| R: CGGCGGATATTCAATAGGAGCT | |||||||
| Pre_33 | F: TGAGGGACGCCATTGCTTAT | (AG)9 | 170–188 | 4 | 0.756 | −0.033 | LC217348 |
| R: CCATTACTTGCTCTGTTCGCT | |||||||
| Pre_36 | F: CTAGCGAGCGAACACAATGC | (CA)9 | 142–152 | 5 | 0.815 | −0.008 | LC217349 |
| R: ATTGAGACTGATGGCGGGAC | |||||||
| Pre_38 | F: AGGCTTTCAACTGAACATAACGG | (AG)9 | 218–226 | 6 | 0.540 | −0.041 | LC217350 |
| R: ATGGTGTTCGTCAGTCCCAC | |||||||
| Pre_40 | F: AGTACCTGTAGTGGAGAGGG | (AG)9 | 202–212 | 5 | 0.687 | −0.047 | LC217351 |
| R: CCCATTAGGTTAACATTCACGGT | |||||||
| Pre_43 | F: GGCATTACCTTAAAGTAAGAGGGT | (GA)9 | 304–308 | 3 | 0.392 | 0.433 | LC217352 |
| R: GGCATTACCTTAAAGTAAGAGGGT | |||||||
| Pre_47 | F: AAGCCATCGATGAAGCTGCT | (ATT)8 | 284–290 | 3 | 0.371 | −0.262 | LC217353 |
| R: CACTCTGCTGTGAGACTCTGA | |||||||
| Pre_51 | F: CCCTGTAATCTACCTCCACGG | (TAT)7 | 152–158 | 3 | 0.595 | −0.154 | LC217354 |
| R: ATCATATCGCATTCCTAAGCATCA | |||||||
| Pre_52 | F: TTGCAGGGCAAGTGAACTCA | (CAG)7 | 242–272 | 5 | 0.567 | −0.047 | LC217355 |
| R: GGCTGAGAAGGAGCAGTTGA | |||||||
| Pre_57 | F: TGGCTGTTTGTGGAATTAGCT | (TCT)7 | 143–152 | 4 | 0.409 | 0.211 | LC217356 |
| R: GTTTGAGTGAGAGGTGGCTCT | |||||||
| Pre_61 | F: TGACTGGATGGGAGGACGAT | (TTC)6 | 266–278 | 4 | 0.489 | 0.148 | LC217357 |
| R: TTGTGTACCGTACCGCAGAG | |||||||
| Pre_73 | F: ACGGATCTTTGTGAGGAAGGAG | (TATG)5 | 140–148 | 2 | 0.125 | −0.034 | LC217358 |
| R: TCCGTCTGTCTGAATTTAGGGT | |||||||
| For P. reinii var. okamotoi | |||||||
| Pok_6 | F: TGGTTCACAATTCACAACCCA | (AG)11 | 160–162 | 2 | 0.474 | 0.116 | LC217359 |
| R: CTGGAGCTGGGCATCTCATC | |||||||
| Pok_8 | F: AAGGCAGTTGAGTCCCTTTCT | (CT)11 | 308–312 | 2 | 0.495 | 0.088 | LC217360 |
| R: TGCGAACAAGATCTAAGGATGT | |||||||
| Pok_11 | F: TGAAGGATAAGTTAGTAATTTGTGCCA | (CT)11 | 192–210 | 2 | 0.354 | 0.123 | LC217361 |
| R: ACTCCGTATTTATTACCTGAACAAGT | |||||||
| Pok_15 | F: ATTTCTATGTTCTTATGCATGAACTCT | (TA)10 | 206–212 | 2 | 0.497 | −0.368 | LC217362 |
| R: TGTTTGGGCCAATTGTACTACG | |||||||
| Pok_24 | F: ACACCATCATTCGGTTTAGTACCT | (GA)10 | 151–157 | 2 | 0.382 | −0.122 | LC217363 |
| R: AACGGAGAGGCGAATAATACG | |||||||
| Pok_25 | F: GGTGTTCCATGAGACCAGAACA | (GA)10 | 155–161 | 3 | 0.325 | 0.181 | LC217364 |
| R: TGGTCTCTGGTTGCTAAGGC | |||||||
| Pok_27 | F: ATCCGTCTCGCATCGTCTTC | (CT)10 | 156–160 | 2 | 0.542 | 0.135 | LC217365 |
| R: TAGAGGCGCCATTGAAGGTC | |||||||
| Pok_31 | F: ACCAATTGCAGCCCAATCAAC | (AGT)9 | 164–176 | 2 | 0.155 | −0.073 | LC217366 |
| R: CCACTAGCTCTGCAGTTCTGA | |||||||
| Pok_32 | F: CGAAACAATATTACCCGACCGG | (CCA)8 | 159–162 | 2 | 0.222 | −0.125 | LC217367 |
| R: CGCTCTTCTGCCTACTCAACA | |||||||
| Pok_39 | F: CATCAAGATGCCACCAAGGG | (GAA)7 | 283–286 | 2 | 0.235 | 0.432 | LC217368 |
| R: CCTTTCCCTAGTTCTGGCCC | |||||||
| Pok_40 | F: GCAAGCAATGAGACGAGTAACT | (TCA)7 | 277–286 | 3 | 0.194 | 0.288 | LC217369 |
| R: TACGTGAGGCGCTTTGTGAA | |||||||
| Pok_45 | F: GGAAACACCCAAGGCTTTCA | (TTA)6 | 167–171 | 2 | 0.487 | 0.079 | LC217370 |
| R: GCTGACAAGGCTCAACTGGA | |||||||
| Pok_58 | F: GCCTTGTGAAACGCCGTTAA | (ATAC)5 | 162–170 | 2 | 0.131 | −0.056 | LC217371 |
| R: ACAATCCCAGCTGAAAGATCCT | |||||||
| Pok_60 | F: TGCCAGGTGTATTATCCGACG | (TTTA)5 | 189–201 | 2 | 0.354 | −0.267 | LC217372 |
| R: ACCAGACTAACACAAACCGGA | |||||||
| For P. reinii var. rhodotricha | |||||||
| Prh_1 | F: AAACGTAGGCAGGAGCAACA | (AT)12 | 254–256 | 4 | 0.514 | 0.089 | LC217373 |
| R: TATGAGCGGTGGACTTAGGGT | |||||||
| Prh_5 | F: GCCGAAAGTGACAAATGAAAGC | (AG)11 | 137–163 | 3 | 0.575 | 0.076 | LC217374 |
| R: TCATGGCCAGATTCTTGTTGC | |||||||
| Prh_6 | F: ACGCAACGGCAAACTTCTTT | (CT)11 | 165–167 | 2 | 0.146 | −0.068 | LC217375 |
| R: ACAGGGACCAAATTGAAACTATTG | |||||||
| Prh_17 | F: GAGGGTGTATCTGAAGATTACTCT | (CT)10 | 212–218 | 2 | 0.418 | −0.078 | LC217376 |
| R: TCGGATTGGGTTAAATTCTGGGT | |||||||
| Prh_22 | F: AGGCGGGTGTGATAAACCG | (AG)10 | 254–256 | 2 | 0.253 | −0.151 | LC217377 |
| R: GGGACCTGTTTGAGTAGAGGC | |||||||
| Prh_30 | F: GAGCCAGGTCATCAACACCC | (CCA)7 | 220–229 | 2 | 0.275 | 0.296 | LC217378 |
| R: TGGATTATTCACGCTGTGAGTGA | |||||||
| Prh_35 | F: TGGTCTGAGGATCAACTGCG | (GAT)6 | 158–167 | 3 | 0.468 | −0.002 | LC217379 |
| R: CACGAATTCCCAGAGGCGAA | |||||||
| Prh_46 | F: GGCCGATCCACATATTCATCA | (GAA)6 | 253–259 | 2 | 0.490 | 0.107 | LC217380 |
| R: CCAACTCGGTTTGATCCAGT | |||||||
| Prh_60 | F: CGTTGATCTACTGTTTCGGCAG | (TGTA)5 | 130–154 | 3 | 0.352 | 0.337 | LC217381 |
| R: TCGATTGGCACACGTATGGA | |||||||
| Prh_64 | F: TGGGTGAAGAATTGGAGAAACT | (TTTG)5 | 261–270 | 3 | 0.454 | 0.243 | LC217382 |
| R: CCCTCGGTCCAGCTTAAAGC | |||||||
| NA, number of alleles; HE, expected heterozygosity; FIS, inbreeding coefficient. | |||||||
The genetic status of var. rhodotricha determined using our newly developed microsatellite markers was nearly identical to that determined using previously established markers (Yamamoto et al., 2017a), whereas our results for var. reinii and okamotoi indicated a relatively lower genetic diversity than that in a previous study using only six loci (estimated HE of 0.620 and 0.412 for vars. reinii and okamotoi, respectively) (Yamamoto et al., 2017a). Therefore, our results imply that the genetic diversities of vars. reinii and okamotoi were overestimated in the previous study, possibly due to an insufficient number of loci.
In this study, we isolated 1799 microsatellite loci from P. reinii and its relatives. A total of 208 primer pairs were used for wild populations of these critically endangered plants, and 43 microsatellite markers were used to assess the genetic diversity of critically endangered primroses and develop effective conservation and management strategies.
Acknowledgments:This research was financially and technically supported by FASMAC Co., Ltd. (Kanagawa, Japan) and The Environment Research and Technology Development Fund (#4-1403).
We are grateful to Chichibu Taiheiyo Cement Co. and Ryoko Lime Industry Co., Ltd. for their help with sample collection.
Doyle J.J., 1990. Isolation of plant DNA from fresh tissue. Focus, 12, 13-15.
|
||
Excoffier L., Lischer H.E., 2010. Arlequin suite ver 3. 5:a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour, 10, 564-567.
|
||
Meglecz E., Costedoat C., Dubut V., Gilles A., Malausa T., Pech N., Martin J.F., 2010. QDD:a user-friendly program to select microsatellite markers and design primers from large sequencing projects. Bioinformatics, 26, 403-404.
DOI:10.1093/bioinformatics/btp670 |
||
Ministry of the Environment. 2017. The Japanese red lists 2017. Available from: URL: http://www.env.go.jp/press/files/jp/105449.pdf.
|
||
Richards, A. J., 2003. Primula. Batsford, London.
|
||
Rozen S., Skaletsky H., 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol, 132, 365-386.
|
||
Yamamoto M., Yasui M., Setoguchi H., Kurata K., 2013. Conservation of an endangered perennial herb, Primula reinii var. rhodotricha. rhodotricha. J. Nat. Restor. Conserv, 6, 23-29.
|
||
Yamamoto M., Kurata K., Setoguchi H., 2017a. Conservation genetics of an ex situ population of Primula reinii var. rhodotricha, an endangered primrose endemic to Japan on a limestone mountain. Conserv. Genet..
DOI:10.1007/s10592-017-0966-2(inpress) |
||
Yamamoto M., Ohtani M., Kurata K., Setoguchi H., 2017b. Contrasting evolutionary processes during Quaternary climatic changes and historical orogenies:a case study of the Japanese endemic primroses Primula sect. Reinii. Ann. Bot..
DOI:10.1093/aob/mcx108(inpress) |
||
Yamazaki, T., 1993. Primulaceae. In: Iwatsuki, K., Yamazaki, T., Bufford, D. E., Ohba, H. (Eds. ), Flora of Japan, vol. 3a. Kodansya, Tokyo, pp. 87-95.
|
||
Zerbino D.R., Birney E., 2008. Velvet:algorithms for de novo short read assembly using de Bruijn graphs. Genome Resour, 18, 821-829.
DOI:10.1101/gr.074492.107 |



