b. University of Chinese Academy of Sciences, Beijing, 100049, China
Nectaries are specialized structures that produce and secrete nectar (Fahn, 1979; Nepi et al., 1996; Nicolson et al., 2007). Generally, the nectary is composed of three tissue types: nectary epidermis, nectary parenchyma and subnectary parenchyma (Nicolson et al., 2007). Nectary epidermis may have trichomes or modified stomata as secretory structures. Extrafloral nectaries may provide rewards for insects to defend fruit and seeds from predators; floral nectaries may also attract agents of pollination (Pacini et al., 2003; Thornburg et al., 2003). In response to these interactions, animals and flowers have co-evolved, leading to a diversity of floral nectary types which may play a crucial role in driving floral evolution and diversity in flowering plants (Thornburg et al., 2003). This variation in floral nectaries (e.g. shape, structure, position) has frequently been used to classify various plant genera in taxonomic and phylogenetic studies (Solereder et al., 1908; Fahn, 1953; Ancibor, 1969; Smets, 1986; Galetto, 1997; Galetto and Bernardello, 2004; Bernardello, 2007).
Euonymus L. (Celastraceae) consists of approximately 129 species which are mainly distributed in East Asia to the Himalayan Region as well as South Asia and Southeast Asia (Ma, 2001). The habitats of Euonymus species are highly diverse; flowers and fruits of this genus are inconspicuous and caducous. Intensive taxonomic studies of most species are limited by the number of specimens available after species descriptions. Infrageneric classification of Euonymus is mainly based on whether the capsules are angular, smooth, echinate or dehiscent (Blume, 1825; Maximowicz, 1881; Sprague, 1908; Wang, 1939; Nakai, 1941; Loesener, 1942; Blakelock, 1951; Ma, 2001; Ma and Funston, 2008). Fruit characters were first used in grouping of Euonymus in 1825 (Blume, 1825). Then, species with echinate capsules were included in Euonymus for the first time in 1908 (Sprague, 1908). The comprehensive revision of Celastraceae in 1942 recorded more than 100 Euonymus species in the world, which established the foundation for further studies on Euonymus (Loesener, 1942). Blakelock's monograph which comprised 176 species was the most important work for the taxonomy of Euonymus (Blakelock, 1951). This genus is divided into five sections in the most recent classification: E. sect. Uniloculares, E. sect. Echinococcus, E. sect. Ilicifolia, E. sect. Melanocarya and E. sect. Euonymus (Ma, 2001; Ma and Funston, 2008). GlyptopetalumThw. (Celastraceae) is a small genus containing about 20 species in tropical and subtropical Asia (Liu and Funston, 2008). There are many significant similarities between Glyptopetalumand Euonymus. Glyptopetalum is treated as a section of Euonymus in some studies, but others believe that it is independent though closely related with Euonymus (Thwaites, 1856; Baillon, 1880; Hou, 1963). The molecular phylogenetic relationships of Euonymus are controversial. It has therefore been suggested that Euonymus be considered paraphyletic and the infrageneric classification unnatural, whereas Euonymus and Glyptopetalum form a monophyletic group together (Meng et al., 2011; Simmons et al., 2012; Simmons and Kappa, 2013; Li et al., 2014).
Euonymus L. (Celastraceae) consists of approximately 129 species which are mainly distributed in East Asia to the Himalayan Region as well as South Asia and Southeast Asia (Ma, 2001). The habitats of Euonymus species are highly diverse; flowers and fruits of this genus are inconspicuous and caducous. Intensive taxonomic studies of most species are limited by the number of specimens available after species descriptions. Infrageneric classification of Euonymus is mainly based on whether the capsules are angular, smooth, echinate or dehiscent (Blume, 1825; Maximowicz, 1881; Sprague, 1908; Wang, 1939; Nakai, 1941; Loesener, 1942; Blakelock, 1951; Ma, 2001; Ma and Funston, 2008). Fruit characters were first used in grouping of Euonymus in 1825 (Blume, 1825). Then, species with echinate capsules were included in Euonymus for the first time in 1908 (Sprague, 1908). The comprehensive revision of Celastraceae in 1942 recorded more than 100 Euonymus species in the world, which established the foundation for further studies on Euonymus (Loesener, 1942). Blakelock's monograph which comprised 176 species was the most important work for the taxonomy of Euonymus (Blakelock, 1951). This genus is divided into five sections in the most recent classification: E. sect. Uniloculares, E. sect. Echinococcus, E. sect. Ilicifolia, E. sect. Melanocarya and E. sect. Euonymus (Ma, 2001; Ma and Funston, 2008). GlyptopetalumThw. (Celastraceae) is a small genus containing about 20 species in tropical and subtropical Asia (Liu and Funston, 2008). There are many significant similarities between Glyptopetalumand Euonymus. Glyptopetalum is treated as a section of Euonymus in some studies, but others believe that it is independent though closely related with Euonymus (Thwaites, 1856; Baillon, 1880; Hou, 1963). The molecular phylogenetic relationships of Euonymus are controversial. It has therefore been suggested that Euonymus be considered paraphyletic and the infrageneric classification unnatural, whereas Euonymus and Glyptopetalum form a monophyletic group together (Meng et al., 2011; Simmons et al., 2012; Simmons and Kappa, 2013; Li et al., 2014).
Flowers of Euonymus have a broad, flat and well-exposed disk surrounding the ovary. The blooming stage of the flowers is from March to August, and the lifespan of a single flower is approximately three days (Ma, 2001). When the flower is fully open, certain areas of the disk are sharply outlined by a great abundance of nectar, which attracts a variety of insects, including bees, ants, beetles, and flies (Berkeley, 1953; Konarska, 2015). In spite of the availability of comprehensive publications dealing with anatomical and morphological aspects of floral nectaries (Pacini et al., 2003), studies focused on floral nectaries in Euonymus are rare. To date, the nectary structure and nectar secretions have only been studied in five species from this genus (Berkeley, 1953; Matthews and Endress, 2005; Konarska, 2015). The nectaries of E. americanus L. and E. japonicus Thunb. are the first to be described: the nectariferous area is usually rose colored and is thus set off from the ovary and the regions around the base of each filament (Berkeley, 1953). Nectaries of E. latifolius (L.) Mill appear between the corolla and the stigma, with stomata sunken in pits (Matthews and Endress, 2005). According to Konarska (2015), the nectaries of Euonymus fortunei (Turcz.) Hand.-Mazz. and E. europaeus L. differ in size, location, abundance of stomata, and nectar content. The nectary disk is typical of Celastraceae, and it is one type of receptacular nectary (Fahn, 1979). Observations in other genera in this family show that floral nectaries are diverse in morphology, anatomy and histology (Berkeley, 1953; Matthews and Endress, 2005; Tan et al., 2007; Gomes and Lombardi, 2013). Clearly, nectary types have evolved in Celastraceae and display an array of adaptations.
Although floral nectaries have aroused interest in Euonymus, there is a paucity of data with regard to nectary morphology. Accordingly, in the present study, we investigate whether nectary features, such as form type and nectarostomata location, are correlated with the most recent classification of Euonymus. For this purpose, we characterized the diversity and development of nectaries in this genus. We also provide a detailed analysis of the origin of the echinate fruit surface.
2. Materials and methodsFloral nectaries were surveyed from flowers field-collected in China. In order to reduce sampling error, 10 flowers from each species were sampled at different developmental stages (before, during, and after anthesis). Twenty-one species of flowers from Euonymus (19 species) and Glyptopetalum (2 species), were randomly selected and fixed in FAA (formalin: acetic acid: 50% ethanol = 5: 5: 90). The source of the species studied for each analysis is given in Table 1. All Euonymus voucher specimens have been stored at the Shanghai Chenshan Herbarium (CSH), and the two Glyptopetalum voucher specimens have been deposited at the Beijing Normal University Herbarium (BNU). The scientific names of species in this study generally follow the recent taxonomic revisions (Ma, 2001; Ma and Funston, 2008).
| No. | Taxa | Section | Voucher specimens | Source | Form of nectary | Nectarostomata location |
| 1 | Euonymus sanguineus Loes. ex Diels | E. sect. Uniloculares | E0208 | Mount Emei, Sichuan | Mix of inter-and extrastaminal | Sunken |
| 2 | Euonymus schensianus Maxim. | E. sect. Uniloculares | 20160313 (Ma J.S.) | Mount Taibai, Shanxi | Mix of inter-and extrastaminal | Level with the epidermis |
| 3 | Euonymus verrucocarpus C. Y. Cheng ex J. S. Ma | E. sect. Echinococcus | DC147 (Du C.) | Tengchong, Yunnan | Mix of inter-and extrastaminal | Raised |
| 4 | Euonymus wilsonii Sprague | E. sect. Echinococcus | DC057 (Du C.) DC293 (Du C.) |
Malipo, Yunnan Malipo, Yunnan |
Mix of inter-and extrastaminal Mix of inter-and extrastaminal |
Raised Raised |
| 5 | Euonymus actinocarpus Franch. | E. sect. Echinococcus | DC075 (Du C.) |
Pingbian, Yunnan | Mix of inter-and extrastaminal | Raised |
| 6 | Euonymus balansae Sprague | E. sect. Echinococcus | DC083 (Du C.) | Xichou, Yunnan | Mix of inter-and extrastaminal | Raised |
| 7 | Euonymus acanthoxanthus Pit. | E. sect. Echinococcus | DC305 (Du C.) | Baise, Guangxi | Intrastaminal | Raised |
| 8 | Euonymus pseudovagans Pit. | E. sect. Ilicifolia | DC093 (Du C.) | Malipo, Yunnan | Intrastaminal | Raised |
| 9 | Euonymus theifolius Wall. ex M. A. Lawson | E. sect. Ilicifolia | DC079 (Du C.) | Xichou, Yunnan | Intrastaminal | Raised |
| 10 | Euonymus fortunei (Turcz.) Hand.-Mazz. | E. sect. Ilicifolia | E0214 | Hengyang, Hunan | Intrastaminal | Raised |
| 11 | Euonymus centidens H. Lév. | E. sect. Melanocarya | E0209 | Mount Emei, Sichuan | Intrastaminal | Sunken |
| 12 | Euonymus alatus (Thunb.) Sieb. | E. sect. Melanocarya | YCY3 (Yao C.Y.) | Xuhui, Shanghai | Mix of inter-and extrastaminal | Sunken |
| 13 | Euonymus hukuangensis Cheng ex Ma | E. sect. Melanocarya | 20150602 (Ma J.S.) | Shaoguan, Guangdong | Mix of inter-and extrastaminal | Sunken |
| 14 | Euonymus yunnanensis Franch. | E. sect. Euonymus | DC282 (Du C.) | Binchuan, Yunnan | Mix of inter-and extrastaminal | Level with the epidermis |
| 15 | Euonymus tingens Wall. | E. sect. Euonymus | E0570 | Lijiang, Yunnan | Intrastaminal | Raised |
| 16 | Euonymus wui Ma | E. sect. Euonymus | DC005 (Du C.) | Wenshan, Yunnan | Mix of inter-and extrastaminal | Level with the epidermis |
| 17 | Euonymus nitidus Benth. | E. sect. Euonymus | DC302 (Du C.) | Anlong, Guizhou | Mix of inter-and extrastaminal | Sunken |
| 18 | Euonymus oblongifolius Loes. & Rehd. | E. sect. Euonymus | E0211 | Mount Emei, Sichuan | Mix of inter-and extrastaminal | Sunken |
| 19 | Euonymus microcarpus (Oliv.) Sprague | E. sect. Euonymus | 20130513 (Ma J.S.) | Mount Taibai, Shanxi | Intrastaminal | Level with the epidermis |
| 20 | Glyptopetalum longipedicellatum (Merr. et Chun) C. Y. Cheng | G. sect. Patelliformia | 09041601A09 (Meng S.Y.) | Yangshuo, Guangxi | Mix of inter-and extrastaminal | Level with the epidermis |
| 21 | Glyptopetalum longepedunculatum Tardieu | G. sect. Patelliformia | 09041201 (Meng S.Y.) | Mount Paotai, Guangxi | Mix of inter-and extrastaminal | Level with the epidermis |
| a All Euonymus voucher specimens have been stored in the Shanghai Chenshan Herbarium (CSH), and the two Glyptopetalum voucher specimens have been deposited in the Beijing Normal University Herbarium (BNU). | ||||||
The presence and position of nectaries on the flower disk were examined under a dissecting microscope (Leica S8APO) after the calyx and corolla were removed. The characteristics of the nectary gland were observed under scanning electron microscope and light microscope. Tissue specimens were first dehydrated in an ethanol series of 50, 60, 70, 80, 95, and 100% (two changes); subsequently, specimens were passed through a mixture of 100% ethanol and t-Butanol in following the proportions, ethanol: t-Butanol (3: 1, 2: 2, 1: 3 v/v) and 100% t-Butanol for 10 min intervals at each step; then the samples were dried in a t-Butanol Freeze Drying Device (VFD-21S). The dried materials were carefully mounted on aluminum stubs under a stereomicroscope (Olympus SZ61). After being coated with gold, the samples were viewed using a scanning electron microscope (FEI Quanta 250) at an accelerating voltage of 12.5 KV.
3. Results 3.1. Floral morphology and form of nectariesEuonymus flowers are tetramerous or pentamerous, actinomorphic, and 5–25 mm in diameter, with white green or purple petals and inconspicuous calyx. Stamens are inserted on a fleshly disk with yellow or purple anthers. The ovary is inferior, and the base of the style is surrounded by the disk.
All examined species had prominent nectaries on the disk (Fig. 1). Organ initiation of the nectary occurred at a primordial floral stage when the flower bud was still quite small. According to the classification of receptacular nectaries developed by Schmid (1988), which was based on the location of the gland relative to stamens, there are two major types of nectaries in Euonymus: a mix of inter-and extrastaminal types, and the intrastaminal type. The nectary disk of inter-and extrastaminal types is located between the corolla and the stigma, and thus also encompasses the stamen bases like a congenitally united collar (Euonymus sanguineus, Euonymus schensianus, Euonymus verrucocarpus, Euonymus wilsonii, Euonymus actinocarpus, Euonymus balansae, Euonymus alatus, Euonymus hukuangensis. Euonymus yunnanensis, Euonymus wui, Euonymus nitidus, Euonymus oblongifolius, Glyptopetalum longipedicellatum, and Glyptopetalum longepedunculatum) (Fig. 1: a, g). The nectary disk of the intrastaminal type is located between the stamen and the stigma, and the stamen bases are at the edge (Euonymus acanthoxanthus, Euonymus pseudovagans, Euonymus theifolius, E. fortunei, Euonymus centidens, Euonymus tingens, and Euonymus microcarpus) (Fig. 1: d).
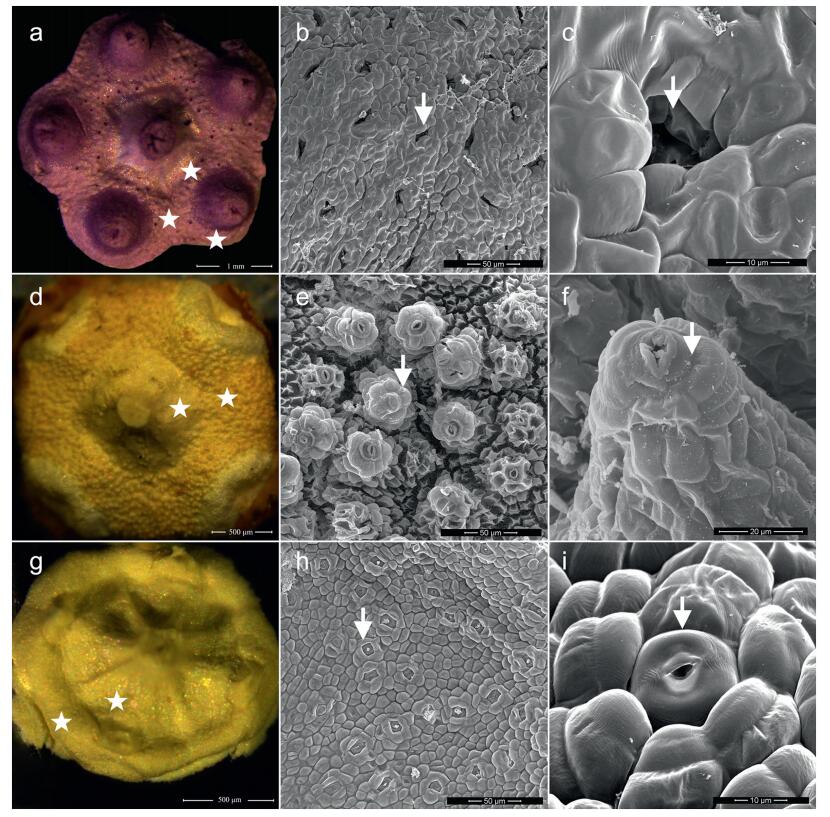
|
| Fig. 1 Floral nectaries (asterisks) and varied nectarostomata (arrows) on the epidermis. a, floral disk with a mix of inter-and extrastaminal nectaries in Euonymus; b and c, pit with sunken nectarostomata; d, floral disk with intrastaminal nectaries in Euonymus; e and f, nectarostomata raised like chimneys; g, floral disk with mix of inter-and extrastaminal nectaries in Glyptopetalum; h and i, nectarostomata level with the epidermis. Species: a, b, c, E. sanguineus. d, e, f, E. theifolius. g, h, i, G. longepedunculatum. |
In Euonymus, nectar is secreted by modified stomata that have lost the capacity to open and close (Fig. 1). Variations in the location of nectarostomata were found on the apex of nectaries. In E. sanguineus, E. centidens, E. alatus, E. hukuangensis, E. nitidus and E. oblongifolius, the nectarostomata are sunken in pits (Fig. 1: b, c). In all species of E. sect. Echinococcus and E. sect. Ilicifolia, as well as E. tingens (E. sect. Euonymus), the nectarostomata are located on distinct convexities, forming structures resembling chimneys or volcanoes (Fig. 1: e, f; Fig. 2). In E. schensianus, E. yunnanensis, E. wui, and E. microcarpus, as well as all species of Glyptopetalum, the nectarostomata are level with adjacent epidermal cells (Fig. 1: h, i).
4. Discussion 4.1. Variation of nectary morphology among sectionsNectaries are commonly similar throughout some families, such as Lamiaceae, Brassicaceae, and Asteraceae (Kumari, 1986; Davis et al., 1998; Mani and Saravanan, 1999). At least two types of floral nectaries exist in Cucurbitaceae (Pacini et al., 2003). A conspicuous nectary disc is common in Celastraceae: the disc extends between the androecium and gynecium in most Celastroideae; and it is restricted to the periphery of the gynecium in some other subfamilies (Berkeley, 1953; Matthews and Endress, 2005; Tan et al., 2007; Gomes and Lombardi, 2013). Though distinct differences in floral nectaries appear among Euonymus sections, nectaries exhibit a number of similarities between these representatives of Euonymus and other species in Celastraceae. The combination of inter-and extrastaminal nectary types is the most representative in Euonymus, where they appear between the corolla and stigma and thus also encompass the stamen bases. The complex type of floral nectaries was observed in most species of Euonymus and Glyptopetalum and was first reported in E. europaeus of E. sect. Euonymus (Konarska, 2015). In contrast, intrastaminal nectaries were found in all species of E.sect. Ilicifolia and some in E. sect. Euonymus, and were described in detail in E. fortunei and E. latifolius a few years ago (Matthews and Endress, 2005; Konarska, 2015). This type is concentrated into a single section, and it may have originated in a gain event to adapt to pollination.
Nectar exudation through nectarostomata is the most typical mode, having been described in many species (Fahn, 1988; Davis and Gunning, 1993; Gaffal et al., 1998; Davis, 2003; Abedini et al., 2013; Papp et al., 2013; Tobe, 2013; Zini et al., 2014). The presence of modified stomata in the nectary is a characteristic shared by Parnassiaceae, Lepidobotryaceae and Celastraceae; thus, it is a potential synapomorphy for Celastrales (Matthews and Endress, 2005). E. sect. Uniloculares was indeed monophyletic among the five sections (Li et al., 2014), but the location of nectarostomata is inconsistent (Table 1). In E. sect. Ilicifolia, the features of floral nectaries are highly consistent, but this section was once thought to be intricately connected to other sections in a Bayesian consensus tree based on the combined nuclear dataset (Li et al., 2014). The incongruence between morphology and molecular phylogeny is common in plants, and the nectary morphology supports the division of E. sect. Ilicifolia. Floral nectaries vary widely within E. sect. Euonymus, and this demonstrates that the section is somewhat confusing.
Species in E. sect. Echinococcus and E. sect. Ilicifolia show great consistency in habits and morphology. They all display the vine habit, which is infrequent in the genus (Ma, 2001). We found that the nectarostomata in these two sections are raised like chimneys or volcanoes, and are indistinguishable from each other. After anthesis, the nectaries in E. sect. Echinococcuscontinue to grow and change to the prickly or tuberculate appendages on the fruit surface; the nectaries in E. sect. Ilicifolia stop developing and ultimately degenerate. Hence, differences in fruit surface should not be used to classify these sections.
4.2. Origin of echinate fruit surfaceThe fruit of E. sect. Echinococcus clearly differs from others in Euonymus. A unique feature is the presence of echinate, prickly or tuberculate fruit surface, whereas the fruits are smooth in the other four sections (Ma, 2001). Our observations of flowers before anthesis, at anthesis, and after anthesis in E. sect. Echinococcus species show for first time that the appendages on fruit surface emerge from raised nectaries on the disk; nectarostoma can clearly be observed on the top of each protrusion there (Fig. 2). Many protrusions are present on the surface of some Sapindaceae fruits (for example, Litchi chinensis). The epidermis of the floral nectaries in L. chinensis is covered with numerous epidermal hairs, with sporadic stomata distributed on the top (Ning and Wu, 2006). Therefore, it is reasonable to speculate that evenly distributed prickles on the fruit surface are derived from the nectary in some species. The nectaries of E.sect. Echinococcus represent a model with which to study the origin of echinate fruit surface. In fact, from an ecological standpoint, this type of nectary is useful to seed propagation after pollination.
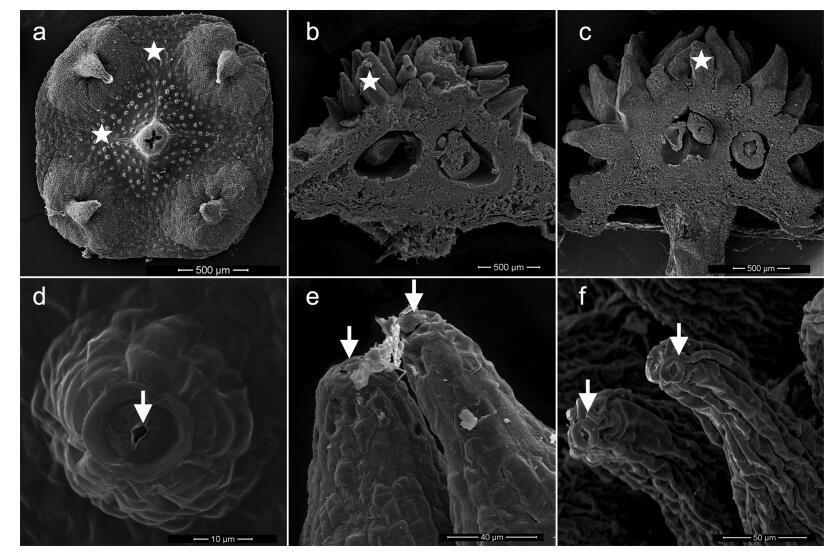
|
| Fig. 2 Representative stages from raised floral nectaries to echinate protrusions on fruit surface in E. sect. Echinococcus. a and d, raised nectaries (asterisks) and nectarostomata on the apex (arrows); b and e, mature nectaries with nectar out of the nectarostomata; c and f, echinate protrusions on fruit surface. The above photos were taken based on continuous observation of E. balansae from flower bud to young fruit. |
The morphology of floral nectaries varies among Euonymus sections, but is relatively constant within each section. The combination of inter- and extrastaminal nectary types is a plesiomorphic characteristic shared by Euonymus and Glyptopetalum, and this is in accordance with recent phylogenetic results showing the two genera form a clade. Furthermore, the prickly or tuberculate appendages on fruit surface originate from floral nectaries, which have nectarostomata raised above the epidermal surface; therefore, the difference in fruit surface is not a good trait to use in the classification of E. sect. Echinococcus and E. sect. Ilicifolia. Although rarely mentioned, floral nectaries may develop into protrusions on fruit surface in flowering plants.
AcknowledgementsThis study was supported by the National Natural Science Foundation of China (Grant No. 31170179) and a grant from the Shanghai Municipal Administration of Landscaping and City Appearance (Grant No. F132428). We would like to thank Professor Quan-Ru Liu of Beijing Normal University for providing Glyptopetalum samples.
Appendix A. Supplementary dataSupplementary data related to this article can be found at https://doi.org/10.1016/j.pld.2017.12.004.
Abedini M., Movafeghi A., Aliasgharpour M., Dadpour M.R., 2013. Anatomy and ultrastructure of the floral nectary in Peganum harmala L. (Nitrariaceae). Plant Spec. Biol, 28, 185-192.
DOI:10.1111/psbi.2013.28.issue-3 |
||
Ancibor E., 1969. Los nectarios florales en leguminosas-mimosoideas. Darwiniana, 15, 128-142.
|
||
Baillon, H. E., 1880. The Natural History of Plants. L. Reeve and Co., London.
|
||
Berkeley E., 1953. Morphological studies in the Celastraceae. J. Elisha Mitchell Sci. Soc, 69, 185-208.
|
||
Bernardello, G., 2007. A systematic survey of floral nectaries. In: Nectaries and Nectar, vol. 33. Springer, Dordrecht, pp. 19-128.
|
||
Blakelock R.A., 1951. A synopsis of the genus Euonymus L. Kew Bull, 2, 210-290.
|
||
Blume C.L., 1825. Bijdragen tot de flora van Nederlandsch Indie. Ter Lands Drukkerij. Batavia, 1146-1148.
|
||
Davis A.R., Pylatuik J.D., Paradis J.C., Low N.H., 1998. Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae. Planta, 205, 305-318.
DOI:10.1007/s004250050325 |
||
Davis A.R., Gunning B.E.S., 1993. The modified stomata of the floral nectary of Vicia faba L. Bot. Acta, 106, 241-253.
DOI:10.1111/plb.1993.106.issue-3 |
||
Davis A.R., 2003. Influence of elevated CO2, and ultraviolet-b radiation levels on floral nectar production:a nectary-morphological perspective. Plant Syst. Evol, 238, 169-181.
DOI:10.1007/s00606-002-0267-0 |
||
Fahn A., 1953. The origin of the banana inflorescence. Kew Bull, 8, 299-306.
DOI:10.2307/4115516 |
||
Fahn A., 1979. Secretory Tissues in Plants. Academic Press, pp :203
-204. |
||
Fahn A., 1988. Secretory tissues in vascular plants. New Phytol, 108, 229-257.
DOI:10.1111/nph.1988.108.issue-3 |
||
Gaffal K.P., Heimler W., El-Gammal S., 1998. The floral nectary of Digitalis purpurea L. structure and nectar secretion. structure and nectar secretion. Ann. Bot, 81, 251-262.
|
||
Galetto L., 1997. Flower structure and nectar chemical composition in three Argentine Apocynaceae. Flora, 192, 197-207.
DOI:10.1016/S0367-2530(17)30778-8 |
||
Galetto L., Bernardello G., 2004. Floral nectaries, nectar production dynamics and chemical composition in six Ipomoea species (Convolvulaceae) in relation to pollinators. Ann. Bot, 94, 269.
DOI:10.1093/aob/mch137 |
||
Gomes S.M.A., Lombardi J.A., 2013. Anatomy of the floral nectaries of some neotropical Salacioideae (Celastraceae). Plant Syst. Evol, 299, 515-528.
DOI:10.1007/s00606-012-0740-3 |
||
Hou D., 1963. Two additional Asiatic species of Glyptopetalum (Celastraceae). Blumea, 12, 57-60.
|
||
Konarska A., 2015. Comparison of the structure of floral nectaries in two Euonymus L. species (Celastraceae). Protoplasma, 252, 1-10.
DOI:10.1007/s00709-014-0745-6 |
||
Kumari D.S., 1986. Evolution of floral nectary in Lamiaceae. Plant Sci, 96, 281-288.
|
||
Li Y.N., Xie L., Li J.Y., Zhang Z.X., 2014. Phylogeny of Euonymus inferred from molecular and morphological data. J. Syst. Evol, 52, 149-160.
DOI:10.1111/jse.v52.2 |
||
Liu, Q. R., Funston, A. M., 2008. Glyptopetalum Thwaites. In: Flora of China, vol. 11. Science Press, Beijing. Missouri Botanical Garden Press, St. Louis, pp. 463-465.
|
||
Loesener, T., 1942. Celastraceae. In: Die Natürlichen Pflanzenfamilien, vol. 20b. Duncker et Humblot, Berlin, pp. 87-197.
|
||
Ma J.S., 2001. A revision of Euonymus (Celastraceae). Thaiszia, 11, 1-264.
|
||
Ma, J. S., Funston, A. M., 2008. Euonymus Linnaeus. In: Flora of China, vol. 11. Science Press, Beijing. Missouri Botanical Garden Press, St. Louis, pp. 440-463.
|
||
Mani M.S., Saravanan J.M., 1999. Pollination Ecology and Evolution in Compositae(Asteraceae). Science Publishers. Science Publishers..
|
||
Matthews M.L., Endress P.K., 2005. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae). Bot. J. Linn. Soc, 149, 129-194.
DOI:10.1111/j.1095-8339.2005.00445.x |
||
Maximowicz C.J., 1881. Diagnoses plantarum novarum Asiaticarum. Bull. Acad. Sci. St.-Petersb, 27, 439-454.
|
||
Meng S.Y., Wang J.L., Liu Q.R., 2011. On the identity of Euonymus pallidifolia(Celastraceae). Ann. Bot. Fenn, 48, 185-187.
DOI:10.5735/085.048.0216 |
||
Nakai T., 1941. Subdivisions of the genus Euonymus. J. Jpn. Bot, 17, 615-619.
|
||
Nepi M., Pacini E., Willemse M.T.M., 1996. Nectary biology of Cucurbita pepo:ecophysiological aspects. Plant Biol, 45, 41-54.
|
||
Nicolson, S. W., Nepi, M., Pacini, E., 2007. Nectaries and nectar. Springer, Netherlands, pp. 167-214.
|
||
Ning X.P., Wu H., 2006. The structural and developmental characteristics of floral nectaries of Litchi chinensis and their biological significance. J. Syst. Evol, 44, 523-537.
DOI:10.1360/aps050100 |
||
Pacini E., Nepi M., Vesprini J.L., 2003. Nectar biodiversity:a short review. Plant Syst. Evol, 238, 7-21.
DOI:10.1007/s00606-002-0277-y |
||
Papp N., Csete S., Farkas A., 2013. Comparative ecomorphology of the cyathial nectaries in eight European Euphorbia species. Acta Biol. Hung, 64, 45-59.
DOI:10.1556/ABiol.64.2013.1.5 |
||
Schmid R., 1988. Reproductive versus extra-reproductive nectaries-historical perspective and terminological recommendations. Bot. Rev, 54, 179-227.
DOI:10.1007/BF02858528 |
||
Simmons M.P., Mckenna M.J., Bacon C.D., Yakobson K., Cappa J.J., Archer R.H., Ford A.J., 2012. Phylogeny of Celastraceae tribe Euonymeae inferred from morphological characters and nuclear and plastid genes. Mol. Phylogenet. Evol, 62, 9-20.
DOI:10.1016/j.ympev.2011.08.022 |
||
Simmons M.P., Cappa J.J., 2013. Wilczekra, A new genus of Celastraceae for a disjunct lineage of Euonymus. Syst. Bot, 38, 148-153.
DOI:10.1600/036364413X661881 |
||
Smets E., 1986. Localization and systematic importance of the floral nectaries in the Magnoliatae (Dicotyledons). Bull. Du Jardin Bot. Natl. De Belgique, 56, 51-76.
DOI:10.2307/3667757 |
||
Solereder H., Boodle L.A., Fritsch F.E., Scott D.H., 1908. Systematic Anatomy of the Dicotyledons.. Clarendon Press, New York. |
||
Sprague T.A., 1908. The prickly-fruited species of Euonymus. Bull. Misc. Inform, 1, 29-36.
|
||
Tan K., Guo Y.H., Nicolson S.W., Radloff S.E., Song Q.S., 2007. Honeybee (Apis cerana) foraging responses to the toxic honey of Tripterygium hypoglaucum(Celastraceae):changing threshold of nectar acceptability. J. Chem. Ecol, 33, 2209-2217.
DOI:10.1007/s10886-007-9384-0 |
||
Thornburg R.W., Carter C., Powell A., Mittler R., Rizhsky L., Horner H.T., 2003. A major function of the tobacco floral nectary is defense against microbial attack. Plant Syst. Evol, 238, 211-218.
DOI:10.1007/s00606-003-0282-9 |
||
Thwaites G.H.K., 1856. Description of new genera and species of Ceylon plants. Hooker's J. Bot. Kew Gard. Misc, 8, 266-271.
|
||
Tobe H., 2013. Floral morphology and structure of Phyllonoma (Phyllonomaceae):systematic and evolutionary implications. J. Plant Res, 126, 709-718.
DOI:10.1007/s10265-013-0556-4 |
||
Wang C.H., 1939. The studies of Chinese Celastraceae. Chin. J. Bot, 1, 1-84.
|
||
Zini L.M., Sol#237;s S.M., Ferrucci M.S., 2014. Anatomical and developmental studies on floral nectaries in Cardiospermum species:an approach to the evolutionary trend in Paullinieae. Plant Syst. Evol, 300, 1515-1523.
DOI:10.1007/s00606-013-0978-4 |



