b. Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming 650201, PR China;
c. University of Chinese Academy of Sciences, Beijing 100049, PR China
Glandular trichomes (GTs) populate the aerial surface of many terrestrial vascular plants (Wang, 2014), and can be subdivided into peltate glandular trichomes (PGTs) and capitate glandular trichomes (CGTs) based on their respective morphological characteristics (Wagner et al., 2004). GTs synthesize, store, and release a wide variety of secondary metabolites such as terpenoids, alkaloids, and phenolic compounds, and thus have been regarded as chemical factories of plants (Schilmiller et al., 2008; Wagner et al., 2004). It is well known that many GT-produced chemicals have important pharmaceutical values, as exemplified by artemisinin and Δ9-tetrahydrocannabinol (Happyana et al., 2013; Kim and Mahlberg, 1997; Wang, 2014; Wang et al., 2015). In nature, these special chemicals have been generally considered as defensive compounds in antagonistic interactions among organisms (Benlarbi et al., 2014; Nyasembe and Torto, 2014). For instance, pyrethrin, which is located in the GTs of Tanacetum cinerariifolium, has been recognized as a potent botanical insecticide (Ramirez et al., 2013; Wang, 2014); sclareol, excreted by the GTs of Salvia sclarea, shows antimicrobial activity and also affects Arabidopsis thaliana seed germination (Caissard et al., 2012; Campbell and Manners, 2003; Jasiński et al., 2001).
Volatile compounds (including terpenoids and phenylpropenoids) produced by the GTs have also been reported to contribute to the defensive properties of many plant species (Gang et al., 2001; Veronese et al., 2001). For example, mint oils are synthesized and stored in the GTs of Mentha arvensis (Ohloff, 1994; Sharma et al., 2003), and play valuable roles in plant defense against herbivores (Harborne, 1993) and attracting predatory and parasitic insects to attack phytophagous pests (Turlings et al., 1995); methylketones in the GTs of the wild tomato Lycopersicon hirsutum serve as defensive compounds against various insects (Williams et al., 1980; Yu et al., 2010); (-)-5, 6-dehydrocamphor in the GTs of Zuccagnia punctata shows antifungal activity (Álvarez et al., 2012).
In order to investigate the bioactive compounds in plant GTs as well as their defensive functions, we previously developed an approach which uses laser microdissection (LMD) coupled with sensitive analytical technology including cryogenic nuclear magnetic resonance and ultra-performance liquid chromatographytandem mass spectrometry (Li et al., 2013). This new approach has identified several defensive plant compounds. We discovered two unique classes of defensive sesterterpenoids with insect antifeedant and antifungal activities in the PGTs of two Lamiaceae plants, Leucosceptrum canum and Colquhounia coccinea var. mollis (Li et al., 2013; Luo et al., 2010), respectively. Three defensive clerodane diterpenoids, which exhibited significant insect antifeedant activity, were also found in the PGTs of another Lamiaceae plant Colquhounia seguinii (Li et al., 2014). In addition, five phytotoxic labdane diterpenoids were identified in the CGTs of Paragutzlaffia henryi (Acanthaceae), which might serve as allelochemicals against other neighboring competitive plants (Wang et al., 2015). This research indicates that the secondary metabolites of plant GTs and their defensive roles may vary greatly among different species.
Oenothera glazioviana Mich., a member of the Onagraceae family, is commonly known as Evening Primrose, and has been an excellent model for studying molecular mechanisms of speciation (Greiner et al., 2008; Massouh et al., 2016). O. glazioviana is widely distributed in subtropical areas throughout the world and is commonly cultivated in northeast and southwest China due to its outstanding commercial value. An essential oil, whose major component is γ-linolenic acid, is produced from the seeds of O. glazioviana and has been used as a nutritional and medicinal supplement due to its anti-inflammatory and antithrombotic properties. In addition, previous reports have shown that this oil reduces lipids as well as enhances smooth muscle relaxation and vasodilation (Guo et al., 2014; Rauwolf et al., 2008; Stonemetz, 2008). We observed that in the wild the pedicels and calyxes of O. glazioviana are covered with abundant CGTs, and that their number gradually declines during the flowering stage. Curiously, the taste of O. glazioviana CGTs is pungent, which prompted us to investigate the identity, chemistry and function of putative chemical compounds of the CGTs. Herein, we report the localization of a volatile compound, 4-hydroxy-4-methylpentan-2-one (= diacetone alcohol) (1), in the CGTs of O. glazioviana, using LMD coupled with gas chromatography-mass spectrometry (GC-MS). We also evaluated the antimicrobial, phytotoxic and insecticidal activities of compound 1.
2. Materials and methods 2.1. Plant materialO. glazioviana GTs were collected for microscopic study and metabolic analysis by LMD from plants grown in Kunming Botanical Garden in July 2015. A. thaliana wild-type seeds (ecotype Colombia) were maintained in our laboratory.
2.2. MicroscopyFresh pedicels and flowers of O. glazioviana were examined under a Leica S8 APO light stereo microscope (Leica Microsystems, Wetzlar, Germany) with bright-field optics. For scanning electron microscope (SEM), samples were processed according to our previously reported protocol (Li et al., 2013), and the specimens were observed using a Hitachi S-4800 scanning electron microscope (SEM) (Hitachi Ltd., Tokyo, Japan) at 10.0 keV accelerating potential. Magnifications ranged from 30× to 500× (Wang et al., 2015).
2.3. Laser microdissection of CGTs and metabolic analysis by GC-MSCGTs were microdissected and collected from fresh pedicels of O. glazioviana with a Leica LMD 7000 system (Leica Microsystems, Wetzlar, Germany) using a procedure similar to one we have previously described (Li et al., 2013). Approximately 800 CGTs were collected in the cap of a microtube. The collected samples were centrifuged at 4 ℃ (12, 000 g, 10 min) to settle the contents, and extracted with acetone (500 μL) by ultrasonication for 10 min. The extract was directly analyzed by Agilent GC model 7890A coupled to a 5975C mass spectrometer (Aglient Technologies, Santa Clara, USA) equipped with a HP-5MS capillary column (50 m × 0.32 mm i.d, 0.52 μm film thickness). A split injection and diversion ratio (10:1) mode was used and 1 μL of sample was injected. Helium carrier gas was used at a constant flow rate of 3 mL/min. The temperatures of injector and mass transfer line were at 250 ℃ and 230 ℃, respectively. The initial temperature was held at 50 ℃ with a programmed increase at 5 ℃/min to 250 ℃ followed by 10 min at 250 ℃. For mass spectral detection, an electron impact (EI) mode with ionization energy of 70 eV was used. Total time of the program running was 50 min. Spectra were obtained over an m/z range of 45-450. Total ion chromatogram (TIC) acquired via GC-MS was used for peak area integration. MSD ChemStation software was used for data acquisition.
2.4. Antimicrobial assayWe tested the antimicrobial properties of compound 1 against bacteria and fungi. Specifically, we used three strains of gram positive bacteria (Bacillus subtilis, Micrococcus luteus, Staphylococcus aureus) obtained from the Research Institute of Resources Insects, Chinese Academy of Forestry; three strains of pathogenic fungi (Rhizoctonia solani, Colletotrichum litchi, C. gloeosporioides) provided by the Department of Plant Pathology, South China Agriculture University; and a fungus that was isolated from diseased leaves of O. glazioviana and identified as Aspergillus niger HQ170509 by sequencing the internal transcribed spacers (ITS) of the ribosomal RNA gene region, were used in this study. The antimicrobial activities were evaluated using a broth dilution method. All experiments were performed in a 96-well microtitre plate with three replicates. The strains were prepared from 24 h broth cultures, and each suspension was standardized to 0.4 McFarland standard turbidity. Then, the test compound was dissolved into methanol to obtain a high concentration (2.20 mM) and series concentrations were acquired (1.10, 0.55, 0.28, 0.14, 0.07 mM) by two-fold dilution. Each well containing 90 μL of LuriaeBertani (LB) broth and 10 μL of the diluted test compound was inoculated with the microbial fluids to a final volume of 200 μL (Peng and Don, 2013). The 96-well microtire plates were further incubated for 6 h at 37 ℃ (bacteria) or 24 h at 28 ℃ (fungi), until McFarland standard turbidity reached the value between 0.6 and 0.8. The results were recorded with a microplate reader (Molecular Devices, USA). Ampicillin and nystatin were used as positive controls for antibacterial and antifungal assays, respectively, and methanol was used as negative control.
| $ Inhibitory\;\;rate\;\;\% = \left({1 - \frac{{{N_T}}}{{{N_C}}}} \right) \times 100 $ |
here, NT is the value of optical density at 600 nm (OD600) at each treatment, and NC is the value of OD600 in control. The half maximal inhibitory concentration (IC50) was obtained by probit analysis using SPSS.
2.5. Insecticidal assayLarvae of Myzus persicae were obtained from Key Laboratory of Economic Plants and Biotechnology in West China, Kunming Institute of Botany, Chinese Academy of Sciences, and reared on Nicotiana tabacum plants. The test compound was dissolved in methanol at a concentration of 0.52 mM, and water containing Triton X-100 (0.1 mg/L) was added to dilute the compound to a series of concentrations (0.26, 0.13, 0.06, 0.03 and 0.015 mM). Leaves of N. tabacum with 20 healthy larvae were dipped into the chemical solutions for 5 s, and the excess liquid was removed with filter paper. After that the leaves were placed on a Petri dish (9.0 cm in diameter). Water containing Triton X-100 (0.1 mg/L) was used as a control. The mortality rate was evaluated at 24 h after treatment. Each treatment was repeated three times.
| $ Corrected\;\;mortality\;\;\% = \left({\frac{{{N_T} - {N_C}}}{{20 - {N_C}}}} \right) \times 100 $ |
here, NT is the number of deaths in each treatment, and NC is the number of deaths in control. The half maximal lethal concentration (LC50) was estimated by probit analysis using SPSS.
2.6. Seed germination assayA. thaliana seeds were vernalized in a refrigerator at 4 ℃ for 3 days before using. The test compound was assayed at 0.86, 0.43, 0.22, 0.11, and 0.055 mM, respectively. To avoid toxic effects of the organic solvent, the final concentration of methanol did not exceed 0.2%. Fifty sterilized seeds were equidistantly sown on Murashige and Skoog medium [0.8% agar (w/v), pH 5.8] supplemented with the test sample at various concentrations in a Petri dish (9.0 cm in diameter). The same volume of methanol was used as a blank control. Three replicates of each treatment were carried out. The seeds were left under 24 h dim light at 25 ℃ in a growth chamber for 4 days, allowing germination. The number of germinated seeds was recorded daily and the inhibition index was calculated until most seeds (≥95%) in the control Petri dishes germinated. The seed germination inhibition rate (IG) was evaluated by the following equation:
| $ Inhibitory\;\;rate\;\;\% = \left({1 - \frac{{{N_T}}}{N}} \right) \times 100 $ |
here, NT is the number of germinated seeds at each treatment, and N is the number of seeds used in the bioassay. The half maximal effective concentration (EC50) was then determined by probit analysis using SPSS.
3. Results and discussion 3.1. Morphology and distribution of trichomes on O. glaziovianaTo study the morphology and distribution of O. glazioviana trichomes, flowers, leaves and young stems were analyzed by stereomicroscope and SEM. CGTs were only present on the pedicels and calyxes, especially on young pedicels (Fig. 1A-B), and the number of CGTs on the pedicels gradually declined with the flowering period. CGT length was about 100-200 μm (Fig. 1C-D). Typical CGTs had a common globular-like storage cavity with 20-25 μm in diameter atop a short stalk (Fig. 1E). Interestingly, double globular-like storage cavities were also occasionally found at the top of one stalk (Fig. 1F). Also, we commonly observed the breakdown of the storage cavities, especially after rain.
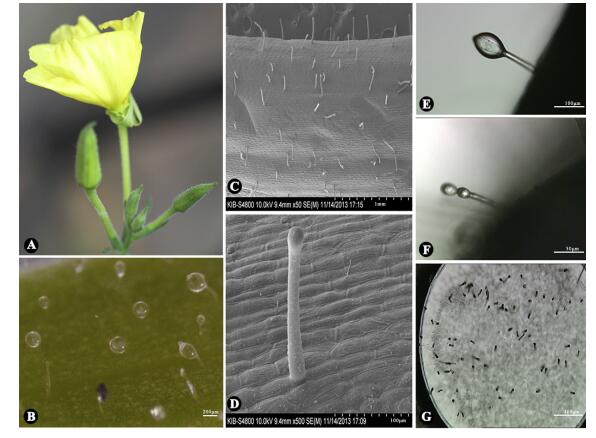
|
| Fig. 1 Morphology and distribution of glandular trichomes (GTs) of Oenothera glazioviana and laser microdissection of capitate glandular trichomes (CGTs). (A) O. glazioviana in flowering. (B) CGTs on the pedicels under optical microscope. Scale bar: 200 μm. (C-D) CGTs on the pedicels under scanning electron microscope. Scale bar: 1.00 mm (C), 100 μm (D). (E) Intact CGTs before dissection. Scale bar: 100 μm. (F) Intact CGTs with double globular-like storage cavities before dissection. Scale bar: 50 μm. (G) Collected CGTs by laser microdissection. Scale bar: 200 μm |
In order to analyze the secondary metabolites present in O. glazioviana CGTs, intact CGTs were carefully collected under a microscope and dissected using LMD, as illustrated in Fig. 1G (Li et al., 2014; Wang et al., 2015). Approximately 800 CGTs were collected in the caps of a 0.5 mL Eppendorf microcentrifuge tubes, which were stored at -80 ℃ immediately after LMD to avoid degradation of secondary metabolites.
The collected CGT samples were extracted with 500 mL of acetone by ultrasonication for 10 min. Since O. glazioviana has been reported to be rich in essential oil (Liu, 1997; Pelc et al., 2005), the CGT extract was analyzed directly by GC-MS. One predominant peak (compound 1) with a retention time of 11.73 min was detected in the chromatogram (Fig. 2A).
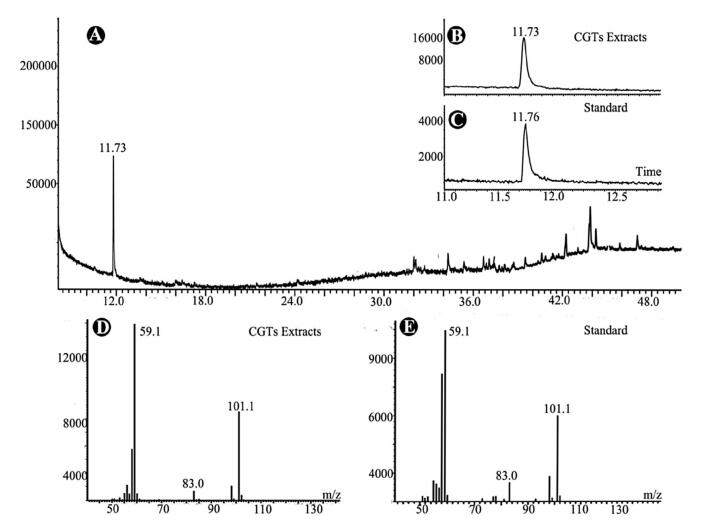
|
| Fig. 2 GC-MS analysis of secondary metabolites in the capitate glandular trichomes (CGTs) of Oenothera glazioviana. (A) Total Ion Chromatogram (TIC) spectrum of CGT extracts. (B) and (C) Comparison of retention times of 4-hydroxy-4-methylpentan-2-one in TICs of CGT extract (B) and commercial standard (C). (D) and (E) MS spectra of 4-hydroxy-4-methylpentan-2-one in CGT extract (D) and commercial standard (E) |
Compound 1 had a molecular weight of 116 according to its mass spectrum. The characteristic fragmental ions were clearly present at m/z 101 and 59 in the mass spectrum, which was very similar to that of a volatile compound, 4-hydroxy-4-methylpentan-2-one, in the NIST library (version 2.0), with a high match value of 968 (out of 1000). We therefore speculated that compound 1 detected in O. glazioviana CGTs might be 4-hydroxy-4-methylpentan-2-one.
Since the CGTs collected by LMD were insufficient for direct isolation of compound 1 for structural elucidation, a commercial sample of 4-hydroxy-4-methylpentan-2-one was obtained as a standard for verification. Through comparison of their retention times and MS spectra (Fig. 2B-E), the identity of compound 1 in the CGT extract of O. glazioviana was confirmed (Fig. 3). In addition, a series of minor peaks with retention times ranging from 32 to 48 min were detected in the total ion chromatogram of the CGT extract. However, these compounds were not identified due to the low match values between their MS spectra and those in the NIST data library.
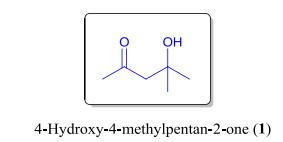
|
| Fig. 3 The chemical structure of 4-hydroxy-4-methylpentan-2-one (1) |
Since the secondary metabolites of plant GTs have been generally considered to contribute to plant defense, we were interested in discovering whether compound 1 played a role in plant defense. Compound 1 was tested for its antimicrobial activity against three strains of gram positive bacteria (B. subtilis, M. luteus, and S. aureus), and three strains of pathogenic fungi (R. solani, C. litchi, and C. gloeosporioides), using a modified broth dilution method (Luo et al., 2010; Peng and Don, 2013; Wiegand et al., 2008). Compound 1 showed possible growth inhibitory activities against B. subtilis, M. luteus, and S. aureus, with IC50 values of 0.51 ± 0.29, 0.16 ± 0.80, and 0.13 ± 0.89 mM, respectively. In addition, compound 1 exhibited potent antifungal activity against the pathogenic fungus R. solani, with an IC50 value of 0.15 ± 0.82 mM (Table 1). The growth of associated fungus A. niger HQ170509 was also significantly inhibited by compound 1, with IC50 value of 0.10 ± 0.99 mM. These results suggest that compound 1 may serve as an antimicrobial chemical for the plant against pathogenic microbes such as bacteria and fungi.
| Test organism | Antimicrobial activities/IC50 (mM) | |
| 4-Hydroxy-4-methylpentan-2-one | Positive control |
|
| Bacteriaa | ||
| Bacillu subtilis | 0.51 ± 0.29 | < 0.0005 |
| Micrococcus luteus | 0.16 ± 0.80 | < 0.0005 |
| Staphylococcus aureus | 0.13 ± 0.89 | < 0.0005 |
| Fungib | ||
| Rhizoctonia solani | 0.15 ± 0.82 | 0.06 ± 0.008 |
| Colletotrichum litchi | NA | < 0.5 |
| C. gloeosporioides | NA | < 0.5 |
| Aspergillus niger | 0.10 ± 0.99 | 0.004 ± 0.19 |
| a Ampicillin was used as a positive control. b Nystatin was used as a positive control; NA = not active. |
||
Compound 1 has been used as a dispersant solvent contributing to the acute toxicity of insecticides, and has been reported to have a poisonous effect on the skin, eyes, and the pulmonary system (Kitulagodage et al., 2008; Lewis, 2004). Given that the above work has shown that O. glazioviana GTs are solely distributed on the pedicels and calyxes, and that the insect M. persicae feeds preferentially in or near plant inflorescences (Ibbotson and Kennedy, 1950; Metcalfe, 2005; Sholes, 1984), we tested the insecticidal activity of compound 1 against the larvae of M. persicae. Interestingly, compound 1 exhibited significant insecticidal activity, with LC50 value of 16.2 ± 0.18 μM.
3.6. Seed germination assayPrevious studies have reported that compound 1 inhibits germination in corn and wheat (Zolotovich et al., 1974). We therefore tested the phytotoxic effect of compound 1 on A. thaliana seed germination. We found that compound 1 was capable of effectively inhibiting seed germination (EC50 = 0.73 ± 0.13 mM).
In summary, we have identified a volatile compound, 4-hydroxy-4-methylpentan-2-one, in the CGTs of O. glazioviana using LMD coupled with GC-MS. Although 4-hydroxy-4-methylpentan-2-one has been frequently detected in plants (e.g. Phlomis frutic, Juniperus phoenicea) and microorganisms (e.g. Mycobacterium tuberculosis) by GC-MS (Elsawi et al., 2007; Mgode et al., 2012; özcan et al., 2011), and has also been isolated from Lonchocarpus laxiflorus and Stipa vaseyi (Igoli et al., 2008; Epstein et al., 1964), this is the first time it has been localized in the GTs of a plant. 4-Hydroxy-4-methylpentan-2-one has been used as a synthetic material in industry and as a wood preservative (Gong and Gong, 1990). Oral transmission of 4-hydroxy-4-methylpentan-2-one to rats (LD50 of 4 g/kg) has been shown to be toxic (Smyth and Carpenter, 1948). However, the function of 4-hydroxy-4-methylpentan-2-one in plants, especially in the GTs, has until now remained unclear. GTs are considered an apparent first defense line for plants, and compounds synthesized and accumulated in GTs usually contribute to plant defense against herbivores and pathogens. As we predicted, 4-hydroxy-4-methylpentan-2-one showed obvious antimicrobial, insecticidal and phytotoxic activities. These results suggest that 4-hydroxy-4-methylpentan-2-one in the GTs of O. glazioviana may have defensive functions for the plant against pathogens and insects. Considering the uneven distribution pattern of GTs on O. glazioviana, 4-hydroxy-4-methylpentan-2-one might be a constitutive defensive chemical specially for the plant to protect its reproductive organs from pathogens and insects. In addition, as breakdown of the storage cavities of O. glazioviana GTs were commonly observed especially after raining, 4-hydroxy-4-methylpentan-2-one may also be released into the environment to function as an allelochemical against the surrounding competitive plants.
4. ConclusionsIn our study, the volatile compound 4-hydroxy-4-methylpentan-2-one was localized in the CGTs of O. glazioviana using LMD coupled with GC-MS. 4-Hydroxy-4-methylpentan-2-one exhibited significant inhibitory activities against three strains of gram positive bacterium (B. subtilis, M. luteus, S. aureus), a strain of pathogenic fungus (R. solani) and a strain of associated fungus of O. glazioviana (A. niger). Moreover, 4-hydroxy-4-methylpentan-2-one displayed strong phytotoxic activity against A. thaliana seed germination and insecticidal activity against M. persicae larvae. These findings suggest that 4-hydroxy-4-methylpentan-2-one in the GTs of O. glazioviana may have defensive functions for the plant against pathogens, insect herbivores, and presumably competitive plants as well.
Acknowledgment: This research was supported financially by the National Science Fund for Distinguished Young Scholars (31525005), the NSFCYunnan Joint Fund (U1202263), the National Basic Research Program of China (973 Program) on Biological Control of Key Crop Pathogenic Nematodes (2013CB127505), and the "Hundred Talents Program" of the Chinese Academy of Sciences (awarded to S.-H. Li).| Álvarez S.L., Cortadi A., Juárez M.A., Petenatti E., Tomi F., Casanova J., van Baren C.M., Zacchino S., Vila R., 2012. (-)-5, 6-Dehydrocamphor from the antifungal essential oil of Zuccagnia punctata. Phytochem. Lett, 5, 194-199. DOI:10.1016/j.phytol.2011.12.008 | ||
| Benlarbi K.H., Elmtili N., Macías F.A., Galindo J.C.G., 2014. Influence of in vitro growth conditions in the production of defence compounds in Mentha pulegium. Phytochem. Lett, 8, 233-244. DOI:10.1016/j.phytol.2014.03.007 | ||
| Caissard C.J., Thomas O., Claire D., Sabine P., Pierre-Philippe G., Arthur A., Nadine V., Sandrine M., Florence N., Jean-Louis M., 2012. Extracellular localization of the diterpene sclareol in clary sage (Salvia sclarea L., Lamiaceae). Plos One, 7, 248-253. | ||
| Campbell E.J., Manners J.M., 2003. Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol, 133, 1272-1284. DOI:10.1104/pp.103.024182 | ||
| Elsawi S.A., Motawae H.M., Ali A.M., 2007. Chemical composition, cytotoxic activity and antimicrobial activity of essential oils of leaves and berries of Juniperus phoenicea L. grown in Egypt. Afr. J. Tradit. Complement. Altern. Med, 4, 417-426. | ||
| Epstein W., Gerber K., Karler R., 1964. The hypnotic constituent of Stipa vaseyi, sleepy grass. Experientia, 20, 390-391. | ||
| Gang D.R., Wang J., Dudareva N., Lewinsohn E., Pichersky E., 2001. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol, 125, 539-555. DOI:10.1104/pp.125.2.539 | ||
| Gong Z.W., Gong S.W., 1990. The synthesis of diacetone alcohol. Fine Chem. Intermed, 2, 57-58. | ||
| Greiner S., Wang X., Rauwolf U., Silber M.V., Mayer K., Meurer J., Haberer G., Herrmann R.G., 2008. The complete nucleotide sequences of the five genetically distinct plastid genomes of Oenothera, subsection Oenothera: I. sequence evaluation and plastome evolution. Nucleic Acids Res, 36, 2366-2378. | ||
| Guo P., Wang T., Liu Y., Xia Y., Wang G., Shen Z., Chen Y., 2014. Phytostabilization potential of evening Primrose (Oenothera glazioviana) for copper-contaminated sites. Environ. Sci. Pollut. Res, 21, 631-640. DOI:10.1007/s11356-013-1899-z | ||
| Happyana N., Agnolet S., Muntendam R., Dam A.V., Schneider B., Kayser O., 2013. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry, 87, 51-59. DOI:10.1016/j.phytochem.2012.11.001 | ||
| Harborne J.B., 1993. Herbivores, their interactions with secondary metabolites. Phytochemistry, 33, 482-252. | ||
| Ibbotson A., Kennedy J.S., 1950. The distribution of aphid infestation in relation to leaf age. Ann. Appl. Biol, 37, 651-679. DOI:10.1111/aab.1950.37.issue-4 | ||
| Igoli J.O., Onyiriuka S.O., Letzel M.C., Nwaji M.N., Gray A.I., 2008. Cassane diterpenoids from Lonchocarpus laxiflorus. Nat. Product. Commun, 3, 5-10. | ||
| Jasiński M.S.Y. Degand H., Purnelle B., Marchand B.J., Boutry M., 2001. A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell, 13, 1095-1107. DOI:10.1105/tpc.13.5.1095 | ||
| Kim E.S., Mahlberg P.G., 1997. Immunochemical localization of tetrahydrocannabinol (THC) in cryofixed glandular trichomes of Cannabis (Cannabaceae). Am. J. Bot, 84, 336-342. DOI:10.2307/2446007 | ||
| Kitulagodage M., Astheimer L.B., Buttemer W.A., 2008. Diacetone alcohol, a dispersant solvent, contributes to acute toxicity of a fipronil-based insecticide in a passerine bird. Ecotoxicol. Environ. Saf, 71, 597-600. DOI:10.1016/j.ecoenv.2007.11.001 | ||
| Lewis R.J., 2004. Sax's Dangerous Properties of Industrial Materials. John Wiley & Sons, Inc, Canada, 1103-1103. | ||
| Li C.H., Jing S.X., Luo S.H., Shi W., Hua J., Liu Y., Li X.N., Schneider B., Gershenzon J., Li S.H., 2013. Peltate glandular trichomes of Colquhounia coccinea var. mollis harbor a new class of defensive sesterterpenoids. Org. Lett, 15, 1694-1697. | ||
| Li C.H., Liu Y., Hua J., Luo S.H., Li S.H., 2014. Peltate glandular trichomes of Colquhounia seguinii harbor new defensive clerodane diterpenoids. J. Integr. Plant Biol, 56, 928-940. DOI:10.1111/jipb.v56.9 | ||
| Liu S., 1997. Oenothera biennis seed rich in γ-linolenic acid. Zhongcaoyao, 28, 105-106. | ||
| Luo S.H., Luo Q., Niu X.M., Xie M.J., Zhao X., Schneider B., Gershenzon J., Li S.H., 2010. Glandular trichomes of Leucosceptrum canum harbor defensive sesterterpenoids. Angew. Chem. Int. Ed, 49, 4471-4475. DOI:10.1002/anie.201000449 | ||
| Massouh A.S.J., Yaneva R.L., Ulbricht J.E.S., Zupok A., Johnson M.T., Wright S., Pellizzer T., Sobanski J., Bock R., Greiner S., 2016. Spontaneous chloroplast mutants mostly occur by replication slippage and show a biased pattern in the plastome of Oenothera. Plant Cell, 28, 911-929. | ||
| Metcalfe D.J., 2005. Hedera helix L. J. Ecol, 93, 632-648. DOI:10.1111/jec.2005.93.issue-3 | ||
| Mgode G.F., Weetjens B.J., Nawrath T., Lazar D., Cox C., Jubitana M., Mahoney A., Kuipers D., Machang R.S., Weiner J., 2012. Mycobacterium tuberculosis volatiles for diagnosis of tuberculosis by cricetomys rats. Tuberculosis, 92, 535-542. DOI:10.1016/j.tube.2012.07.006 | ||
| Nyasembe V.O., Torto B., 2014. Volatile phytochemicals as mosquito semiochemicals. Phytochem. Lett, 8, 196-201. DOI:10.1016/j.phytol.2013.10.003 | ||
| Ohloff G., 1994. The fascination of odors and their chemical perspectives. Scent Fragr, 8, 238-241. | ||
| Özcan M.M., Chalchat J.C., Bagci Y., Dural H., Figueredo G., Savran A., 2011. Chemical composition of essential oils of Phlomis grandiflora H. S. Thomposon var. grandiflora flowers and leaves of Turkish origin. J. Food Biochem, 35, 125-132. | ||
| Pelc M., Kosakowska O., Weglarz Z., Przybyl J., Geszprych A., 2005. Sterols and fatty acids in the seeds of evening Primrose (Oenothera sp.) and willow herb (Epilobium sp.). Herba Pol, 51, 20-24. | ||
| Peng T.Y., Don M.M., 2013. Antifungal activity of in-vitro grown Earliella scabrosa, a malaysian fungus on selected wood-degrading fungi of rubberwood. J. Phys. Sci, 24, 21-33. | ||
| Ramirez A.M., Saillard N., Yang T., Franssen M.C.R., Bouwmeester H.J., Jongsma M.A., 2013. Biosynthesis of sesquiterpene lactones in pyrethrum (Tanacetum cinerariifolium). Plos One, 8, 59-59. | ||
| Rauwolf U., Golczyk H., Meurer J., Herrmann R.G., Greiner S., 2008. Molecular marker systems for Oenothera genetics. Genetics, 180, 1289-1306. DOI:10.1534/genetics.108.091249 | ||
| Schilmiller A.L., Last R.L., Pichersky E., 2008. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J, 54, 702-711. DOI:10.1111/j.1365-313X.2008.03432.x | ||
| Sharma S., Sangwan N.S., Sangwan R.S., 2003. Developmental process of essential oil glandular trichome collapsing in menthol mint. Curr. Sci, 84, 544-550. | ||
| Sholes O.D.V., 1984. Responses of arthropods to the development of goldenrod inflorescences (Solidago: Asteraceae). Am. Midl. Nat, 112, 1-14. DOI:10.2307/2425450 | ||
| Smyth Jr., H. F., Carpenter C.P., 1948. Further experience with the range finding test in the industrial toxicology laboratory. J. Ind. Hyg. Toxicol, 30, 63-68. | ||
| Stonemetz D., 2008. A review of the clinical efficacy of evening Primrose. Holist. Nurs. Pract, 22, 171-174. DOI:10.1097/01.HNP.0000318026.45527.07 | ||
| Turlings T.C., Loughrin J.H., Mccall P.J., Röse U.S., Lewis W.J., Tumlinson J.H., 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. U. S. A, 92, 4169-4174. DOI:10.1073/pnas.92.10.4169 | ||
| Veronese P., Li X., Niu X., Weller S.C., Bressan R.A., Hasegawa P.M., 2001. Bioengineering mint crop improvement. Plant Cell Tissue Organ Cult, 64, 133-144. DOI:10.1023/A:1010649207445 | ||
| Wagner G.J., Wang E., Shepherd R.W., 2004. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot, 93, 3-11. DOI:10.1093/aob/mch011 | ||
| Wang G., 2014. Recent progress in secondary metabolism of plant glandular trichomes. Plant Biotechnol, 31, 353-361. DOI:10.5511/plantbiotechnology.14.0701a | ||
| Wang Y., Luo S.H., Hua J., Liu Y., Jing S.X., Li X.N., Li S.H., 2015. Capitate glandular trichomes of Paragutzlaffia henryi harbor new phytotoxic labdane diterpenoids. J. Agric. Food Chem, 63, 10004-10012. DOI:10.1021/acs.jafc.5b04113 | ||
| Wiegand I., Hilpert K., Hancock R.E., 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc, 3, 163-175. DOI:10.1038/nprot.2007.521 | ||
| Williams W.G., Kennedy G.G., Yamamoto R.T., Thacker J.D., Bordner J., 1980. 2-Tridecanone: a naturally occurring insecticide from the wild tomato Lycopersicon hirsutum f. glabratum. Science, 207, 888-889. DOI:10.1126/science.207.4433.888 | ||
| Yu G., Nguyen T.T., Guo Y., Schauvinhold I., Auldridge M.E., Bhuiyan N., Ben I.I., Iijima Y., Fridman E., Noel J.P., 2010. Enzymatic functions of wild tomato methylketone synthases 1 and 2. Plant Physiol, 154, 67-77. DOI:10.1104/pp.110.157073 | ||
| Zolotovich, G. D. , Mikhailova-Koleva, S. , Georgieva, M. V. , 1974. Biological effect of some alcohols isolated from concrete of fresh water cultivated microalgae. In: 6th Int. Congr. Essent. Oils, [Pap. ]; Allured Publ. Corp. : Oak Park, IL (CAN 84: 55230); 6, pp. 22-26. |



