b. Shandong Provincial Engineering and Technology Research Center for Wild Plant Resources Development and Application of Yellow River Delta, Faculty of Life Science, Binzhou University, Binzhou, 256603, Shandong, People's Republic of China;
c. Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, People's Republic of China
Acanthaceae is a pantropical family consisting of about 230 genera and 4000 species( Mabberley, 2008 ; Hu et al., 2011). Recent phylogenetic studies of Acanthaceae have resulted in the recognition of four subfamilies, viz., Nelsonioideae, Thunbergiodeae, Avicennioideae and Acanthoideae. Acanthoideae differs from the other three subfamilies in having reticulata and is further divided into seven tribes, Acantheae, Ruellieae, Justicieae, Barlerieae, Neurantheae, Andrographideae and Whitifieldieae( Reveal, 2012). Four lineages were recognized by McDade et al.(2000) in Justicieae, viz., the Justicia lineage, Tetramerium lineage, Isoglossinae and Pseuderanthemum lineage.
Justicia L. is the largest genus in the family Acanthaceae and belongs to tribe Justicieae. It comprises approximately 600 species and is distributed in the tropical and subtropical regions of the world( Graham, 1988 ; Mabberley, 2008 ; Hu et al., 2011). Two divergent trends were suggested by previous studies of the genus, resulting either in the recognition of a large number of small segregate genera or in the adoption of a very broad definition of Justicia . In the former, Bremekamp(1948) separated Justicia s.l. into several genera and his treatment was followed by many authors(e.g., Hu and Tsui, 2002). In contrast, Graham(1988) adopted a very broad definition of Justicia and divided the genus into 16 sections and 7 subsections. Graham's treatment was followed by the most recent works( Hu et al., 2011 ; Wood, 2014). The genus Justicia is characterized by several characters, including the 2-lipped corolla with the bilobed upper lip and trilobed lower lip, two bithecous stamens, usually one thecae above the other, and the lower one with a spur at the base( Graham, 1988 , Hu et al., 2011). However, recent molecular evidence has indicated that Justicia in the broad sense of Graham(1988) is paraphyletic( McDade et al., 2000 ; Gao, 2010).
In China, 43 species were recognized in the recently published volume 19 of the Flora of China( Hu et al., 2011). Among these, Justicia microdontaW.W. Smith(1918) is quite different in that each anther-theca has two spurs at the base, whereas the lower anther-theca has but one spur in other Chinese Justicia . The number of spurs of each theca seems to be an important character for delimitating generic boundary in the Acanthaceae. For example, three genera in tribe Ruellieae were found to have two spurs at the base of each theca. Diceratotheca is a genus described from Thailand in recent years( Wood et al., 2012). Stenothyrsus C.B. Clarke was described from the Malaysian state of Perak( Clarke, 1908). The recent studies indicated that Chinese species of Echinacanthus differ from the type of Echinacanthus, Echinacanthus attenuatus Wall., in having two spurs at the base of each theca and represent a new undescribed genus(Nees von Esenbeck, 1832 ; Lo and Fang, 1985 ; Hu and Tsui, 2002 ; Deng et al., 2010 ; Hu et al., 2011 ; Tripp et al., 2013). Thus, it is also necessary to re-evaluate the phylogenetic and taxonomic position of J. microdonta W. W. Smith.
In the present study, we determine the phylogenetic position of J. microdonta based on morphological data, especially the characters of anther, pollen and seed examined using scanning electron microscope(SEM), and molecular data.
2 Material and methods 2.1 Plant materialSamples of pollen grains and seeds of J. microdonta were removed from Deng Yunfei et al . 25860 collected from Butuo Xian, Sichuan Province, China during our field investigations in October, 2014 . Voucher specimens were deposited with the Herbarium of the South China Botanical Garden, Chinese Academy of Sciences(IBSC)and the Herbarium of the Kunming Institute of Botany, Chinese Academy of Sciences(KUN).
In order to determine phylogenetic relationships in Justicieae using molecular markers, we sampled 35 taxa in 18 genera. Leaf material was dried in silica gel, and specimens from our field work were deposited at IBSC. To complete the dataset, additional DNA sequences of 39 species in 39 genera were obtained from GenBank(Table 1).
| Taxon | Voucher specimens | Voucher location | GenBank | |||
| ITS | trnL-F | trnS-G | rps16 | |||
| Asystasia gangetica (L.) T. Anderson | Y.F.Deng 1836 (IBSC) | Yunnan, China | KP744316 | KP744151 | KP744233 | KP744187 |
| Asystasiella neesiana (Wallich) Nees | Y. F. Deng 1915 (iBSc) | Hunan, China | KP744317 | KP744152 | KP744234 | KP744188 |
| Clinacanthus nutans (N. L. Burman) L. | Y. F. Deng 2007002 (IBSC) | Guangdong, China | KP744318 | KP744153 | KP744235 | KP744189 |
| Cosmianthemum viriduliflorum (C. Y. Wu & H. S. Lo) H. S. Lo | Y. Tong 13082342 (IBSC) | Hainan, China | - | KP744224 | KP744230 | KP744227 |
| Crossandra infundibuliformis (L.) Nees | Y. F. Deng 2007006 (IBSC) | Guangdong, China | - | KP744154 | KP744236 | KP744190 |
| Dicliptera chinensis (L.) Jussieu | Y. F. Deng 18025 (IBSC) | Guangdong, China | KP744319 | KP744155 | KP744237 | KP744191 |
| Eranthemum tetragonum A. Diereich ex Nees | Y. F. Deng 18140 (IBSC) | Yunnan, China | KP744320 | KP744156 | KP744238 | KP744192 |
| Hygrophila ringens (L.) R. Brown exSteudel | Y. F. Deng 19358 (IBSC) | Guangxi, China | KP744321 | KP744157 | KP744239 | KP744193 |
| Hypoestes phyllostachya Baker | Y. F. Deng 200711 (IBSC) | Guangdong, China | KP744322 | KP744158 | KP744240 | KP744194 |
| Isoglossa collina (T. Anderson) B. Hansen | Y. G. Wei 06450 (IBSC) | Guangxi, China | KP744323 | KP744159 | KP744241 | KP744195 |
| Justicia acutangula H. S. Lo & D. Fang | Y. G. Wei 06262 (IBSC) | Guangxi, China | KP744324 | KP744160 | KP744242 | KP744196 |
| Justicia adhatoda L. | Y. F. Deng18220 (IBSC) | Yunnan, China | KP744325 | KP744161 | KP744243 | KP744197 |
| Justicia bentonica Linnaeus | Y. F. Deng 17927 (IBSC) | Guangdong, China | KP744326 | KP744162 | KP744244 | KP744198 |
| Justicia brandegeeana Wasshausen & L.B. Smith | Y. F. Deng 20591 (IBSC) | Guangdong, China | KP744327 | KP744163 | KP744245 | KP744199 |
| Justicia demissa N.H. Xia & Y. F. Deng | 2000 MO-IBSC expedition to Hainan 122 (IBSc) | Hainan, China | KP744329 | KP744165 | KP744246 | KP744201 |
| Justicia gendaruss N. L. Burm. | Y. F. Deng 17547 (iBSC) | Hainan, China | KP744338 | KP744174 | KP744255 | KP744210 |
| Justicia grossa C. B. Clarke | Q. L. Wang s. n. (IBSC) | Hainan, China | KP744328 | KP744164 | - | KP744200 |
| Justicia latiflora Hemsley | Y. F. Deng 19854 (IBSC) | Hunan, China | KP744330 | KP744166 | KP744247 | KP744202 |
| Justicia leptostachya Hemsl. | N. H. Xia s. n.(IBSC) | Guangdong, China | KP744331 | KP744167 | KP744248 | KP744203 |
| Justicia microdonta W. W. Smith | Y. F. Deng 25860 (IBSC) | Sichuan, China | KP744315 | KP744150 | KP744232 | KP744186 |
| Justicia microdonta W. W. Smith | Y. F. Deng 25796 (IBSC) | Sichuan, China | KP744222 | KP744223 | KP744229 | KP744226 |
| Justicia patentiflora Hemsley | Y. F. Deng 18515 (iBSc) | Yunnan, China | KP744332 | KP744168 | KP744249 | KP744204 |
| Justicia procumbens L. | Y. F. Deng 18044 (iBSc) | Yunnan, China | KP744333 | KP744169 | KP744250 | KP744205 |
| Justicia pseudospicata H. S. Lo & D. Fang | Y. F. Deng 19922 (IBSC) | Guangxi, China | KP744334 | KP744170 | KP744251 | KP744206 |
| Justicia quadrifaria (Nees) T. Anderson | Y. F. Deng 19148 (IBSC) | Hunan, China | KP744335 | KP744171 | KP744252 | KP744207 |
| Justicia thunbergioides | Wood et al. 22799 (OXF) | Bolivia | KP744336 | KP744172 | KP744253 | KP744208 |
| Justicia vagabunda Benoist | Y.F.Deng21099 (IBSC) | Yunnan, China | - | KP744225 | KP744231 | KP744228 |
| Justicia ventricosa Wallich ex Hooker | Y.F.Deng 17534 (iBSc) | Hainan, China | KP744337 | KP744173 | KP744254 | KP744209 |
| Leptostachya wallichii Nees | Y. F. Deng 20851 (IBSC) | Sichuan, China | KP744349 | KP744185 | KP744266 | KP744221 |
| Mackaya tapingensis (W.W. Sm.) Y. F. Deng & C. Y. Wu | Y. F. Deng 18452 (IBSC) | Yunnan, China | KP744339 | KP744175 | KP744256 | KP744211 |
| Pachystachys lutea Nees | Y. F. Deng 20593 (IBSC) | Guangdong, China | KP744340 | KP744176 | KP744257 | KP744212 |
| Peristrophe japonica (Thunberg) Bremekamp | 2000 MO-IBSC expedition to Hainan 114(IBSc) | Hainan, China | KP744341 | KP744177 | KP744258 | KP744213 |
| Pseuderanthemum graciliflorum | Y. F. Deng 17910 (IBSC) | Yunnan, China | KP744342 | KP744178 | KP744259 | KP744214 |
| Pseuderanthemum latifolium (Vahl) B. Hansen | Y. F. Deng17909 (IBSC) | Yunnan, China | KP744343 | KP744179 | KP744260 | KP744215 |
| Pseuderanthemum polyanthum (C. B. Clarke ex Oliver) | Y. F. Deng 17923 (IBSC) | Yunnan, China | KP744344 | KP744180 | KP744261 | KP744216 |
| Rhinacanthus nasutus (L.) Kurz | Y.F.Deng 17820 (IBSC) | Yunnan, China | KP744345 | KP744181 | KP744262 | KP744217 |
| Rungia chinensis Bentham | Y. F. Deng 19254 (IBSC) | Guangdong, China | KP744346 | KP744182 | KP744263 | KP744218 |
| Rungia mina H. S. Lo | Y. F. Deng 18217 (iBSc) | Yunnan, China | KP744347 | KP744183 | KP744264 | KP744219 |
| Strobilanthes fluviatilis (C. B. Clarke ex W. W. Smith), Moylan & Y. F. Deng | Y. F. Deng 18215 (iBSc) | Yunnan, China | KP744348 | KP744184 | KP744265 | KP744220 |
| Angkalanthus oligophylla Balf. | MillerM10292 (UPS) | Yemen | EU0874781 | EU0875671 | EU0811051 | EU0875331 |
| Anisacanthus brasiliensis Lindau | Silva 2333 (US) | Brazil | EU0874571 | EU0875521 | EU0810761 | EU0875091 |
| Anisotes madagascariensis Benoist | Daniel & Butterwick 6736 (CAS) | Madagascar | AF2897722 | AF2897332 | - | - |
| Aphanosperma sinaloensis (Leonard & Gentry) T. F. Daniel | Daniel 4070cv (CAS) | American | EU0874541 | EU0875501 | EU0810721 | EU0875031 |
| Brachystephanus africanus S. Moore | Luke et al. 6704 (US) | Tanzania | DQ3724693 | - | DQ3724913 | EU087537 |
| Calycacanthus magnusianus K. Schum | Daniel 10072 (CAS) | Sydney | EU0874811 | - | EU0811081 | EU0875361 |
| Carlowrightia haplocarpa Robinson & greenm | Manktelow 715 (UPS) | Mexico | EU0874501 | EU0875481 | EU0810671 | EU0875001 |
| Chalarothyrsus amplexicaulis Lindau | Daniel & Bartholomew 4842gh (CAS) | American | AF2897802 | AF2897402 | EU0810741 | EU0875051 |
| Chorisochora transvaalensis (A. Meeuse) Vollesen | Daniel 9379 (CAS) | South Africa | EU0874741 | EU0875651 | EU0811001 | EU0875281 |
| Chileranthemum pyramidatum (Lindau) T. F. Daniel | Breedlore & Daniel 70767 (CAS) | Mexico | AF2897972 | AF2897522 | - | - |
| Chlamydocardia buettneri Lindau | Accession No. 95-0034-44 (BR) | Belgium | EU0874801 | EU0875691 | EU0811071 | EU0875351 |
| Ecbolium syringifolium (Vahl) Vollesen | Daniel & Butterwick 6733 (CAS) | Madagascar | AF2897862 | AF2897432 | DQ3724803 | EU0875291 |
| Forcipella sp. | Daniel et al. (PH) | Madagascar | EU5288871 | EU5289201 | EU5289581 | EU5290211 |
| Fittonia albivenis (Lindl. Ex Veitch) Brummitt | McDade 1178 (ARIZ) | American | AF2897812 | AF2897412 | EU0810791 | EU0875131 |
| Gypsacanthus nelsonii E. J. Lott, V. Jaram. & Rzed | Daniel 8357 (CAS) | Mexico | AF2897792 | AF2897392 | EU0810701 | EU0875061 |
| Habracanthus charien Leonard | Wood 4547 (US) | Colombia | DQ3724763 | - | DQ3725033 | - |
| Harpochilus neesianus Mart. ex. Nees | Souza et al. 5413(CAS) | Brazil | AF2897622 | AF2897212 | - | - |
| Henrya insularis Nees ex Benth | Jenkins 89-432 (ARIZ) | Mexico | AF1698432 | AF0631252 | EU0810711 | EU0875071 |
| Herpetacanthus stenophyllus Gomez-Laur. & Grayum | Herrera 3855 (ARIZ) | Costa Rica | AF2897952 | AF2897502 | - | - |
| Hoverdenia speciosa Nees | Deniel & Baker 3739 (CAS) | Mexico | AF2897772 | AF2897382 | EU0810891 | EU0875191 |
| Kalbreyeriella rostellata Lindau | McDade 1007 (DUKE) | Colombia | DQ3724733 | - | DQ3724983 | - |
| Kudoacanthus albonervosus Hosok | W. C. Leong 2939 (HAST) | China | EU5288891 | EU5289241 | EU5289651 | EU5290291 |
| Megalochlamys revoluta (Lindau) Vollesen | MaDade & Balkwill 1264 (!) | South Africa | EU0874731 | EU0875641 | EU0810991 | EU0875271 |
| Megaskepasma erythrochlamys Lindau | McDade 253 (DUKE) | Costa Rica | AF1698402 | AF0631262 | EU5289801 | EU5290411 |
| Metarungia galpinii (Baden) C. Baden | Deniel 9322 (CAS) | South Africa | AF2897762 | AF2897372 | EU5289841 | EU5290461 |
| Mexacanthus mcvaughii T. F. Daniel | Van Devender 94-23 (CAS) | Mexico | EU0874331 | EU0875391 | EU0810471 | EU0874841 |
| Mirandea hyssopus (Nees) T. F. Daniel | Diaz B. and Carranza 7498 (CAS) | Mexico | EU0874591 | EU0875551 | EU0810941 | EU0875121 |
| Odontonema tubiforme (Bertol) Kuntze | McDade 1182 (ARIZ) | American | AF1697482 | AF0631272 | DQ0592973 | DQ0592153 |
| Oplonia microphylla (Lam.) Stearn | Ornduff 7814cv (CAS) | American | AF2897982 | AF2897532 | - | - |
| Oreacanthus mannii Benth. | Leeuwenberg 8925 (BR) | Cameroon | DQ3724713 | - | DQ3724953 | - |
| Poikilacanthus macranthus Lindau | Haber 707 (MO) | Costa Rica | AF1698382 | AF0670662 | EU5289941 | EU5290541 |
| Populina richardii Baill. | Kerardren 1671 (P) | Madagascar | EU0874771 | EU0875661 | EU0811041 | EU0875321 |
| Ptyssiglottis pubisepala (Lindau) B. Hansen | Daniel 6630 (CAS) | Papua New Guinea | AF2897872 | AF2897442 | DQ3724833 | EU5290551 |
| Razisea spicata Oerst | Hammel 7974 (DUKE) | Costa Rica | AF1698482 | AF0631312 | DQ3725023 | EU5290561 |
| Ruspolia seticalyx (C. B. Clarke) Milne-Redh | Daniel & Butterwick 6635 (CAS) | American | AF2898002 | AF2897552 | - | - |
| Ruttya fruticosa Lindau | Danile s. n. (CAS) | American | AF2898012 | AF2897562 | - | - |
| Schaueria calicotricha (Link & Otto) Nees | Foote s. n. (CAS) | American | AF2897822 | EU0875561 | EU0810801 | EU0875141 |
| Spathacanthus parviflorus Leonard | Daniel et al. 8403 (CAS) | Mexico | AF2898032 | AF2897572 | - | - |
| Stenostephanus chiapensis T. F. Daniel | Breedlove & Burns 72688cv (CAS) | American | AF2897922 | AF2897472 | DQ3725063 | - |
| Streblacanthus cordatus Lindau | Daniel et al. 8203 (CAS) | Panama | AF2897842 | AF2897422 | EU0810841 | EU0875171 |
| Justicia caudata A. Gray | Faivre 64 (ARIZ) | Mexico | AF1698372 | AF0631342 | EU5289641 | EU5290281 |
| Justicia longii Hilsenb | Van Devender 87-307 (ARIZ) | American | AF1698392 | AF0631352 | - | - |
| Tetramerium nervosum Nees | McDade & Jenkins 1154 (ARIZ) | American | AF1698472 | AF0631332 | EU0810581 | EU0874931 |
| Trichaulax mwasumbii Vollesen | Mwasumbi 14238 (CAS) | Tanzania | EU0874711 | EU0875621 | EU0810971 | EU0875251 |
| Yeatesia mabryi Hilsenb. | Deniel & Baker 3698 (CAS) | American | EU0874601 | EU0875541 | EU0810781 | EU0875111 |
The corolla samples were opened and observed under light microscope and the number of stamens and staminodes were observed immediately. The anther samples were taken from the flower and observed under SEM.
2.3 Pollen and seed morphology observationPollen grains were acetolysed following the modified method of Erdtman(1969) . The sampled pollen grains and seeds were mounted on stubs and coated with gold for 110 s in JFC-1600 sputter coaters(JEOL Ltd, Tokyo, Japan). A JSM-6360LV scanning electron microscope(SEM)(JEOL Ltd, Tokyo, Japan)was used for detailed examination. Measurements of polar axis(P)and equatorial diameter(E)were made on digital SEM images(20-30 pollen grains)with JEOL's Smile View 2.2.6.1 software(JEOL Ltd, Tokyo, Japan). Shape classes of pollen were determined following Erdtman(1969) . The terminology for pollen descriptions follows Punt et al.(2007) , while that for seeds follows Graham(1988) and Liu et al.(2004) .
2.4 DNA amplificationTotal genomic DNA was extracted from silica gel-dried leaves following the method of Doyle and Doyle(1990) . Four DNA fragments(trnS-G, trnL-F, rps16 , ITS)were amplified and sequenced. Each gene was amplified by polymerase chain reaction(PCR)using a total volume of 25 μl containing 1 μl DNA(100 ng/ml), 2.5 μl dNTP(250 mm), 1 μl each primer(0.2 mm), 2.5 μl 10 × buffer, 0.25 μl Taq polymerase(0.2U), and 17.5 μl ddH 2 O. The PCR cycling conditions were exactly the same for all markers, [96 ℃ 3 min, (94 ℃ 1 min, 52 ℃ or 50 ℃ 1 min, 72 ℃ 1 min)× 32 cycles, 72 ℃ 10 min]. The PCR products were purified on a column of sephadex and sequence reactions were read on an ABI 3730 or ABI 3730xl genetic analyzer.
2.5 Phylogenetic analysesSequences for each region were edited by SeqMen(Lasergene 7), aligned with ClustalX( Thompson et al., 1997), and then adjusted by EditPlus manually. All sequences obtained in this study have been deposited in Genbank(Table 1). Maximum parsimony(MP)and Bayesian inference(BI)analyses were used on the individual and combined datasets. MP analyses were run with Paup * v.4.0b10( Swofford, 2003). Heuristic search was performed with 1000 replicates of random addition sequence, tree bisection-reconnection(TBR)branch-swapping, retaining up to a single most parsimonious tree at each replicate. Strict consensus trees were constructed from the most parsimonious trees. Bootstrap analyses(BP)were used to examine the support for individual clades( Felsenstein, 1985). Bootstrap proportions >70% are considered well supported( Hillis and Bull, 1993). Bayesian analyses were performed using MrBayes version 3.1.2( Ronquist and Huelsenbeck, 2003). These were produced using Modeltest 3.7( Posada and Crandall, 1998)by Hierarchical likelihood ratio test(hLRTs)and Akaike information criterion(AIC)( Posada and Buckley, 2004). The Bayesian analyses started from random trees sampling on tree every 100th generation with four incremental heated chains. The Markov chain Monte Carlo(MCMC)algorithm was run for 2000000-4000000 generations for each dataset. The first 1000 trees corresponding to the "burn-in” period were discarded, and the remaining trees were used to construct a majority-rule consensus tree. We consider posterior probabilities(PP)> 0.95 to indicate significant probability for each clade.
3 Results 3.1 Morphological charactersJ. microdonta is subshrub(Fig. 1: B)with the following characteristics: leaves opposite, glabrous on both surfaces; inflorescence a terminal spike with 2-4 flowers per node(Fig. 1: C); bracts linearlanceolate; bracteoles 2, similar to bracts; calyx 5-lobed almost to base, lobes linear-lanceolate(Fig. 1: E); corolla 2-lipped, lower lip 3-lobed, upper lip 2-lobed, tube short, limb longer than tube(Fig. 1: D); stamens 2(Fig. 1: F), filaments pilose, anther bithecate, thecae oblong, each with two white spurs at base(Fig. 1: G); staminodes 2(Fig. 1: F); ovary with 2 ovules in each locule(Fig. 1: H), style pilose; capsule 4-seeded, stalked at the base(Fig. 1 : H).
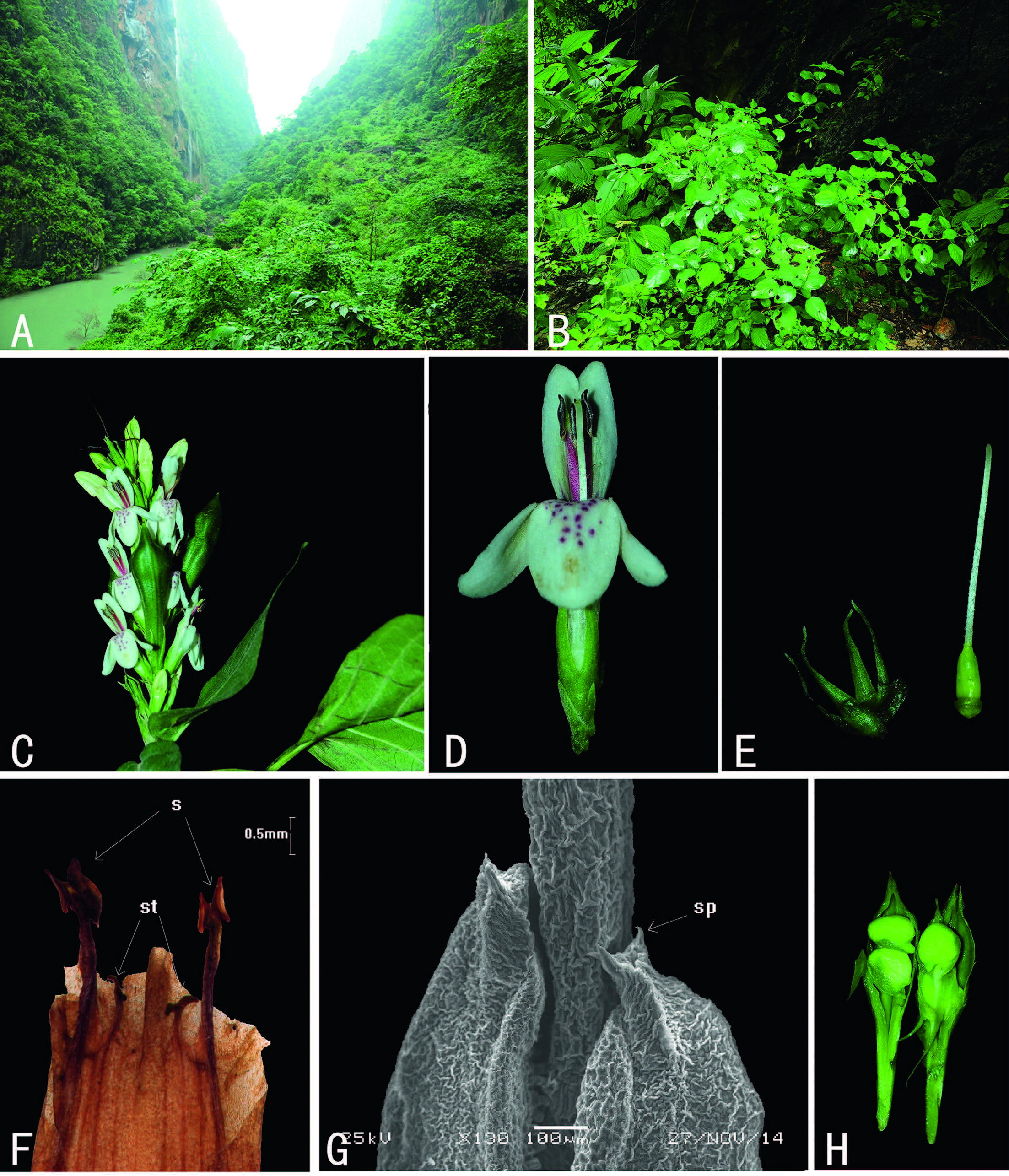
|
| Fig. 1 Wuacanthus microdontus (W.W. Sm.) Y.F. Deng, N.H. Xia & H. Peng. A. Habitat; B. Habit; C. Inflorescence; D. Flower; E. Calyx and pistil; F. Opened corolla showing androecium; G. Anther base showing spurs; H. Opened capsule. s: stamen; st: staminode; sp: spur . |
The morphological observations showed that J. microdonta has an androecium of two stamens and two staminodes(Fig. 1: F), with parallel bithecate anthers, with two very small spurs at the base of each theca(Fig. 1: G).
Pollen grains of J. microdonta are oblate-spheroidal(P/E = 1.15), 37.8(31-34)× 33.0(26-38)μm, triangular in polar view, ellipsoidal in equatorial view, tricolporate, with 2 pseudocolpi per mesocolpium that are shorter than the colpi, perforate exine, and verrucate ornamentation(Fig. 2: A-D).
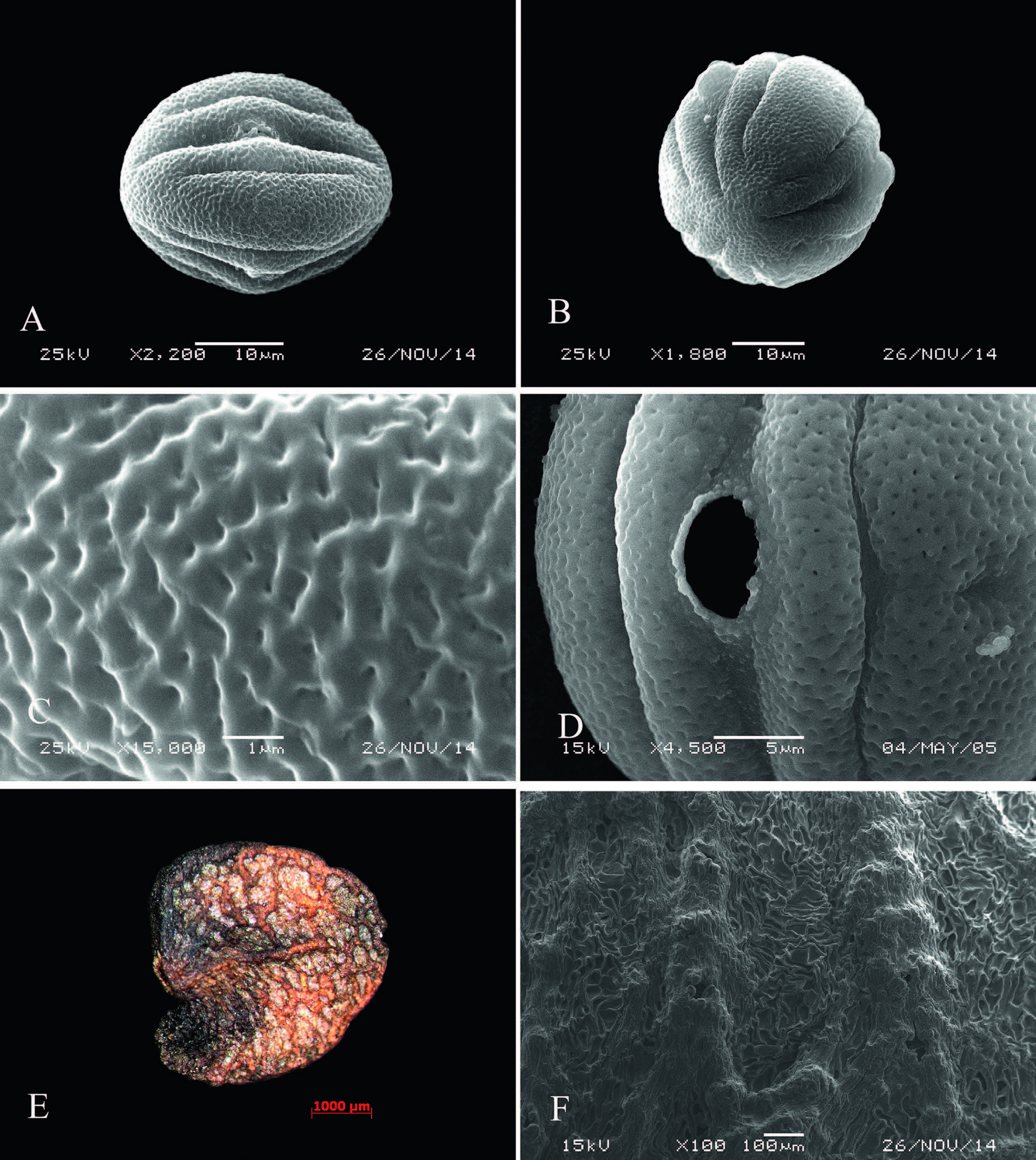
|
| Fig. 2 Pollen and seed morphology of Wuacanthus microdontus (W.W. Sm.) Y.F. Deng, N.H. Xia & H. Peng. A. Equatorial view of pollen grain; B. Polar view of pollen grain; C. Pollen sexine ornamentation; D. & E. Seed under SEM and LE, respectively; F. Testa sculpture. |
Seeds of J. microdonta are broadly ovoid, compressed, 3.85-5.56 × 3.32-4.17 × 1.2-1.89 mm, with favulariate testa folded with granules, and reticulate(Fig. 2: E-F).
3.2 Phylogenetic analysesThe tree topologies produced with maximum parsimony(MP)and Bayesian inference(BI)were similar, and Fig. 2 shows the maximum parsimony tree with the addition of bootstrap support values.
The MP tree formed four main clades(Fig. 3). The result is in accordance with the previous work of McDade et al.(2000) , who recognized four lineages in Justicieae, i.e., the Justicia lineage, Tetramerium lineage, Isoglossinae and Pseuderanthemum lineage. Species of Justicia were separated into three different lineages:(1)the majority of Justicia is accommodated in the Justicia lineage and nested with Dicliptera , Peristrophe , Hypoestes, Codonacanthus, Rhinacanthus, Poikilacanthus, Harpochilus, Megaskepasma, Anisotes, Rungia and Metarungia ;(2)Justicia grossa is included in "Tetramerium" lineage and is closely related to Clinacanthus (PP = 1.00); the Justicia lineage is strongly supported and is a sister group to the "Tetramerium" lineage(PP = 1.00);(3)J. microdonta resolved with the "Pseuderanthemum"lineage, forming a sister taxon with Pseuderanthemum (PP = 1.00).
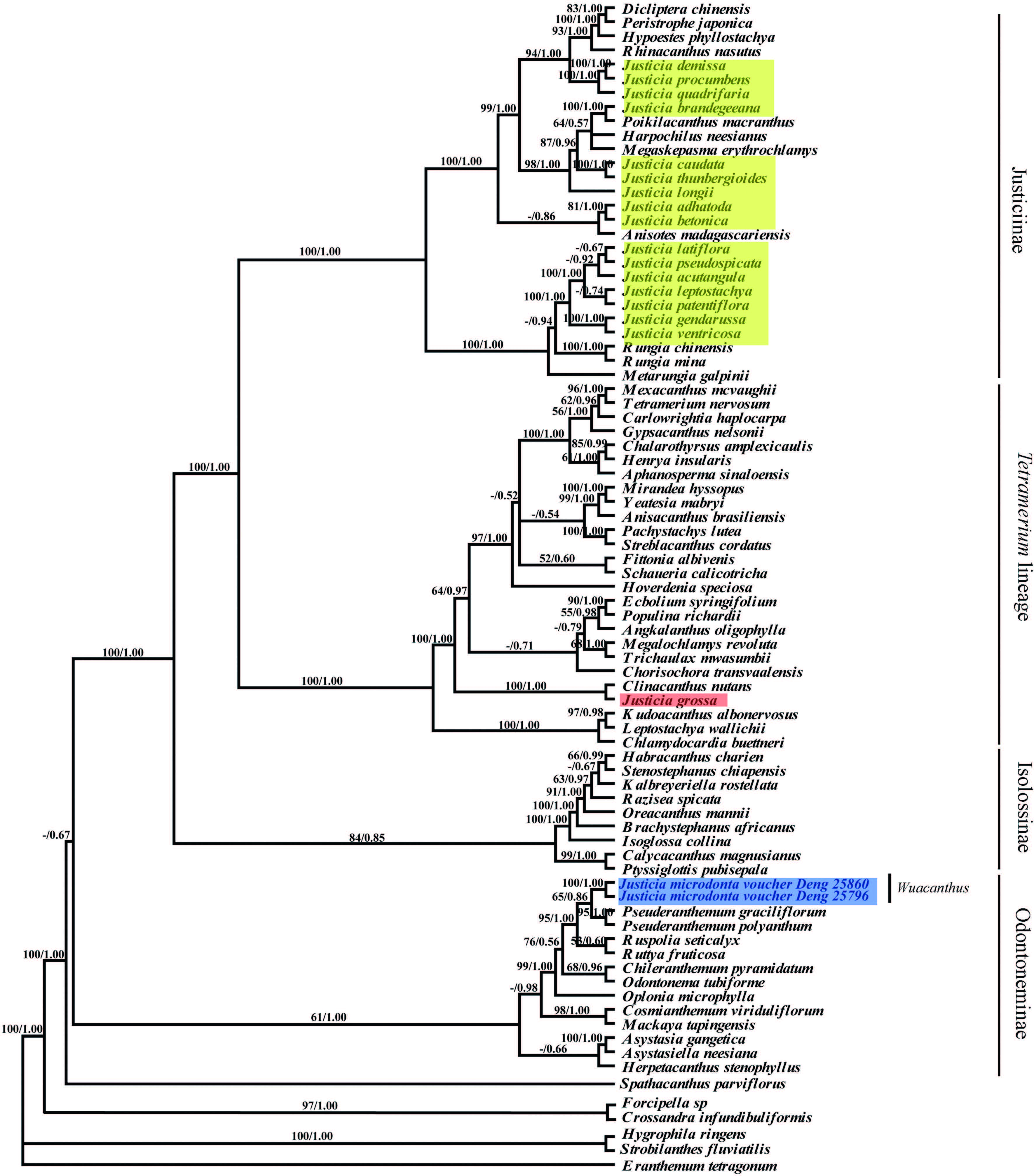
|
| Fig. 3 Maximum parsimony tree based on nuclear ITS and plastid trnL-K, trnS-G and rps16 regions. Numbers above branches indicate bootstrap percentages. |
Justicia is the largest genus in the family Acanthaceae and comprises about 600 species distributed in the tropical and subtropical regions of the world( Graham, 1988 ; Daniel, 1998 ; Mabberley, 2008 ; Hu et al., 2011). Species placed in Justicia have the 2-lipped corolla, two stamens, superposed anther-thecae, the upper one muticous and lower one spurred or not. As with earlier studies( McDade et al., 2000 , 2008), our results show that the genus Justicia is not monophyletic. All sampled taxa of Justicia by McDade et al.(2000 , 2008)were nested in the Justicia lineage. In our analyses, at least seven clades are recognized and all taxa belong to Justicia lineage, except J. microdonta and J. grossa . Morphologically, Justicia is one of the most diverse genera in Acanthaceae. Characters of the anther and pollen are important for delimiting generic boundaries. In Justicia , the two thecae vary from being superposed to parallel and spurred or not at the base. Pollen morphology is one of the important characters for defining generic boundary in the family Acanthaceae( Lindau, 1893 , 1895 ; Bremekamp, 1944 ; Daniel, 1998). Pollen grains of Justicia vary from tricolporate to bicolporate and the pollen have been divided into ten major types by Graham(1988) . The seeds of Justicia vary in shape from spherical to discoid, and the ornamentation of seed surfaces is also variable. Graham(1988) divided the seeds of Justicia into eleven types based on the testa morphology. These characters of androecium, pollen and seeds indicate that the genus Justicia may not be monophyletic. Justicia microdonra and J. grossa were nested in the Pseuderanthemum and Tetramerium lineages, respectively. Both differ from other species of Justicia by both anther-theca being spurred at the base. J. grossa belongs to Justicia sect. Grossa( Hansen, 1987). Justicia sect. Grossa consists of three species and is distributed in S. China, Vietnam, Laos, Thailand and Peninsular Malaysia( Hansen, 1987 , Hu et al., 2011). It might represent another undescribed new genus and will be treated after further studies because only one taxon was sampled in the present study. Because of the limited taxon sampling for Justicia , further studies on generic delimitation will be necessary.
4.2 Morphological evidencePalynologically, the genus Justicia is one of the most diverse genera of Acanthaceae( Lindau, 1895 ; Raj, 1961 ; Graham, 1988 ; Daniel, 1998 ; Hu et al, . 2005 ; Rueangsawang et al., 2013). Lindau(1895) recognized eleven pollen types in the family Acanthaceae and classified the pollen of Justicia as being "Knöotchenpollen", that is, characterized by having two or three pores and an aperture area, with 1-3 rows of insulae on each side of the pore. Graham(1988) recognized ten major pollen types in the genus Justicia. Her types 1-9 belong to the "Knöotchenpollen" category of Lindau(1895) and type 1 is accordance with "Spangenpollen" in being tricolporate with six pseudocolpi( Lindau, 1895 ; Raj, 1961 ; Bremekamp, 1965). Rueangsawang et al.(2013) separated Thai Justicia species into two groups based on pollen morphology based on whether pollen grains had pseudocolpi. One group has the tricolporate pollen grain with six pseudocolpi, which is the same as the Spangenpollen of Lindau(1895) . Another group has bi- to tricolporate pollen grains, equivalent to the "Knöotchenpollen" of Lindau(1895) . "Spangenpollen" was also recognized as a typical character of subtribe Odontoneminae( Lindau, 1895 ; Raj, 1961 ; Bremekamp, 1965). The ornamentation of "Spangenpollen" in Justicia observed by Graham(1988) and Rueangsawang et al.(2013) is microreticulate or reticulate with granules in muri. Pollen grains of J. microdonta were tricolporate with six pseudocolpi and belong to the "Spangenpollen", but differ from other species in having "Spangenpollen" with verrucate ornamentation. The pollen of J. microdonta resemble those of most genera in subtribe Odontoneminae as shown by previous studies( Lindau, 1895 ; Raj, 1961 ; Bremekamp, 1965 ; Stern, 1971 ; Ensermu et al., 1992 ; Daniel, 1993 , 1995 , 1998 ; Scotland and Vollesen, 2000 ; Hu et al., 2005 ; Deng and Wu, 2009). The pollen morphology implied a close relationship between Justicia and subtribe Odontoneminae.
Traditionally, the genus Justicia was recognized by having simple spicate or compound inflorescences, solitary or in sessile clusters, a tubular and bilabiate corolla, two stamens and bithecate and basally spurred anthers(Graham, 1998 ; Darbyshire et al., 2010 ; Hu et al., 2011 ; McDade et al., 2012 ; Rueangsawang et al., 2012 , 2013). The characters of two stamens and two staminodes suggest that Justicia micrantha might be removed from Justicia and more related to the subtribe Odontoneminae ( Lindau, 1895 ; Bremekamp, 1965).
Morphology of the testa cells of the seeds of Acanthaceae is variable and has been used in circumscribing taxa at different ranks( Kippist, 1842 ; Schaffnit, 1906 ; Oehm, 1932 ; Bremekamp, 1944 ;Hedréen, 1988 , 1989 ; Graham, 1988 ; Ensermu et al., 1992 ; Immelman, 1990 ; Balkwill et al., 1996 ; Balkwill and Getliffie Norris, 1988 ; Balkwill and Campbelg-Young, 1999 ; Darbyshire, 2008 ; Rodriguez Greuter and Rankin , 2010 ; Darbyshire et al., 2011 ; Ruengsawang et al., 2012 ; Tripp et al., 2013 ; Kiel and McDade, 2014 ; Al-Hakimi and Latiff, 2015). For example, seeds of Ruellieae have spiral hygroscopic mucilaginous hairs( Kippist, 1842 ; Greuter and Rankin Rodriguez, 2010 ; Duan, 2013). Clarke(1885) described the seeds of Justicia as ovoid and strongly compressed or compressed, and the testa being tubercular, scaly or glochidiate-subspinescent. Balkwill and Getliffie Norris(1988) re-appraised tribal and subtribal limits of the Acanthaceae of southern Africa and considered that seed coats lacking hairs were definitive of the Justicieae and some genera. However, some species of Justicia with hairy seeds were included in later studies( Graham, 1988 ; Ruengsawang et al., 2012). Hedréen(1988) described the seed surface as "tuberculate” in his paper on the Justicia capensis Thunb. group. Later, Hedréen(1989) proposed six testa types in Justicia sect. Harnieria from tropical Africa. Graham(1988) recognized three distinct groups and some 20 seed types using a low-power binocular microscope, and noted that the testa is an important character for distinguishing the sections. Ensermu(1990) observed 12 species of Justicia sect. Anellia from Ethiopia and described the testa of all species as doublereticulate. Immelman(1990) described the seed surface of southern African Justicia and pointed out that seed surface characteristics can be used for delimiting some sections in Justicia . Ruengsawang et al.(2012) observed 30 species of Justicia from Thailand and recognized five types and four subtypes. The seed of J. microdonta resembles some species classified as Type 1 of Graham(1988) and Ruengsawang et al.(2012) , but differs by the reticulate testa sculpture being favulariate folded with granules. However, the seed testa sculpture of J. microdonta is more similar to that of some species of Pseuderanthemum ( Duan, 2013). Seed morphology implied a close relationship between J. microdonta and Pseuderanthemum .
4.3 The new genus Wuacanthus and its systematic positionOur results support the exclusion of J. microdonta from Justicia and its affinity with the Pseuderanthemum lineage, which is defined as the subtribe Odontoneminae by Bremekamp(1965) . The Pseuderanthemum lineage differs from another three lineages of McDade et al.(2000) by having four stamens or two stamens and two staminodes, and was recognized as a basal lineage in Justicieae( McDade et al., 2000). Morphologically, Wuacanthus differs from other Justicia species in having two stamens and two staminodes, which identify it with the Pseuderanthemum lineage. Spur number at the base of each theca seems to be a very useful character for delimiting basic taxonomic boundaries in the family Acanthaceae. Some sister genera are mainly differentiated based on the number of spurs. Chroesthes is separated from Lepidagathismainly by the spurred anther thecae, which are multicous in Lepidagathis ( Hansen, 1983). Three genera in tribe Ruellieae were found to have two spurs at the base of each theca. Diceratotheca J.R.I.Wood et al. is distributed in Thailand( Wood et al., 2012). Stenothyrsus C.B. Clarke was described from the Malaysian state of Perak( Clarke, 1908). Recent studies have shown that Chinese species of Echinacanthus differ from the type of Echinacanthus, E. attenuatus Wall., in having two spurs at the base of each theca and represent another undescribed genus( Nees von Esenbeck, 1832 ; Lo and Fang, 1985 ; Hu and Tsui, 2002 ; Hu et al., 2011 ; Tripp et al., 2013). J. microdonta differs from other genera in the Pseuderanthemum lineage and can be recognized as an independent genus, Wuacanthus , within the Pseuderanthemum lineage.
There are two main clades in the Pseuderanthemum lineage, the Pseuderanthemum and Asystasia clades. The Pseuderanthemum clade is characterized by two stamens and two staminodes and includes Wuacanthus, Pseuderanthemum, Ruspolia, Ruttya, Chileranthemum, Odontonema, Oplonia and Mackaya . The Asystasia clade is characterized by four stamens without staminodes and includes Asistasia and Asystasiella . Wuacanthus formed sister groups with Pseuderanthemum in our studies, and share the characters of the flowers arranged in terminal thyrses, 2-lipped limbs, erect 2-lobed upper lip, reflexed 3-lobed lower lip, two stamens and two staminodes, basally solid 4-seeded capsules., Wuacanthus and Pseuderanthemum formed sister groups with Ruspolia and Ruttya , but differ from the monothecate condition of the latter two genera in having bithecate anthers. Wuacanthus differs from Pseuderanthemum by the shorter corolla tube and bi-spurred theca base of the anther. In Pseuderanthemum , the corolla is salverform with a long slender tube that is not or scarcely apically enlarged, and the antherthecae are multicous at the base( Hu et al., 2011).
5 Taxonomic treatmentWuacanthus Y.F. Deng, N.H. Xia & H. Peng, gen. nov. - Type: Wuacanthus microdontus (W.W.Sm.)Y.F. Deng, N.H. Xia & H. Peng.
Shrubs. Leaves opposite, petiolate, blade margin entire, penninerved. Spikes terminal, with 2-4 flowers per node; bracts linearlanceolate; bracteoles 2, similar to bracts. Calyx 5-lobed almost to base; lobes linear-lanceolate. Corolla 2-lipped, upper lip 2-lobed, lower lip 3-lobed; tube short, limb longer than tube. Stamens 2, included; filaments pilose; anther bithecate, thecae oblong, each with two white spurs at the base. Staminodes 2, minute. Ovary glabrous, 2 ovules in each locule; style densely pilose. Capsule 4- seeded, stalked at the base. Seeds reddish brown, ovoid.
Distribution . Monospecific; endemic to the Jinshajiang Valley, China, occurring in Sichuan and Yunnan Provinces. Jinshajiang Valley is located at the basal zone of the Hengduan Mountain Region, which is situated within the Indo-Burma hotspot, one of 25 biodiversity hotspots defined by Myers et al.(2000) . About 13 genera of seed plants are endemic to the Hengduan Mountain Region, including Acanthochlamys P.C. Kao, Dipoma Franch., Metanemone W.T. Wang, Salweenia E.G. Baker, Sinadoxa C.Y. Wu et al., Smithiorchis T. Tang & F.T.Wang, etc.( Wu, 1988 ; Li and Li, 1993 ; Wu and Wu, 1996). Jinshanjiang Valley has a special and vulnerable ecosystem characterized by aridity, high temperatures, semisavanna vegetation and relatively few plants. Jinshajiang Valley is rich in endemic taxa and four generawere previously recognized as endemic there, viz., Anemoclema (Franch.)W.T. Wang, Nouelia Franch., Musella(Franch.)C.Y.Wu & H.W. Li, and Trailliaedoxa W.W. Sm. & G. Forrest( Jin et al., 1994 ; Guan et al., 2013).
Etymology. The generic name honors ProfessorWu Zhengyi(Wu Chengyih)(1916-2013). Prof. Wu has devoted over 70 years to the study of the flora and vegetation of China. In 2007, he was awarded the State Preeminent Science and Technology Award of China for his outstanding achievements in the fields of systematic botany and plant geography, as well as plant diversity, conservation, and sustainable use of plant resources( Peng et al., 2013 ; Deng et al., 2013). Previously, three generic names, Baijiania A.M. Lu & J.Q. Li( Cucurtibitaceae, Li, 1993), Sinobaijiania C. Jeffrey & W.J. de Wilde(Cucurtibitaceae, Jeffrey and de Wilde, 2006) and Zhengyia T. Deng, D.G. Zhang & H. Sun( Urticaceae, Deng et al., 2013), and nineteen species names were published in honor of Prof. Wu Zhengyi(Kunming Institute of Botany, Chinese Academy of Sciences, 2013 ; Peng et al., 2014).
Wuacanthus microdontus (W.W.Sm.)Y.F. Deng, N.H. Xia & H. Peng, comb. nov .≡Justicia microdonta W.W. Sm. in Not. Bot. Gard. Edinb. 10: 183. 1918. ≡ Mananthes microdonta (W.W. Sm.)C.Y.Wu & C.C. Hu in C. C. Hu, Fl. Reipubl. Popularis Sin. 70: 296. 2002. - Type: CHINA. Yunnan : mountains of the Chungtien Plateau [27°30′N], July 1914, G. Forrest 12822(Lectotype E! here designated; isolectotype K!). Fig. 4 .
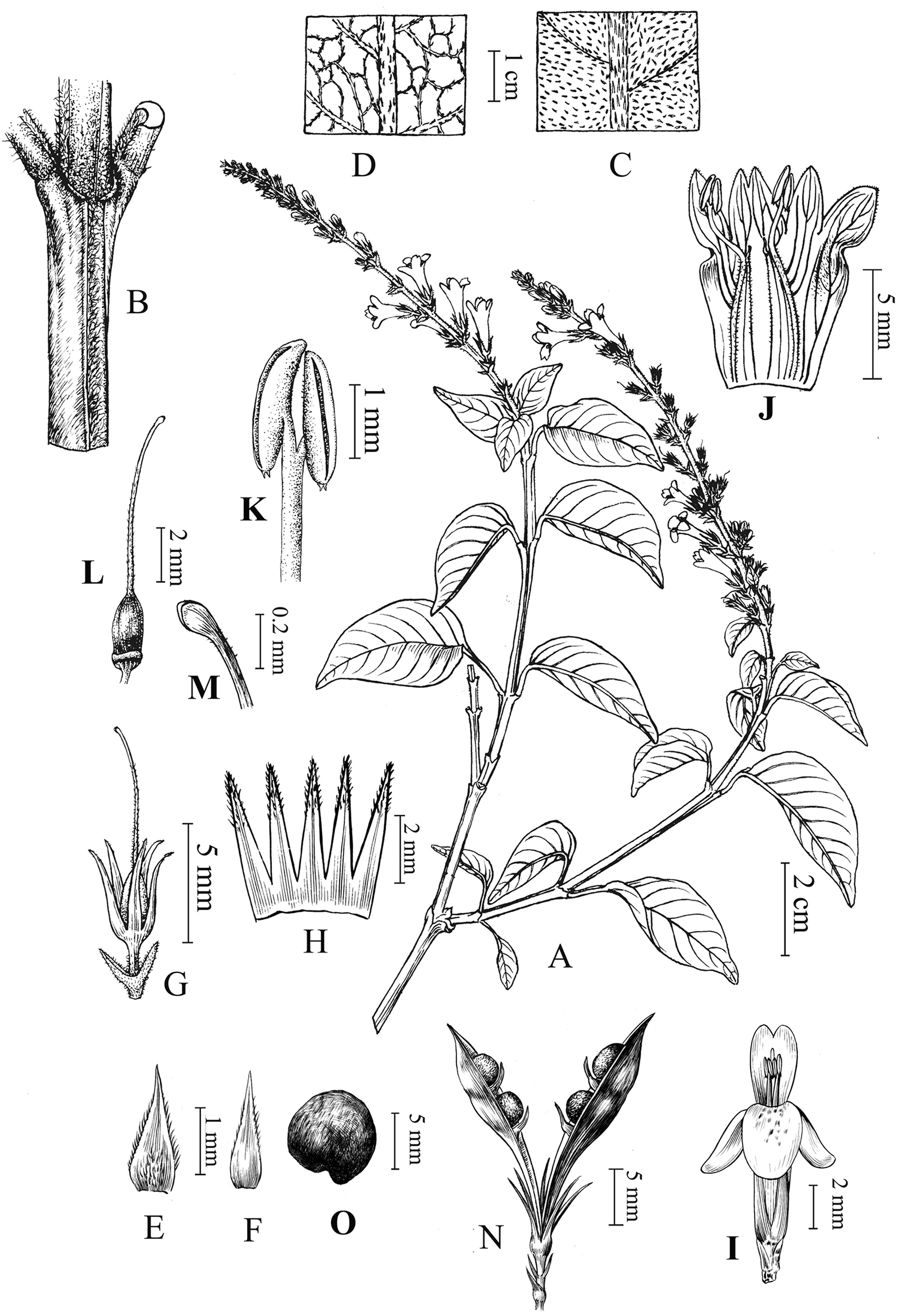
|
| Fig. 4 Wuacanthus microdontus (W.W. Sm.) Y.F. Deng, N.H. Xia & H. Peng. A. Flowering branch; B. Portion of stem; C. Upper leaf surface; D. Lower leaf surface; E. Bract; F. Bracteole; G. Calyx; H. Opened calyx; I. Flower; J. Opened corolla showing androecium; K. Anther; L. Pistil; M. Stigma; N. Capsule; O. Seed. AeG, IeM drawn from F. Ducloux 5741 (P) by Yun-Xiao Liu, GeH, NeO drawn by Ding-Han Cui from Y.F. Deng et al. 25860 (IBSC). |
Shrubs to 2 m tall, much branched. Branches quadrangular, sparsely pilose. Petiole 1-2 cm; leaf blade papery, ovate, (2-)3-5.5 × 1.5-2.5 cm, abaxially light green and pilose along midvein, adaxially green and pilose especially along veins, base broadly cuneate and decurrent onto petiole, margin entire, apex shortly acuminate to acute, lateral veins 5-8 pairs. Spikes terminal, 5-7(-10)cm, unbranched, nodes distant, with 2-4 flowers per node; peduncle short; rachis densely pilose; bracts linear-lanceolate, 3-5 cm; bracteoles similar to bracts. Calyx ca. 7 mm, 5-lobed almost to base; lobes linear-lanceolate, 5-6 mm, pilose along the midveins. Corolla white, with purplish-red dots on lower lip inside, 1-1.3 cm, outside somewhat pubescent to glabrescent; tube short; limb longer than tube; lower lip 4-5 mm, 3-lobed, lobes pilose inside and ciliate on margin; upper lip 2-lobed. Stamens 2, included; filaments pilose, purple; anther bithecate, thecae oblong, ca. 2 mm long, each with two white spurs at base. Staminodes 2, minute. Ovary glabrous; style densely pilose. Capsule ca. 2 cm, 4- seeded. Seeds reddish brown, circular in outline, 3-4 mm in diameter, favulariate folded with granules, reticulate.
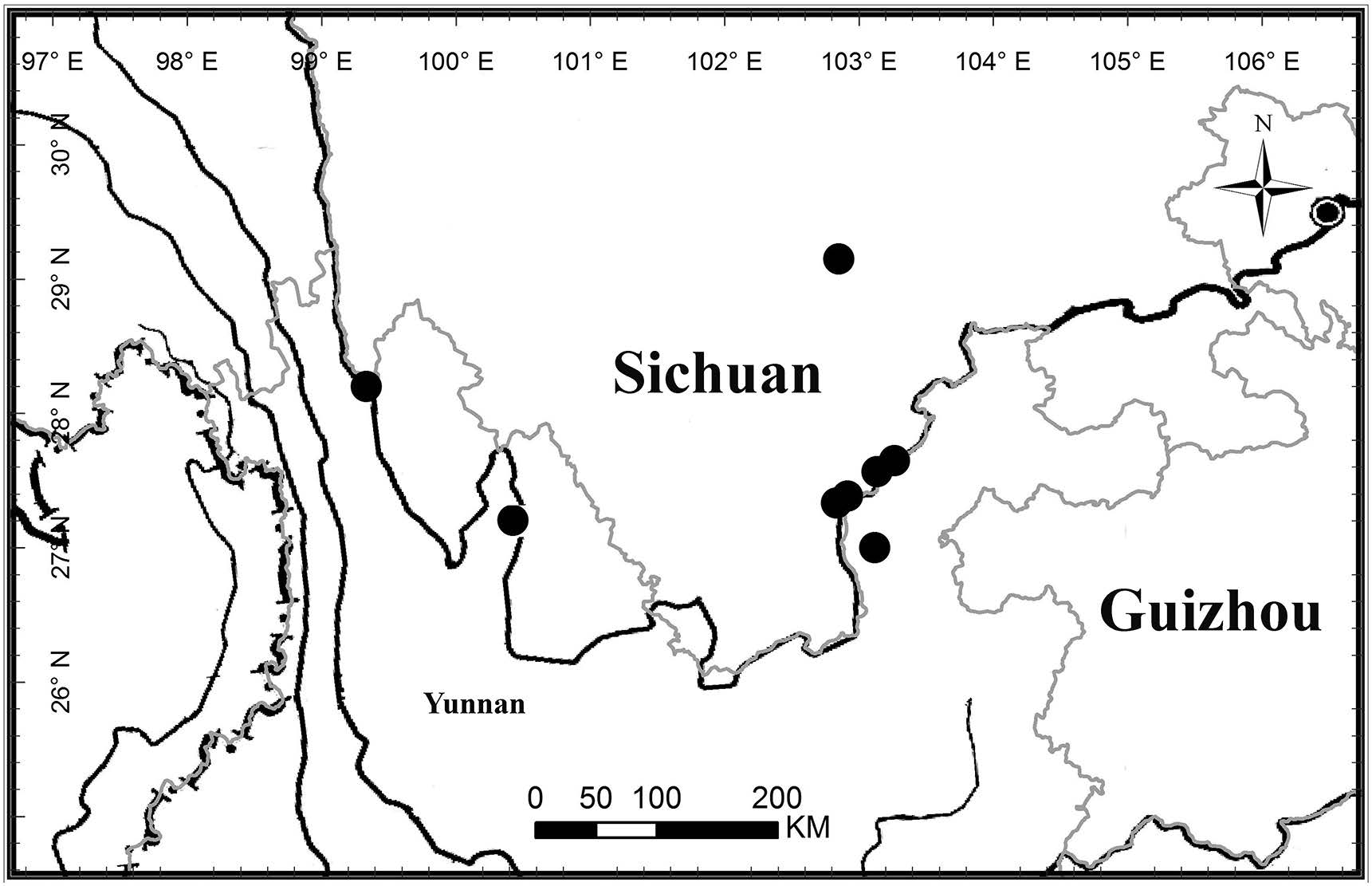
Distribution and habitat . Endemic to the Hengduan Mountains, China, occurring inNW Yunnan and SW Sichuan(Fig. 5). It grows in thickets at rocky sites in dry valleys at 690-1500 m.
|
| Fig. 5 Distribution of Wuacanthus microdontaus (W.W. Sm.) Y.F. Deng, N.H. Xia & H. Peng (solid circles). |
Conservation status . This species is only known from nine collections from seven localities. Amongst these, three collections from Yunnan Province were collected more than 100 years ago. Before the authors re-collected the species at the two localities of Jinyang and Butuo in Sichuan Province in 2014, others were collected more than 30 years ago. Only several collections were collected from a few localities but it would potentially occur in the Jinshajiang Valley. Apart from habitat destruction, no other specific threats are known. The provisional conservation status of the species could therefore be considered as Endangered EN B2ab(iii), based on the IUCN Red List Categories and Criteria( IUCN, 2012 , 2014). On the other hand, for the present, it may be better to consider this as Data Deficient(DD)because there have been no specific field searches for the population of the species.
Phenology . It was observed flowering from July to October and fruiting from October to next February.
Typification . In his protologue, Smith(1918) cited two collections(G. Forrest 12822 and 13162 )and both of them are syntypes according to Art. 9.5 of the ICN( McNeill et al., 2012). Both are in accordance with the original description and can be candidates for being lectotype. Here we choose G. Forrest 12822 as the lectotype.
Additional specimens examined . CHINA. Sichuan : Butuo Xian, Jiaojihe Qu, Dadiba, hilltop, pathside, in the thickets, fl. white, Aug. 20, 1959, Chuan Jing Liang 5636(KUN); Butuo Xian, Niujiaowan Xiang, bank of Xixihe, 690 m, 11 October 2014, Y.F. Deng with H. Peng, Y. Tong & Y.P. Chen 25860 (IBSC, KUN); Ganluo Xian, near Suxiong Qu, 800 m, Sept. 24, 1976, unknown collector 14291 (CDBI); Jinyang xian, Tiandiba Zhen, 810 m, in the thicket, ravine, 9 October 2014, Y.F. Deng with H. Peng, Y. Tong & Y.P. Chen 25796 (IBSC, KUN); Jinyang Xian, Tiandiba Zhen, Jinshajiang Valley, 800 m, Oct. 26, 1985, H. Li 249 (KUN); Jinyang Xian, on the way from Lugao to Pailai, 1100 m, on slope, roadside, Aug. 19, 1964, T.P. Zhu et al 295 (CDBI). Yunnan : Deqên Xian, Yangtze Valley at Paung-tzu-la [28°12′N], 7000 ft, Aug 1914, G. Forrest 13162 (E, K); Yulong Naxizu Zizhixian, Baoshan Xiang, Shitoucheng, October 2014, Z.K. Wu s.n .(IBSC); Qiaojia Xian, Siao Fa Ka dans la region de Kiao Kia, 4 Febuary 1908, F. Ducloux 5741 (P).
Acknowledgements: This study was supported by the National Natural Science Foundation of China(grant nos. 30670124,31270247,31470302,31400184,31670191,31110103911)and Science and Technology BasicWork(grant no. 2013FY112100).We sincerely thank Mrs. Yun- Xiao Liu(IBSC)and Mr. Ding-Han Cui(IBSC)for preparing the line drawings; Dr. Sheng-Xiang Yu for preparing the distribution map; Prof. Wong Khoon Meng(SING)for improving the manuscript; Dr. Yihua Tong(IBSC)and Ms. Ya-Ping Chen(KUN)for their assistance with field work; and the curators of the herbaria CDBI,E,IBSC,KUN and P for facilitating access to their collections.| Al-Hakimi, A.S. Latiff, A, 2015. Pollen and seed morphology of Ruellia L. Phaulopsis Willd. and Dyschoriste Nees (Acanthaceae: Ruellioideae: Ruellieae) of Yemen. Plant Syst. Evol, 301, 1 -13. DOI:10.1007/s00606-014-1035-7 | ||
| Balkwill, K. Campbell-Young, G, 1999. Taxonomic studies in Acanthaceae: testa microsculpturing in southern African species of Thunbergia. Bot.J. Linn. Soc, 131, 301 -325. DOI:10.1111/boj.1999.131.issue-3 | ||
| Balkwill, K. , Getiliffe Norris F, 1988. Classification of the Acanthaceae: a southern African perspective. Monogr. Syst. Bot, 25, 503 -516. | ||
| Balkwill, K. Getiliffe Norris, F. Balkwill, M, 1996. Systematic studies in the Acan- thacea: Dicliptera in southern Africa. Kew Bull, 51, 1 -16. DOI:10.2307/4118744 | ||
| Bremekamp, C.E.B, 1944. Materials for a monograph of the Strobilanthinae (Acan- thaceae). Verh. Kon. Akad. Wetensch. Afd. Natuurk. Sect, 41, 1 -305. | ||
| Bremekamp, C.E.B, 1948. Notes on the Acanthaceae ofJava. Verk. K. Ned. Akad. Natuurk. Sect, 45(2), 1 -78. | ||
| Bremekamp, C.E.B, 1965. Delimitation and subdivision of the Acanthaceae. Bull. Bot. Surv, 7, 21 -30. | ||
| Clarke, C.B, 1885. Acanthaceae. In: Hooker, J.D. (Ed.), The Flora of British India, vol. 4. Reeve & Co, London, 387 -558. | ||
| Clarke, C.B, 1908. Family LXXXIX. Acanthaceae. In: King, G. (Ed.), Materials for the flora of the Malayan Peninsula. J. Asiat. Soc. Bengal, 74, 628 -698. | ||
| Daniel, T.F, 1993. New and Reconsidered Mexican Acanthaceae V, vol. 19. Contr. Univ. Michigan Herb, 271 -291. | ||
| Daniel, T.F, 1995. Revision of Odontonema (Acanthaceae) in Mexico, vol. 20. Con- trib. Univ. Michigan Herb, 147 -171. | ||
| Daniel, T.F, 1998. Pollen morphology of Mexican Acanthaceae: diversity and sys-tematic significance. Proc. Calif. Acad. Sci, 50, 217 -256. | ||
| Daniel, T.F. MaDade, L.A. Manktelow, M ., et al., 2008. The “Tetramerium Lineage” (Acanthaceae: Acanthoideae: Justicieae): delimitation and infra-lineage re-lationships based on cp and nrITS sequence data. Syst. Bot, 33, 416 -436. DOI:10.1600/036364408784571581 | ||
| Darbyshire, I. Vollesen, K. Ensermu, K, 2010. Flora of Tropical East Africa, Acan- thaceae (Part 2). oyal Botanic Gardens, Kew, UK, 287 -755. | ||
| Darbyshire, I. Pearce, L. Banks, H, 2011. The genus Isoglossa (Acanthaceae) in west A.ica. Kew Bull, 66, 1 -15. DOI:10.1007/s12225-011-9254-3 | ||
| Darbyshire, I, 2008. Notes on the genus Dicliptera (Acanthaceae) in Eastern Africa. Kew Bull, 63, 361 -383. DOI:10.1007/s12225-008-9053-7 | ||
| Deng, T. Kim, C.K. Zhang, D. G., et al., 2013. Zhengyia shengnongensis: a new bul- biliferous genus and species of the nettle family (Urticaceae) from central China exhibiting parallel evolution of the bulbil trait. Taxon, 62, 89 -99. | ||
| Deng, Y.F. Wu, Z .Y, 2009. A new combination in Mackaya (Acanthaceae), with lectotypification for Mackaya tapingensis. Novon, 19, 307 -309. DOI:10.3417/2007009 | ||
| Deng, Y.F. Hai, D.V. Xuyen, D .T, 2010. Echinacanthus Nees (Acanthaceae), a newly recorded genus from Vietnam. J. Trop. Subtrop. Bot, 18, 40 -42. | ||
| Doyle, J.J. , Doyle J.L, 1990. A rapid total DNA preparation procedure for fresh plant tissue. Focus, 12, 13 -15. | ||
| Duan, P.N., 2013. Seed Morphology of Acanthaceae and its Systematic Implications. M. Sc. Thesis. Nanjing Forestry University, Nanjing. | ||
| Ensermu, K, 1990. Justicia sect. Ansellia (Acanthaceae). Symb. Bot. Upsal, 29(2), 1 -99. | ||
| Ensermu, K. , Brummitt R.K., Furness C.A, 1992. A reconsideration of Asystasiella Lindau (Acanthaceae). Kew Bull, 47, 669 -675. DOI:10.2307/4110705 | ||
| Erdtman, G, 1969. The Acetolysis method. A revised description. Sven. Bot. Tids, 54, 561 -564. | ||
| Felsenstein, J, 1985. Phylogenies and the comparative method. Am. Nat, 125, 1 -15. DOI:10.1086/284325 | ||
| Gao, C.M, 2010. Phylogenetic Relationship Among Acanthaceae from China. | ||
| Graham, V.A.W, 1988. Delimitation and infra-generic classification of Justicia (Acanthaceae). Kew Bull, 43, 551 -624. DOI:10.2307/4129957 | ||
| Greuter, W. , Rankin R.R, 2010. Notes on some endemic Cuban species of Ruelliinae (Acanthaceae), on their seeds, pollen morphology and hygroscopic features. Willdenowia, 40, 285 -304. DOI:10.3372/wi.40.40210 | ||
| Guan, M.M. , Ma R., Gong X, 2013. Conservation genetics of an endemic plant, Anemoclema glaucifolium, in the Jinsha River Valley. Plant Diver. Res, 35, 555 -562. | ||
| Hansen, B, 1983. Notes on the genus Chroesrhes (Acanthaceae). Nord. J. Bot, 3, 207 -211. DOI:10.1111/j.1756-1051.1983.tb01067.x | ||
| Hansen, B, 1987. Justicia sect. Grossa sect. nov. (Acanthaceae). Nord. J. Bot, 7, 505 -509. DOI:10.1111/j.1756-1051.1987.tb02017.x | ||
| Hedrén, M, 1988. The Justicia capensis species group (sect. Harnieria, Acanthaceae) in Tropical Africa. Kew Bull, 43, 349 -359. DOI:10.2307/4113744 | ||
| Hedrén, M, 1989. Justicia sect. Harnieria (Acanthaceae) in tropical Africa, vol. 29. Acta Univ. Upsal. Symb. Bot. Upsal, 1, 1 -141. | ||
| Hillis, D.M. , Bull J.J, 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol, 42, 182 -192. DOI:10.1093/sysbio/42.2.182 | ||
| Hu, J.Q., Deng, Y.F., Daniel, T.F., 2011. Justicia. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds.), Flora of China (Cucurbitaceae-Valerianaceae with Annonaceae and Berberidaceae), vol. 19. Science Press, Beijing, and Missouri Botanical Garden Press, St. Louis, pp. 449-461. | ||
| Hu, C.C., Tsui, H.P., 2002. Acanthaceae. Flora Reipublicae Popularis Sinicae. Tomus 70. Science Press, Beijing. | ||
| Hu, C.C. Tsui, H.P. Xi, Y. Z., et al., 2005. Pollen morphology of one genus in Lep- idagathideae, two in Andrographideae and eight in Justicieae (Acanthaceae) from China. Acta Phytotax. Sin, 43, 151 -162. DOI:10.1360/aps020124 | ||
| Immelman, K.L, 1990. Studies in the southern African species of Justicia and Siphonoglossa (Acanthaceae): seeds. Bothalia, 20, 49 -59. | ||
| IUCN, 2012. IUCN Red List Categories and Criteria. Version 3.1, second ed. Prepared by IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cam-bridge, UK, p. 35. | ||
| IUCN, 2014. Guidelines for Using the IUCN Red List Categories and Criteria. Version 11. Prepared by the Standards and Petitions Subcommittee of the IUCN Species Survival Commission. Download from. http://www.iucnredlist.org/documents/RedListGuidelines.pdf (Accessed 30 November 2014). | ||
| Jeffrey, C. de , Wilde W.J.J.O, 2006. A review of the subtribe Thladianthinae (Cucurbitaceae). Bot. Zhurn. (St. Petersburg), 91, 766 -776. | ||
| Jin, Z.Z. , Ou X.K., Ou P.D., et al., 1994. A preliminary study on the floristic characteristics of seed plants in the dry hot river valley ofJinshajiang. Acta Bot. Yunnan, 16, 1 -16. | ||
| Kiel, C.A., McDade L.A., Daniel T.F., et al., 2006. Phylogenetic delimitation of Iso- glossinae (Acanthaceae: Justicieae) and relationships among constituent genera. Taxon, 55, 683 -694. DOI:10.2307/25065644 | ||
| Kiel, C.A., McDade L.A, 2014. The Mirandea clade (Acanthaceae, Justicieae, Tetra- merium lineage); Phylogenetic signal from molecular data and micromor?phology makes senseof taxonomic confusion caused by remarkeble diversity of floral form. Syst. Bot, 39, 950 -964. DOI:10.1600/036364414X681446 | ||
| Kippist, R, 1842. On the existence of spiral cells in the seeds of Acanthaceae. Trans. Linn. Soc. Lond, 19, 65 -76. DOI:10.1111/j.1096-3642.1842.tb00077.x | ||
| Kunming Institute of Botany, Chinese Academy of Sciences, 2014. In Memory of Wu Zhengyi. Yunnan Science and Technology Publishing House, Kunming. | ||
| Li, J.Q, 1993. A revision of the genus Siraitia Merr. and two new genera of Cucur- bitaceae. Acta Phytotax. Sin, 31, 45 -55. | ||
| Li, X.W., Li J, 1993. A preliminary floristic study on the seed plants from the region of Hengduan Mountain. Acta Bot. Yunnan, 15, 217 -231. | ||
| Lindau, G, 1893. Beitrage zur Systematik der Acanthaceen. Bot. Jahrb. Syst, 18, 36 -64. | ||
| Lindau, G, 1895. canthaceae. In: Engler, A. Prantl, K. (Eds.), Die naturlichen Pflanzenfamilien, vol. 4. Engelmann, Leipzig, 274 -353. | ||
| Liu, C.J., Lin Q., He J.X, 2004. Methods and terminology of study on seed morphology from China. Acta Bot. Boreal.-Occident. Sin, 24, 178 -188. | ||
| Lo, H.S., Fang D, 1985. Echinacanthus - An acanthaceous genus new to the Chinese flora. Acta Bot. Yunnan, 7, 137 -142. | ||
| Mabberley, D.J., 2008. Mabberley's Plant-book: a Portable Dictionary ofPlants, Their Classification and Uses. Cambridge University Press, Cambridge. | ||
| McDade, L.A., Daniel T.F., Masta S.E., et al., 2000. Phylogenetic relationships within the tribe Justicieae (Acanthaceae): evidence from molecular sequences, morphology, and cytology. Ann. Missouri Bot. Gard, 87, 435 -458. DOI:10.2307/2666140 | ||
| McDade, L.A., Daniel T.F., Kiel C.A, 2008. Toward a comprehensive understading of phylogenetic relationships among lineages of Acanthaceae s.l. (Lamiales). Am. J. Bot, 95, 1136 -1152. DOI:10.3732/ajb.0800096 | ||
| McDade, L.D., Daniel T.F., Kiel C.A., et al., 2012. Phylogenetic placement, delimita?tion, and relationships among genera of the enigmatic Nelsonioideae (Lamiales: Acanthaceae). Taxon, 61, 637 -651. | ||
| McNeill, J., Barrie, F.R., Buck, W.R., et al., 2012. International Code of Nomenclature for Algae, Fungi, and Plants (Melbourne Code), Adopted by the Eighteenth In-ternational Botanical Congress Melbourne, Australia, July 2011. [Regnum Veg- etabile 154]. Koeltz Scientific Books, Konigstein. | ||
| Myers, N., Mittermeier R.A., Mittermeier C.G., et al., 2000. Biodiversity hotspots for conservation priorities. Nature, 403, 853 -858. DOI:10.1038/35002501 | ||
| Nees von Esenbeck, C.G.E, 1832. Acanthaceae. In: Wallich, N. (Ed.), Plantae asiaticae rariores, vol. 3. Richter, London, 70 -117. | ||
| Oehm, G, 1932. Beitrag zur Morphologie und Anatomie einiger Acanthaceen- Fruchte und-Samen. Beih. Bot. Centralbl, 49, 413 -444. | ||
| Peng, C.I., Wang H., Kono Y., Yang H.A, 2014. Begonia wui-senioris (sect. Platy- centrum, Begoniaceae), a new species from Myanmar. Bot. Stud, 55, 13 . DOI:10.1186/1999-3110-55-13 | ||
| Peng, H., Yang Q.E., Li D.Z, 2013. In memory of Wu Zheng-Yi (Wu Cheng-Yih) (1916-2013). Plant Diver. Res, 35, 533 -535. | ||
| Posada, D., Buckley T.R, 2004. Model selection and model averaging in phyloge?netics: advantages of akaike information criterion and Bayesian approached over likelihood ratio tests. Syst. Biol, 53, 793 -808. DOI:10.1080/10635150490522304 | ||
| Posada, D., Crandall K.A, 1998. Modeltest: testing the model of DNA substitution. Bioinformatics, 14, 817 -818. DOI:10.1093/bioinformatics/14.9.817 | ||
| Punt, W., Hoen P.P., Blackmore S., Nilsson S., Le Thomas A, 2007. Glossary of pollen and spore terminology. Rev. Palaeobot. Palyn, 143, 1 -81. DOI:10.1016/j.revpalbo.2006.06.008 | ||
| Raj, B, 1961. Pollen morphological studies in the Acanthaceae. Grana Palynol, 3, 3 -108. | ||
| Reveal, J.M., 2012. An outline of a classification scheme for extant flowering plants. Phytoneuron 2012-37, 1-221. | ||
| Ronquist, F., Huelsenbeck J.P, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572 -1574. DOI:10.1093/bioinformatics/btg180 | ||
| Rueangsawang, K., Chantaranothai P., Simpson D.A, 2012. Contributions to the seed morphology and taxonomy of Justicia (Acanthaceae) from Thailand. J. Syst. Evol, 50, 153 -162. DOI:10.1111/jse.2012.50.issue-2 | ||
| Rueangsawang, K., Chantaranothai P., Simpson D.A, 2013. Pollen morphology of Justicia L. (Acanthaceae) from Thailand and its taxonomic value. Grana, 52, 275 -288. DOI:10.1080/00173134.2013.819526 | ||
| Schaffnit, E, 1906. Beitrage zur Anatomie der Acanthaceen-Samen. Beih. Bot. Cen- tralbl, 19, 453 -521. | ||
| Scotland, R.W., Vollesen K, 2000. Classification of Acanthaceae. Kew Bull, 55, 513 -589. DOI:10.2307/4118776 | ||
| Smith, W.W, 1918. Diagnoses specierum novarum in herbario Horti Regii Botanici Edinburgensis congnitarum (Species asiaticae) CCCLI-CCCC. Notes Roy. Bot. Gard. Edinb, 10, 167 -204. | ||
| Stern, W.L, 1971. A survey of the tropical genera Oplonia and Psilanthele (Acan- thaceae). Bull. Br. Mus. (Nat. Hist.) Bot, 7, 261 -323. | ||
| Swofford, D.L., 2003. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0b10. Sinauer, Sunderland, Massachusetts. | ||
| Thompson, J.D., Gibson T.J., Plewniak F., et al., 1997. The Clustal X windows inter-face: flexible strategies for multiple sequence alignment by qualitu analysis tools. Nucl. Acids. Res, 24, 4875 -4882. | ||
| Tripp, E.A., Daniel T.F., Fatimah S., et al., 2013. Phylogenetic relationships within Ruellieae (Acanthaceae) and a revised classification. Int. J. Plant Sci, 174, 97 -137. DOI:10.1086/668248 | ||
| Wood, J.R.I., Williams B.P., Scotland R.W, 2012. Diceratotheca, a new genus of Acanthaceae from Thailand. Kew Bull, 67, 1 -9. DOI:10.1007/s12225-012-9328-x | ||
| Wood, J.R.I, 2014. New names and combinations in Indian Acanthaceae. Novon, 23, 385 -395. DOI:10.3417/2013046 | ||
| Wu, Z.Y, 1988. Hengduan mountain flora and her significance. J. Jpn. Bot, 63, 297 -311. | ||
| Wu, Z.Y., Wu, S.G., 1996. A proposal for a new floristic kingdom (Realm) - The E. Asiatic Kingdon, its delineation and characteristics. In: Zhang, A.L., Wu, S.G. (Eds.), Floristic Characteristics and Diversity of East Asian Plants, Proceedings of the First International Symposium on Floristic Characteristics and Diversity of East Asian Plants. Higher Education Press, Beijing, pp. 3-42. |


