Conservationists have long recognized that the recovery of an imperiled plant species may require augmentation of existing populations or the creation of new ones and hundreds of such introductions have been carried out in recent decades (Maschinski et al., 2012). However, assessing the success of plant introductions is problematic for several reasons. First, defining success is often difficult because of lack of knowledge of the biology, autecology or genetics of the target species. Moreover, “success” is often based on short-term results, poorly defined criteria and inadequate monitoring (Menges, 2008). Few results are available beyond ten years post-introduction (Godefroid et al., 2011; Dalrymple et al., 2012) and initial successes may not persist (Drayton and Primack, 2012). In addition, the published literature inflates the success rate of plant introductions (Godefroid et al., 2011): practitioners are generally reluctant to report and journals reluctant to publish accounts of introduction failures. Nonetheless, enough information has accumulated on plant introductions to make some generalizations, such as the advantages of using transplants rather than seeds or seedlings (Albrecht and Maschinski, 2012), the advantages of larger founder sizes (Albrecht and Maschinski, 2012), and the need to carefully consider favorable sites that match the species' niche (Dalrymple et al., 2012; Knight, 2012).
Plant (re) introductions for conservation goals echo classic reciprocal transplant experiments documenting the genetic basis of adaptive morphological differences across ecological gradients (Clausen et al., 1941). When properly designed as ecological experiments (Guerrant and Kaye, 2007; Menges, 2008), even introductions that are not biologically successful may be successful in answering critical questions about the biology or autecology of an imperiled species. The most common hypotheses used in experimental introductions involve comparisons of habitats and microhabitats (Guerrant, 2012; Dunwiddie and Martin, 2016). The suitability of microhabitats for transplants may vary with plant stage and year (Wendelberger and Maschinski, 2016) but, in general, can be particularly useful in informing subsequent introductions, in a process of adaptive introductions (Menges, 2008). However, underreporting of failed introductions may bias our understanding. Recent reviews of plant introductions by Godefroid et al. (2011) and Guerrant (2012) agree on the need for practitioners to report failure as well as success.
In evaluating success of introductions, it may be arbitrary to set quantitative levels of survival, reproduction, etc. One way to place introductions into context is to compare them to wild populations. Comparisons can provide insights into the levels of vital rates, environmental conditions affecting populations, and the effects of disturbance regimes on introductions. However, few studies have compared wild vs. introduced populations. Those that have made such comparisons (e.g., Bell et al., 2003; Maschinski and Duquesnel, 2007; Colas et al., 2008) have found it provides additional insights. For example, Colas et al. (2008) found that introduced populations, which received additional care, had higher plant survival than wild populations, but had reduced fecundity. To counter low colonization, they recommended repeated introductions over time.
In this paper, we evaluate a series of experimental introductions of an endangered shrub, Florida ziziphus Pseudoziziphus (Condalia, Ziziphus) celata. These introductions sequentially tested a series of hypotheses about the effects of microhabitat requirements, fire, and propagule source on the vital rates of transplants and seeds introduced into various protected areas. The sequence of introductions was visualized in an “adaptive introduction” framework (Menges, 2008), where ongoing results of prior introductions suggested new experimental treatments for new introductions. We also contrasted the vital rates of these introductions with long-term data on wild plants collected over more than a decade. We hypothesized that introduced populations would have more favorable vital rates than wild populations because they target the best habitat and have additional care initially. Finally, we calculated the percent of seeds that result in surviving plants when introduced into the field, contrasting plants raised in a botanical garden and transplanted to those resulting from the germination of seeds placed directly into the field.
2 Study speciesFlorida ziziphus (Pseudoziziphus celata (Judd and D.W. Hall) (Hauenschild et al., 2016); Condalia celata (Judd and D.W. Hall) M.B. Islam (Islam and Guralnick 2015); most commonly known as Ziziphus celata (Judd and Hall 1984)) is a federally endangered (USFWS, 1999) long-lived clonal shrub (Rhamnaceae) that is narrowly endemic to south-central Florida's Lake Wales Ridge. When the species was described (Judd and Hall, 1984), it was thought to be extinct, but in subsequent years several populations have been discovered (DeLaney et al., 1989; Weekley, 2009). Florida ziziphus is found only on xeric yellow sands which formerly supported sandhill vegetation; of all the federally listed plants studied, it had the strongest affinities for these soils (Menges et al., 2007). Most extant populations occur in grazed pastures with little or no woody plant cover. Florida ziziphus is self-incompatible (Weekley and Race, 2001), most populations are uniclonal, and as a whole the species encompasses fewer than 45 wild genotypes (Godt et al., 1997; Weekley et al., 2002; Gitzendanner et al., 2012). Many genotypes are cross-incompatible due to a SI breeding system (Weekley et al., 2002). Ten of the 15 wild populations comprise a single genetic individual and only five populations occur on publicly protected lands. Few fruits occur in the wild and we have never identified a seedling in wild populations. The establishment of genetically diverse and sexually reproductive populations on appropriate conservation lands is the central goal of the Florida ziziphus recovery plan (USFWS, 1999). To accomplish this, a collaboration of Archbold Biological Station, Bok Tower Gardens, and the US Fish and Wildlife Service (along with other scientists and landowners) have accomplished ten introductions and augmentations (see below).
3 MethodsWe studied the ecology of Florida ziziphus from 15 wild populations, two of which have been augmented, and from four introduced populations (six introductions) (Tables 1 and 2). All populations are found on xeric yellow sands and occur in pasture or sandhill vegetation. Wild populations are largely on private lands, while augmentations and introductions have occurred on protected lands. Most wild populations are uniclonal and have not produced fruits. Augmentations and introductions have used multiple genotypes but only one, to date, has produced fruit.
| Wild population | Habitat | Multi-genotype | Fruiting | First year | Total# plants 2015 | # Flowering 2015 | Subsequent fires |
| Mt.Lake Sandhill | DS | no | no | 1995 | 15 | 6 | 1998, 1999 |
| LWRSF-(RSF1) | S | no* | no | 1995 | 5 | 2 | 1996, 2005 |
| Mitigation Site | DS | no* | no | 1996 | 1 | 0 | 2000 |
| Friedlander | P | no | no | 1996 | 138** | 66 | 1998, 1999, 2004, 2009 |
| Avon Pines-1 | P | no | no | 2000 | 48 | 26 | 2005, 2007 |
| Avon Pines-2 | P | no | no | 2000 | 4 | 1 | 2011 |
| Avon Pines-3 | P | no | no | 2000 | 31 | 11 | 2005, 2007, 2011 |
| Avon Pines-4 | P | no | yes | 2001 | 17 | 11 | 2005 |
| Mt. Lake Disturbed | DS | no | no | 2002 | 7 | 2 | None |
| Masterpiece South | P | yes | yes | 2008 | 429** | 163 | None |
| Masterpiece North | P | yes | no | 2008 | 232** | 69 | None |
| LWRSF-2 | S | no | yes | 2008 | 4 | 3 | 2015 |
| LWRWEA/Carter North-1 (west) | S | yes | yes | 2008 | 22 | 3 | 2010 |
| LWRWEA/Carter Creek North-2 (east) | S | yes | no | 2008 | 6 | 4 | 2010, 2014 |
| Alico | S | no | no | 2013 | 3 | 3 | None |
| Location | Intro. Year | I/A | # Plants | # Seeds | Expt intro? | Subsequent fires |
| LWRSF (RSF1) | 1998 | A | 12 | None | No | 2005 |
| LWRNWR/Carter Creek | 2002 | I | 144 | 1728 | Yes | 2007, 2009, 2015 |
| Mitigation Site | 2003 | A | 64 | None | No | None |
| TNC/Tiger Creek | 2005 | I | 286 | 3000 | Yes | 2009, 2012, 2013, 2014, 2015 |
| LWRSF (RSF1) | 2006 | A | 30 | None | No | None |
| Mitigation Site | 2006 | A | 30 | None | No | None |
| TNC/Tiger Creek | 2007 | I | 110 | 1200 | Yes | 2008, 2013, 2014 |
| LWRNWR/Carter Creek | 2009 | I | 38a | 480 | Yes | 2015 |
| LWRSF (GF14) | 2010 | I | 141 | 720 | Yes | None |
| LWRWEA/Silver Lake | 2012 | I | 200 | 2400 | Yes | 2014 |
| All Sites | - | - | 1055 | 9528 | ||
| a Only 33 with survival data to 2015. | ||||||
From 2002 to 2012, we (and our collaborators) have carried out ten introductions, six of which have been experimental, as well as four augmentations of wild populations (Table 2). These efforts were done as a collaboration of Archbold Biological Station, Bok Tower Gardens, The Natives, Inc., land managing agencies (Florida Forest Service, The Nature Conservancy, the US Fish and Wildlife Service, and Florida Fish and Wildlife Conservation Commission) and funding sources (Florida Forest Service, US Fish and Wildlife Service, and others). All told, we introduced over 1000 plants and over 9500 seeds (Table 2).
Several non-experimental augmentations have been attempted (Table 2). The earliest augmentation occurred in 1998 into an existing population at the Lake Wales Ridge State Forest, and included only 12 plants of unknown age. Material for this augmentation were originally collected as root cuttings from a single genotype and subsequently propagated as root cuttings between 1992 and 1997 in Bok Tower Gardens. Although this genotype thrived in an ex situ setting at Bok Tower Gardens, and were irrigated for several months after outplanting, most died in the wild.
An additional augmentation to this site occurred in June 2006. Material for this augmentation consisted of 30 1-year old seedlings representing five maternal genotypes. Seedlings were propagated from seed collected from the ex situ population. After transplanting, seedlings were provided supplemental irrigation as needed for four months.
Two augmentations were made to the Mitigation site (2003, 2006) as part of a large restoration project. Both augmentations cloned material of unknown ages collected from the ex situ population. The 2006 augmentation was comprised of 30 1-year old seedlings. Both augmentations received supplemental irrigation for the first few months as needed. These outplantings have been more successful. The land has now passed into federal hands (in 2015) and is managed by the US Fish and Wildlife Service.
Six later introductions were designed as experiments to test hypotheses about propagule efficiency, demographic performance, fire response, and microhabitat requirements (Table 3). To produce plants, we germinated seeds at Archbold Biological Station or Bok Tower Gardens. Plants were grown in pots filled with sand from similar sites for 6 months -2 years. Outplanting was generally accomplished in the summer. In each case, plants were randomly or stratified randomly (by size) assigned to pre-determined planting locations well before transplant day.
| Introduction and (Month) year | Expt. Factor | Levels | MU | Last fire before outplanting |
| Carter Creek (June) 2002 | Land Management | Fire + canopy felling | Not defined | 2001 |
| Fire only | 2001 | |||
| Control | Long unburned | |||
| Tiger Creek (June) 2005 | Site Quality | Good | Cehi18 | 2004 |
| Fair | Pf8A-S | 2004 | ||
| Poor | Pf8A-N | Long unburned | ||
| Poor | Pf3 | 2005 | ||
| Poor | Pf6 | 2005 | ||
| Tiger Creek (October) 2007 | Fire | Burned | Pf4A | 2007 |
| Unburned | Pf6A | 2005 | ||
| Carter Creek (July) 2009 | Fire | Burned | West | 2009 |
| Unburned | East | 2007 | ||
| LWRSF (GF14) (July) 2010 | Microsites | Open | GF14 | Long unburned |
| Wiregrass | ||||
| Canopy shade | ||||
| Silver Lake (July) 2012 | Shade + Irrigation | Shadecloth only | 4 | 2012 |
| Shadecloth + irrigation | ||||
| Irrigation only | ||||
| Control |
Site selection for the introductions varied by site and experimental design (Table 3). The first introduction at Carter Creek (CCS02) was imbedded within a larger project to investigate restoration dynamics of long-unburned sandhill. Propagules at this site were introduced nearly a year after the initial management treatments, spread evenly across three treatments: burned only, chainsaw felling of oak subcanopy followed by burning (saw & burn), and an untreated control. At Tiger Creek (TCP05), planting sites were chosen among five management units that fell into three stages of sandhill restoration (high quality (minimal subcanopy/shrub cover and extensive wiregrass cover), intermediate quality (sparse canopy, open gaps), and poor quality (dense subcanopy/shrub cover, little herbaceous cover)). At TCP07 and CCS09, propagules were placed between two adjacent sites that differed in recent management history. At TCP07, Pf4A received extensive removal of the hardwood canopy and was burned five months prior to the introduction. In contrast, Pf6A was last burned in 2005 and had a relatively closed hardwood canopy. At CCS09 the west site burned one month prior to the introduction, while the east site last received fire in 2007. At the Lake Wales Ridge State Forest (RSF10), propagules were placed among three microhabitat conditions: wiregrass/no shade (wiregrass); no wiregrass/shade (shade); no wiregrass no shade (open). The Silver Lake (SL12) introduction was designed to investigate microhabitat requirements by using a full factorial design with two main factors, shade and irrigation, and their combination, resulting in four treatment combinations. At this site, planting points were randomly chosen within open areas devoid of pine overstory and shrubs, and treatments were assigned randomly. At the time of planting, we required propagules to be at least 1 m apart.
Seeds in all introductions were sown in arrays encompassing 24 seeds in a 6-column 4-row grid measuring 28 × 18 cm. Seeds were dibbled into the ground at a depth of about 1 cm. Both seeds and transplants were caged to reduce disturbance by animals. Caging and fencing have been used in other introductions to increase survival and other vital rates (Fenu et al., 2016). In all introductions, we irrigated using a citrus style irrigation system, whenever there were more than about three days without rains throughout the summer and fall. An exception was the SL12 introduction, where irrigation was an experimental factor and propagules were irrigated regularly until the end of December, five months after the introduction (Table 3).
We monitored both wild and introduced plants and seeds using the same protocol. We sampled annually in January (at the time of peak flowering), collecting data from individually tagged and mapped plants. Variables included survival, stage (new root shoot, resprout, small vegetative < 50 cm tall, large vegetative, flowering, died back, top-killed by fire or mowing), size (height, maximum crown length, number of stems), and number of flowers (base 10 scale). We collected data on number of fruits later in the spring (often May). Because Florida ziziphus is clonal, we defined an individual plant as a group of stems separated by 25 cm or more from any other stems. For introductions, plant survival and growth was followed at shorter intervals than for wild plants, ranging from biweekly to monthly during the first six months, then quarterly to annually thereafter. We also collected additional data on survival and fire severity (whether plant was scorched or consumed) within a month after each prescribed burn.
Within the six experimental introductions we calculated annual and annualized (calculated as the nth root of cumulative survival, where n is the number of years from the first January after the introduction to 2015) survival of transplants. To compare transplant and seedling cumulative survival and seed germination among experimental treatments, we used chi square tests (for a single treatment) or binary logistical regression (for multiple treatments). We used analysis of variance (ANOVA) or t-tests to analyze how relative growth rate (calculated on the basis of height) of transplants and seedlings varied among treatments, with Tukey's posthoc tests to assess pairwise differences among treatment levels. For all analyses except SL12, we considered vital rates through 2015. For SL12, we analyzed treatment effects on vital rates through 2014, after which a wildfire disrupted the experimental treatments.
We compared wild vs. introduced transplants for several vital rates: annual survival, annualized survival (calculated as the nth root of cumulative survival, where n is the number of years from the first January after the introduction to 2015), relative growth rate (based on heights), transition to reproductive size (height≥50 cm), and transitions to flowering, flowering, and fruit production. We used binary logistic regression to test for annual survival differences among origin (wild vs. introduced), prior life history stages (resprouts, small vegetatives, and large vegetatives), calendar year (2006-2015), and 2-way interactions. We did not test for population effects as they would be redundant with origin. We used general linear models to analyze relative growth rates (based on heights) using the same predictors as for survival for years 2006e2011, but excluded plants in the year they burned as RGR was significantly lower in the year of fire (t-tests, t3162=24.159, p < 0.001). All analysis was conducted in SPSS version 22. Figures were created in SigmaPlot version 11.0.
Finally, for introductions, we calculated the proportion of seeds that resulted in established plants (plants surviving until the first January after outplanting) through the alternative pathways of direct seeding vs. outplanting of transplants that had been germinated and raised in a botanical garden setting. The calculations involved multiplying percent seed germination by percent survival to transplanting (for outplanted seedlings) by percent survival in the field (through January 2014).
4 Results 4.1 Transplant survivalFlorida ziziphus transplants had generally high survival. Among introductions, cumulative survival through 2015 has varied among introductions from over 85% for the two most recent introductions to as low as 37% in an old introduction (Table 4). Declines in cumulative survival have been fairly consistent for most introductions in most years (Fig. 1). Annualized survival has varied less widely, from 90 to 99% among introductions. The highest survival has been at the RSF10 introduction.
| Introduction and year | Transplants | Seeds | Germinants | ||||||
| % CS | % AS | RGR (SD) | % FPS | Germ % | % CS | % AS | % FPS | RGR (SD) | |
| Carter Creek (CCS02) 2002 | 50.0 | 94.4 | +0.005 (0.063) | 9.7 | 3.6 | 1.6 | 70.9 | 0.0 | -0.046 (0.456) |
| Tiger Creek (TCP05) 2005 | 37.1 | 89.6 | +0.030 (0.091) | 11.2 | 4.8 | 15.2 | 81.1 | 4.5 | +0.066 (0.468) |
| Tiger Creek (TCP07) 2007 | 78.2 | 96.5 | -0.018 (0.103) | 3.4 | 2.8 | 33.3 | 85.5 | 0.0 | +0.105 (0.408) |
| Carter Creek (CCS09) 2009 | 60.6 | 90.5 | -0.046 (0.082) | 0 | 5 | 15.8 | 69.1 | 0.0 | +0.143 (0.313) |
| Lake Wales Ridge State Forest (RSF10) 2010 | 97.2 | 99.3 | +0.069 (0.092) | 15.3 | 6.4 | 38.5 | 78.8 | 0.0 | +0.254 (0.347) |
| Silver Lake (SL12) 2012 | 85.4 | 92.4 | +0.011 (0.254) | 0.6 | 2.4 | 25.9 | 50.9 | 0.0 | +0.268 (0.537) |
| Introduction/Year Mean | 68.1 | 93.8 | +0.008 (0.114) | 6.7 | 4.2 | 21.7 | 72.7 | 0.075 | +0.132 (0.422) |
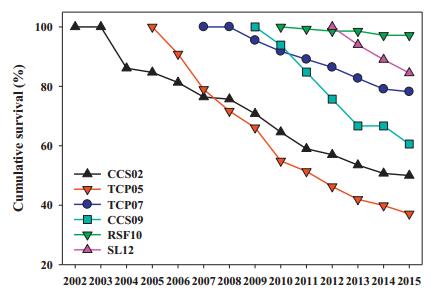
|
| Fig. 1 Cumulative survival of Florida ziziphus transplants through 2015 in six introductions. Populations are organized by age from the 2002 Carter Creek (CCS02) introduction to the 2012 Lake Wales Ridge Wildlife and Environmental Area/Silver Lake introduction (SL12). Two-digit number attached to each site abbreviation indicates the year the translocation took place. |
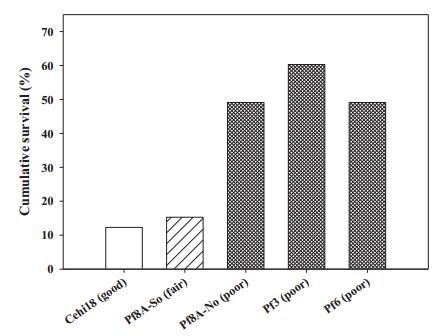
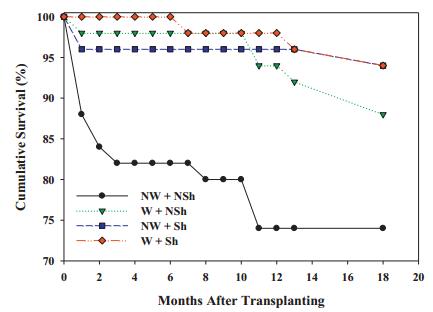
Transplant survival through 2015 often varied among experimental treatments. At CCS02, survival varied marginally (chi square=5.17, df=2, p=0.076) and was highest in the control, intermediate in the burn, and lowest in the saw & burn. At TCP05, survival was strongly affected by site quality (chi square=45.41, df=2, p < 0.001) and was higher in poor quality sites than other sites (Fig. 2). At TCP07, survival was higher in the unburned than burned site (chi square=1.92, df=1, p < 0.017). At CCS09, survival varied between two sites, being higher in the burned site (chi square=3.96, df=1, p=0.055). At RSF10, survival did not vary among the three microsites (chi square=3.60, df=2, p=0.165). Survival through January 2014 for SL12 transplants was markedly lower for plants receiving no shade or irrigation, compared to receiving one or both (Fig. 3). Survival was marginally increased by irrigation (Logistic regression, Wald=0.57, df=1, p=0.085) but not by shade or the interaction of irrigation and shade at SL12.
|
| Fig. 2 Percent cumulative survival through the 2015 census, for Florida ziziphus transplants in the 2005 Tiger Creek Preserve experimental introduction. Based on The Nature Conservancy's habitat quality rankings, Cehi18 was good quality sandhill, Pf8ASouth was intermediate quality sandhill, and the other three sites were poor quality sandhill at the time of the introduction. |
|
| Fig. 3 Cumulative monthly survival (%) of Florida ziziphus transplants at Lake Wales Ridge Wildlife and Environmental Area/Silver Lake for the first 18 months postplanting (July 2012eJanuary 2014). Survival is divided among four treatment combinations: W=water added, NW=no water added, Sh=shaded, NSh=not shaded (naturally sunny). The irrigation treatment ceased on 31 December 2012, five months after the introduction. |
Transplant growth through January 2015 has generally been inconsistent. Relative growth rates (RGR; based on height) being within a standard deviation of zero (no growth) for each of the six experimental introductions (Table 4). Growth has been greatest for the two most recent introductions (RSF10 and SL12) and has been most negative for the second Carter Creek (CCS09) introduction.
Growth rates of transplants varied among microsites in some introductions. In the oldest experimental introduction (CCS02), RGR from 2005 to 2015 did not vary among control, burn-only, and saw & burn treatments (ANOVA, F=0.1, df=2, p=0.902), despite reasonable sample sizes (total n=72). At TCP05, transplant RGR varied among the five sites (ANOVA, F=5.4, df=4, p=0.001) with growth being negative at the good quality site (-0.109, n=6), low at the intermediate quality site (0.019, n=9), and low to high at the three poor quality sites (0.017, n=28; 0.046, n=35; 0.058, n=27). The good site had lower RGR than either intermediate or poor (Tukey HSD posthoc tests, p < 0.05), but intermediate and poor sites were similar. RGR for plants in the TCP07 introduction did not vary (t-test, t84=0.4, p=0.691) between the burned (Pf4A, -0.022, n=40) and unburned (Pf6A, -0.014, n=46) site. RGR for plants at CCS09 did not vary between burned (-0.033, n=13) and unburned (-0.070, n=7) locations (t-test, t18=1.0, df=1, p=0.350). RGR for transplants at RSF10 varied significantlyamong microsites (ANOVA, F=3.1, df=2, p=0.049), being significantly (p < 0.05) higher for plants in wiregrass (0.094, n=46), than for shaded plants (0.048, n=44) and intermediate for plants in open conditions (0.064, n=47; not significantly different from the other two treatments). RGR from 2013 to 2014 for transplants at SL12 did not vary significantly among treatments (2 way ANOVA with shade [p=0.372], irrigation [p=0.506] and their interaction [p=0.452]).
4.3 Seedling germination, survival, and growthMean percent field germination ranged from 2.4 to 6.4% (mean 4.5±1.4%) among introductions (Table 4). Most (94.8%) germination took place in the first few months, before the annual January census. Germination was highest for the RSF10 introduction and lowest for the SL12 introduction.
Percent germination varied between or among treatments in four of the six introductions and in two cases germination was higher in shaded vs. unshaded sites. Germination was similar among the three treatments at CCS02 (17e23 germinants, chi square=1.0, df=2, p=0.595) and between the twoTCP07 sites (15 each, chi square=0.0, df=1, p=0.854). At TCP05, more seedlings germinated in the three poor quality sites (49, 39, and 22) than in the intermediate (20) or the good quality site (15). The differences due to site quality were significant (chi square=16.4, df=2, p < 0.001). Germination was higher in the burned (16) than the unburned (3) site for the CCS09 introduction (chi square=7.9, df=1, p=0.005). At RSF10, germination was much higher in shaded (26 germinants) than the open (8) or wiregrass (5) microsites; these microsites had significantly different germination (chi square=21.0, df=1, p < 0.001). At SL12, germination was higher in irrigated (39) than unirrigated (15) treatments (chi square=10.0, df=1, p=0.002) but similar in shaded (29) and unshaded (25) treatments (chi square=0.2, df=1, p=0.680).
For four of the six introductions, there were enough data to test for treatment effects of seedling survival. At TCP05, seedling survival was marginally affected by site quality (chi square=5.60, df=2, p=0.061), with all but one surviving seedling in poor quality sites. Seedling survival did not vary with fire treatment at TCP07 (chi square=0.44, df=1, p=0.700) but varied marginally with microsite at RSF10 (chi square=5.08, df=2, p=0.079), with survival decreasing from open to shaded to wiregrass microsites. At SL12, seedling survival through 2014 was not affected by shade (Wald=2.7, df=1, p=0.10), irrigation (Wald=0.05, df=1, p=0.82) or their interaction (Wald=3.5, df=1, p=0.55). For the two Carter Creek introductions (CCS02 and CCS09), there were too few seedlings to conduct statistical tests on seedling survival.
Seedling relative growth rates were higher than transplant relative growth rates. Growth rates for seedlings surviving through January 2015 varied among the six introductions, ranging from -0.046 to 0.268 (Table 4). Seedling growth rates through 2015 varied among microsites in two of three introductions for which we could analyze. RGR for seedlings at TCP07 were higher for seedlings in the initially unburned unit Pf6A (0.21, n=6) than for the initially burned unit Pf4A (0.05, n=4); this difference was significant (ttest, t8=2.9, p=0.018) despite subsequent fires at this site (Table 2). RGR for seedlings at RSF10 through 2015 were greater for seedlings in the open (0.43, n=5) than seedlings in the shade (0.23, n=10); this difference was significant (t-test, t13=2.78, p=0.016). RGR for seedlings at SL12 from 2013 to 2014, did not vary with shade (ANOVA, F=1.09, df=1, p=0.316), irrigation (ANOVA, F=0.07, df=1, p=0.79), or their interaction (ANOVA, F=0.15, df=1, p=0.70).
4.4 Flowering size, flowering, and fruitingDespite reasonable survival, sexual reproduction of introduced Florida ziziphus has rarely occurred. Few on-site germinants have grown into flowering plant size, and only in one of the older experimental introductions (TCP05; Table 4). In the RSF10 introduction, 15.3% of transplants reached flowering size within five years, but the Carter Creek (CCS02 and CCS09) and Tiger Creek (TCP05 and TCP07) introductions have had < 12% of transplants reaching flowering plant size through 2015 and none have flowered despite being outplanted for 6e13 years. None of the transplants or on-site germinants in our six experimental introductions have produced fruit.
In contrast, transplants used in two non-experimental augmentations, the Mitigation Site (2003 and 2006), began flowering in 2010, with over 60% of augmented plants flowering in both 2014 and 2015. Fruit production among the 2003 augmentation population has been documented each year since 2010. After sowing 55 fruits on-site in 2011, we observed a single seedling in January 2013, the first time we have seen second generation recruitment in a Florida ziziphus introduction or augmentation.
4.5 Effects of fireSome plants in 11 of the 15 wild populations were affected by prescribed fire since 1998 (Table 1). Six of the 11 of these burned wild populations experienced multiple fires, some up to three times. Repeated fires have also occurred in introduced populations: at Carter Creek following the 2002 introduction (burns in 2007 and 2009) and at Tiger Creek following the 2005 (burns in 2009, 2012, 2013, and 2014 to parts of our introductions) and 2007 (burns in 2008, 2013 and 2014 to a subset of plants; Table 4) introductions. Fires were often patchy, with some plants unburned, scorched, or consumed. For introduced plants, burned plants had similar survival (93.1%) to unburned plants (91.7%) in the year encompassing fire (chi square=1.00, df=1, p=0.318). Not surprisingly, for the year burned, RGR was much lower among burned plants (mean=-1.01±0.48SD) than unburned (mean=0.057±0.01SD).
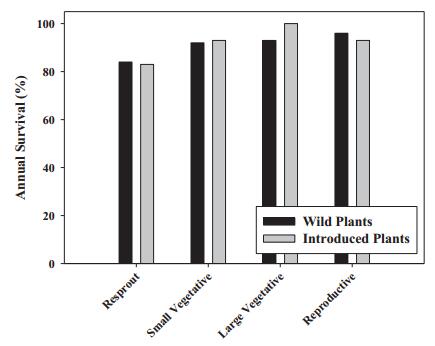
4.6 Comparing vital rates between introduced and wild populationsAnnual survival was quite high for both introduced (94%) and wild (95%) plants across a range of non-seedling life history stages (Fig. 4). In binary logistic regressions utilizing 7517 annual survival records, annual survival varied strongly among years (Wald=26.194, df=1, p < 0.001), but not by prior life history stage (Wald=0.243, df=2, p=0.886), or by whether plants were from wild or introduced populations (“origin”, Wald=0.001, df=1, p=0.978). No interactions were statistically significant. In most years annual survival was >90%, with the exception of 2006 and 2007, where survival was 83.9% and 89.2% respectively.
|
| Fig. 4 Mean annual survival of plants of different stages, for wild and introduced plants. Stages shown are resprout from dieback, small vegetative < 50 cm tall, large vegetative at least 50 cm tall, and reproductive (flowering) plants. |
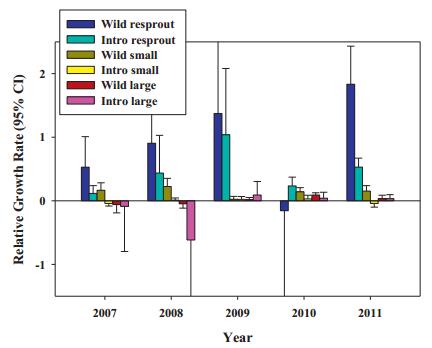
In contrast to survival results, growth in many introduced populations was minimal, and the mean relative growth rate of introduced transplants was only a tenth of wild plants (0.005 vs. 0.052). In analyses with origin, prior life history stage, and year, year alone (ANOVA, F=25.779, df=1, p < 0.001) and all interactions were statistically significant at predicting RGR (Fig. 5). RGRs were particularly low in 2010. Year interacted with stage and origin in that larger plants and introduced had lower growth in early than later years. Stage and origin interacted in that introduced plants that were resprouts underperformed wild resprouts, but wild and introduced plants in other stages grew at more similar rates. A significant three-way interaction manifests as particularly negative growth for large introduced plants in 2008, and particularly strong positive growth for wild resprouts in 2011 (Fig. 5).
|
| Fig. 5 Relative growth rates (±95% CI) by year, prior life history stage and whether plants were wild or introduced. The X axis values refer to the second year of the annual RGR; e.g. “2007” indicates 2006-2007. |
Introduced plants showed slow annual transitions to flowering plant size and to flowering. For introduced plants, on average, only 2.2% of small vegetative plants transitioned to large vegetative plants (vs. 17.1% for wild plants) and only 14.9% of large vegetative plants transitioned to flowering plants (vs. 23.6% for wild plants). No introduced plants spread clonally, but new clonal plants made up 2.7% of wild plants.
4.7 Comparing the efficacy of seeds vs. transplantsWe tracked the fates of seeds to established outplanted individuals via one of two routes: from direct seed sowing vs. transplants that were germinated ex situ and cared for in a botanical garden setting. Only 1.2% of seeds germinated and survived when direct-sown. In contrast, 1.5% of seeds that went through botanical garden care survived long enough to be used as outplantings, and this percentage was 4.2% for fresh seeds.
5 DiscussionOur series of introductions and augmentations of Florida ziziphus have shown some success. Transplants have had similar survival and fire tolerance to wild populations and a substantial number of surviving introduced populations remain after over a decade. On the other hand, transplant growth has been generally slow and inconsistent and flowering has been minimal. Fruit production and second generation seedling recruitment has occurred only in one non-experimental augmentation.
Florida ziziphus annual survival varied widely among years, but was similarly high (generally > 0%) for both introduced and wild plants. This stability in the introduced population is a strong indicator that reintroductions and augmentations may be successful in the long run. Despite high annual survivals, cumulative survival declines each year, and without recruitment (clonal or seedling) there may be a need for additional augmentation to retain population size and genetic structure.
On the other hand, transplant growth has been erratic, often negative, and about a tenth the rate of wild plants. In some years this variation is largely explained by poor growth in plants that were resprouts in the prior year. Growth varied widely among years, and with complex interactions with prior life history stage and origin. Variation in weather, irrigation, outplanting site, and plant age are all potential reasons that growth rates varied among introductions made in different years. The better performance of the first TCP introduction, relative to the second, argues for strong year effects, as seen in other introductions (e.g. Colas et al., 2008). The strong year effect is also an argument for achieving introduction success with multiple years of introductions, as a bet hedging strategy against poor weather and other factors specific to individual years.
In comparing wild to introduced plants, an obvious difference is the size and age of plants. In most years, growth among wild plants surpassed that of introduced regardless of stage class. Wild Florida ziziphus plants have large root systems that include deeply rooted taproots. Introduced plants may take many years to accumulate this root mass. Nonetheless, growth appears to slow down in wild plants as they reach larger sizes. Growth rates of introduced plants have become more positive as they age and grow. Introduced plants are also resilient to fire, resprouting and surviving at high rates. Plants that are able to protect buds from fire in the soil, such as many resprouting shrubs in Florida scrub (Menges and Kohfeldt, 1995; Maguire and Menges, 2011), are good choices for restoration in pyrogenic ecosystems (Pyke et al., 2010).
The experimental nature of these introductions has helped us understand what conditions favor survival and growth. More open conditions have generally been associated with lower transplant and seedling survival, and low seed germination percentages. Growth responses have been more variable. Shade from tree canopies, at least over the range at our transplant sites, has not been detrimental. In fact, vital rates have been generally higher in more shaded sites ranked as poor quality in terms of ecological restoration. The better performance with shade contrasts with the treeless condition of many of the wild Florida ziziphus populations, which most often occur in pastures or open sandhill vegetation. Whether the shaded microsites that seem to be good for survival and growth of small plants will also be favorable for older plants remains an open question.
The advantage of shady sites for early survival may be due to more favorable water status of transplants in these areas. An experiment combining artificial shading with irrigation showed the primary positive effect of irrigation and a weaker effect of shading; the combination had the greatest effect on transplant survival. Tree canopies may provide the advantage of shade reducing leaf temperatures and transpirations. Dense shrub competition probably does not offer this advantage, as shrubs may increase transpiration from the soil (Weekley et al., 2007) while still exposing transplants to high light.
One feature of our introductions of Florida ziziphus are that sites have been continued to be managed using frequent prescribed fire. Fire causes top-kill of both wild and introduced Florida ziziphus plants. Relative growth rates in the year of the fire were strongly negative, as resprouting plants were generally much smaller than pre-fire shrubs. However, these small plants made relatively large proportional growth in the following year. Our research shows that individual transplants have similar survival whether burned or not, and similar burn responses to older wild plants. Early allocation to belowground roots and rhizomes may allow transplants to efficiently resprout. Even seedlings often survive fire, although their overall survival has been very low.
The strong postfire resprouting of Florida ziziphus transplants means that introductions need not affect ongoing fire management. However, frequent fire may slow growth as plants will be smaller for several years post-fire. Fires also affect vegetation structure and this could have indirect effects on introduced populations. Fires top-kill competing shrubs which could benefit Florida ziziphus. Low severity fires that allow tall hardwoods and oaks to survive without resprouting may retain a partial canopy that promotes Florida ziziphus survival.
Our experiments also show that transplants are the preferred introduction propagule. Although there is more labor and expense associated with rearing plants for subsequent introduction than to direct seeding, plant introductions make better use of limited seed availability. In addition, transplants are larger and have higher survival than seedlings emerging from outplanted seeds. Because it appears to take decades for even large transplants to become reproductively mature, using seeds which would take much longer does not seem a good strategy. Transplants are generally thought to have an advantage over seeds for many rare plant introductions (Albrecht and Maschinski, 2012).
Future conservation work with Florida ziziphus will certainly include additional introductions and augmentations, as none of the extant introductions has been shown to be viable. In particular, none have produced a second generation of plants, an important yardstick for determining the success of introductions (Menges, 2008). Future introductions will be able to take advantage of the recent discovery of new genetic material (Weekley, 2009) that will allow more diverse and (hopefully) reproductively successful new populations.
This study shows that information collected from careful monitoring of introductions can potentially be quite useful in allowing for adaptive introductions, improving the success of subsequent introductions (Menges, 2008). We also argue that using data from wild populations provides important measuring sticks for evaluating the success or failure of introductions.
6 AcknowledgementsSusan Wallace and Tamera Race did early research and conservation on Florida ziziphus that helped pave the way for our work. Cheryl Peterson and her crew at Bok Tower Gardens raised nearly all the plants for introductions and augmentations and were also very helpful in collecting seeds and outplanting. We also appreciate the efforts of land managers who have cooperated with us at introduction sites, including Steve Morrison (The Nature Conservancy), Dorn Whitmore (US Fish and Wildlife Service), Dave Butcher (Florida Forestry Service) and their agency colleagues. Special thanks to Nancy Bissett for her insights. The project would not have proceeded without continuing funding and support from the Rare Plant Conservation program of the Florida Forestry Service, managed by Dennis Hardin and Mike Jenkins, from the National Science Foundation (DEB98-15370, DEB02-33899, DEB08-12717, DEB-1347843), from the US Fish and Wildlife Service, and from Archbold Biological Station. In particular, we appreciate the strong support of David Bender (US Fish and Wildlife Service). The Florida ziziphus Ad-Hoc Recovery Team has provided important leadership for introductions and other aspects of Florida ziziphus conservation. We also thank the private landowners and their managers for allowing us access to their properties. Finally, numerous volunteers, interns, and others helped with the hard work: caging, irrigating, outplanting and measuring this thorny shrub in conditions that were generally hot and humid.
| Albrecht, M.A., Maschinski, J., 2012. Influence of founder population size, propagule stages, and life history on the survival of reintroduced populations. In: Maschinski, J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate. Island Press, Washington, pp. 171-188, 1-432. | ||
| Bell T.J., Bowles M.L., McEachern A.K., 2003. Projecting the success of plant population restoration with viability analysis. In: Brigham C.A., Schwartz, M.W. (Eds.), Population Viability in Plants. Springer-Verlag, Berlin, 313 -348. | ||
| Clausen J., Keck D.D., Hiesey W.M., 1941. Regional differentiation in plant species. Am. Nat, 75(758), 231 -250. DOI:10.1086/280955 | ||
| Colas B., Kirchner F., Riba M., Olivieri I., Mignot A., Imbert E., Beltrame C., Carbonelli D., Freville H., 2008. Restoration demography: a 10-year demographic comparison between introduced and natural populations of the endemic Centaurea corymbosa (Asteraceae). J. Appl. Ecol, 45(5), 1468 -1476. DOI:10.1111/jpe.2008.45.issue-5 | ||
| Dalrymple S.E., Banks E., Stewart G.B., Pullin A.S., 2012. A meta-analysis of threatened plant reintroductions from across the globe. In: Maschinski J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate. Island Press, Washington: 1 -432. | ||
| DeLaney K.R., Wunderlin R.P., Hansen B.E., 1989. Rediscovery of ziziphus celata (Rhamnaceae). Sida Contributions Bot, 13, 325 -330. | ||
| Drayton B., Primack R.B., 2012. Success rates for reintroductions of eight perennial plant species after 15 years. Restor. Ecol, 20(3), 299 -303. DOI:10.1111/rec.2012.20.issue-3 | ||
| Dunwiddie, P.W., Martin, R.A., 2016. Microsites matter: improving the success of rare species. PLoS One 11 (3), e0150417. http://dx.doi.org/10.1371/journal.pone.0150417. | ||
| Fenu G., Cogoni D., Bacchetta G., 2016. The role of fencing in the success of threatened plant species translocation. Plant Ecol, 217(2), 207 -217. DOI:10.1007/s11258-015-0517-1 | ||
| Gitzendanner M.A., Weekley C.W., Germain-Aubrey C.C., Soltis D.E., Soltis P.S., 2012. Microsatellite evidence for high clonality and limited genetic diversity in Ziziphus celata (Rhamnaceae), an endangered, self-incompatible shrub endemic to the Lake Wales Ridge, Florida, USA. Conserv. Genet, 13, 223 -234. DOI:10.1007/s10592-011-0287-9 | ||
| Godefroid S., Piazza C., Ross G., Buord S., Stevens A.D., Aguraiuja R., Cowell C., Weekley C.W., Vogg G., Iriondo J.M., Johnson I., Dixon B., Gordon D., Magnanon S., Valentin B., Bjureke K., Koopman R., Vicens M., Virevaire M., Vanderborght T., 2011. How successful are plant species reintroductions?. Biol.Conserv, 144(2), 672 -682. DOI:10.1016/j.biocon.2010.10.003 | ||
| Godt M.J.W., Race T., Hamrick J.L., 1997. A population genetic analysis of Ziziphus celata, an endangered Florida shrub. J. Hered, 88(6), 531 -533. DOI:10.1093/oxfordjournals.jhered.a023151 | ||
| Guerrant E.O., 2012. Characterizing two decades of rare plant reintroductions. In: Maschinski J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate. Island Press, Washington: 1 -432. | ||
| Guerrant, E.O., Kaye, T.N., 2007. Reintroduction of rare and endangered plants: common factors, questions and approaches. Aust. J. Bot. 55 (3), 362-370. http://dx.doi.org/10.1071/BT06033. | ||
| Hauenschild F., Matuszak M.S., Muellner-Riehl A.N., Favre A., 2016. Phylogenetic relationships within the cosmopolitan buckthorn family (Rhamnaceae) support the resurrection of Sarcomphalus and the description of Pseudoziziphus gen. nov. Taxon, 65(1), 47 -64. DOI:10.12705/651.4 | ||
| Islam M.B., Guralnick R.P., 2015. Generic placement of the former Condaliopsis (Rhamnaceae) species. Phytotaxa, 236(1), 25 -39. DOI:10.11646/phytotaxa.236.1 | ||
| Judd W.S., Hall D.W., 1984. A new species of Ziziphus (Rhamnaceae) from Florida. Rhodora, 86, 381 -387. | ||
| Knight T.M., 2012. Using population viability analysis to plan reintroductions. In: Maschinski J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate. Island Press, Washington: 1 -432. | ||
| Maguire A.J., Menges E.S., 2011. Post-fire growth strategies of resprouting Florida scrub species. Fire Ecol, 7(3), 12 -25. DOI:10.4996/fireecology | ||
| Maschinski, J., Duquesnel, J., 2007. Successful reintroductions of the endangered long-lived Sargent's cherry palm, Pseudophoenix sargentii, in the Florida Keys. Biol. Conserv. 134 (1), 122-129. http://dx.doi.org/10.1016/j.biocon.2006.07.012. | ||
| Maschinski J., Haskins K.E., Raven P.H., 2012. Plant Reintroduction in a Changing Climate. Washington: Island Press, 1 -432. | ||
| Menges E.S., 2008. Restoration demography and genetics of plants: when is a translocation successful?. Aust. J. Bot, 56(3), 187 -196. DOI:10.1071/BT07173 | ||
| Menges E.S., Kohfeldt N.M., 1995. Life history strategies of Florida scrub plants in relation to fire. Bull. Torrey Botanical Club, 122, 282 -297. DOI:10.2307/2996320 | ||
| Menges E.S., Weekley C.W., Hamze S.I., Pickert R.L., 2007. Soil preferences for federally-listed plants on the Lake Wales Ridge in Highlands County, Florida. Fla. Sci, 70(1), 24 -39. | ||
| Pyke D.A., Brooks M.L., D'Antonio C., 2010. Fire as a restoration tool: a decision framework for predicting the control or enhancement of plants using fire. Restor. Ecol, 18(3), 274 -284. DOI:10.1111/rec.2010.18.issue-3 | ||
| US Fish and Wildlife Service, 1999. Florida ziziphus. In: Multi-species Recovery Plan for the Threatened and Endangered Species of South Florida. US Fish and Wildlife Service, Atlanta, GA, pp. 1986-1999. | ||
| Weekley C.W., 2009. Recent developments boost recovery prospects of Florida ziziphus. The Palmetto, 26(1), 8 -11. | ||
| Weekley C.W., Kubisiak T.L., Race T.M., 2002. Genetic impoverishment and cross-incompatibility in remnant genotypes of Ziziphus celata (Rhamnaceae), a rare shrub endemic to the Lake Wales Ridge, Florida. Biodivers. Conserv, 11(11), 2027 -2046. DOI:10.1023/A:1020810800820 | ||
| Weekley C.W., Gagnon D., Menges E.S., Quintana-Ascencio P.F., Saha S., 2007. Variation in soil moisture in Florida scrub. EcoScienc, 14(3), 377 -386. DOI:10.2980/1195-6860(2007)14[377:VISMIR]2.0.CO;2 | ||
| Weekley C.W., Race T., 2001. The breeding system of Ziziphus celata Judd and D.W. Hall (Rhamnaceae), a rare endemic plant of the Lake Wales Ridge, Florida, USA. Biol. Conserv, 100, 207 -213. DOI:10.1016/S0006-3207(01)00024-6 | ||
| Wendelberger K.S., Maschinski J., 2016. Assessing microsite and regeneration niche preferences through experimental reintroduction of the rare plant Tephrosia angustissima var. corallicola. Plant Ecol, 217(2), 155 -167. DOI:10.1007/s11258-015-0521-5 |



