b. Nanjian Administration Bureau of Wuliangshan National Nature Reserve, Nanjian 675700, China
Lysimachia L. belongs to the tribe Lysimachieae Reich. and is commonly recognized as a primitive group of traditional Primulaceae (Chen and Hu, 1989; Fang, 2003). Based on the subsequent molecular phylogenetic analyses and morphological studies, Lysimachia and other genera of the tribe Lysimachieae have been transferred to the traditional family Myrsinaceae (Anderberg and Ståhl, 1995; Anderberg et al., 1998, 2002, 2007; Käallersjöo et al., 2000; Mast et al., 2001), and then moved back to Primulaceae but as members of subfamily Myrsinoideae (Stevens, 2016).
Lysimachia is the largest genus of the tribe Lysimachieae and contains approximately 140e200 species worldwide (Hu and Kelso, 1996; Ståhl and Anderberg, 2004; Heywood et al., 2007; Mabberley, 2008). China, as one of its famous diversity centers, has about 140 species (Chen and Hu, 1979, 1989; Hu and Kelso, 1996; Zhou et al., 2015). The paraphyly of traditional Lysimachia has been reported by several studies (Martins et al., 2003; Hao et al., 2004; Anderberg et al., 2007; Zhou et al., 2015). For monophyly, the other genera of the tribe Lysimachieae (i.e. Glaux L., Trientalis L., Anagallis L., Asterolinon Hoffmanns. & Link, Pelletiera A. St.-Hil.) need to be merged in (Manns and Anderberg, 2009), leaving Lysimachia s.l. as the sole genus in this tribe.
During a plant survey of Nanjian Yi Autonomous country (Yunnan Province, China) in 2015, a distinct flowering population was found. The whole plant, especially the upper part of the stem and inflorescence, was coated with distinct multicellular nodiferous hairs. Moreover, several other characters made taxonomic assignment, even at the family level, extremely difficult. These characters include: leaves in whorls of 3, the terminal racemes, distinct bracts, 7-merous flowers, only one layer of half-divided tepals and long exserted stamens. Fortunately, we later found another two conspecific fruiting specimens collected in Xinping Yi Autonomous County (Yunnan) in 2009. These specimens indicate that they may belong to the family Primulaceae. Further studies of morphological characters and molecular phylogenetic relationships showed that the newly found population represented an undescribed species of Lysimachia s.l., which is described and illustrated below.
2 Materials and methodsThe morphological description of the new species was based on careful examination of the types. The comparison between it and the twomorphologically related species (Lysimachia thyrsiflora and Glaux maritima) was based on studies of the herbarium species in KUN.
The pollen grains and seeds were directly mounted on aluminum stubs coated with gold in a sputter coater and examined using scanning electron microcopy (SEM) as in Xiang et al.(2013). Pollen terminology follows Erdtman (1960), Bennell and Hu (1983). Seed terminology follows Oh et al.(2008).
Because the new species exhibited distinct morphological characters, we adopted a molecular approach to shed light on the position of L. septemfida within Lysimachia s.l. Bayesian and Maximum Likelihood analyses were used to construct a phylogenetic tree based on ITS sequences. GenBank sequences were selected to represent all the subgenus names of Lysimachia s.str., and cover all the three Chinese species of the other three genera (Glaux, Trientalis, Analgalis) of the tribe Lysimachieae. DNA extraction, PCR amplification, sequence alignment and phylogenetic reconstruction follow protocols detailed in Wang et al.(2013).
3 Results 3.1 Taxonomic treatmentLysimachia septemfida Z.H.Wang & E.D. Liu, Sp.nov.(Fig. 1)七齿珍珠菜(qi chi zhen zhu cai).
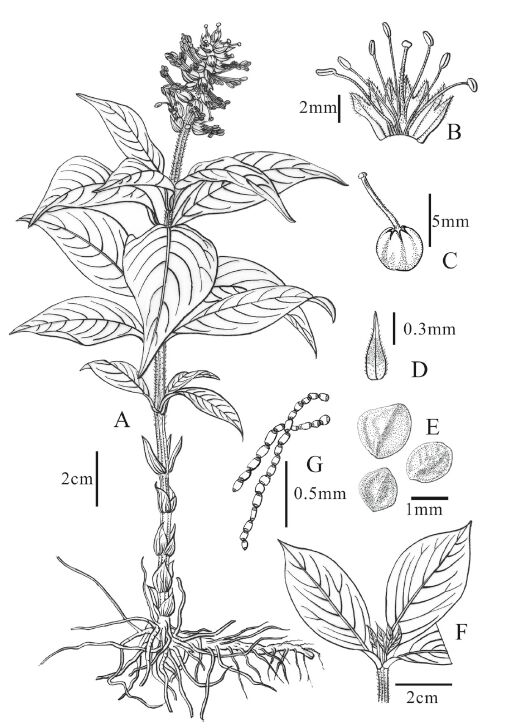
|
| Fig. 1 Lysimachia septemfida. A. Flowering plant; B. Opened flower; C. Fruit with persistent style; D. Bract; E. Seeds; F. Leaves in whorls of 3; G: Multicellular nodiferous hairs. |
Type: CHINA. Yunnan province, Nanjian Yi Autonomous county, Baohua town, Yongzheng village, Mt. Wuliang, peak of Dazhongshan. 24°50′08.56′′N, 100°25017.9000E. Alt. 2790 m. 2015- 06-28, Liu En-de et al. 4178(holotype KUN; isotypes KUN, IBSC).
Perennial herbs or subshrubs, 25-150 cm tall, horizontal rhizomes densely with roots. Stems erect, usually simple, lower part glabrous, upper part densely with yellowish transparent multicellular nodiferous hairs. Leaves on lower part opposite, reduced to be scalelike, glabrous, and gradually become whorls of 3 and normal upwards. Leaves sessile, rarely indistinctly petiolate; leaf blade ovate-lanceolate to elliptic-lanceolate, 4-9 × 1.5-3.5 cm, with black glandular spots punctuated on both sides and yellowish transparent multicellular nodiferous hairs on the adaxial veins, lower part tapering toward semi-clasping base, apex acute to acuminate, margin entire. Racemes terminal, dense and floriferous, 2-3 cm long, elongating to 7 cm in fruit; peduncle 0.3-2 cm, with yellowish transparent multicellular nodiferous hairs; flowers sessile. Bracts ovate-lanceolate, 5-7 mm, with sparse yellowish glandular punctuate and marginal fringe-like multicellular nodiferous hairs abaxially. Calyx yellowish white, sub-transparent, shorter than bract; Calyx tube 3 mm; lobes 7, triangular, 1.5 mm, apex acuminate, sparsely with yellowish glandular spots outside and marginal fringe-like multicellular nodiferous hairs. Corolla absent. Stamens 7, long exserted, almost twice as long as calyx; anthers elliptic, dorsifixed, ca. 1-1.5 mm; pollen spheroidal, tricolporate, medium-sized [27.24(24.28-29.29)× 23.04(21.54-24.50)μm], P/E = 1.07(Fig. 2A-D). Ovary and style both with yellowish transparent multicellular nodiferous hairs; style 4-5 mm in length, persistent. Capsule globose, ca. 3 mm in diam., dehiscing by valves. Seed dorsiventrally and laterally flattened (Fig. 2 E & G). Flowering in June, fruiting in September.
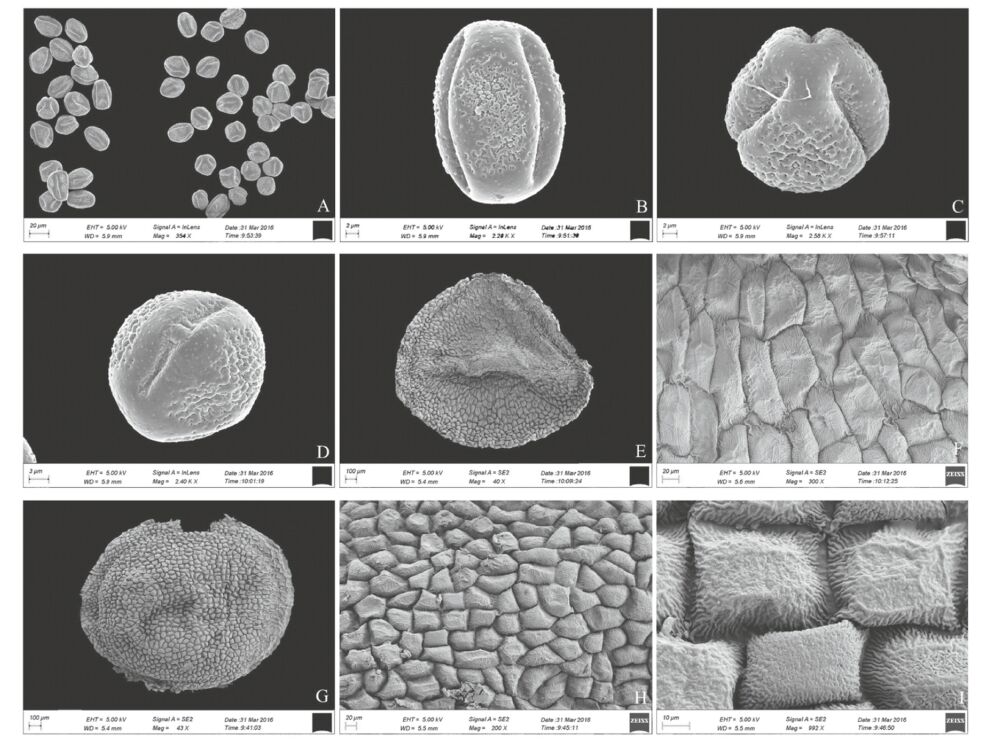
|
| Fig. 2 Scanning electron microscope (SEM) images of Lysimachia septemfida. A-D: Pollen grain (A. Overall view; B. Equatorial view; C. Polar view; D. Aperture). EeI: Seed (E. Ventral view; F. Ornamentation of ventral side; G. Dorsal view; H & I. Ornamentation of dorsal side). |
The newspecies shares most characters with Glaux (G. maritima) and Lysimachia subgen. Naumburgia(L. thyrsiflora)(Table 1). It is probably related to G. maritima by their apetalous flowers (i.e. corolla absent, calyx half divided, persistent and surrounded the capsule), but differs from the latter by its 7-merous flowers, leaves in whorls of 3 and the distinct multicellular nodiferous hairs. Otherwise, given their shared characters of non-5-merous flowers and furry fells, it is probably related to L. thyrsiflora, but the absent corolla, terminal raceme and the arrangement of leaves in whorls of 3 makes it readily distinguishable from the latter (Chen and Hu, 1989; Hu and Kelso, 1996; Fang, 2003).
| Taxa (NO. of species in China) | Stem1 | Phyllotaxy2 | Inflorescence3 | Flower merous | Pedicel | Calyx4 | Corolla | S/C5 | |
| Anagallis (1) | (+) | O, W | S | 5 | Obvious | Green, B | Present | -1 | |
| Glaux (1) | 0 | A, O | S | 5 | Almost absent | White/Pink, M | Absent | 0 | |
| Lysimachia | Subgen. Idiophyton (38) | (+) | A, O, W | S, TR, AR | 5 | Obvious | Green, B | Present | -1 |
| Subgen. Lysimachia (56) | (+)/+ | A, O, W | TR | 5 | Obvious | Green, B | Present | 0 | |
| Subgen. Palladia (36) | (+)/+ | A, O, W | TR | 5 | Obvious | Green, B | Present | 0 | |
| Subgen. Heterostylandra (1) | + | O, basal scalelike | AR | 42528 | Almost absent | Green, B | Present | 1 | |
| Trientalis (1) | 0 | F | S | 7 | Obvious | Green, B | Present | 0 | |
| Lysimachia septemfida(1) | + | W, basal scalelike | TR | 7 | Almost absent | Yellowish white, M | Absent | 1 | |
| Note: 1. Stem: “0” completely glabrous; “(+)” generally glabrous; “+” with multicellular nodiferous hairs. 2. Phyllotaxy: “A” alternate; “O” opposite; “W” whorl; “F” fascicled; “R” rosulate. 3. Inflorescence: “S” solitary; “TR” terminal raceme; “AR” axillary raceme. 4. Calyx: “B” split to the base; “M” almost half lobated. 5. S/C=Comparison of stamen and corolla length: “-1” stamen obviously shorter than corolla; “0” stamens almost equal to corolla; “+1” stamen obviously longer than corolla. | |||||||||
CHINA. Yunnan province, Xinping Yi Autonomous county, Gasa town, Mt. Ailao, Yuenanjin. 23°59′59′′N, 101°33′36′′E. Alt. 2400 m, 2009-09-14, Wang Jia-he XPALSC518(KUN, Paratype).
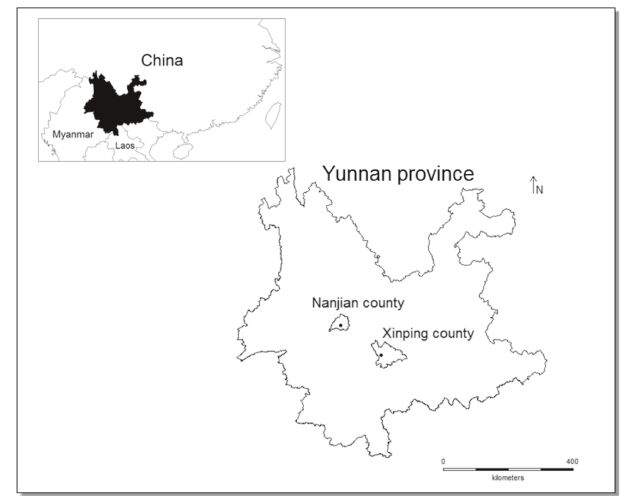
3.1.3 Distribution and habitatLysimachia septemfida is currently known from two separate localities in Yunnan, China (Fig. 3). It grows among shrubs on the peaks of mountains (Fig. 4) or in mountain valleys.
|
| Fig. 3 Distribution map of Lysimachia septemfida. |

|
| Fig. 4 Lysimachia septemfida in the wild (Mt.Wuliang, Nanjian Yi Autonomous county, Yunnan province, China), photographed by SHANGGUAN Fa-zhi. A: microhabitat; B: plant; C:inflorescens. |
The specific epithet “septemfida” is derived from the 7-merous flowers of the new species, indicating the calyx is 7-lobed. The “septem-” is a Latin prefix, which means “seven”; the “-fidus” is a Latin suffix, which means “half lobated”.
3.1.5 Conservation statusTo date, Lysimachia septemfida has only been collected from two separate locations in Yunnan, China. Its distribution range still needs further investigation to assess whether it is endangered. It is, therefore, classified as Data Deficient (DD) in the IUCN Red List (Petition IUCN Standards and Subcommittee, 2014).
3.2 Phylogenetic reconstructionBoth Bayesian and Maximum Likelihood analysis generated congruent results and the Bayesian 50% consensus tree is shown in Fig. 5. The new species is deeply nested in Lysimachia s.str., clustering with Glaux maritima and one subclade of L. subgen. Lysimachia(Clade C2), with weak support value (ML<50; BI=0.9).

|
| Fig. 5 The Bayesian consensus tree of Lysimachia s.l. based on the ITS region. The Bayesian posterior probabilities are shown below the branches and Maximum Likelihood(ML)bootstrap values above. The subgenera of Lysimachia s.str.(Hao et al., 2004)are labeled on the right side with different color bar. All the sequences downloaded from the GenBankhave species names followed by their accession number. |
Our present data confirmed the paraphyly of both Lysimachia s.str. and its present subgenus classification, which is in accordance with previous studies based on both morphology and molecular data (Anderberg and Ståhl, 1995; Käallersjöo et al., 2000; Hao and Hu, 2001; Martins et al., 2003; Hao et al., 2004; Anderberg et al., 2007; Zhou et al., 2015).
The Chinese Lysimachieae contains four genera (i.e. Lysimachia s.str., Glaux, Trientalis and Anagallis), with the latter three being monotypic (Chen and Hu, 1989; Hu and Kelso, 1996). The largest Lysimachia s.str. can be divided into 5 subgenera, i.e. L. subgen. Idiophyton Hand.-Mazz., L. subgen. Lysimachia, L. subgen. Palladia(Moench) Hand.-Mazz., L. subgen. Heterostylandra(Hand.-Mazz.) Chen et C. M. Hu and L. subgen. Naumburgia(Moench) Hand.-Mazz (Chen and Hu, 1989; Fang, 2003). Our molecular phylogenetic study based on ITS sequence (Fig. 5) shows that Lysimachia s.str. is mixed with Anagallis, Trientalis and Glaux and it could be divided into four clades (Clade A-D). Anagallis and Trientalis form one small clade with weak support values (Fig. 5; ML<50; BI ¼ 0.93), which is sister to all Lysimachia s.str. except the small clade A. The two sequences of Glaux maritima form a well-supported subclade (ML=100; BI=1), which is deeply nested in Lysimachia Clade C and groups with the taxa of L. subgen. Lysimachia (Clade C2), with weak support values (ML<50; BI=0.9). For the subgenus classification, our present data do not corroborate the current subgenus classification of Lysimachia s.str., especially L. subgen. Lysimachia, which was revealed as a heterogeneous assembly of at least five lineages: part of the clade A and B, part of the subclade C2 and the whole subclade C3, conforming its polyphyletic status to previous studies (Hao et al., 2004; Anderberg et al., 2007; Zhou et al., 2015).Further efforts are needed to improve the infrageneric classification of Lysimachia s.l.
4.2 Systematic position of the new speciesMolecular phylogenetic study based on ITS sequence shows that the new species is deeply nested within the Lysimachia s.str. Clade C and groups with Glaux maritima and the taxa of L. subgen. Lysimachia (Clade C2), but with weak support values (Fig. 5; ML, <50; BI=0.9).
Lysimachia s.str. pollen morphology has previously been reported (Huynh, 1970, 1971; Bennell and Hu, 1983; Ren et al., 1994; Shao and Zhang, 2005). Bennell and Hu (1983)recognized ten major pollen-types and four subtypes. We found that L. septemfida pollen is spheroidal, tricolporate, tectum imperforate, and medium-sized [27.24(24.28-29.29)×23.04(21.54-24.50)µm, P/ E=1.07]. Furthermore, the pollen morphology of L. septemfida is most similar to that of Glaux maritima (Halbritter, 2010), which suggests the two species are closely related. Our phylogenetic analysis (Fig. 5) and comparative morphological studies (Table 1) support the close relationship between the two species.
Two seed microfeature types were first recognized for Chinese Lysimachia s.str. by Shao et al.(2006)using light microscopy (LM) and scanning electron microscopy (SEM). Oh et al.(2008)investigated the seed morphology of Lysimachia s.l. systematically and included 34 species of Lysimachia s.str. and 14 species and 2 subspecies of its six related genera (Anagallis, Ardisiandra, Asterolinon, Glaux, Pelletiera, Trientalis), identifying three types of seed shape and six types of seed surface pattern. The sectoroid seed shape is the most common type in Lysimachia s.str., which has also been found in Anagallis, Glaux maritima, and Asterolinon adoense. L. septemfida seed shape is dorsiventrally and laterally compressed, which is similar to the sectoroid type, but much thinner and more flattened. The seed surface sculpturing of the new species may belong to the colliculate type, which is very rare and has only been found in L. insignis thus far. However, L. septemfida differs from L. insignis in that its seed top surface is much flatter.
Based on the above discussion, we can only preliminarily conclude that Lysimachia septemfida is closely related to Glaux maritima and some species of L. subgen. Lysimachia. Due to the incorporation of Glaux, Trientalis, Anagallis, Asterolinon and Pelletiera, the relationships within Lysimachia s.l. remain unresolved. The combination of several distinct characters (Table 1) of L. septemfida bar it from being assigned to current subgenera or sections of Lysimachia s.str. Therefore, further studies are needed to unveil the systematic position of the new species in Lysimachia.
| Anderberg A.A, Manns U, Kallersjo M, 2007. Phylogeny and floral evolution of the Lysimachieae (Ericales, Myrsinaceae): evidence from ndhF sequence data. Willdenowia, 37(2), 407 -421. DOI:10.3372/wi.37.37202 | ||
| Anderberg A.A, Rydin C, Kallersjo M, 2002. Phylogenetic relationships in the order Ericales s. l.: analyses of molecular data from five genes from the plastid and mitochondrial genomes. Am. J. Bot., 89(4), 677 -687. | ||
| Anderberg A.A, Stahl B, 1995. Phylogenetic interrelationships in the order Pri- mulales, with special emphasis on the family circumscriptions. Can. J. Bot., 73(11), 1699 -1730. DOI:10.1139/b95-184 | ||
| Anderberg A.A, Stahl B, Kallersjo M, 1998. Phylogenetic relationships in the Primulales inferred from rbcL sequence data. Plant Systtematics Evololution, 211(1), 93 -102. | ||
| Bennell A.P, Hu C.M, 1983. The pollen morphology and taxonomy of Lysimachia. Notes R. Botanic Gard. Edinb., 40(3), 425 -458. | ||
| Chen F.H, Hu C.M, 1979. Taxonomic and phytogeographic studies on Chinese species of Lysimachia. Acta Phytotaxon. Sin., 17(4), 21 -53. | ||
| Chen, F.H., Hu, C.M., 1989. Primulaceae. In: Wu, C.Y. (Ed.), Flora Reipublicae Popu- laris Sinica, vol. 59. Science Press, Beijing, pp. 1-134 (1). | ||
| Erdtman G, 1960. The Acetolysis method. A revised description. Sven. Bot. Tidskr., 54, 561 -564. | ||
| Fang, R.Z., 2003. Lysimachia L. In: Wu, C.Y. (Ed.), Flora Yunnanica, vol. 15. Science Press, Beijing, pp. 327-382. | ||
| Halbritter, H., 2010. Glaux maritima. In: PalDat (2010-11-01) - a Palynological Database. Published on the Internet. https://www.paldat.org/pub/Glaux_maritima/110624(accessed19.07.16). | ||
| Hao G, Hu C.M, 2001. Phylogenetic relationships in Lysimachia (Primulaceae): a cladistic analysis. J. Trop. Subtropical Bot., 9, 93 -100. | ||
| Hao G, Yuan Y.M, Hu C.M, Ge X.J, Zhao N.X, 2004. Molecular phylogeny of Lysimachia (Myrsinaceae) based on chloroplast trnL-F and nuclear ribosomal ITS sequences. Mol. Phylogenetics Evol., 31(1), 323 -339. DOI:10.1016/S1055-7903(03)00286-0 | ||
| Heywood, V.H., Brummitt, R.K., Culham, A., Seberg, O., 2007. Flowering Plant Families of the World. Royal Botanic Gardens, England. | ||
| Hu, C.M., Kelso, S., 1996. Primulaceae. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds.), Flora of China (Primulaceae), vol. 15. Science Press and St Louis: Missouri Botanical Garden Press, Beijing, pp. 39-78. | ||
| Huynh K.L, 1970. Le pollen et la systematique chez le genre Lysimachia (Primula- ceae). I: morphologie genera le du pollen etpalynotaxonomie. Candollea, 25, 267 -296. | ||
| Huynh K.L, 1971. Le pollen et la systeematique chez le genre Lysimachia (Primula- ceae). II: considerations geeneerale. Candollea, 26, 279 -295. | ||
| IUCN Standards and Pertition Subcommittee, 2014. Guidelines for Using the IUCN Red List Categories and Criteria. Version 11. Prepared by the Standards and Petitions Subcommittee. Available. http://www.iucnredlist.org/documents/RedListGuidelines.pdf(accessed30.03.16). | ||
| Käallersjöo M, Bergqvist G, Anderberg A.A, 2000. Generic realignment in primuloid families of the Ericales s. l.: a phylogenetic analysis based on DNA sequences from three chloroplast genes and morphology. Am. J. Bot., 87(9), 1324 -1341. | ||
| Mabberley D.J, 2008. Mabberley's Plant-book: a Portable Dictionary ofPlants, Their Classification and Uses.third ed. Cambridge: Cambridge University Press | ||
| Manns U, Anderberg A.A, 2009. New combinations and names in Lysimachia (Myrsinaceae) for species of Anagallis, Pelletiera and Trientalis. Willdenowia, 39(1), 49 -54. DOI:10.3372/wi.39.39103 | ||
| Martins L, Oberprieler C, Hellwig F.H, 2003. A phylogenetic analysis of Primu- laceae s. l. based on internal transcribed spacer (ITS) DNA sequence data. Plant Syst. Evol., 237(1), 75 -85. | ||
| Mast A.R, Kelso S, Richards J, Lang D.J, Feller D.M.S, Conti E, 2001. Phylogenetic relationships in Primula L. and related genera (Primulaceae) based on non?coding chloroplast DNA. Int. J. Plant Sci., 162(6), 1381 -1400. | ||
| Oh I.C, Anderberg A.L, Schoanenberger J, Anderberg A.A, 2008. Comparative seed morphology and characterevolution in the genus Lysimachia (Myrsinaceae) and related taxa. Plant Syst. Evol., 271(3-4), 177 -197. DOI:10.1007/s00606-007-0625-z | ||
| Ren X.F, Zhou S.B, Guo X.H, Chen Y, 1994. Studies on the pollen morphology of 13 species of Lysimachia in Anhui. J. Anhui Normal Univ. Nat. Sci., 17(4), 61 -68. | ||
| Shao J.W, Zhang X.P, 2005. Pollen morphology of Lysimachia and its systematic implication. Acta Micropalaeontologica Sin., 22(1), 78 -86. | ||
| Shao J.W, Zhang X.P, Zhu G.P, 2006. The microcharacteristics of seed surface in Lysimachia (Primulaceae) and its systematic implications. Acta Bot. Yunnanica, 28(4), 378 -382. | ||
| Stahl, B., Anderberg, A.A., 2004. Myrsinaceae. In: Kubitzki, K. (Ed.), The Families and Genera of Vascular Plants, vol. 6. Springer, Berlin, pp. 266-281. | ||
| Stevens, P.F., 2016. Angiosperm phylogeny website. 2012-onward; Version 12. Available. http://www.mobot.org/MOBOT/research/APweb/(accessed30.06.16). | ||
| Wang, Z.H., Peng, H., Kilian, N., 2013. Molecular phylogeny of the Lactuca alliance (Cichorieae Subtribe Lactucinae, Asteraceae) with focus on their Chinese center of diversity detects potential events of reticulation and chloroplast capture. Plos One 8, E82692. http://dx.doi.org/10.1371/journal.pone.0082692. | ||
| Xiang C.L, Funamoto T, Evangelista E.V, Zhang Q, Peng H, 2013. Pollen morphology of the East Asiatic genus Chelonopsis (Lamioideae: Lamiaceae) and allied genera, with reference to taxonomic implications and potential pollination ecology. Plant Biosyst., 147(3), 620 -628. DOI:10.1080/11263504.2012.748099 | ||
| Zhou J.J, Yu X.L, Deng Y.F, Yan H.F, Lin Z.L, 2015. Lysimachia huangsangensis (Primulaceae), a new species from Hunan,. China. PLoS ONE, 10(7), e0132713 . DOI:10.1371/journal.pone.0132713 |



