b. Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, China;
c. Department of Biology, Honghe University, Mengzi, 661100, China;
d. Department of Biology, Honghe University, Mengzi, 661100, China
Over-watering is a type of stress commonly encountered by plants. Plants in floodplains, riparian zones, and swamps are intermittently subject to submergence, and crops can exhibit reduced yield or die as a result. The essential problem associated with submergence is that the underground parts, or the whole plant, are submerged, limiting the availability of oxygen for the roots (Fukao and Bailey-Serres, 2004). This causes plant-specific responses at the morphological, physiological, and biochemical levels (Glenz et al., 2006). Upon submergence, plants form hypertrophied lenticels to take up, oxygen and develop aerenchyma and adventitious roots to promote oxygen transport (Glenz et al., 2006); they also reduce their rates of respiration, photosynthesis, and growth, and use unsaturation of very-long-chain ceramides to protect themselves from hypoxia-induced damage (Xie et al., 2015a). In addition, oxidative phosphorylation is blocked, glycolysis is accelerated, and the plants' metabolism switches from aerobic to anaerobic (Zhang et al., 2006; Kolb and Joly, 2009). Waterlogged plants also synthesise anaerobic peptides and degrade aerobic proteins. The expression of genes related to glycolysis, energy metabolism, and lipid metabolism is also changed (Zhang et al., 2006). It has been reported that oxygen limitation decreases the barrier function of membranes (Blokhina et al., 2003) and induces lipid changes (Xie et al., 2015b), but the details of the responses of membrane lipid composition to submergence are still unknown.
Plant membranes mainly consist of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidic acid (PA), phosphatidylglycerol (PG), monogalactosyldiacylglycerol (MGDG), and digalactosyldiacylglycerol (DGDG). The two classes of galactolipids are the major components of plastidic membranes. Membrane lipid composition is sensitive to the environment. To adapt or respond to temperature or osmotic stress, plants have the capacity to remodel their lipids to optimise membrane function or, under extreme conditions, to maintain membrane integrity (Welti et al., 2002; Zhang et al., 2013; Li et al., 2014a; Yu et al., 2015). Changes of membrane lipids are also critical for plant development and growth, such as germination and leaf senescence (Yu et al., 2015; Jia et al., 2013). Types of lipid alteration include changes to the contents of lipid classes and changes to the number of double-bonds and the length of acyl chains. These changes directly affect membrane fluidity and integrity (Zheng et al., 2011; Li et al., 2014b). One major change to membrane lipids occurs through phospholipase D (PLD)-mediated hydrolysis. PLDs hydrolyse phospholipids into PA and a head group. This reaction plays an important role in many biological processes (Wang et al., 2006). For example, there is a marked induction of PLD activity in response to desiccation, freezing, and salt stress (Sang et al., 2001; Bargmann et al., 2009; Munnik et al., 2000; Li et al., 2008, 2014a), in which cells suffer high osmotic stress (low water potential), and cellular water deficiency is their core stress. Submergence involves low osmotic and hypoxic stresses. However, the issue of how membrane lipids respond to low intracellular water potential has yet to be investigated.
The aim of the present study is therefore to explore the changes of membrane lipids during submergence. We completely submerged leaves and whole plants of Arabidopsis thaliana in water and observed the symptoms that resulted from this. Using electrospray ionisation mass spectrometry (ESI-MS/MS) (Welti et al., 2002), we profiled the molecular species of membrane lipids in the submerged plants. We analysed the lipid contents and composition under stress conditions from which plants could or could not recover. We also examined the changes in the number of double-bonds and in the acyl chain lengths of lipids during submergence. Our findings provide new insight into the cellular responses of plants to submergence.
2 Materials and methods 2.1 Plant materials, growth conditions and treatmentsSeeds of wild-type Arabidopsis ecotype Columbia were cultivated in soil with fluorescent lighting at 120 mmol m-2 s-1, a 12-h photoperiod, and a relative humidity of 60%, at 23 ℃ (day) and 19 ℃ (night). All plants were selected from a single synchronously growing population. Well-developed plants and leaves with an adult morphology were selected. To induce submergence stress, the leaves were placed with their adaxial surfaces facing upwards in Petri dishes that contained water. The petri dishes were filled with water to make sure that the leaves were completely submerged. The plants were transplanted into containers that were filled with water until the plants were completely submerged. The control was given suitable water as needed. The plants and leaves were incubated for the indicated time at 22 ℃ with a 12-h photoperiod and light at 120 mmol m-2 s-1.
2.2 Measurement of chlorophyll fluorescenceChlorophyll (Chl) fluorescence was measured using an imaging chlorophyll fluorometer, the MAXI-Imaging Pulse-Amplitude (PAM) instrument (Walz, Effeltrich, Germany). The value of Fv/Fm was obtained after the plant had adapted to the dark for 20 min and then exposed to a saturating pulse (>1800 mmol photons m-2 s-1). The initial fluorescence level (F0) and the maximal fluorescence level (Fm) were determined, and the results obtained using Fv/Fm=(Fm -F0)/Fm. Thus was the maximal quantum yield of photosystem Ⅱ (PS Ⅱ) produced. False-colour images of the Fv/Fm parameter were obtained using Imaging Win software.
2.3 Lipid extraction, ESI-MS/MS, and data processingLipid extraction, ESI-MS/MS analysis, and data processing followed a procedure as detailed previously with some minor modifications (Welti et al., 2002). Leaves were harvested at the sampling time and transferred immediately into 3 mL of isopropanol with 0.01% butylated hydroxytoluene (BHT) in a 75 ℃ water bath to inhibit lipolytic activities. The tissue was extracted several times with chloroform: methanol (2:1) containing 0.01% BHT until the remaining leaves appeared white. The remaining plant tissue was heated overnight at 105 ℃ and weighed to give the dry weight of the plant. Triple quadrupole MS/MS equipped for ESI was applied to analyse lipid samples. Data processing was performed as described previously (Zheng et al., 2011; Welti et al., 2002). The lipids in each class were quantified in comparison with two internal standards of the class. We analysed five replicates for each treatment. The Q-test was performed on the replicates of the total amount of each lipid class with different head groups and data from discordant samples were removed. One-way analysis of variance (ANOVA) was perform using SPSS 19.0 to analyse the data. Statistical significance was tested using Fisher's least significant difference (LSD) method. The double-bond index (DBI) was calculated using the following formula: DBI=(Σ[N × mol% lipid])/ 100, where N is the total number of double bonds in each lipid molecule (Zheng et al. 2011). Acyl chain length (ACL) was calculated using a formula derived from the DBI calculation above: ACL=(Σ[NC × mol% lipid])/100, where NC is the number of acyl carbon atoms in each lipid molecule (Li et al., 2014b).
3 Results and discussion 3.1 Submergence symptoms in Arabidopsis and experimental designTo investigate the effects of submergence stress on plants, we completely submerged detached leaves and whole plants of Arabidopsis in water to observe their symptoms. We found that detached leaves did not show serious yellowing until 7 d after submergence (Fig. 1A). In contrast, 1-d-treated plants showed a slight yellowing of their leaves, which were able to recover to normal growth after removal of the overwatering stress (de-submergence). However, 3-d-treated plants exhibited significant yellowing and wilting in their leaves and eventually died even after removal of the overwatering stress and after growth under normal conditions for 1 d (Fig. 1B). These observations by the naked eye were confirmed by chlorophyll fluorescence (Fig. 2). Most leaves of 1-d-treated plants showed an Fv/Fm of approximately 0.7, meaning that photosynthesis was proceeding normally, whereas all the leaves of 3-d-treated plants showed an Fv/Fm of zero, which indicates a lack of photosynthesis. This implies that the cellular damage induced by submergence at the whole plant level progresses faster than that at the leaf level, suggesting that the cellular responses for detached leaves differ from those of whole plants differ. We therefore harvested the aboveground part of completelysubmerged plants in order to study the effects of submergence on membrane lipids.

|
| Fig. 1 A, submergence symptoms of Arabidopsis leaves. B, submergence symptoms of Arabidopsis whole plants. SM denotes submergence; de-SM, de-submergence. The colour bar on the right indicates Fv/Fm values. |

|
| Fig. 2 Hierarchical clustering analysis of Arabidopsis lipid molecular species during submergence. The colour bar within a column represents the lipid molecular species (nmol/mg dry weight) in the corresponding treatments. The colour of each bar expresses the relative abundance of each lipid species, which represents the relative variation from the mean of each lipid species within all treatments. Lipid species in the indicated lipid classes were organised by class (as indicated), total number of acyl carbons (in ascending order within a class), and total number of double bonds (in ascending order with class and total acyl carbons). Values are means (n=4 or 5). |
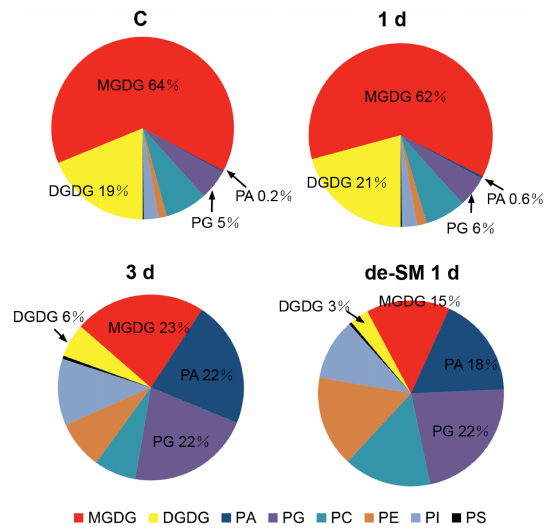
We used ESI-MS/MS to profile the changes of membrane lipids by identifying 140 molecular species belonging to six head-group classes of phospholipids (PC, PE, PI, PS, PA, and PG) and two head-group classes of galactolipids (MGDG and DGDG). Sixteen lyso-lipid species were also identified and were present at very low levers; we therefore discuss these separately. Overall, both the content (nmol/mg dry weight, Fig. 2) and the composition (mol%, Fig. 3) of major lipids indicated that significant changes took place during submergence. Cluster analysis showed that there were only small differences in lipid content between the control and the samples treated for 1 d, and that a dramatic difference in lipid content occurred between 1 d and 3 d of submergence (Fig. 3). This is consistent with the timing of the threshold between survival and death and suggests that the cellular membranes exhibited two types of response to submergence.
|
| Fig. 3 Changes in molar percentages of lipid classes during submergence in Arabidopsis. Values are means (n=4 or 5). |
We first examined the differences in the lipids between the control and the samples treated for 1 d. We found that the levels of two classes, MGDG and PC significantly decreased; four classes: DGDG, PG, PE, and PI, remained unchanged; and two classes, PS and PA, increased, after 1 d of submergence (Table 1). The greatest hange occurred for PA, with 58% relative changes. The total lipids decreased subtly and the lipid composition was similar (Fig. 3). This suggests that the lipid changes might be reversible at this stage and that plants can recover to normal growth after removal of the overwatering stress. It also suggests that the degradation might go through the PA pool during 1 day of submergence.
| Lipid class | Lipid/dry weight (nmol/mg) | RC to C (%) | ||||
| C | 1 d | 3 d | 3 d + de-SM 1 d | 1 d | 3 d | |
| DGDG | 40.23±1.97 | 38.80±2.93 | 0.44±0.04* | 0.28±0.04* | - | -99 |
| MGDG | 137.31±8.04 | 118.73±7.31* | 1.87±0.09* | 1.15±0.06* | -14 | -99 |
| PG | 11.48±1.06 | 10.33±0.14 | 1.87±0.17* | 2.04±0.58* | - | -82 |
| PC | 15.62±1.00 | 13.78±0.56* | 0.64±0.08* | 1.43±0.57* | -12 | -91 |
| PE | 3.13±0.41 | 3.25±0.42 | 0.73±0.16* | 1.53±0.76* | - | -51 |
| PI | 5.65±0.44 | 5.07±0.46 | 1.03±0.02* | 0.96±0.18* | - | -83 |
| PS | 0.37±0.04 | 0.43±0.03* | 0.05±0.02* | 0.04±0.03* | 16 | -89 |
| PA | 0.52±0.04 | 0.82±0.06* | 1.92±0.21* | 1.71±0.95* | 58 | 229 |
| Total lipids | 214.38±10.13 | 191.10±8.16 | 8.73±0.52* | 9.25±2.98* | - | -96 |
| The relative change (RC) in lipids (%) from control (C) to 1 d is the difference between the values of the control and 1 d divided the value of the control; of C to 3 d is the significant difference between the values of 3 d and the control divided by the value of the control. Values are means±SD (n=4 or 5). An asterisk indicates that the value is different from that of the control (p < 0.05). de-SM denotes de-submergence. | ||||||
We then examined the changes of lipids between 1 d and 3 d of treatment. There were remarkable decreases for all lipids except PA after 3 d of submergence (Table 1). For example, MGDG decreased from 118.73 to 1.87 nmol/mg and PC decreased from 13.78 to 0.64 nmol/mg. Total lipids decreased from 191.10 to 8.73 nmol/mg, representing a decrease of 96%. The rate of lipid loss was even higher than those reported for extreme water deficiency (Li et al., 2014a) and freezing at -8 ℃ (Li et al., 2008). These results indicate that the membranes underwent very rapid and severe deterioration and confirm the finding mentioned above that the plants died after 3 d of submergence. Combining the findings that anoxia is the dominant stress in submerged whole plants and detached leaves and that lipid degradation (Jia et al., 2013) and death progressed much more rapidly (Fig. 1A and B) in whole plants than in detached leaves, our evidence suggests that anoxia had a more rapid effect in whole plants than in leaves. In other words, anoxia could be the primary cause of massive degradation of membrane lipids upon submergence.
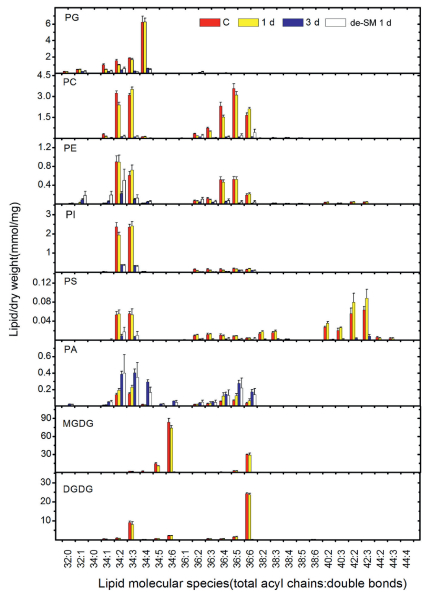
On the other hand, PA increased from 0.82 to 1.92 nmol/mg and its proportion increased from 0.6% to 22% (Fig. 3). Further examination found that increases of PA molecular species corresponded to decreases of other lipid molecular species (Fig. 4). For example, the decreases in the ratio of 34:4 (total number of carbon: number of double bonds) for PG and 34:6 for MGDG corresponded to the increases of 34:4 for PA and 34:6 for PA, respectively. This implies that these PA species were produced by turnover from other lipid species, which confirms that the degradation of lipids goes through the PA pool. For the 3-d-treated plants, de-submergence and growth under normal conditions for 1 d caused both their lipids and their membranes to continue to degrade (Table 1 and Fig. 3).
|
| Fig. 4 Changes in lipid molecular species during submergence in Arabidopsis. Values are means±standard deviations (n=4 or 5). |
The above findings show that there were two types of lipid change during submergence. The first type involved subtle decreases in lipids and occurred at the initial stage of submergence; the second showed dramatic lipid degradation and occurred at the later stage of submergence. To explore the detailed characteristics of these types, we considered the changes in the PG molecular species. The PG class includes four molecular species: 34:1 PG, 34:2 PG, 34:3 PG, and 34:4 PG (Welti et al., 2002). Among these, 34:1 PG and 34:2 PG are extraplastidic lipids, 34:3 PG is located in both the plastidic and extraplastidic membranes, and 34:4 PG is located in the plastidic membrane (Marechal et al., 1997; Miquel et al., 1998). A comparison of their changes could indicate a difference in the sensitivity of plastidic and extraplastidic membranes to stress (Jia et al., 2013). We found that, during 1 d of submergence, the levels of 34:1 PG and 34:2 PG significantly decreased, 34:3 PG slightly decreased, but 34:4 PG was maintained (Table 2). In other words, for the PG head-group class, only the extraplastidic species changed at this stage. In contrast, the levels of all species dramatically decreased during 3 d of submergence. These results indicate that the initial responses of plastidic lipids involved degradation later than in extraplastidic ones. This suggests that plastidic membranes might be less sensitive than extraplastidic membranes to submergence. The response patterns of PG molecular species to submergence differ from those to stresses involving cellular dehydration, such as water deficiency, high temperature, and freezing, in which the degradation of plastidic and extraplastidic lipids occurs simultaneously (Table 3). The changes in the patterns of PG molecular species in response to submergence also differed from those in senescence (Jia et al., 2013), in which plastidic lipids were shown to degrade sooner than extraplastidic ones. This evidence is consistent with the different observations of detached leaves and whole plants as described above (Fig. 1A and B).
| Species | Cellular location | PG/dry weight (nmol/mg) | RC to C (%) | |||||
| Plastidic | Extraplastidic | C | 1 d | 3 d | 3 d + de-SM 1 d | 1 d | 3 d | |
| 34:1 | √ | 1.03±0.11 | 0.47±0.05* | 0.17±0.03* | 0.28±0.08* | -54 | -83 | |
| 34:2 | √ | 1.56±0.13 | 1.02±0.08* | 0.39±0.03* | 0.59±0.18* | -35 | -75 | |
| 34:3 | √ | √ | 1.85±0.03 | 1.65±0.16 | 0.25±0.04* | 0.17±0.01* | - | -86 |
| 34:4 | √ | 6.22±0.74 | 6.26±0.45 | 0.59±0.02* | 0.44±0.15* | - | -91 | |
| The relative change (RC) in lipids (%) from the control (C) to 1 d is the difference between the values of the control and 1 d divided by the value of the control; that of C to 3 d is the significant difference between the values of 3 d and the control divided by the value of the control. Values are means±SD (n=4 or 5). An asterisk indicates that the value is different from that of the control (p < 0.05). de-SM denotes de-submergence. | ||||||||
| Species | PG/dry weight (nmol/mg) | ||||||||
| Left panel (Li et al. 2014a) | Middle panel (Tang et al. 2016) | Right panel (Li et al. 2008) | |||||||
| Control | Dehydration | Rehydration | Control | 35 ℃ for 2 d | 35 ℃ for 8 d | Control | Freezing | Thawing | |
| 34:1 | 1.89±0.48 | 1.51±0.63 | 0.30±0.06* | 1.11±0.20 | 1.51±0.15* | 0.16±0.00* | 1.41±0.15 | 0.66±0.41* | 0.74±0.09* |
| 34:2 | 2.08±0.35 | 1.02±0.33* | 0.26±0.02* | 1.92±0.30 | 2.00±0.27 | 0.44±0.05* | 3.22±0.20 | 1.90±0.41* | 1.77±0.21* |
| 34:3 | 3.34±0.40 | 0.53±0.15* | 0.44±0.06* | 3.36±0.14 | 3.51±0.35 | 1.07±0.20* | 7.02±0.38 | 4.43±0.77* | 4.14±0.41* |
| 34:4 | 13.78±1.53 | 0.08±0.03* | 1.63±0.06* | 11.64±0.44 | 6.58±0.28* | 0.54±0.10* | 10.88±0.82 | 7.53±0.96* | 6.77±0.77* |
| Values are means±SD (n=4 or 5). An asterisk indicates that the value is significantly different from that of the control (p < 0.05). Data were obtained from our previous experiments as indicated. | |||||||||
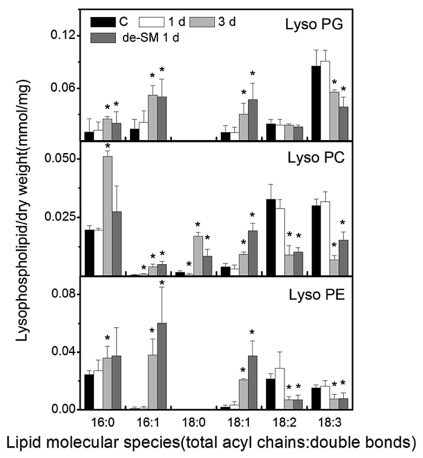
Lyso-phospholipids are derived from phospholipase A-mediated hydrolysis at the sn-1 or sn-2 position of phospholipids (Wang et al., 2002). It is reported that they dramatically increase in response to freezing stress (Welti et al., 2002) or severe dehydration (Li et al., 2014a). We examined the changes of lyso-phospholipids here to see how they respond to submergence. Lyso-phospholipids remained unchanged during 1 d of submergence but changed significantly during 3 d of submergence (Fig. 5). It is interesting that the lyso-phospholipids exhibited various patterns of change induced by submergence. The levels of lyso-phospholipids 16:0, 16:1, 18:0, and 18:1 increased, whereas those of 18:2 and 18:3 decreased during 3 d of submergence (Fig. 5). This is different from the findings obtained for freezing and dehydration.
|
| Fig. 5 Changes in lyso-phospholipid molecular species during submergence in Arabidopsis. Values are means±standard deviation (n=4 or 5). An asterisk indicates that the value is significantly different from that of the control (p < 0.05). |
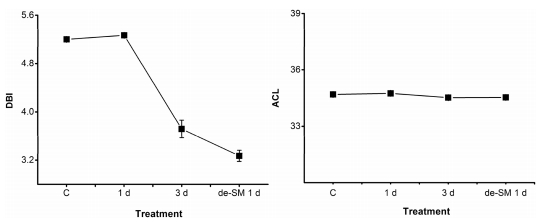
Membrane fluidity is an important property enabling cells to adapt to their environment. It can be characterised by the number of double-bonds and the acyl chain length (ACL) of lipids. Decreases in the number of double-bonds number and ACL confer high fluidity. To explore the effect of low intracellular water potential on membrane fluidity, we calculated the average double-bond number (double bond index, DBI) and the ACL of lipids during submergence (Fig. 6). We found that DBI was maintained after 1 d and dramatically decreased after 3 d of submergence, and that ACL did not change during the whole treatment. The decreases in DBI probably resulted from lipid degradation, rather than as a direct effect of submergence. The evidence suggests that submergence did not affect membrane fluidity. This differs from the findings obtained under temperature stress and water deficiency.
|
| Fig. 6 Double-bond index (DBI) and acyl chain length (ACL) of membrane lipids in A. thaliana during submergence. Values are means±standard deviations (n=4 or 5). |
Membrane change is thought to be one of the initial cellular responses to abiotic stresses. The changes of membrane lipids in response to high temperature, low temperature, drought, and nutrition deficiency have been well documented (Li et al., 2004, 2014a; Welti et al., 2002; Tang et al., 2014; Yu et al., 2015; Li et al., 2006; Wang et al., 2014). The compositions of membrane lipids in tissues and their changes during senescence have also been reported (Jia et al., 2013; Devaiah et al., 2006, 2007). To the best of our knowledge, this is the first profiling of the molecular species of membrane lipids upon submergence. Membrane lipids were slightly degraded but maintained their basic relative contents during early submergence. The decrease of MDGD and the increase of PA were the most significant changes at this stage. After 3 d of submergence, dramatic lipid degradation took place and the membrane integrity deteriorated. The submergence-induced changes of membrane lipids involved PA-mediated degradation, but had three unique characteristics in comparison to temperature stress and water deficiency. The first was that extraplastidic PG species degraded sooner than plastidic ones. The second was that the lyso-phospholipids exhibited various patterns of change. The third was that the DBI remained unchanged.
The core difference at the cellular level between submergence and temperature stress/water deficiency is water potential. Intracellular water potential is low and water flows into a cell making it swell under submergence, whereas intracellular water potential is high and water flows out of a cell and making it shrink under temperature stress or water deficiency. Cellular swelling and shrinkage have different effects on membranes. The different water potential also has different effects on the hydrophobic force of lipids, making them form laminar membranes. These could be the reasons why the changes of membrane lipids under submergence are different from those under temperature stress and water deficiency. Our results also suggest that anoxic damage to cells took place more rapidly at the level of the whole plant than that of the leaves.
AcknowledgementsThe authors thank Mary Roth (Kansas Lipidomics Research Center) for her assistance with the lipid analysis, Dr. Yanxia Jia for the use of the IMAGING-PAM chlorophyll fluorometer.
| Bargmann B.O., Laxalt A.M., ter Riet B., et al., 2009. Multiple PLDs required for high salinity and water deficit tolerance in plants[J]. Plant Cell Physiol, 50, 78 -89. DOI:10.1093/pcp/pcn173 | ||
| Blokhina O., Virolainen E., Fagerstedt K.V., 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review[J]. Ann. Bot, 91, 179 -194. DOI:10.1093/aob/mcf118 | ||
| Devaiah S.P., Pan X., Hong Y., et al., 2007. Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis[J]. Plant J, 50, 950 -957. DOI:10.1111/j.1365-313X.2007.03103.x | ||
| Devaiah S.P., Roth M.R., Baughman E., et al., 2006. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dalpha1 knockout mutant[J]. Phytochemistry, 67, 1907 -1924. DOI:10.1016/j.phytochem.2006.06.005 | ||
| Fukao T., Bailey-Serres J., 2004. Plant responses to hypoxia e is survival a balancing act?[J]. Trends Plant Sci, 9, 449 -456. DOI:10.1016/j.tplants.2004.07.005 | ||
| Glenz C., Schlaepfer R., Iorgulescu I., et al., 2006. Flooding tolerance of Central European tree and shrub species[J]. For Ecol Manage, 235, 1 -13. DOI:10.1016/j.foreco.2006.05.065 | ||
| Jia Y., Tao F., Li W., 2013. Lipid profiling demonstrates that suppressing Arabidopsis phospholipase D retards ABA-promoted leaf senescence by attenuating lipid degradation[J]. PloS One 8, e65687 . | ||
| Kolb R.M., Joly C.A., 2009. Flooding tolerance of Tabebuia cassinoides: metabolic, morphological and growth responses[J]. Flora, 204, 528 -535. DOI:10.1016/j.flora.2008.07.004 | ||
| Li A., Wang D., Yu B., et al., 2014a. Maintenance or collapse: responses of extraplastidic membrane lipid composition to desiccation in the resurrection plant Paraisometrum mileense[J]. PloS One 9, e103430 . | ||
| Li M., Welti R., Wang X., 2006. Quantitative profiling of Arabidopsis polar glyc-erolipids in response to phosphorus starvation[J]. Roles of phospholipases D zeta1 and D zeta2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol, 142, 750 -761. | ||
| Li W., Li M., Zhang W., et al., 2004. The plasma membrane-bound phospholipase Ddelta enhances freezing tolerance in Arabidopsis thaliana[J]. Nat. Biotechnol, 22, 427 -433. DOI:10.1038/nbt949 | ||
| Li W., Wang R., Li M., et al., 2008. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana[J]. J. Biol. Chem, 283, 461 -468. DOI:10.1074/jbc.M706692200 | ||
| Li Y., Zheng G., Jia Y., et al., 2014b. Acyl chain length of phosphatidylserine is correlated with plant lifespan[J]. PloS One 9, e103227 . | ||
| Marechal E., Block M.A., Dorne A.J., et al., 1997. Lipid synthesis and metabolism in the plastid envelope[J]. Physiol. Plant, 100, 65 -77. DOI:10.1111/ppl.1997.100.issue-1 | ||
| Miquel M., Cassagne C., Browse J., 1998. A new class of Arabidopsis mutants with reduced hexadecatrienoic acid fatty acid levels[J]. Plant Physiol, 117, 923 -930. DOI:10.1104/pp.117.3.923 | ||
| Munnik T., Meijer H.J., Ter Riet B., et al., 2000. Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate[J]. Plant, 22, 147 -154. DOI:10.1046/j.1365-313x.2000.00725.x | ||
| Sang Y., Zheng S., Li W., et al., 2001. Regulation of plant water loss by manipulating the expression of phospholipase Dalpha[J]. Plant J, 28, 135 -144. DOI:10.1046/j.1365-313X.2001.01138.x | ||
| Tang T., Liu P., Zheng G., et al., 2016. Two phases of response to long-term mod-erate heat: variation in thermotolerance between Arabidopsis thaliana and its relative Arabis paniculata[J]. Phytochemistry, 122, 81 -90. DOI:10.1016/j.phytochem.2016.01.003 | ||
| Tang T., Zheng G.W., Li W.Q., 2014. Adaptation to extremely high temperature in an Alpine environment: systemic termotolerance in Arabis paniculata[J]. Plant Divers. Resour, 36, 683 -697. | ||
| Wang D.D., Zheng G.W., Li W.Q., 2014. Plants adapt to long-term Potassium deficiency by accumulation of membrane lipids in leaves and maintenance of lipid composition in roots[J]. Plant Divers. Resour, 36, 163 -176. | ||
| Wang X., Devaiah S.P., Zhang W., et al., 2006. Signaling functions of phosphatidic acid[J]. Prog. Lipid Res, 45, 250 -278. DOI:10.1016/j.plipres.2006.01.005 | ||
| Wang X., Wang C., Sang Y., et al., 2002. Networking of phospholipases in plant signal transduction[J]. Physiol. Plant, 115, 331 -335. DOI:10.1034/j.1399-3054.2002.1150301.x | ||
| Welti R., Li W., Li M., et al., 2002. Profiling membrane lipids in plant stress re-sponses[J]. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem, 277, 31994 -32002. | ||
| Xie L.J., Chen Q.F., Chen M.X., et al., 2015a. Unsaturation of very-long-chain ceramides protects plant from hypoxia-induced damages by modulating ethylene signaling in Arabidopsis[J]. PLoS Genet. 11, e1005143 . | ||
| Xie L.J., Yu L.J., Chen Q.F., et al., 2015b. Arabidopsis acyl-CoA-binding protein ACBP3 participates in plant response to hypoxia by modulating very-long-chain fatty acid metabolism[J]. Plant J, 81, 53 -67. DOI:10.1111/tpj.12692 | ||
| Yu X., Li A., Li W., 2015. How membranes organize during seed germination: three patterns of dynamic lipid remodelling define chilling resistance and affect plastid biogenesis[J]. Plan. Cell Environ, 38, 1391 -1403. DOI:10.1111/pce.2015.38.issue-7 | ||
| Zhang X.D., Wang R.P., Zhang F.J., et al., 2013. Lipid profiling and tolerance to low-temperature stress in Thellungiella salsuginea in comparison with Arabidopsis thaliana[J]. Biol. Plant, 57, 149 -153. DOI:10.1007/s10535-012-0137-8 | ||
| Zhang Z.X., Zou X.L., Tang W.H., et al., 2006. Revelation on early response and molecular mechanism of submergence tolerance in maize roots by microarray and suppression subtractive hybridization[J]. Environ. Exp. Bot, 58, 53 -63. DOI:10.1016/j.envexpbot.2005.06.016 | ||
| Zheng G., Tian B., Zhang F., et al., 2011. Plant adaptation to frequent alterations between high and low temperatures: remodelling of membrane lipids and maintenance of unsaturation levels[J]. Plant Cell Environ, 34, 1431 -1442. DOI:10.1111/pce.2011.34.issue-9 |



