2. Plant Germplasm and Genomics Center, Germplasm Bank of Wild Species in Southwest of China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, China
3. State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, 100101, China
4. University of Chinese Academy of Sciences, Beijing, 100049, China
1. Introduction
Although many plants have evolved various strategies to cope with arid conditions, drought remains one of the most severe abiotic stresses for plants (Morgan, 1984; Turgut and Kadioglu, 1998; Johanson et al., 2001; Chaves et al., 2003; Nelson et al., 2007; Nicotra and Davidson, 2010; Terzi et al., 2013). Drought stress can affect plant growth, inhibit the productivity of plant populations or communities, and even cause plant death and vegetation degradation (Yordanov et al., 2000; Aranjuelo et al., 2011). In the Tibetan Plateau, plants are faced with drought conditions. Because of the characteristics of atmospheric circulation and the special terrain, there is a marked decrease in precipitation from east to west in the Tibetan Plateau (Klein et al., 2004; Shen et al., 2008). Thus, plants distributed in different areas are subjected to diverse water conditions. Because global climate change can cause temporal and spatial changes of precipitation (Zhuang et al., 2010), such change may indirectly exacerbate the severity of drought in some regions of the Tibetan Plateau, which is considered one of the most sensitive areas to global climate change (Carlyle et al., 2014; Wang et al., 2014). The dominant species of the alpine steppe, Stipa pur-purea, is widely distributed across this large water gradient. This indicates S.purpurea can adapt to a wide range of water conditions. However, because of global warming and human activities, grassl and degradation processes such as desertification continue to intensify (Cai et al., 2007). Severely or extremely degraded grassl and s need artificial grass for recovery (He et al., 2008). One of the keys to grassl and recovery is overcoming the water problem. To solve this problem, we first need to underst and the responses or adaptive mechanisms of S.purpurea to drought environments.
In recent years, several studies have focused on the interactions between S.purpurea and drought. Yang et al.(2015b) first studied the morphological and structural differences, physiological and biochemical changes, and transcriptional differentiation of five natural S.purpurea populations. Their results showed that S.purpurea had developed a series of adaptive characteristics including thickened cell walls in roots, diminished stomata and increased production of resistance-related substances and expression of genes to cope with increasing drought. To exclude the interference of other environmental factors, Yang et al.(2015a) studied the responses of S.purpurea from the same population to different drought degrees in the greenhouse through physiological, biochemical and proteomics approaches. The results showed that an increase in betaine, soluble sugar and abscisic acid, and an up-regulation of several groups of proteins such as antioxidant enzymes and heat shock proteins (HSPs) played an important role in the S.purpurea response to drought treatment. Without doubt, these findings greatly improved our underst and ing of the S.purpurea response to drought conditions. However, because S.purpurea is widely distributed across the Tibetan Plateau, the ability of different populations to resist drought may have diverged. We assumed that S.purpurea from more arid regions were more resistant to drought stress. Our recent study verified this hypothesis, in which S.purpurea from a western population were more resistant to drought than those from an eastern population (Li et al., 2015). The results suggested that several closely-related drought-responsive genes were involved in the S.purpurea response to drought stress (Li et al., 2015). After our previous study, we wanted to know how S.purpurea responds to drought stress at a molecular level and what molecular mechanisms underlie drought resistance differences among populations. In the present study, we used a comparative proteomics approach to identify putative protein classes involved in drought stress response in two S.purpurea populations. This study provides more information about the interaction between S.purpurea and drought environments, which is the theoretical basis for the protection and restoration of alpine steppe in the Tibetan Plateau.
2. Materials and methods 2.1. Seed collection and seedling breedingMature S.purpurea seeds were collected in August 2013 at the time of seed release (Phillips et al., 1983) from two populations of western Gar County (GR) and eastern Nagqu County (NQ) in the Tibetan Plateau (Fig. 1). After being brought to the laboratory, the seeds were dried under constant conditions at 15 ℃ and 15% air humidity for 1 wk.
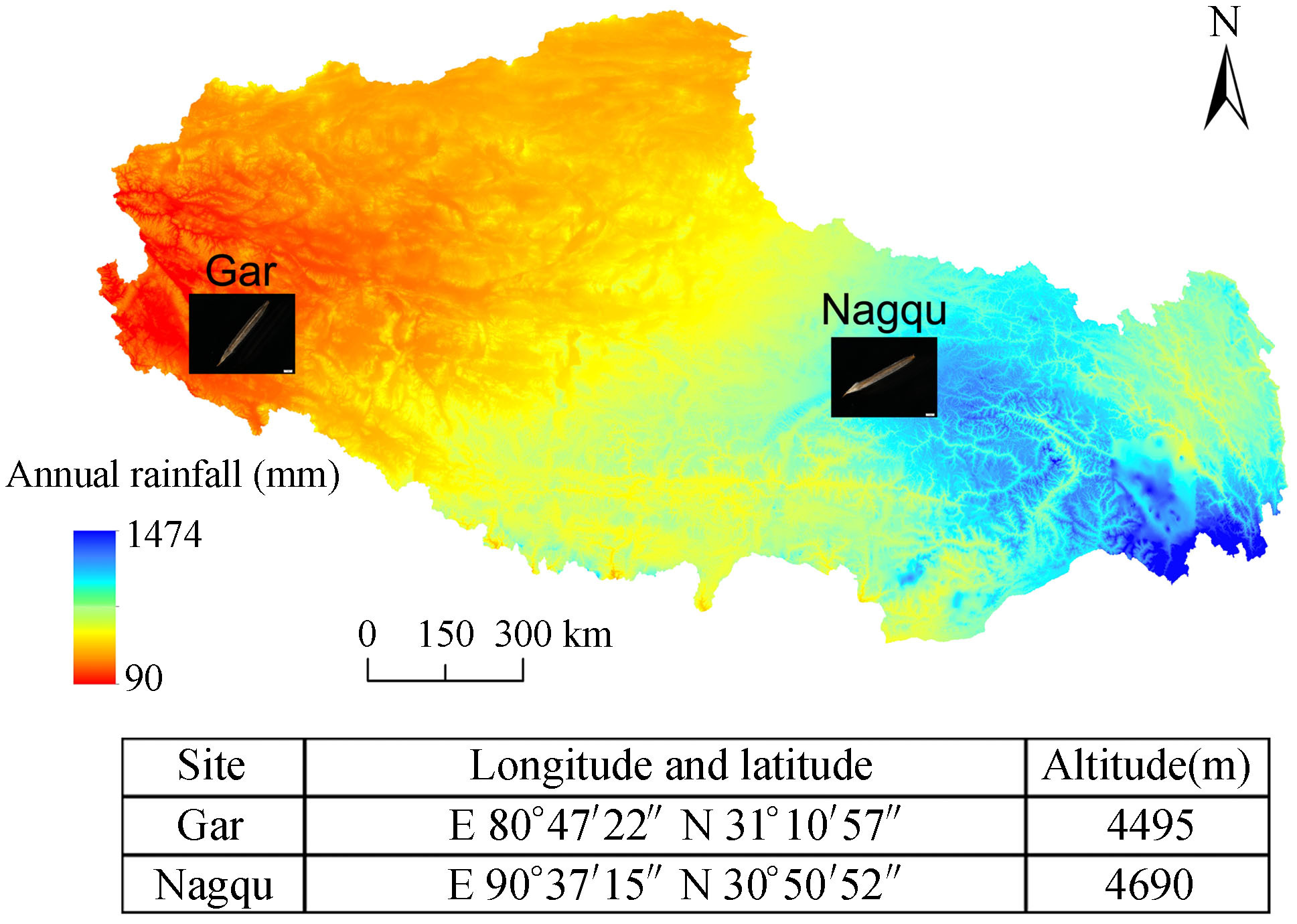
|
| Fig.1 Geographical location of the two seed collection sites on a precipitation distribution map. |
Mature seeds of the two populations were chosen r and omly and the seed awns were removed. The seeds were then sown in flowerpots (d=9.4 cm, h=8 cm) with equal amounts of humus soil, and about 0.5 cm of covering soil. Each pot was sown with 30 seeds, and 120 pots were prepared for each population. The samples were first sufficiently watered and then placed in a greenhouse (12-h light/12-h dark cycle; 28 ℃/20 ℃, day/night; 40%-60% air humidity). Every day at 17∶00, when the soil moisture content (SMC) had decreased by approximately 50%, each flowerpot was watered with 15 mL water to keep the SMC constant.
2.2. Drought treatmentWhen they reached the trefoil stage (about 3 wk growth), the plants of each population were treated with two stages of drought. Water was withheld from half of the seedlings of each population for 7 d and then they were re-watered for another 7 d, during which the seedlings were fully watered once initially and then watered with 15 mL water each day when the SMC was about 50%. Water was withheld from the remaining seedlings for 14 d (the plants of one population showed apparent death), and then they were re-watered for another 14 d. The samples (flag leaves) under drought for 0, 7, and 14 d and rehydration for 7 and 14 d were collected for subsequent measurements. Specifically, the samples (flag leaves) under drought for 0 and 7 d and rehydration for 7 d were collected for protein extraction and isobaric tag for relative and absolute quantitation (iTRAQ) analysis.
2.3. Morphological changesThe seedlings were photographed at each sampling time, and plant mortality in the two populations was recorded when the seedlings were re-watered for 7 or 14 d.
2.4. Leaf water content measurementThe relative water content (RWC) of the leaves was determined as: RWC=[Fresh weight-Dry weight (DW)]/(Turgid weight-DW). To measure turgid weight, leaves were kept in distilled water in darkness at 4 ℃ to minimize respiration losses until they reached a constant weight (Rivero et al., 2007). The DW of leaves was determined after 48 h at 70 ℃ in an air oven (Rivero et al., 2007). Three biological replicates were performed for the measurement.
2.5. Analysis of chlorophyll fluorescenceChlorophyll fluorescence was measured as previously described (Bai et al., 2011), using a pulse-amplitude modulation chlorophyll fluorometer (Heinz Walz GmbH, Effeltrich, Germany). Briefly, S.purpurea seedlings were dark-adapted for 30 min at the time of sampling to measure the maximum quantum yield of photosystem Ⅱ (PS Ⅱ; Fv/Fm). The maximum fluorescence (Fm) was recorded by a 0.8 s pulsed light at 8 000 μmol s-1 m-2, and minimal fluorescence was recorded during the weak measuring pulses. Three biological replicates were performed for the measurement.
2.6. Protein extractionProtein extraction was performed using TRIzol reagent as previously described (Young and Truman, 2012). Briefly, approximately 1 g of fresh leaves was chopped with 5 mL TRIzol for 5 min. Next, 1 mL chloroform was added and the mixture was allowed to st and at -20 ℃ for 5 min. Following centrifugation at 4 ℃ and 12 000 × g for 10 min, the supernatant was removed. The lower phase was then mixed with isometric isopropanol and allowed to st and at -20 ℃ for 2 h. Next, the mixture was centrifuged at 4 ℃ and 12 000 × g for 10 min, after which the supernatant was removed. The precipitate was then washed three times with isopropanol and dried at room temperature, after which it was dissolved in lysate [8 mol·L-1 urea, 2 mol·L-1 thiourea, 4% CHAPS and 60 mmol·L-1 DTT] for 1 h with intermittent shaking.
2.7. iTRAQ analysisiTRAQ analysis was performed as previously described (Kong et al., 2014). Briefly, all samples containing 75 mg protein were prepared for iTRAQ labeling. Six groups of replicate samples, consisting of three groups of S.purpurea samples from the GR population (i.e., GR-C, GR-D, and GR-R) and three groups of S.purpurea samples from the NQ population (i.e., NQ-C, NQ-D, and NQ-R) were used, and each replicate group consisted of three samples; thus, at least 18 samples were required for labeling. The pelleted protein was first dissolved in 1% SDS and 100 mmol·L-1 triethylammonium bicarbonate (pH 8.5), and then successively subjected to reduction, alkylation, trypsin digestion, and labeling with 8-pl ex iTRAQ reagent kits, based on the manufacturer′s instructions (AB Sciex, USA). After labeling, the samples were combined and lyophilized, and the peptide mixture was dissolved in strong cation exchange solvent A (25% acetonitrile, 10 mmol·L-1 ammonium formate, pH 2.8). The peptides were fractionated on an Agilent HPLC system 1 100 with a polysulfoethyl A column (2.1 mm × 100 mm, 5 μm, 300 A, PolyLC, Columbia, MD, USA). The peptides were eluted at a ow rate of 200 μL/min with a linear gradient of 0-20% solvent B (25% acetonitrile, 500 mmol·L-1 ammonium formate) over 50 min, and then were ramped up to 100% solvent B in 5 min and held for 10 min. The absorbance at 214 nm was monitored, and 12 fractions were collected. Each strong cation exchange fraction was lyophilized and dissolved in solvent A (3% acetonitrile, 0.1% formic acid), and submitted for analysis with a Q-Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Finnigan Scientific, San Jose, CA, USA). The samples were separated on a Hypersil Gold C18 column (100 mm × 2.1 mm, 1.9 μm)(Thermo Fisher Scientic, Pittsburgh, PA, USA). The peptides were eluted with a linear gradient of acetonitrile/0.1% formic acid from 3 to 50% in 90 min at a ow rate of 250 nL/min, and then sprayed into the orice of the Q-Exactive MS/MS system with a spray voltage of 2.2 kV. Full-scan mass spectra were recorded over 200-1 800 m/z at high resolution at 60 000. At least the four most intense precursor ions were selected for collision-induced fragmentation in the linear ion trap with 50-2 000 m/z and 30-2 000 ms at a resolution of 7500. Dynamic exclusion was employed within 40 s to prevent repetition.
2.8. Protein identificationMascot 2.3.02 was used for protein identification based on the protein database (including 42, 809 sequences) that was sequenced, assembled and translated from the transcriptome. The mass spectrometry results were submitted to Mascot, the database was selected, and then the database search was performed according to the parameters shown in Supplementary Table S1.
2.9. Analysis of differentially expressed proteinsAbundance changes of 1.50- or 0.67-fold were used as criteria to indicate up-regulation or down-regulation of proteins, and P < 0.05 was used to indicate significance. We counted and analyzed the differential proteins of the two S.purpurea populations during the drought and recovery treatments compared with their respective controls, as well as the differentially expressed proteins between the controls of the two populations.
2.10. Statistical analysisStatistical analyses were performed using SPSS version 18.0. One-way ANOVA was used to identify differences among treatments (P < 0.05).
3. Results 3.1. Drought resistant traits in two populations of S.purpureaTo examine drought resistance differences in two populations of S.purpurea, we measured mortality rate and relative water content (RWC) of seedlings after two stages of drought stress followed by subsequent re-watering. When treated with drought for 7 d, no significant changes in morphology were observed between the two S.purpurea populations (Fig. 2A), but after re-watering, some plants of both populations began to die (Fig. 2A), with mortality rates of 11.6% in GR and 19.6% in NQ plants (Fig. 2C). However, after 14-d drought treatment, the plants of both S.purpurea populations exhibited withering, with a more pronounced change in the NQ population (Fig. 2A). When re-watered for 14 d, only some plants of the GR population (30.3%) died, whereas most plants of the NQ population (84.1%) died (Fig. 2C). These morphological changes directly demonstrated that S.purpurea from the GR population were more resistant to drought stress than those from the NQ population.

|
| Fig.2 Effects of drought and subsequent recovery on morphology, relative water content and mortality of S.purpurea from two sites.(A) Changes in plant morphology.(B) Changes in relative water content of leaves. Error bars indicate SE. Means denoted by different letters are significantly different (P < 0.05).(C) Changes of plant mortality rate. Error bars indicate SE. Means denoted by different letters are significantly different (P < 0.05). |
When subjected to drought for 7 d, the leaf relative water content (RWC) of S.purpurea from GR and NQ declined from 84.5% and 85.9% to 75.1% and 52.8%, respectively, with a significant difference between the two populations (Fig. 2B). After re-watering for 7 d, the leaf RWC of GR and NQ returned to 79.9% and 74.0%, respectively (Fig. 2B). After 14-d treatment, the leaf RWC of GR and NQ was reduced to 25.9% and 10.1%, respectively, with a significant difference between the two populations (Fig. 2B). When re-watered for 14 d, the leaf RWC of GR returned to 51.5% while that of NQ reached 27.6%(Fig. 2B). These differences in leaf water retention and rehydration capacity also showed that the drought resistance of S.purpurea from GR was stronger than that of S.purpurea from NQ.
3.2. Physiological changes after drought treatments between the two S.purpurea populationsWe then compared changes in chlorophyll fluorescence to show the effect of drought stress on the physiological activity of S.purpurea. The two populations exhibited different responses during the drought and recovery treatments (Fig. 3A). Drought stress for 7 d significantly decreased the Fv/Fm of S.purpurea from NQ, whereas the Fv/Fm remained high in S.purpurea from GR (Fig. 3B). After recovery for 7 d with re-watering, the Fv/Fm values of GR and NQ plants returned to 0.81 and 0.79, respectively (Fig. 3B). After 14-d drought stress, the Fv/Fm values of GR and NQ plants were reduced to 0.67 and 0.12, respectively, with a significant difference between the two populations (Fig. 3B). After re-watering for 14 d, the Fv/Fm ratio for plants from GR returned to 0.71, whereas the Fv/Fm ratio increased to only 0.15 in plants from NQ (Fig. 3B). These results further indicated S.purpurea from GR were more resistant to drought stress than plants from NQ.

|
| Fig.3 Effects of drought and subsequent recovery on chlorophyll fluorescence of S.purpurea from two sites.(A) Change of chlorophyll fluorescence. The pseudocolor code depicted at the bottom of the image ranges from 0 (red) to 1.0 (purple).(B) Changes of Fv/Fm values. Fv/Fm was determined for whole plants. Error bars indicate SE. Means denoted by different letters are significantly different (P < 0.05). |
In our study, a total of 182, 208 peptides were detected, 10, 162 peptides were identified, and 3, 235 proteins were ultimately determined (Supplementary Fig. S1).
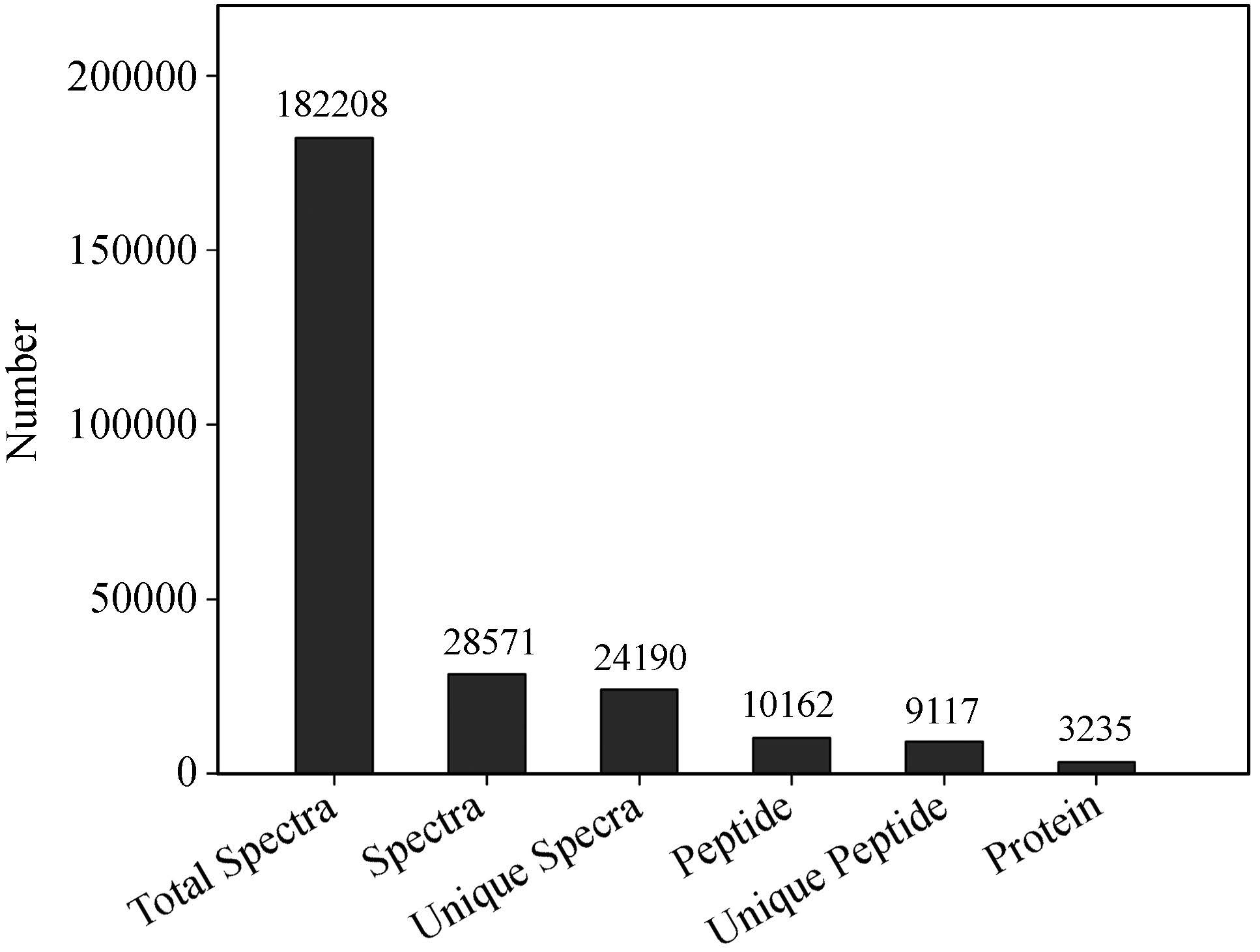
|
| Supplementary Fig. S1. Graph of the basic statistical information for mass spectrometric identification. ‘Total Spectra’ represents the total two-level spectrograms. ‘Spectra’ represents the matched spectrograms. ‘Unique Spectra’ represents the unique matched spectrograms. ‘Peptide’ represents the identified peptides. ‘Unique Peptide’ represents the unique identified peptides. ‘Protein’ represents the identified proteins. |
To explore the underlying mechanisms of drought resistance in S.purpurea from GR, we first examined differential protein expression between GR and NQ populations of S.purpurea under control conditions. We called the proteins that showed differential expression between the controls inherently differentially expressed proteins. A total of 117 inherently differentially expressed proteins were detected between the two S.purpurea populations, with 56 proteins showing higher abundance and 61 showing lower abundance in GR compared with NQ S.purpurea(Supplementary Fig. S2A, Supplementary Tables S2 and S3). Among the identified proteins with higher expression in GR plants, 28 were not responsive to drought stress (Supplementary Fig. S2B and Table 1). Of the remaining, expression of 20 proteins from GR (14 up-regulated and 6 down-regulated) and 9 proteins from NQ (3 up-regulated and 6 down-regulated) were altered under drought stress (Supplementary Fig. S2B). Similarly, among the identified proteins with higher expression in NQ plants, 35 were not responsive to drought stress (Supplementary Fig. S2B and Table 2), while 19 (17 up-regulated and 2 down-regulated) and 10 proteins (6 up-regulated and 4 down-regulated) were differentially expressed in the GR and NQ populations, respectively, during the drought treatment (Supplementary Fig. S2B).

|
| Supplementary Fig.S2. Number of inherently differentially expressed proteins and their expression patterns during drought treatment and subsequent recovery in the two S.purpurea populations. (A) Number of inherently differentially expressed proteins between the two S.purpurea populations. (B) Expression patterns of the inherently differentially expressed proteins during drought treatment and subsequent recovery. “+”represents up-regulated proteins. “-”represents down-regulated proteins. |
| Protein accession | Fold change | Score | Description | Species | Functional category |
| GR-C/NQ-C | |||||
| P82659 | 2.26 | 75 | Defensin SD2 | Helianthus annuus | T |
| Q9ZP21 | 1.52 | 1927 | Thioredoxin M-type, chloroplastic | Aegilops tauschii | O |
| Q9LEH3 | 1.74 | 759 | peroxidase 54-like | Brachypodium distachyon | B |
| O04066 | 1.66 | 138 | Acyl-CoA-binding protein | Ricinus communis | E |
| P51414 | 1.75 | 395 | 60S ribosomal protein L26-1-like | Setaria italica | S |
| Q67YC9 | 1.62 | 225 | Uncharacterized protein At4g14100 | Arabidopsis thaliana | Y |
| P36213 | 2.14 | 144 | photosystem I reaction center subunit Ⅱ | Zea mays | P |
| Q7XKD0 | 1.56 | 235 | Thioredoxin X, chloroplastic | Oryza sativa | T |
| Q9SJ12 | 2.01 | 547 | mitochondrial ATP synthase precursor | Triticum aestivum | H |
| Q9CAB7 | 3.72 | 99 | peroxygenase | Arabidopsis thaliana | T |
| P93788 | 2.21 | 209 | remorin-like isoform 1 | Brachypodium distachyon | T |
| Q0JPA6 | 1.72 | 95 | Salt stress root protein RS1 | Oryza sativa | T |
| P93447 | 2.46 | 379 | elongation factor 1-delta 1-like | Brachypodium distachyon | E |
| P13240 | 4.61 | 88 | disease resistance response protein 206-like | Setaria italica | T |
| Q9LEV3 | 2.08 | 395 | CBS domain-containing protein CBSX3, mitochondrial-like | Brachypodium distachyon | C |
| P21569 | 1.68 | 792 | cyclophilin | Triticum aestivum | R |
| P21569 | 1.89 | 507 | CYCLOPHILIN 1 | Oryza brachyantha | R |
| Q96520 | 2.13 | 690 | cationic peroxidase SPC4-like isoform X2 | Setaria italica | B |
| P84516 | 2.02 | 137 | peroxidase 12 precursor | Zea mays | B |
| P93303 | 6.17 | 71 | ATPase subunit 8 | Sorghum bicolor | H |
| P12331 | 1.62 | 615 | chlorophyll a-b binding protein 2 | Zea mays | P |
| Q9XF89 | 1.53 | 746 | chlorophyll a/b-binding apoprotein CP26 precursor | Zea mays | P |
| P40880 | 2.28 | 591 | Carbonic anhydrase, chloroplastic | Hordeum vulgare | D |
| P52758 | 2.15 | 104 | ribonuclease UK114-like | Setaria italica | N |
| Q8S0J7 | 2.28 | 1257 | membrane-associated 30 kDa protein, chloroplastic-like | Brachypodium distachyon | M |
| P41344 | 1.61 | 5064 | ferredoxin--NADP reductase, leaf isozyme, chloroplastic-like | Brachypodium distachyon | P |
| Q7F8S5 | 1.88 | 1320 | peroxiredoxin-2E-2, chloroplastic-like | Brachypodium distachyon | B |
| gi|475527099 | 1.86 | 646 | Heat shock cognate 70 kDa protein 1 | Aegilops tauschii | T |
The inherently differentially expressed proteins with higher expression in S.purpurea from GR were divided into 17 categories based on their functions (Fig. 4A). Most of these proteins were involved in photosynthesis, protein synthesis and transport, the antioxidant system, stress response, and energy production and conversion (Fig. 4A and Supplementary Table S2). Among them, the drought-nonresponsive proteins were divided into 13 functional categories, including stress response, antioxidant system, and photosynthesis (Fig. 4B and Table 1). The inherently differentially expressed proteins with higher expression in NQ S.purpurea were divided into 19 categories based on their functions (Fig. 4A). Most were involved in protein synthesis and transport, photosynthesis, the antioxidant system, stress response, and chromatin structure and dynamics (Fig. 4A and Supplementary Table S3). Among them, the drought-nonresponsive proteins were divided into 17 functional categories, mainly including protein synthesis and transport, antioxidant system, and photosynthesis (Fig. 4B and Table 2).
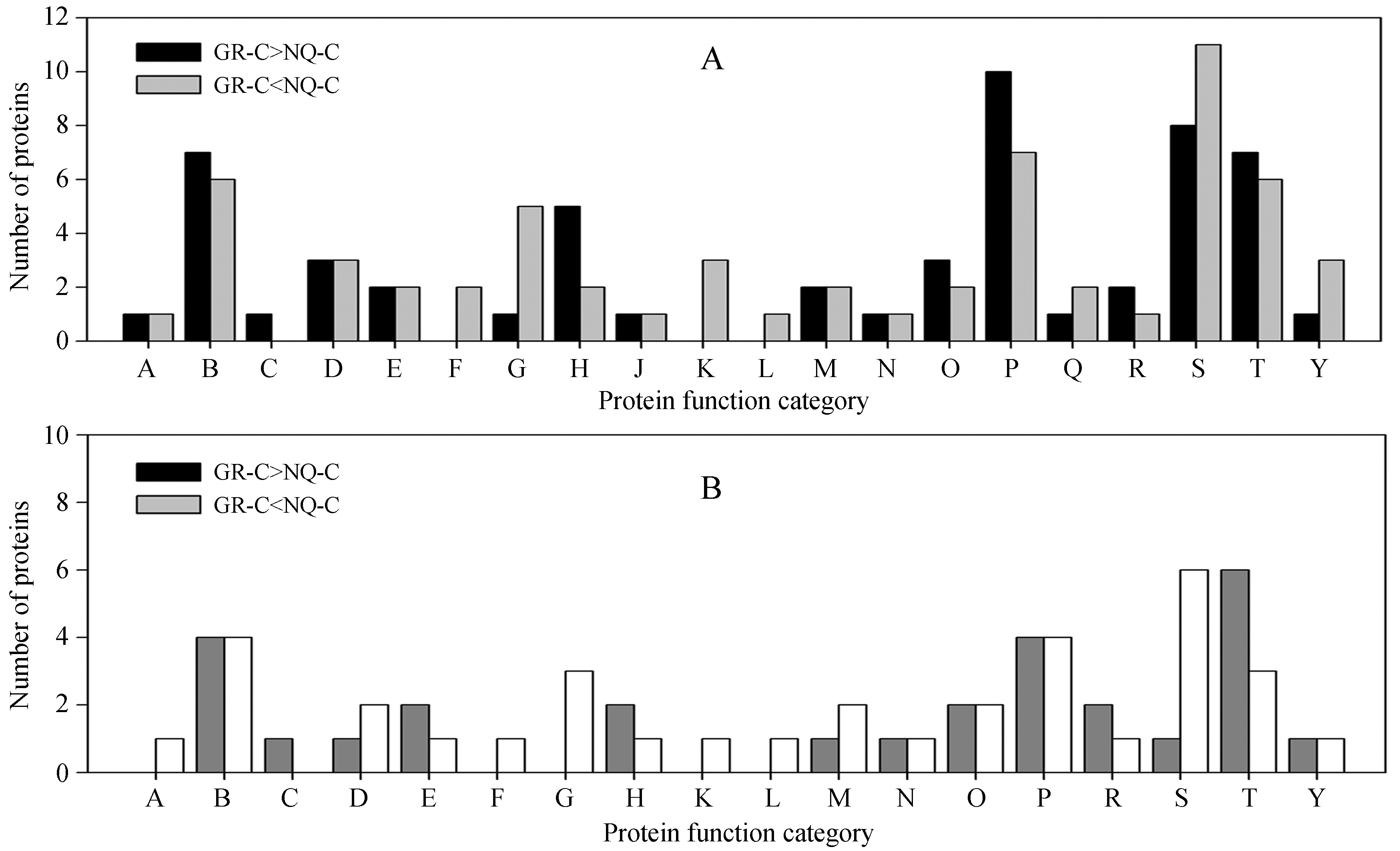
|
| Fig.4 Functional classification of the inherently differentially expressed proteins in the two S.purpurea populations.(A) Comparison of functional classifications of the inherently differentially expressed proteins with higher abundance in each S.purpurea population.(B) Comparison of functional classifications of the inherently differentially expressed proteins with no response to drought treatment in each S.purpurea population. The letters correspond to the protein functional categories shown as follows: A, amino acid transport and metabolism; B, antioxidant system; C, biosynthesis and biotransformation; D, carbohydrate metabolism; E, cell structure and activity; F, molecular chaperone; G, chromatin structure and dynamics; H, energy production and conversion; J, inorganic ion transport and metabolism; K, kinase; L, lipid transport and metabolism; M, membrane-associated; N, nucleotide transport and metabolism; O, oxidation-reduction process; P, photosynthesis; Q, post translational modification; R, osmotic regulation; S, protein synthesis and transport; T, response to stimulus; Y, others. |
| Protein accession | Fold change | Score | Description | Species | Functional category |
| GR-C/NQ-C | |||||
| Q93VR4 | 0.66 | 440 | salt tolerant protein | Triticum aestivum | T |
| P14009 | 0.63 | 620 | 14 kDa proline-rich protein | Daucus carota | R |
| Q8LQ68 | 0.54 | 158 | hexokinase-6-like | Setaria italica | D |
| P20143 | 0.42 | 468 | photosystem I reaction center subunit VI, chloroplastic-like | Solanum lycopersicum | P |
| Q9M573 | 0.62 | 87 | 60S ribosomal protein L31-like isoform 1 | Brachypodium distachyon | S |
| B1XL18 | 0.52 | 362 | Trigger factor | Aegilops tauschii | F |
| Q84MP7 | 0.61 | 107 | Histone H2A | Medicago truncatula | G |
| Q03958 | 0.65 | 113 | Prefoldin, chaperonin cofactor | Oryza sativa Japonica Group | Y |
| P12783 | 0.64 | 579 | phosphoglycerate kinase, cytosolic-like | Setaria italica | K |
| A2Y720 | 0.64 | 916 | group3 late embryogenesis abundant protein | Triticum aestivum | T |
| Q8L803 | 0.36 | 719 | 50S ribosomal protein L9, chloroplastic-like isoform 1 | Brachypodium distachyon | S |
| P42798 | 0.51 | 461 | 40S ribosomal protein S15a-1-like | Brachypodium distachyon | S |
| P36213 | 0.66 | 187 | 1Oryza sativa Japonica Group | photosystem I subunit Ⅱ | P |
| P19683 | 0.47 | 847 | 31 kDa ribonucleoprotein, chloroplastic-like isoform 1 | Brachypodium distachyon | E |
| Q43312 | 0.59 | 162 | protein H2A.7-like isoform 2 | Glycine max | G |
| Q07300 | 0.66 | 556 | ATP synthase delta chain, chloroplastic-like | Brachypodium distachyon | H |
| Q0JPA6 | 0.56 | 76 | DREPP4 protein | Zea mays | T |
| P46485 | 0.60 | 33 | Glycine cleavage system H protein, mitochondrial | Flaveria trinervia | A |
| Q7TP48 | 0.60 | 324 | adipocyte plasma membrane-associated protein-like | Brachypodium distachyon | M |
| Q949H0 | 0.53 | 646 | ribosomal protein S7 | Triticum aestivum | S |
| P50162 | 0.59 | 72 | tropinone reductase 1-like | Brachypodium distachyon | O |
| P27521 | 0.33 | 575 | chlorophyll a-b binding protein 4, chloroplastic-like | Brachypodium distachyon | P |
| Q06030 | 0.61 | 341 | 50S ribosomal protein L12, chloroplastic-like | Setaria italica | S |
| P46274 | 0.62 | 669 | mitochondrial outer membrane porin-like | Brachypodium distachyon | M |
| Q10HD0 | 0.57 | 975 | chlorophyll a-b binding protein, chloroplastic-like | Setaria italica | P |
| Q8S9M1 | 0.56 | 177 | plastid-lipid-associated protein 13, chloroplastic | Arabidopsis thaliana | L |
| A5H452 | 0.55 | 670 | pox1 | Triticum aestivum | B |
| Q948T6 | 0.48 | 1013 | Lactoylglutathione lyase | Triticum urartu | B |
| P55142 | 0.21 | 323 | glutaredoxin-C6-like | Brachypodium distachyon | O |
| Q6L500 | 0.60 | 167 | Protein H2A.6 | Aegilops tauschii | G |
| Q06396 | 0.55 | 582 | ADP-ribosylation factor 1-like isoform 1 | Fragaria vesca subsp. vesca | N |
| Q40977 | 0.64 | 552 | Monodehydroascorbate reductase | Pisum sativum | B |
| P18566 | 0.53 | 1394 | Ribulose bisphosphate carboxylase small chain A, chloroplastic | Oryza sativa subsp. japonica | D |
| Q8LE52 | 0.65 | 308 | glutathione S-transferase DHAR3, chloroplastic-like | Brachypodium distachyon | B |
| P35685 | 0.50 | 448 | 60S ribosomal protein | Oryza sativa subsp. japonica | S |
Compared with control levels, 141 proteins in S.purpurea from GR showed differential expression (99 up-regulated and 42 down-regulated) during the drought treatment (Supplementary Fig. S3). These proteins belonged to 21 functional categories. Most were involved in stress response, protein synthesis and transport, the antioxidant system, photosynthesis, and carbohydrate metabolism (Fig. 5). The up-regulated proteins were mainly involved in stress response, protein synthesis and transport, the antioxidant system, and photosynthesis (Fig. 6A left). The down-regulated proteins were mainly related to carbohydrate metabolism, stress response, photosynthesis, and RNA metabolic processes (Fig. 6A left). There were 38 differentially expressed proteins (20 up-regulated and 18 down-regulated) in S.purpurea from GR during the recovery process (Supplementary Fig. S3). These proteins were divided into 14 functional categories, including stress response, biosynthesis and biotransformation, protein synthesis and transport, chromatin structure and dynamics, and photosynthesis (Fig. 5). The up-regulated proteins were mainly involved in stress response, chromatin structure and dynamics, protein synthesis and transport, photosynthesis, and kinase activity (Fig. 6A right). The down-regulated proteins were mainly related to biosynthesis and biotransformation, stress response, photosynthesis, and protein synthesis and transport (Fig. 6A right).
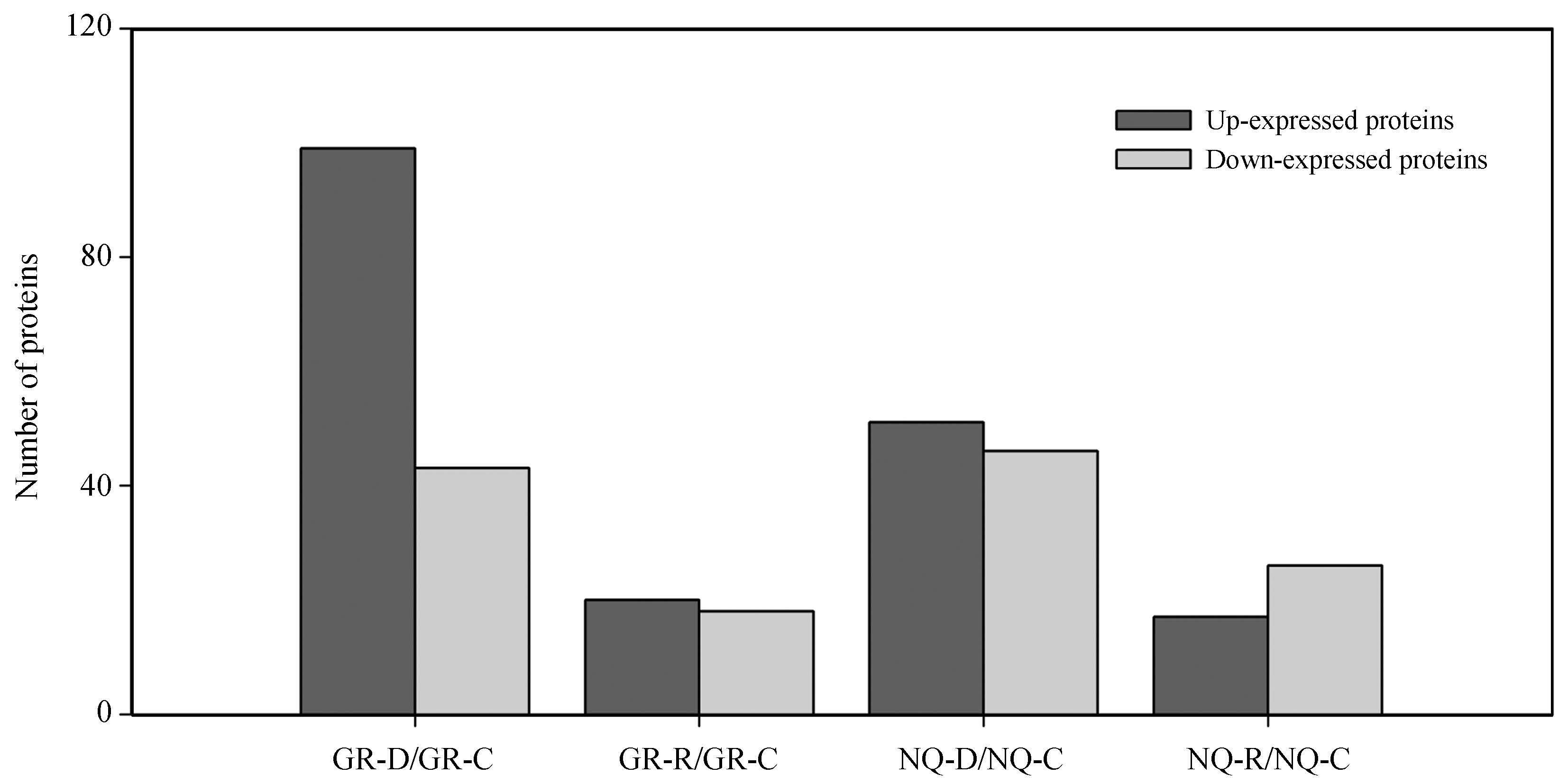
|
| Supplementary Fig.S3. Number of differentially expressed proteins during drought treatment and subsequent recovery in the two S.purpurea populations. |

|
| Fig.5 Functional classification of identified proteins and the number of proteins with various functions under drought stress and subsequent recovery in the two S.purpurea populations. The letters correspond to the protein functional categories shown as follows: A, amino acid transport and metabolism; B, antioxidant system; C, biosynthesis and biotransformation; D, carbohydrate metabolism; E, cell structure and activity; F, molecular chaperone; G, chromatin structure and dynamics; H, energy production and conversion; I, growth regulation; J, inorganic ion transport and metabolism; K, kinase; L, lipid transport and metabolism; M, membrane-associated; N, nucleotide transport and metabolism; O, oxidation-reduction process; P, photosynthesis; Q, post translational modification; R, osmotic regulation; S, protein synthesis and transport; T, response to stimulus; V, RNA metabolic process; X, transcription and translation; Y, others. |

|
| Fig.6 Functional classification of differentially expressed proteins during drought stress and subsequent recovery in the two S.purpurea populations.(A) Comparison of functional classifications of the differentially expressed proteins during drought treatment (left) and subsequent recovery (right) in GR population.(B) Comparison of functional classifications of the differentially expressed proteins during drought treatment (left) and subsequent recovery (right) in NQ population. The letters correspond to the protein functional categories shown as follows: A, amino acid transport and metabolism; B, antioxidant system; C, biosynthesis and biotransformation; D, carbohydrate metabolism; E, cell structure and activity; F, molecular chaperone; G, chromatin structure and dynamics; H, energy production and conversion; I, growth regulation; J, inorganic ion transport and metabolism; K, kinase; L, lipid transport and metabolism; M, membrane-associated; N, nucleotide transport and metabolism; O, oxidation-reduction process; P, photosynthesis; Q, post translational modification; R, osmotic regulation; S, protein synthesis and transport; T, response to stimulus; V, RNA metabolic process; W, signal transduction; X, transcription and translation; Y, others. |
There were 129 proteins (91 up-regulated and 38 down-regulated) that showed specific differential expression under drought treatment (Fig. 7A, Supplementary Tables S4 and S5), and 26 proteins (13 up-regulated and 13 down-regulated) that specifically changed during the recovery process (Fig. 7A, Supplementary Tables S6 and S7). Twelve differentially expressed proteins showed various changes in pattern during both drought and recovery (Fig. 7A and Supplementary Table S8).
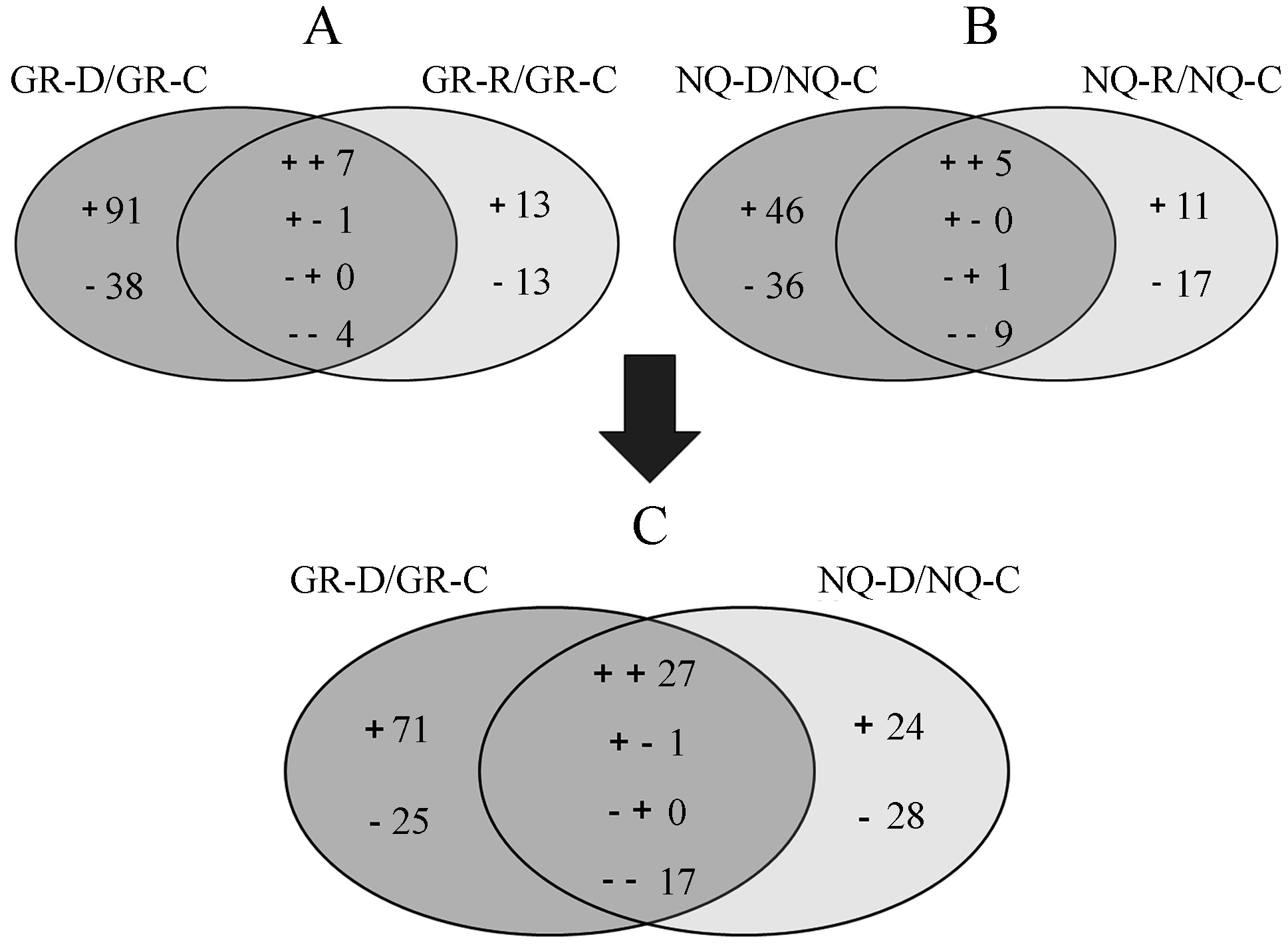
|
| Fig.7 Differentially expressed proteins in S.purpurea from two populations under drought stress and subsequent recovery.(A) Expression patterns and number of differentially expressed proteins during drought treatment and subsequent recovery in the S.purpurea population from GR.(B) Expression patterns and number of differentially expressed proteins during drought treatment and subsequent recovery in the S.purpurea population from NQ.(C) Expression patterns and number of differentially expressed proteins during drought treatment between the two S.purpurea populations. |
Compared with control levels, 97 proteins in S.purpurea from NQ showed differential expression (51 up-regulated and 46 down-regulated) during the drought treatment (Supplementary Fig. S3). These proteins belonged to 21 functional categories. Most were involved in stress response, the antioxidant system, cell structure and activity, and photosynthesis (Fig. 5). The up-regulated proteins were mainly involved in stress response, the antioxidant system, and cell structure and activity (Fig. 6B left). The down-regulated proteins were mainly related to stress response, photosynthesis, cell structure and activity, and carbohydrate metabolism (Fig. 6B left). There were 43 differentially expressed proteins (17 up-regulated and 26 down-regulated) in NQ S.purpurea during the recovery process (Supplementary Fig. S3). These proteins could be divided into 16 functional categories, including photosynthesis, stress response, antioxidant system, and cell structure and activity (Fig. 5). The up-regulated proteins were mainly involved in antioxidant system, stress response, and biosynthesis and biotransformation (Fig. 6B, right). The down-regulated proteins were mainly related to photosynthesis and cell structure and activity (Fig. 6B, right).
There were 82 proteins (46 up-regulated and 36 down-regulated) that showed specific differential expression under drought treatment (Fig. 7B, Supplementary Tables S9 and S10), and 28 proteins (11 up-regulated and 17 down-regulated) that specifically changed during the recovery process (Fig. 7B, Supplementary Tables S11 and S12). There were 15 proteins differentially expressed during both drought and recovery that showed various changes in pattern during these treatments (Fig. 7B and Supplementary Table S13).
3.7. Differential expression of proteins between the two S.purpurea populations during drought stressComparing proteomic profiles of both populations during drought stress, we identified proteins that displayed differential expression in only one population and proteins that showed differential expression in both populations. Seventy-one proteins were specifically up-regulated in S.purpurea from GR (Fig. 7C and Table 3). These proteins belonged to 16 functional categories, with most involved in protein synthesis and transport, stress response, photosynthesis, the antioxidant system, and energy production and conversion (Fig. 8A and Table 3). There were 25 proteins specifically down-regulated in S.purpurea from GR (Fig. 7C and Supplementary Table S14); these were associated with 15 functional categories mainly including antioxidant system and response to stimulus (Fig. 8B and Supplementary Table S14).
| Protein accession | Fold change | Score | Description | Species | Functional category |
| GR-D/GR-C | |||||
| Q9FKC0 | 1.62 | 137 | 60S ribosomal protein L13a-4 | Arabidopsis thaliana | S |
| gi|514817276 | 1.89 | 162 | PREDICTED: 25.3 kDa heat shock protein, chloroplastic-like | Setaria italica | T |
| P14009 | 2.25 | 207 | 14 kDa proline-rich protein DC2.15 | Daucus carota | R |
| gi|357126982 | 1.53 | 225 | PREDICTED: cytochrome b5-like | Brachypodium distachyon | E |
| Q9FJU9 | 1.51 | 391 | Glucan endo-1, 3-beta-glucosidase 13 | Arabidopsis thaliana | D |
| Q9LZF6 | 1.72 | 83 | Cell division control protein 48 homolog E | Arabidopsis thaliana | E |
| gi|357144469 | 1.61 | 526 | PREDICTED: 60S acidic ribosomal protein P1-like | Brachypodium distachyon | S |
| gi|475527602 | 1.61 | 520 | 40S ribosomal protein S5-1 | Aegilops tauschii | S |
| P46522 | 1.50 | 25 | Late embryogenesis abundant protein Lea5-D | Gossypium hirsutum | T |
| Q03968 | 1.73 | 1570 | Late embryogenesis abundant protein, group 3 | Triticum aestivum | T |
| gi|357113396 | 1.62 | 791 | PREDICTED: photosystem I reaction center subunit ⅡI, chloroplastic-like | Brachypodium distachyon | P |
| gi|357146891 | 1.60 | 682 | PREDICTED: thylakoid lumenal 17.4 kDa protein, chloroplastic-like | Brachypodium distachyon | P |
| gi|357160594 | 1.86 | 494 | PREDICTED: photosystem I reaction center subunit N, chloroplastic-like | Brachypodium distachyon | P |
| gi|357132055 | 1.51 | 401 | PREDICTED: ubiquitin-40S ribosomal protein S27a-like | Brachypodium distachyon | Q |
| gi|357127182 | 1.53 | 1306 | PREDICTED: 60S ribosomal protein L11-like | Brachypodium distachyon | S |
| gi|357160171 | 1.82 | 118 | PREDICTED: 60S ribosomal protein L10a-3-like | Brachypodium distachyon | S |
| P13940 | 1.56 | 47 | Late embryogenesis abundant protein D-29 | Gossypium hirsutum | T |
| gi|357114184 | 1.98 | 174 | PREDICTED: thioredoxin-like protein HCF164, chloroplastic-like | Brachypodium distachyon | O |
| gi|226494734 | 1.72 | 329 | 60S ribosomal protein L12 | Zea mays | S |
| Q9CA23 | 1.52 | 80 | Ubiquitin-fold modifier 1 | Arabidopsis thaliana | Q |
| gi|354805214 | 1.80 | 132 | peroxiredoxin | Oryza nivara | B |
| gi|357158024 | 2.74 | 79 | PREDICTED: ras-related protein RABA1f-like | Brachypodium distachyon | Y |
| P26360 | 1.55 | 415 | ATP synthase subunit gamma, mitochondrial | Ipomoea batatas | H |
| A1EA30 | 1.63 | 188 | 30S ribosomal protein S18, chloroplastic | Agrostis stolonifera | S |
| Q7XTE8 | 1.91 | 489 | 14-3-3-like protein GF14-B | Oryza sativa subsp. japonica | T |
| Q10N21 | 1.50 | 795 | L-ascorbate peroxidase 1, cytosolic | Oryza sativa subsp. japonica | B |
| Q05212 | 1.98 | 213 | DNA-damage-repair/toleration protein DRT102 | Arabidopsis thaliana | N |
| Q0JPA6 | 1.52 | 956 | Salt stress root protein RS1 | Oryza sativa subsp. japonica | T |
| gi|357112035 | 1.59 | 239 | PREDICTED: 40S ribosomal protein S9-2-like | Brachypodium distachyon | S |
| gi|474097201 | 1.55 | 732 | Glycine cleavage system H protein, mitochondrial | Triticum urartu | A |
| P50156 | 1.89 | 28 | Probable aquaporin TIP1-1 | Oryza sativa subsp. japonica | T |
| gi|357111884 | 1.68 | 125 | PREDICTED: elongation factor 1-delta 2-like isoform 1 | Brachypodium distachyon | E |
| P46225 | 1.72 | 1789 | Triosephosphate isomerase, chloroplastic | Secale cereale | D |
| gi|357164666 | 1.53 | 303 | PREDICTED: 60S ribosomal protein L14-1-like | Brachypodium distachyon | S |
| gi|514802463 | 1.53 | 207 | PREDICTED: 60S ribosomal protein L23A-like | Setaria italica | S |
| gi|475555669 | 1.51 | 432 | 40S ribosomal protein S12 | Aegilops tauschii | S |
| Q2R2W2 | 1.51 | 296 | 14-3-3-like protein GF14-D | Oryza sativa subsp. japonica | T |
| gi|357163385 | 1.72 | 1042 | PREDICTED: 2-Cys peroxiredoxin BAS1, chloroplastic-like | Brachypodium distachyon | B |
| P34788 | 1.61 | 744 | 40S ribosomal protein S18 | Arabidopsis thaliana | S |
| gi|357111775 | 1.50 | 122 | PREDICTED: 60S ribosomal protein L13a-4-like isoform 1 | Brachypodium distachyon | S |
| gi|357144256 | 1.73 | 239 | PREDICTED: monothiol glutaredoxin-S10-like | Brachypodium distachyon | B |
| O48646 | 1.62 | 128 | Probable phospholipid hydroperoxide glutathione peroxidase 6, mitochondrial | Arabidopsis thaliana | B |
| Q6Z7L3 | 1.60 | 101 | Thioredoxin-like 3-1, chloroplastic | Oryza sativa subsp. japonica | O |
| Q94F47 | 1.62 | 43 | Ubiquitin-conjugating enzyme E2 28 | Arabidopsis thaliana | Q |
| gi|514825357 | 1.86 | 374 | PREDICTED: ATP synthase epsilon chain, chloroplastic-like | Setaria italica | H |
| O22860 | 1.51 | 64 | 60S ribosomal protein L38 | Arabidopsis thaliana | S |
| P49310 | 1.53 | 445 | Glycine-rich RNA-binding protein GRP1A | Sinapis alba | V |
| gi|357124925 | 1.82 | 823 | PREDICTED: 20 kDa chaperonin, chloroplastic-like | Brachypodium distachyon | F |
| gi|357165619 | 2.25 | 734 | PREDICTED: thylakoid lumenal 29 kDa protein, chloroplastic-like | Brachypodium distachyon | P |
| gi|195612180 | 1.51 | 361 | 50S ribosomal protein L5 | Zea mays | S |
| Q5QN75 | 1.72 | 80 | Mitogen-activated protein kinase kinase 1 | Oryza sativa subsp. japonica | T |
| Q10CE7 | 1.61 | 459 | Probable glutathione S-transferase GSTU1 | Oryza sativa subsp. japonica | B |
| gi|354832242 | 1.62 | 46 | dehydration responsive element binding protein 1 | Hordeum brevisubulatum | T |
| gi|357128479 | 1.60 | 196 | PREDICTED: ras-related protein Rab7-like isoform 1 | Brachypodium distachyon | Y |
| gi|357137744 | 2.13 | 1060 | PREDICTED: ATP synthase subunit b′, chloroplastic-like | Brachypodium distachyon | H |
| Q84PB7 | 1.62 | 990 | Protein THYLAKOID FORMATION1, chloroplastic | Oryza sativa subsp. japonica | P |
| gi|357112354 | 1.53 | 778 | PREDICTED: thylakoid lumenal protein At1g03610, chloroplastic-like | Brachypodium distachyon | P |
| Q9C519 | 1.82 | 32 | WRKY transcription factor 6 | Arabidopsis thaliana | T |
| gi|195622012 | 1.56 | 70 | membrane-associated 30 kDa protein | Zea mays | M |
| P26302 | 1.98 | 3637 | Phosphoribulokinase, chloroplastic | Triticum aestivum | P |
| gi|508701745 | 1.72 | 62 | Glyceraldehyde-3-phosphate dehydrogenase A | Theobroma cacao | D |
| gi|357125896 | 1.72 | 1527 | PREDICTED: sedoheptulose-1, 7-bisphosphatase, chloroplastic-like | Brachypodium distachyon | P |
| P53683 | 1.61 | 155 | Calcium-dependent protein kinase isoform 2 | Oryza sativa subsp. japonica | T |
| O22773 | 1.50 | 590 | Thylakoid lumenal 16.5 kDa protein, chloroplastic | Arabidopsis thaliana | P |
| Q5VRL3 | 1.62 | 37 | Superoxide dismutase [Fe] 1, chloroplastic | Oryza sativa subsp. japonica | B |
| O65037 | 1.60 | 299 | 50S ribosomal protein L27, chloroplastic | Oryza sativa subsp. japonica | S |
| Q42438 | 2.13 | 37 | Calcium-dependent protein kinase 8 | Arabidopsis thaliana | T |
| gi|15788943 | 1.62 | 465 | ferritin | Hordeum vulgare subsp. vulgare | J |
| gi|474016592 | 1.86 | 503 | Malate dehydrogenase [NADP] 1, chloroplastic | Triticum urartu | H |
| P0C1M0 | 1.51 | 2842 | ATP synthase subunit gamma, chloroplastic | Zea mays | H |
| gi|508781655 | 1.86 | 549 | Uridylyltransferase-related | Theobroma cacao | N |

|
| Fig.8 Functional classification of proteins differentially expressed in only one or in both S.purpurea populations during drought stress.(A) Functional classification of proteins specifically up-regulated between the two S.purpurea populations.(B) Functional classification of proteins specifically down-regulated between the two S.purpurea populations.(C) Functional classification of proteins up-regulated in both S.purpurea populations. The legends in different colors represent up-regulated proteins with higher fold changes in the two S.purpurea populations.(D) Functional classification of proteins down-regulated in both S.purpurea populations. The legends in different colors represent down-regulated proteins with higher fold changes in the two S.purpurea populations. The letters correspond to the protein functional categories shown as follows: A, amino acid transport and metabolism; B, antioxidant system; C, biosynthesis and biotransformation; D, carbohydrate metabolism; E, cell structure and activity; F, molecular chaperone; G, chromatin structure and dynamics; H, energy production and conversion; J, inorganic ion transport and metabolism; K, kinase; L, lipid transport and metabolism; M, membrane-associated; N, nucleotide transport and metabolism; O, oxidation-reduction process; P, photosynthesis; Q, post translational modification; R, osmotic regulation; S, protein synthesis and transport; T, response to stimulus; V, RNA metabolic process; X, transcription and translation; Y, others. |
There were 24 proteins specifically up-regulated in NQ S.purpurea(Fig. 7C and Table 4). These proteins belonged to 15 functional categories. Most were involved in the antioxidant system and stress response (Fig. 8A and Table 4). Twenty-eight proteins were specifically down-regulated in NQ S.purpurea(Fig. 7C and Supplementary Table S15) that belong to 11 functional categories including stress response, cell structure and activity and photosynthesis (Fig. 8B and Supplementary Table S15).
| Protein accession | Fold change | Score | Description | Species | Functional category |
| NQ-D/NQ-C | |||||
| O24303 | 1.59 | 58 | Protein TIC110, chloroplastic | Pisum sativum | S |
| Q9FJZ9 | 1.52 | 70 | Peroxidase 72 | Arabidopsis thaliana | B |
| gi|357160378 | 1.56 | 179 | PREDICTED: adenylate kinase A-like | Brachypodium distachyon | N |
| gi|473954044 | 2.08 | 295 | Blue copper protein | Triticum urartu | J |
| gi|495464900 | 1.53 | 430 | phosphoglycerate kinase | Moorea producens | K |
| gi|514801671 | 1.68 | 257 | PREDICTED: 23.2 kDa heat shock protein-like | Setaria italica | T |
| gi|357118142 | 1.78 | 75 | PREDICTED: basic blue protein-like | Brachypodium distachyon | J |
| gi|374428670 | 1.81 | 561 | fusion protein of histone 2A and enhanced yellow fluorescence protein | Cloning vector pSolycp00001 | G |
| gi|357113535 | 1.62 | 487 | PREDICTED: mitochondrial outer membrane protein porin of 36 kDa-like | Brachypodium distachyon | M |
| gi|357115675 | 1.55 | 218 | PREDICTED: zeamatin-like | Brachypodium distachyon | T |
| P12782 | 1.81 | 1593 | Phosphoglycerate kinase, chloroplastic | Triticum aestivum | K |
| A1EA21 | 1.81 | 1611 | Apocytochrome f | Agrostis stolonifera | P |
| gi|657955704 | 1.62 | 731 | PREDICTED: peroxidase 4-like | Malus domestica | B |
| Q8H8U5 | 1.81 | 747 | Protein IN2-1 homolog B | Oryza sativa subsp. japonica | Y |
| gi|193074369 | 1.53 | 1234 | class ⅡI peroxidase | Triticum aestivum | B |
| gi|475611020 | 1.73 | 337 | Peroxidase 1 | Aegilops tauschii | B |
| Q9LS40 | 1.54 | 100 | Protein ASPARTIC PROTEASE IN GUARD CELL 1 | Arabidopsis thaliana | A |
| gi|353249092 | 1.56 | 74 | related to pyridoxine 4-dehydrogenase | Piriformospora indica DSM 11827 | C |
| gi|357111206 | 1.53 | 209 | PREDICTED: cytochrome b-c1 complex subunit 7-like | Brachypodium distachyon | E |
| gi|357112622 | 2.75 | 599 | PREDICTED: peroxisomal (S)-2-hydroxy-acid oxidase GLO1-like | Brachypodium distachyon | B |
| Q7M443 | 2.94 | 285 | Chitinase 2 | Tulipa bakeri | T |
| P19177 | 1.90 | 1367 | Histone H2A | Petroselinum crispum | G |
| Q9FLQ4 | 1.55 | 88 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex 1, mitochondrial | Arabidopsis thaliana | D |
| A8Y9H7 | 1.69 | 5885 | ATP synthase subunit beta, chloroplastic | Lolium perenne | H |
There were 45 differentially expressed proteins in both S.purpurea populations that showed diverse expression patterns (Fig. 7C). There were 27 proteins up-regulated in both S.purpurea populations (Fig. 7C). Among them, 14 proteins showed higher fold increases in S.purpurea from GR (Table 5); these belonged to the functional categories amino acid transport and metabolism, antioxidant system, cell structure and activity, membrane-associated, post translational modification, osmotic regulation, and response to stimulus (Fig. 8C). There were 13 proteins showing higher fold increases in S.purpurea from NQ (Table 5); these were associated with the antioxidant system, carbohydrate metabolism, cell structure and activity, post-translational modification, osmotic regulation, and stress response (Fig. 8C). There were 17 proteins down-regulated in both S.purpurea populations (Fig. 7C). Among these, eight proteins showed higher fold decreases in S.purpurea from GR (Supplementary Table S16) and belong to the functional categories amino acid transport and metabolism, carbohydrate metabolism, cell structure and activity, energy production and conversion, inorganic ion transport and metabolism, post translational modification, and stress response (Fig. 8D and Supplementary Table S16). Nine proteins showed higher fold decreases in S.purpurea from NQ (Supplementary Table S16) and were involved in biosynthesis and biotransformation, carbohydrate metabolism, chromatin structure and dynamics, photosynthesis, protein synthesis and transport, responses to stimulus, RNA metabolic processes, and transcription and translation (Fig. 8D and Supplementary Table S16). Notably, only one protein involved in stress response showed up-regulated expression in S.purpurea from GR but was down-regulated in plants from NQ (Fig. 7C and Supplementary Table S17).
| Protein accession | Fold change | Score | Description | Species | Functional category | |
| GR-D/GR-C | NQ-D/NQ-C | |||||
| Higher expression in the S.purpurea of GR population | ||||||
| Q6Z7V2 | 1.61 | 1.59 | 124 | 24.1 kDa heat shock protein, mitochondrial | Oryza sativa subsp. japonica | T |
| Q43206 | 1.86 | 1.81 | 555 | Catalase-1 | Triticum aestivum | B |
| Q54ND5 | 1.73 | 1.69 | 192 | Multiple inositol polyphosphate phosphatase 1 | Dictyostelium discoideum | Q |
| gi|475534643 | 1.65 | 1.57 | 303 | Aspartic proteinase nepenthesin-2 | Aegilops tauschii | A |
| Q8LGG8 | 1.65 | 1.56 | 33 | Universal stress protein A-like protein | Arabidopsis thaliana | T |
| Q9Z2Y8 | 1.61 | 1.52 | 90 | Proline synthase co-transcribed bacterial homolog protein | Mus musculus | R |
| O04226 | 2.01 | 1.87 | 56 | Delta-1-pyrroline-5-carboxylate synthase | Oryza sativa subsp. japonica | R |
| gi|357134821 | 1.89 | 1.65 | 1074 | PREDICTED: glutathione S-transferase DHAR2-like isoform 1 | Brachypodium distachyon | B |
| gi|433359116 | 2.01 | 1.70 | 51 | cell wall invertase | Triticum aestivum | E |
| gi|474401794 | 2.13 | 1.75 | 320 | Glutathione S-transferase | Triticum urartu | B |
| Q9U639 | 2.01 | 1.63 | 433 | Heat shock 70 kDa protein cognate 4 | M and uca sexta | T |
| Q9LW57 | 2.01 | 1.63 | 415 | Probable plastid-lipid-associated protein 6, chloroplastic | Arabidopsis thaliana | M |
| Q5Z9J0 | 2.13 | 1.72 | 43 | Mitogen-activated protein kinase 12 | Oryza sativa subsp. japonica | T |
| Q07078 | 2.13 | 1.62 | 3447 | Heat shock protein 81-3 | Oryza sativa subsp. japonica | T |
| Higher expression in the S.purpurea of NQ population | ||||||
| A7NY33 | 1.51 | 1.81 | 369 | Peroxidase 4 | Vitis vinifera | B |
| gi|474305749 | 1.53 | 1.81 | 308 | Pathogenesis-related protein 1 | Triticum urartu | T |
| gi|444289170 | 1.65 | 1.89 | 60 | secretory phospholipase A2 | Triticum durum | E |
| gi|514711790 | 1.52 | 1.69 | 213 | PREDICTED: glutathione S-transferase omega-like 2-like | Setaria italica | B |
| P23283 | 1.51 | 1.68 | 49 | Desiccation-related protein PCC3-06 | Craterostigma plantagineum | T |
| Q9X0Y1 | 1.62 | 1.78 | 76 | Phosphorylated carbohydrates phosphatase TM_1254 | Thermotoga maritima | Q |
| Q8H7Y6 | 1.50 | 1.62 | 16 | Heat stress transcription factor A-2d | Oryza sativa subsp. japonica | T |
| P84516 | 1.53 | 1.65 | 160 | Cationic peroxidase SPC4 | Sorghum bicolor | B |
| Q6Q1P4 | 1.62 | 1.67 | 68 | Structural maintenance of chromosomes protein 1 | Arabidopsis thaliana | G |
| P53303 | 1.51 | 1.53 | 209 | Zinc finger protein ZPR1 | Saccharomyces cerevisiae | T |
| Q9FEB5 | 1.73 | 1.75 | 186 | Phosphoglucan phosphatase DSP4, chloroplastic | Arabidopsis thaliana | D |
| Q42479 | 1.61 | 1.62 | 139 | Calcium-dependent protein kinase 3 | Arabidopsis thaliana | T |
| Q9S795 | 1.52 | 1.52 | 40 | Betaine aldehyde dehydrogenase 1, chloroplastic | Arabidopsis thaliana | R |
Similar to the results of a previous study (Li et al., 2015), we found that the morphological and physiological changes of two S.purpurea populations during drought and recovery supported the hypothesis that S.purpurea from GR were more resistant to the same drought stress. However, the mode of response at the molecular level was still unknown in this species. Thus, the iTRAQ quantitative proteomics method was used to analyze the differential responses of the two different S.purpurea populations to drought stress at the protein level.
The proteins responsive to drought and recovery that we identified in this study are basically consistent with previous findings on plant proteomic responses to drought or other abiotic stresses (Alvarez et al., 2014; Li et al., 2014b; Liu et al., 2014), which were mainly involved in responses to stimulus, photosynthesis, and various metabolism. Among all of the drought-responsive proteins, only a few were differentially expressed during the recovery process compared with control levels, indicating that most drought-responsive proteins returned to control levels (91.5% in GR and 84.5% in NQ)(Fig. 7A and B). Because the "resilience" of genes or proteins is thought to reflect the level of plant resistance to abiotic stresses (Seneca and Palumbi, 2015), the results indicated that S.purpurea of both populations had great drought resistance, which is consistent with previous studies (Yang et al., 2015a, b). However, S.purpurea plants from GR showed greater protein resilience, thus demonstrating stronger drought resistance.
Although most drought-responsive proteins returned to control levels in both S.purpurea populations, a few proteins were still differentially expressed during the recovery process. S.purpurea from GR showed 20 up-regulated and 18 down-regulated proteins during the drought treatment (Fig. 7A). Among them, 13 were specifically up-regulated during the recovery process, whereas 13 were specifically down-regulated (Fig. 7A, Supplementary Tables S6 and S7). There were 17 up-regulated and 26 down-regulated proteins during the drought treatment in S.purpurea from NQ (Fig. 7B). Among them, 11 were specifically up-regulated during the recovery process, whereas 17 were specifically down-regulated (Fig. 7B, Supplementary Tables S11 and S12). Similar results were reported in previous studies (Liu et al., 2014). It is possible that recovery after drought treatment is a complex process and might be a stimulus for the "adapted" proteins during drought; thus, some proteins showed responses to re-watering. However, the stability of proteins during the re-watering period may be related to plant resistance to drought. In the present study, GR plant proteins specifically up- and down-regulated during the recovery process accounted for 14.3% and 34.2% of the specifically up- and down-regulated proteins during the drought treatment, respectively (Fig. 7A), whereas the values were 23.9% and 47.2%, respectively, in S.purpurea from NQ (Fig. 7B). These results showed that proteins in S.purpurea from GR were more stable; therefore, the plants from this population were more resistant to drought stress.
4.2. Inherently differentially expressed proteins between the two S.purpurea populationsUnder normal growth conditions, some inherently differentially expressed proteins were detected in the two S.purpurea populations. This may have resulted from their adaptation to different habitats in the wild. The inherently differentially expressed proteins that showed higher abundance in S.purpurea from GR were mainly involved in photosynthesis, protein synthesis and transport, the antioxidant system, stress response, and energy production and conversion (Fig. 4A and Supplementary Table S3). Those with higher expression in S.purpurea from NQ are mainly related to protein synthesis and transport, photosynthesis, the antioxidant system, stress response, and chromatin structure and dynamics (Fig. 4A and Supplementary Table S4). These results indicated that many proteins related to these life processes had formed heritable expression differences in the different S.purpurea populations during the long-term process of adaptation and evolution. The unique higher-expression inherently proteins in each population suggested that the same environmental factor might affect S.purpurea from different populations to different degrees and in diverse ways.
About half of the inherently differentially expressed proteins were responsive to drought treatment in both S.purpurea populations (Supplementary Fig. S2); their functions are carefully analyzed below. Here, we focus mainly on the proteins that are drought-nonresponsive. The drought-nonresponsive proteins with higher expression in S.purpurea from GR were mainly involved in stress response, the antioxidant system, and photosynthesis (Fig. 4B and Table 1); most drought-nonresponsive proteins with higher expression in S.purpurea from NQ were related to protein synthesis and transport, the antioxidant system, and photosynthesis (Fig. 4B and Table 1). These results suggest two things. On one h and , these drought-nonresponsive proteins might play important roles in some other life processes rather than drought resistance. Although proteins involved in stress response and the antioxidant system function universally in plant resistance to various environmental stresses (Li et al., 2014a, b; Yang et al., 2015a), the function of these proteins might have changed in different populations of S. purpurea to respond to different environmental stresses. This is because the Tibetan Plateau is famous for a variety of harsh environmental factors (Li et al., 2014a). Additionally, some proteins in this study might have specific functions, for example, two proteins named PREDICTED: disease resistance response protein 206-like and PREDICTED: salt stress root protein RS1-like might function in response to plant disease and salt stress, respectively (Table 1). Gar and Nagqu Counties are located in the west and east of the Tibetan Plateau, respectively, with considerable differences in climatic conditions. The average temperature is higher, and day length longer in Gar Country, but precipitation is greater in Nagqu County. Thus, photosynthetic conditions for S.purpurea from the two populations have different limitations. This may explain why many proteins related to photosynthesis were differentially expressed between the two populations. On the other h and , although these proteins with inherently differential expression, including some proteins involved in stress response and the antioxidant system, were nonresponsive to drought treatment, their initially high expression levels in the two S.purpurea populations may indicate that they play a role in response to drought. In particular, many proteins involved in stress response and the antioxidant system that showed higher expression in S.purpurea from GR might play significant roles in improving the drought resistance of this population (Table 1). Additionally, the nonresponsiveness of some proteins with inherently differential expression between these two populations might be related to the degree of drought treatment.
4.3. Population-specific differential expression of proteins in S.purpurea during drought stressThe molecular basis for the differential drought resistance between the two S.purpurea populations was mainly reflected in the differential expression of proteins in plants under drought treatment. During drought treatment, the number of proteins differentially expressed in S.purpurea from GR (141 proteins) was much greater than in S.purpurea from NQ (97 proteins)(Supplementary Fig. S3). In particular, nearly twice as many proteins were up-regulated in GR (99 proteins) than in NQ (51 proteins) plants (Supplementary Fig. S3). These results indicated that S.purpurea from GR had a stronger ability to regulate proteins compared with S.purpurea from NQ. The various functions of the great many up-regulated proteins during drought treatment might be the basis of the stronger drought resistance in S.purpurea from GR.
Comparing the two S.purpurea populations, we found proteins that showed population-specific differential expression and proteins that were differentially expressed in both populations (Fig. 7C). All of these results may be related to the differential drought resistance between the two populations. Here, we first analyzed the proteins with differential expression in only one population of S.purpurea during drought stress.
In the present study, the number of proteins specifically up-regulated in S.purpurea from GR (71 proteins) was nearly three times that in S.purpurea from NQ (24 proteins)(Fig. 7C). The proteins specifically up-regulated in GR were divided into 16 functional categories while those in NQ were divided into 15 categories (Fig. 8A). Among them, there were 12 categories in common between the two S.purpurea populations, including amino acid transport and metabolism, antioxidant system, carbohydrate metabolism, cell structure and activity, energy production and conversion, inorganic ion transport and metabolism, membrane-associated, nucleotide transport and metabolism, photosynthesis, protein synthesis and transport, stress response and ‘others’(Fig. 8A). However, the protein numbers in various functional categories in S.purpurea from GR were generally greater than in NQ (Fig. 8A). Proteins in five functional categories were specifically up-regulated in S.purpurea from GR, including molecular chaperone, oxidation-reduction process, post translational modification, osmotic regulation, and RNA metabolic process (V)(Fig. 8A). In S.purpurea from NQ, proteins involved in ‘biosynthesis and biotransformation’, ‘chromatin structure and dynamics’, as well as ‘kinases’ were specifically up-regulated (Fig. 8A). The number of proteins specifically down-regulated in GR plants (25 proteins) was nearly the same as in NQ plants (28 proteins)(Fig. 7C). The proteins specifically down-regulated in GR plants were divided into 15 functional categories while those in NQ plants were divided into 12 categories (Fig. 8B). Among them, there were nine categories in common between the two S.purpurea populations, including amino acid transport and metabolism, carbohydrate metabolism, cell structure and activity, molecular chaperone, inorganic ion transport and metabolism, photosynthesis, post translational modification, stress response, and RNA metabolic process (Fig. 8B). However, the protein numbers in various functional categories in S.purpurea from NQ were generally greater than GR plants (Fig. 8B). Proteins in five functional categories were specifically down-regulated in GR plants, including antioxidant system, biosynthesis and biotransformation, energy production and conversion, kinase, and nucleotide transport and metabolism (Fig. 8B). Proteins in two functional categories were specifically down-regulated in NQ plants, including lipid transport and metabolism and ‘others’(Fig. 8B).
Many studies have demonstrated that proteins related to stress response play very important roles in plant resistance to environmental stresses (Bindschedler et al., 2008; Sergeant and Renaut, 2010; Kosova et al., 2011; Hossain et al., 2012; Kushalappa and Gunnaiah, 2013; Ghosh and Xu, 2014; Ngara and Ndimba, 2014). In the present study, 13 proteins related to stress response were specifically up-regulated in GR plants, whereas only three were specifically up-regulated in NQ plants (Fig. 8A and Table 3), which might be an important basis for the stronger drought resistance in S.purpurea from GR. The late embryogenesis abundant (LEA) family proteins play significant roles in plant resistance to drought stress (Zhang and Zhao, 2003). For instance, previous studies showed that LEA genes from barley and wheat could significantly improve the drought resistance of other plants (Xu et al., 1996; Cheng et al., 2002; Lal et al., 2008; Wang et al., 2009). In the present study, three LEA family proteins (i.e., Late embryogenesis abundant protein Lea5-D, Late embryogenesis abundant protein, group 3, and Late embryogenesis abundant protein D-29) were specifically up-regulated in GR plants (Table 3), indicating they have important roles in improving the drought resistance of S.purpurea from GR. Plant 14-3-3 family proteins are positively associated with plant growth and resistance to environmental stresses (Mayfield et al., 2012), and proteins of the calcium-dependent protein kinase (CDPK) and mitogen-activated protein kinase (MAPK) families also play important roles in plants responses to abiotic stresses (Jiang et al., 2013; Zhang et al., 2014). Alvarez et al.(2014) found that several proteins of the 14-3-3, CDPK and MAPK families were significantly up-regulated in a drought-tolerant wheat variety compared with a drought-sensitive variety during drought treatment. Consistent with these results, we found two 14-3-3 proteins (14-3-3-like protein GF14-B and 14-3-3-like protein GF14-D), two CDPK proteins (calcium-dependent protein kinase isoform 2 and calcium-dependent protein kinase 8), and one MAPK protein (mitogen-activated protein kinase kinase 1) were specifically up-regulated in NQ plants (Table 3). These results suggest that these proteins greatly contribute to the drought resistance of S.purpurea from NQ. The up-regulation of plant HSPs can improve plant resistance to drought, high temperature and salt stresses by preventing protein aggregation and maintaining the stability of organellar precursor proteins (Yang et al., 2015a). HSPs have been reported to participate in S.purpurea responses to drought (Yang et al., 2015a). In the present study, we found one HSP each was specifically up-regulated in GR (PREDICTED: 25.3 kDa heat shock protein, chloroplastic-like) and NQ plants (PREDICTED: 23.2 kDa heat shock protein-like)(Tables 3 and 4), which further indicated that HSPs are important for S.purpurea responses to drought. Additionally, a previous study showed that plant aquaporin, WRKY and dehydration responsive element binding protein (DREB) family proteins helped S.purpurea resist drought stress (Li et al., 2015). Similarly, one protein from each of these three families (Probable aquaporin TIP1-1, WRKY transcription factor 6, and dehydration responsive element binding protein 1, respectively) was specifically up-regulated in GR plants (Table 3), indicating the contributions of these proteins to the drought resistance of S.purpurea from GR. In addition to the HSP protein mentioned above, two other proteins related to stimulus responses (PREDICTED: zeamatin-like and Chitinase 2) were specifically up-regulated in S.purpurea from NQ (Table 4), but these proteins are mainly involved in responses to biotic stresses. This may be because plant diseases and insect pests are rare in the Tibetan Plateau because of the harsh environment, so the functions of some proteins responsive to biotic stresses may have changed during the long-term process of evolution. Although proteins related to stimulus responses usually play positive roles under drought treatment, we found that and six stimulus-response proteins (Disease resistance response protein 206, Glycine-rich protein 2, PREDICTED: heat shock protein 83-like, Subtilisin-chymotrypsin inhibitor-2A, 17.3 kDa heat shock protein, and Heat shock 70 kDa protein) were specifically down-regulated (Supplementary Tables S14 and S15). The negative expression changes of these proteins in NQ plants, especially the three HSPs (Supplementary Table S15), would undoubtedly weaken the drought resistance of this S.purpurea population. In summary, the specific up-regulation of many proteins related to drought resistance in S.purpurea from GR explains its stronger drought resistance.
Reactive oxygen species (ROS) metabolism plays an important role in plant responses to environmental stresses. Under normal conditions, ROS are used as signal molecules in plant growth and development as well as stress responses (Mittler et al., 2011). The ROS content in plant cells usually increases sharply when plants are subjected to environmental stresses (Li and van Staden, 1998; Gururani et al., 2013). Excessive ROS can cause peroxidation of proteins, DNA and lipids, damaging metabolic processes and eventually causing cell death. However, the plant antioxidant system can rapidly remove excess ROS, thus protecting plant cells (Yang et al., 2012; Li et al., 2014b). The plant antioxidant system includes antioxidant enzymes and substances, such as superoxide dismutase, glutathione reductase, ascorbate peroxidase, catalase, peroxidase and glutathione peroxidase (Li and van Staden, 1998). Thus, antioxidant enzyme activities and antioxidant substance contents can reflect a plant′s ability to resist environmental stresses. Previous studies have shown that the antioxidant system plays important roles in the S.purpurea response to drought stress (Li et al., 2015; Yang et al., 2015a). In the present study, we found seven antioxidant proteins from GR plants (peroxiredoxin, L-ascorbate peroxidase 1, cytosolic, PREDICTED: 2-Cys peroxiredoxin BAS1, chloroplastic-like, PREDICTED: monothiol glutaredoxin-S10-like, Probable phospholipid hydroperoxide glutathione peroxidase 6, mitochondrial, Probable glutathione S-transferase GSTU1, and Superoxide dismutase [Fe] 1, chloroplastic) and five antioxidant proteins from NQ plants (Peroxidase 72, PREDICTED: peroxidase 4-like, class ⅡI peroxidase, Peroxidase 1, and PREDICTED: peroxisomal (S)-2-hydroxy-acid oxidase) were specifically up-regulated in S.purpurea(Tables 3 and 4). These results further demonstrated that the antioxidant system is closely correlated with the drought resistance of S.purpurea, and the greater number of specifically up-regulated antioxidant proteins in S.purpurea from GR contributes to stronger drought resistance.
When faced with drought stress, the osmotic adjustment ability of plants directly affects their drought resistance (Ashraf and Foolad, 2007). The accumulation of osmotic adjustment substances such as proline can maintain cell turgor pressure and volume (Ashraf and Foolad, 2007). Proline can also help thylakoid membranes maintain photosynthetic efficiency, cellular redox potential, and antioxidant free radical levels (Jones and Turner, 1978; Porcel and Ruiz-Lozano, 2004). Several studies have found that drought treatment can induce proline accumulation and up-regulation of proline synthase genes (Li et al., 2015; Yang et al., 2015b). Not surprisingly, we found a protein related to proline metabolism (14 kDa proline-rich protein DC2.15) was specifically up-regulated in GR (Table 3), suggesting a role in maintaining the strong drought resistance of this S.purpurea population.
Protein post-translational modifications, including ubiquitination, phosphorylation, and methylation, play an important role in the plant life cycle (Hu et al., 2005). These modifications can make protein structures more complex and protein functions more powerful, leading to more sophisticated and specific protein regulation (Hu et al., 2005). One of the important functions of post-translational modification is regulating the responses of plant cells to environmental conditions (Hu et al., 2005). In this study, three proteins related to ubiquitin (PREDICTED: ubiquitin-40S ribosomal protein S27a-like, Ubiquitin-fold modifier 1, and Ubiquitin-conjugating enzyme E2, 28) were specifically up-regulated in GR plants (Table 3); this might be closely related to the stronger drought resistance in this S.purpurea population.
Environmental stresses usually decrease plant photosynthesis as well as the expression of related proteins (Li et al., 2014b). However, some proteins can maintain higher expression through the protection and restoration of resistance substances and proteins (Li et al., 2014a; Li et al., 2014b). Meanwhile, the material and energy metabolism processes that accompany photosynthesis are affected in different ways, and related proteins show down- or up-regulation (Li et al., 2014a; Li et al., 2014b). Some studies have suggested that plants consume more energy during stress; thus, proteins related to energy generation would be stimulated (Li et al., 2014a). At the same time, proteins related to biosynthesis are also up-regulated to provide a material basis for resistance to environmental stresses (Li et al., 2014a). Interestingly, similar results were observed in the present study. However, overall, the numbers and categories of down-regulated proteins generally reflected the influence of environmental stress. Although the total numbers of specifically down-regulated proteins were almost equal between the two S.purpurea populations, two proteins related to stimulus responses were specifically down-regulated in GR plants compared with six in NQ plants (Supplementary Tables S14 and S15). Additionally, two proteins related to photosynthesis (Cytochrome b6-f complex iron-sulfur subunit, chloroplastic and Oxygen-evolving enhancer protein 3-1, chloroplastic) were specifically down-regulated in GR plants compared with five (Chlorophyll a-b binding protein CP24 10A, chloroplastic, chloroplast photosystem Ⅱ type I chlorophyll a/b-binding protein, Photosystem Ⅱ 22 kDa protein, chloroplastic, Photosystem Ⅱ 22 kDa protein, chloroplastic, and Protein PROTON GRADIENT REGULATION 5, chloroplastic) in NQ plants (Supplementary Tables S14 and S15). Differences in these important biological processes determine the tolerance ability of the two S.purpurea populations to drought stress.
4.4. The differentially expressed proteins in both S.purpurea populationsIn addition to the differentially expressed proteins specific to each S.purpurea population, some proteins were up- or down-regulated in both populations during the drought treatment. However, the ranges of change for these proteins were different, which might contribute to the differential drought resistance between the two S.purpurea populations. Among the proteins up-regulated in both populations, five proteins related to stress response displayed higher up-regulation in GR plants, including 24.1 kDa heat shock protein, mitochondrial, Heat shock 70 kDa protein cognate 4, Heat shock protein 81-3, Mitogen-activated protein kinase 12, and Universal stress protein A-like protein (Fig. 12A and Table 5). Five proteins related to stress response also showed higher up-regulation in NQ plants, including Zinc finger protein ZPR1, Calcium-dependent protein kinase 3, Desiccation-related protein PCC3-06, Heat stress transcription factor A-2d, and Pathogenesis-related protein 1 (Fig. 12A and Table 5). These results indicated not only the universal functions of these resistance proteins in S.purpurea but also the important roles of some proteins with higher fold changes such as HSPs in the improved drought resistance in S.purpurea from GR. Each population had three proteins related to the antioxidant system with higher up-regulation (Fig. 8C and Table 5). In addition to these antioxidant enzymes, glutathione S-transferase can also be included in the antioxidant system because it functions in the antioxidant metabolism of glutathione-ascorbate cycles (Foyer and Noctor, 2011; Yang et al., 2012). The strength of ROS control by antioxidant proteins would directly affect the drought resistance of the two S.purpurea populations. Betaine, like proline, is an important osmotic adjustment substance (Makela et al., 1998). A previous study reported that genes related to betaine synthesis were up-regulated with exacerbated drought in S.purpurea(Yang et al., 2015b), suggesting a role for betaine in response to drought in S.purpurea. In the present study, two proteins related to proline synthesis (Proline synthase co-transcribed bacterial homolog protein and Delta-1-pyrroline-5-carboxylate synthase) were up-regulated more in GR plants (Table 5). A protein related to betaine synthesis (Betaine aldehyde dehydrogenase 1, chloroplastic) was up-regulated more in NQ plants (Table 5). These results indicate that the osmotic process plays an important role in S.purpurea drought response, and increased effectiveness in this process may have strengthened drought resistance in S.purpurea from GR. Additionally, an HSP protein (heat shock protein 90) was up-regulated in GR but down-regulated in NQ plants (Supplementary Table S17), indicating this protein is closely correlated with the stronger drought resistance in S.purpurea from GR.
During the drought treatment, 17 proteins were down-regulated in both S.purpurea populations (Supplementary Table S16), indicating that these proteins are sensitive to drought stress. Eight proteins were down-regulated more in GR while nine were more highly down-regulated in NQ plants (Fig. 8D and Supplementary Table S17). The results showed that these proteins were differentially affected by drought treatment, but generally, those in S.purpurea from NQ were affected more. Different functional proteins showed various changes in the two S.purpurea populations, suggesting that response to drought stress in S.purpurea is a complex process.
5. DiscussionIn the present study, we found that S.purpurea from a more arid region (GR) showed stronger drought resistance than from a more humid region (NQ). To underst and the underlying mechanisms of drought resistance, we used iTRAQ quantitative proteomics to analyze the protein expression changes in S.purpurea samples that were treated with drought for 7 d and then re-watered for 7 d. The results showed that there were inherently differentially expressed proteins between the two populations, some of which were responsive to drought treatment. During the drought treatment and recovery process, we detected proteins that show differential expression specific to each population, and proteins that are differentially expressed in both populations. According to our analysis, a great many proteins involved in stress response, the antioxidant system, post-translational modification, and osmotic regulation showed specific up-regulation or higher abundance in S.purpurea from GR, which may contribute to the stronger drought resistance in this population. These findings improve our underst and ing of drought-resistance differences among different S.purpurea populations. They also may help us to underst and the adaptation of S.purpurea from different populations to local water conditions.
Abbreviations
CDPK: calcium-dependent protein kinase; DREB: dehydration responsive element binding protein; DW: dry weight; GR: Gar Country; GR-C/-D/-R: GR-Control/-Drought/-Recovery; HSP: heat shock protein; iTRAQ: isobaric tag for relative and absolute quantitation; LEA: late embryogenesis abundant protein; MAPK: mitogen activated protein kinase; NQ: Nagqu Country; NQ-C/-D/-R: NQ-Control/-Drought/-Recovery; ROS: reactive oxygen species; RWC: relative water content.
Author contributions
YPY and YQY conceived and designed the experiments. XL, SHY and YQY collected the seeds. XL, SHY, XY, YQY and XDS performed the experiments. XL, SHY, YJZ and YQY analyzed the data. XL wrote the manuscript. YPY, YQY and XDS revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (NSFC)(41271058) and Chinese Academy of Sciences (Grant No. XDA05130702) to Yongping Yang.
Supplementary Table S1. The parameters ofmascot search.
Supplementary Table S2. The inherently differentially-expressed proteins with higher expression in the S.purpurea from GR.
Supplementary Table S3. The inherently differentially-expressed proteins with higher expression in the S.purpurea from NQ.
Supplementary Table S4. Proteins specifically up-regulated in S.purpureafrom GR under drought stress.
Supplementary Table S5. Proteins specifically down-regulated in S.purpureafrom GR under drought stress.
Supplementary Table S6. Proteins specifically up-regulated in S.purpureafrom GR during the recovery process.
Supplementary Table S7. Proteins specifically down-regulated in S.purpureafrom GR during the recovery process.
Supplementary Table S8. The differentially-expressed proteins in S.purpureafrom GR during both the drought and recovery processes.
Supplementary Table S9. Proteins specifically up-regulated in S.purpureafrom NQ under drought stress.
Supplementary Table S10. Proteins specifically down-regulated in S.purpureafrom NQ under drought stress.
Supplementary Table S11. Proteins specifically up-regulated in S.purpurea from NQ during the recovery process.
Supplementary Table S12. Proteins specifically down-regulated in S.purpurea from NQ during the recovery process.
Supplementary Table S13. The differentially-expressed proteins in S.purpurea from NQ during both the drought and recovery processes.
Supplementary Table S14. Proteins specifically down-regulated in S.purpurea from GR underdrought stress.
Supplementary Table S15. Proteins specifically down-regulated in S.purpurea from NQ during drought stress.
Supplementary Table S16. Proteins down-regulated in both S.purpurea populations during drought stress.
Supplementary Table S17. The diverse differentially-expressed proteins between two S.purpurea populations during the drought process.
Download link of the supplementary tables:
http://journal.kib.ac.cn/UserFiles/File/Supplemental%20tables.zip
| Alvarez, S., Choudhury, S.R., Pandey, S., 2014. Comparative Quantitative Proteomics Analysis of the ABA Response of Roots of Drought-Sensitive and Drought-Tolerant Wheat Varieties Identifies Proteomic Signatures of Drought Adaptability. J. Proteome Res, 13, 1688-1701. |
| Aranjuelo, I., Molero, G., Erice, G., et al., 2011. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J. Exp. Bot, 62, 111-123. |
| Ashraf, M., Foolad, M.R., 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot, 59, 206-216. |
| Bai, X.G., Yang, L.M., Yang, Y.Q., et al., 2011. Deciphering the Protective Role of Nitric Oxide against Salt Stress at the Physiological and Proteomic Levels in Maize. J. Proteome Res, 10, 4349-4364 . |
| Bindschedler, L.V., Palmblad, M., Cramer, R., 2008. Hydroponic isotope labelling of entire plants (HILEP) for quantitative plant proteomics; an oxidative stress case study. Phytochemistry, 69, 1962-1972. |
| Cai, X.B., Zhang, Y.Q., Shao, W., 2007. Degredation and Mechanism of Grassland of North Tibet Alpine Prairie. Soils, 39, 855-858. |
| Carlyle, C., Fraser, L.H., Turkington, R., 2014. Response of grassland biomass production to simulated climate change and clipping along an elevation gradient. Oecologia, 174, 1065-1073. |
| Chaves, M.M., Maroco, J.P., Pereira, J.S., 2003. Understanding plant responses to drought from genes to the whole plant. Funct. Plant Biol, 30, 239-264. |
| Cheng, Z.Q., Targolli, J., Huang, X.Q., et al., 2002. Wheat LEA genes, PMA80 and PMA1959, enhance dehydration tolerance of transgenic rice (Oryza sativa L.). Mol. Breeding, 10, 71-82. |
| Foyer, C.H., Noctor, G., 2011. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol, 155, 2-18. |
| Ghosh, D., Xu, J., 2014. Abiotic stress responses in plant roots: a proteomics perspective. Frontiers Plant Sci, 5, 6. |
| Gururani, M.A., Upadhyaya, C.P., Baskar, V., et al., 2013. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum tuberosum Through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J. Plant Growth Regul, 32, 245-258. |
| He, Y.L., Zhou, H.K., Zhao, X.Q., et al., 2008. Alpine Grassland Degradation and Its Restoration on Qinghai-Tibet Plateau. Prataculture & Animal Husbandry, 11, 1-9. |
| Hossain, Z., Nouri, M.Z., Komatsu, S., 2012. Plant Cell Organelle Proteomics in Response to Abiotic Stress. J. Proteome Res, 11, 37-48. |
| Hu, J., Guo, Y.T., Li, Y.M., 2005. Research progress in protein post-translation modification. Chinese Sci. Bull, 50, 1061-1072. |
| Hu, J., Guo, Y.T., Li, Y.M., 2005. Research progress in protein post-translation modification. Chinese Sci. Bull, 50, 1061-1072. |
| Johanson, U., Karlsson, M., Johansson, I., et al., 2001. The complete set of genes encoding major intrinsic proteins in arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol, 126, 1358-1369. |
| Jones, M.M., Turner, N.C., 1978. Osmotic Adjustment in Leaves of Sorghum in Response to Water Deficits. Plant Physiol, 61, 122-126. |
| Klein, J.A., Harte, J., Zhao, X.Q., 2004. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol. Lett, 7, 1170-1179. |
| Kong, X.X., Ma, L., Yang, L.M., et al., 2014. Quantitative Proteomics Analysis Reveals That the Nuclear Cap-Binding Complex Proteins Arabidopsis CBP20 and CBP80 Modulate the Salt Stress Response. J. Proteome Res, 13, 2495-2510. |
| Kosova, K., Vitamvas, P., Prasil, I.T., et al., 2011. Plant proteome changes under abiotic stress-Contribution of proteomics studies to understanding plant stress response. J. Proteomics, 74, 1301-1322. |
| Kushalappa, A.C., Gunnaiah, R., 2013. Metabolo-proteomics to discover plant biotic stress resistance genes. Trends Plant Sci, 18, 522-531. |
| Lal, S., Gulyani, V., Khurana, P., 2008. Overexpression of HVA1 gene from barley generates tolerance to salinity and water stress in transgenic mulberry (Morus indica). Transgenic Res, 17, 651-663. |
| Li, L., van Staden, J., 1998. Effects of plant growth regulators on the antioxidant system in callus of two maize cultivars subjected to water stress. Plant Growth Regulation, 24, 55-66. |
| Li, L., van Staden, J., 1998. Effects of plant growth regulators on the antioxidant system in callus of two maize cultivars subjected to water stress. Plant Growth Regulation, 24, 55-66. |
| Li, X., Yang, S.H., Yang, Y.Q., et al., 2015. Comparative Physiological and Molecular Analyses of Intraspecific Differences of Stipa purpurea (Poaceae) Response to Drought. Plant Divers Resour. 37, 439-452.. |
| Li, X., Yang, Y.Q., Ma, L., et al., 2014a. Comparative Proteomics Analyses of Kobresia pygmaea Adaptation to Environment along an Elevational Gradient on the Central Tibetan Plateau. Plos One, 9, e98410. doi: 10.1371/journal.pone.0098410. |
| Li, X., Yang, Y.Q., Sun, X.D., et al., 2014b. Comparative Physiological and Proteomic Analyses of Poplar (Populus yunnanensis) Plantlets Exposed to High Temperature and Drought. Plos One, 9, e107605. |
| Liu, G.T., Ma, L., Duan, W., et al., 2014. Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. Bmc. Plant Biol, 14, 10 . |
| Makela, P., Munns, R., Colmer, T.D., et al., 1998. Effect of foliar applications of glycinebetaine on stomatal conductance, abscisic acid and solute concentrations in leaves of salt- or drought-stressed tomato. Aust J. Plant Physiol, 25, 655-663. |
| Mayfield, J.D., Paul, A.L., Ferl, R.J., 2012. The 14-3-3 proteins of Arabidopsis regulate root growth and chloroplast development as components of the photosensory system. J. Exp. Bot, 63, 3061-3070. |
| Mittler, R., Vanderauwera, S., Suzuki, N., et al., 2011. ROS signaling: the new wave? Trends Plant Sci, 16, 300-309. |
| Morgan, J.M., 1984. Osmoregulation and Water-Stress in Higher-Plants. Annu. Rev. Plant Phys, 35, 299-319. |
| Nelson, D.E., Repetti, P.P., Adams, T.R., et al., 2007. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. P. Natl. Acad Sci. USA, 104, 16450-16455. |
| Ngara, R., Ndimba, B.K., 2014. Model plant systems in salinity and drought stress proteomics studies: a perspective on Arabidopsis and Sorghum. Plant Biol, 16, 1029-1032. |
| Nicotra, A.B., Davidson, A., 2010. Adaptive phenotypic plasticity and plant water use. Funct. Plant Biol, 37, 117-127. |
| Phillips, R.C., Mcmillan, C., Bridges, K.W., 1983. Phenology of Eelgrass, Zostera-Marina L, Along Latitudinal Gradients in North-America. Aquat Bot, 15, 145-156. |
| Porcel, R., Ruiz-Lozano, J.M., 2004. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot, 55, 1743-1750. |
| Rivero, R.M., Kojima, M., Gepstein, A., et al., 2007. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. P. Natl. Acad Sci. USA, 104, 19631-19636. |
| Seneca, F.O., Palumbi, S.R., 2015. The role of transcriptome resilience in resistance of corals to bleaching. Mol. Ecol, 24, 1467-1484. |
| Sergeant, K., Renaut, J., 2010. Plant Biotic Stress and Proteomics. Curr. Proteomics, 7, 275-297. |
| Shen, C.M., Liu, K.B., Morrill, C., et al., 2008. Ecotone shift and major droughts during the mid-late holocene in the central Tibetan Plateau. Ecology, 89, 1079-1088. |
| Terzi, R., Saruhan Guler, N., Kutlu Caliskan, N., et al., 2013. Lignification response for rolled leaves of Ctenanthe setosa under long-term drought stress. Turk J. Biol, 37, 614-619. |
| Turgut, R., Kadioglu, A., 1998. The effect of drought, temperature and irradiation on leaf rolling in Ctenanthe setosa. Biol. Plantarum, 41, 629-633. |
| Wang, L.J., Li, X.F., Chen, S.Y., et al., 2009. Enhanced drought tolerance in transgenic Leymus chinensis plants with constitutively expressed wheat TaLEA (3). Biotechnol. Lett, 31, 313-319. |
| Wang, Q.X., Fan, X.H., Wang, M.B., 2014. Recent warming amplification over high elevation regions across the globe. Clim Dynam, 43, 87-101. |
| Xu, D.P., Duan, X.L., Wang, B.Y., et al., 1996. Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol, 110, 249-257. |
| Yang, Y.Q., Chen, J.H., Liu, Q., et al., 2012. Comparative Proteomic Analysis of the Thermotolerant Plant Portulaca oleracea Acclimation to Combined High Temperature and Humidity Stress. J. Proteome Res, 11, 3605-3623. |
| Yang, Y.Q., Dong, C., Yang, S.H., et al., 2015a. Physiological and Proteomic Adaptation of the Alpine Grass Stipa purpurea to a Drought Gradient. Plos One, 10, e0117475. |
| Yang, Y.Q., Li, X., Kong, X.X., et al., 2015b. Transcriptome analysis reveals diversified adaptation of Stipa purpurea along a drought gradient on the Tibetan Plateau. Funct. Integr Genomic, 15, 295-307. |
| Yordanov, I., Velikova, V., Tsonev, T., 2000. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica, 38, 171-186. |
| Young, C., Truman, P., 2012. Proteins isolated with TRIzol are compatible with two-dimensional electrophoresis and mass spectrometry analyses. Anal Biochem, 421, 330-332. |
| Zhang, L.S., Zhao, W.M., 2003. LEA Protein Functions to Tolerance Drought of the Plant. Plant Physiol. Commun, 39, 61-66. |
| Zhang, Z.C., Liang, Y., Li, C., 2014. Review on plant MAPK cascades and their functions. J. Northwest A & F Univers (Nat. Sci. Rd.). 42, 207-214. |
| Zhuang, Q., He, J., Lu, Y., et al., 2010. Carbon dynamics of terrestrial ecosystems on the Tibetan Plateau during the 20th century: an analysis with a process-based biogeochemical model. Global Ecol. Biogeogr, 19, 649-662. |



