2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Laboratory of Tobacco Agriculture, Zhengzhou Tobacco Research Institute of CNTC, Zhengzhou 450001, China
Water flooding, or hypoxia, is a common form of environmental stress that reduces crop yields. Global warming has resulted in an increase in flooding events(Morard et al., 2004; Bailey-Serres and Voesenek, 2008). Short-term exposure to flooding can cause physiological changes in plants, such as a reduction in oxidative phosphorylation, a switch to anaerobic respiration, a reduction in nucleoside triphosphates, and an increase in the ratio of NADH to NAD+. Moreover, hypoxia perturbs normal physiological metabolism in plants by reducing the amount of available energy(Li et al., 2012; Wang et al., 2014). However, the mechanism by which hypoxia influences physiological processes in plants remains poorly understood.
The four carbon non-protein amino acid, gamma-aminobutyric acid(GABA), is an important compound in most prokaryotic and eukaryotic organisms(Bouche and Fromm, 2004; Roberts, 2007; Fait et al., 2008). GABA plays a dual role in regulating the C∶N balance and nitrogen metabolism and is involved in many physiological processes, such as carbon flux in the tricarboxylic acid(TCA)cycle and the antioxidant effect. Furthermore, GABA acts as an important signal that triggers a series of downstream responses, such as cold or salt stress tolerance, regulates cytosolic pH, and controls programmed cell death(Kinnersley and Turano 2000; Oppenheim et al., 2003). In plants, GABA is generated via glutamic acid decarboxylation or polyamine degradation(Yang et al., 2013; Ford et al., 1996). GABA is mainly metabolized through a short pathway composed of three enzymes, namely glutamate decarboxylase(GAD), the mitochondrial enzyme GABA transaminase(GAD-T), and succinic semiadehyde dehydrogenase(SSADH). The GABA biosynthesis pathway is illustrated in Figure 1. A previous study showed that exogenous GABA alleviated injury resulting from hypoxia-induced stress in melons(Fan et al., 2015); however, the details underlying this mechanism remain largely unknown.
 |
Fig.1 The gamma-aminobutyric acid (GABA) shunt metabolic pathway and its regulation in plants. The glutamine- synthetase/ glutamate-synthase (GS/GOGAT) cycle is the principal nitrogen assimilation pathway into glutamate and amino acids in plants. The glutamate dehydrogenase (GDH) is thought to function primarily in glutamate catabolism but can also function in the opposite direction. The GABA shunt is composed of three enzymes (purple). Glutamate decarboxylase (GAD) is a cytosolic enzyme regulated (green) by the Ca2t-calmodulin (CaM) complex, which catalyses the irreversible decarboxylation of glutamate to produce GABA. GABA is transported into the mitochondria, where it is converted into succinic semialdehyde by GABA transaminases using either a-ketoglutarate (by GABA-TK) or pyruvate (by GABA-TP) as amino acid acceptors. Succinic semialdehyde is then reduced by succinic semialdehyde dehydrogenase (SSADH) to form succinate, which enters the tricarboxylic acid (TCA) cycle. |
Tobacco(Nicotiana tabacum)generates an array of alkaloids that play essential roles in the plant defense response against herbivore and insect attack(Kessler and Baldwin, 2002; Steppuhn et al., 2004). Nicotine constitutes approximately 0.6%-3% of the tobacco(N.tabacum L.)leaf dry weight and is the main alkaloid produced by cultivated tobacco. Nicotine is synthesized in the root from ornithine and arginine by way of putrescine(Leete, 1980). Putrescine is either metabolized to higher polyamines, such as spermidine and spermine, or conjugated with cinnamic acid derivatives or fatty acids in all higher plants; however, it is also converted into N-methylputrescine in plants that produce nicotine or tropane alkaloids(Smith, 1981). Thus, putrescine N-methyltransferase(PMT; EC2.1.1 .53)participates in the first committed step of alkaloid biosynthesis. N-Methylputrescine is then oxidized by a diamine oxidase(EC1.4.3.6) and cyclized spontaneously to the l-methyl-A′-pyrrolinium cation, which is condensed with nicotinic acid or its derivative(Hibi et al., 1992; Hashimoto and Yamada, 1993). Quinolinic acid phosphoribosyltransferase(QAPRT; EC2.4.2.19)serves as the entry point enzyme in the pyridine nucleotide cycle, which generates nicotinic acid. After biosynthesis in the tobacco root, nicotine is translocated to the leaf via the xylem and then stored in the leaf vacuole with the help of a tonoplast-localized transporter. Nicotine can be demethylated in both leaves and roots, but is primarily demethylated in senescing leaves(Chou and Kutchan, 1998). The accumulation of nicotine in tobacco is affected by environmental factors, cultural(agricultural)practices, and plant hormone levels. For example, the application of nitrogen fertilizer or jasmonic acid markedly increases nicotine biosynthesis(Shoji et al., 2000; Facchini, 2006; Paschold et al., 2007; Shoji et al., 2008). Previous study in vertebrate examined the effect of nicotine on the spontaneous release of GABA from nerve terminals in the chick lateral spiriform nucleus(Zhu and Chiappinelli, 2002). But the relationship between nicotine biosynthesis and GABA were not reported in tobacco before. In this study, we found that flooding treatment markedly induced the accumulation of nicotine and promoted the generation of GABA. Further experiments showed that GABA triggers nicotine biosynthesis under flooding stress. Our study provides new insights into the molecular regulatory mechanism underlying nicotine biosynthesis in tobacco.
1 . Materials and Methods 1.1. Plant materialsSterilized tobacco(N.tobacum)seeds were germinated and grown to seedlings under continuous illumination on half-strength Gamborg B5 medium solidified with 2% gellan gum and supplemented with 0.3% sucrose at 24 ℃. Two-week-old plants were transferred to perlite saturated with half-strength Gamborg B5 medium, and grown for another two weeks in the greenhouse at 24 ℃ before flooding treatment(Niroula et al., 2012). For flooding treatment, the 4-week-old seedlings were fully immersed in water for the indicated periods, as previously described(Niroula et al., 2012). For GABA treatment, 2-week-old tobacco plants were transferred to perlite saturated with half-strength Gamborg B5 medium containing the indicated concentration of GABA.
1.2. Nicotine content measurementThe nicotine content was measured as previously described(Shoji et al., 2008). After exposure to various treatments, 0.5 g of tobacco roots was collected, frozen in liquid nitrogen, lyophilized, homogenized, and then soaked in 4 mL of 0.1 mol·L-1 H2SO4. The homogenate was sonicated for 60 min and centrifuged at 2 000 g for 15 min. The supernatant was neutralized by adding 0.4 mL 25% NH4OH. The mixture was loaded onto an Extrelut-1 column and eluted with 6 mL of chloroform. The eluent was dried at 37 ℃. The dry residues were dissolved in ethanol and analyzed by GC/MS chromatography. The chromatography column temperature was held at 100 ℃ for 10 min, and then increased to 260 ℃ over a 35-min period, at a gradient of 8 ℃/min. Each peak was assigned and calibrated with authentic st and ards, including nicotine.
1.3. Real-time quantitative PCR analysisTotal RNA was isolated from tobacco seedlings after exposure to different treatments using a Qiagen Total RNA Isolation Kit(Qiagen). After total RNA was digested with DNaseI(Fermentas), reverse transcription was carried out according to the manufacturer′s protocol(Fermentas). One microgram of total RNA was used for cDNA synthesis. mRNAs were then converted into cDNAs using M-MLV reverse transcriptase(Fermentas)with an Oligo(dT)18 primer. PCR was performed as described(Hu et al., 2014). For quantification, ten microliters of 2 X PreMix ExTaq(Takara)plus SYBR Green was used for a 20-mL qPCR reaction. The PCR program consisted of one cycle(95 ℃, 3 min), 45 cycles(95 ℃, 10 s; 60 ℃, 10 s; 72 ℃, 10 s), and one cycle(72 ℃, 5 min), followed by a melting curve program(55 to 90 ℃ in 1 ℃ increments). Normalization was achieved using ACT2 amplification as a constitutive control. Three technical repeats were performed for each reaction. Two or three biological repeats were conducted and one representative biological repeat was shown. The primer sequences used in this study are as follows:
For NtADC: Forward primer: 5′-ctttggttgcaaggaagctc-3′, reverse primer: 5′-cttcttcaccacacgaaca-3′; For NtODC: Forward primer: 5′-acggcaccagaaaagtcatc-3′, reverse primer: 5′-gacggatatttgggagagca-3′; For NtPMT: Forward primer: 5′-tatgcacacaggctgaaagc-3′, reverse primer: 5′-agtcaacttctggcccttca-3′; For NtMPO: Forward primer: 5′-acagtgaggctgatgctcct-3′, reverse primer: 5′-atcgacccctcctcttgtct-3′; and For NtACTIN2: Forward primer: 5′-caggaatggttaaggctgga-3′, reverse primer: 5′-ccatatcgtcccagttgctt-3′.
1.4. GABA content and GAD activity determinationGABA content was determined as previously reported(Renault et al., 2010). Briefly, the sample(0.3-0.5 g)was pulverized in liquid nitrogen immediately after sample collecting, and , after evaporation of the liquid nitrogen, was extracted with 5 mL of 100% methanol to inactivate the enzyme. Subsequently, 10 mL of chloroform and 5 mL of water were added to the samples while stirring, and the samples were centrifuged at 2800 g for 10 min to separate the aqueous and organic phases. The GABA-containing aqueous phase was removed, dried, dissolved in 10 mmol·L-1 Tris-HCl buffer(pH 8.0), and passed through an AG 50W resin column(Bio-RAD). GABA was eluted with 4 mol·L-1 NH4OH, and the eluents from 2 to 6 mL inclusive was collected, dried, and dissolved in 0.1 mol·L-1 potassium pyrophosphate buffer(pH 8.6). The GABA content was analyzed using GABase(Sigma).
To measure GAD activity, protein extraction was performed in extraction buffer containing 100 mmol·L-1 Tris-HCl(pH 7.5), 1 mmol·L-1 EDTA, 1% protease inhibitor cocktail(Sigma, #P9599), and 10% glycerol. Enzyme assays were performed with 15 μL of protein extract(~30 μg of protein)in a reaction buffer containing 150 mmol·L-1 potassium phosphate(pH 5.8), 0.1 mmol·L-1 PLP, and 20 mmol·L-1 L-glutamate in a final volume of 150 μL. Control assays were conducted as previously described. After incubation at 30 °C for 60 min, samples were heated at 97 °C for 7 min to stop the reaction. GAD activity was evaluated by quantifying the amount of GABA produced by enzymatic assays using GABase(Sigma). GABase assays were performed using 20 μL of GAD in an assay mix containing 75 mmol·L-1 potassium pyrophosphate(pH 8.6), 3.3 mmol·L-1 2-mercaptoethanol, 1.25 mmol·L-1 b-NADP+, 5 mmol·L-1 2-ketoglutarate, and 0.02 units of Pseudomonas fluorescens GABase(Sigma, #G7509)in a final volume of 200 μL. The increase in absorbance at OD340 was recorded using a 96-well microplate reader. Protein concentrations were determined by the Bradford method with bovine serum albumin as st and ard.
2 . Results 2.1. Flooding treatment promotes GABA production and GAD activity under floodingTo underst and the role of GABA in tobacco under floo-ding stress, we firstly exposed 2-week-old tobacco seedlings to different periods of flooding stress and found that flooding markedly induced the accumulation of GABA, with levels peaking after 48 h of treatment. By contrast, GABA levels remained low in tobacco seedlings not subjected to flooding treatment(Figure 2A). In plants, GAD is the main enzyme responsible for GABA biosynthesis. We found that flooding treatment increased GAD activity. These results suggest that GABA possibly plays an important role to tobacco in response to flooding treatment.
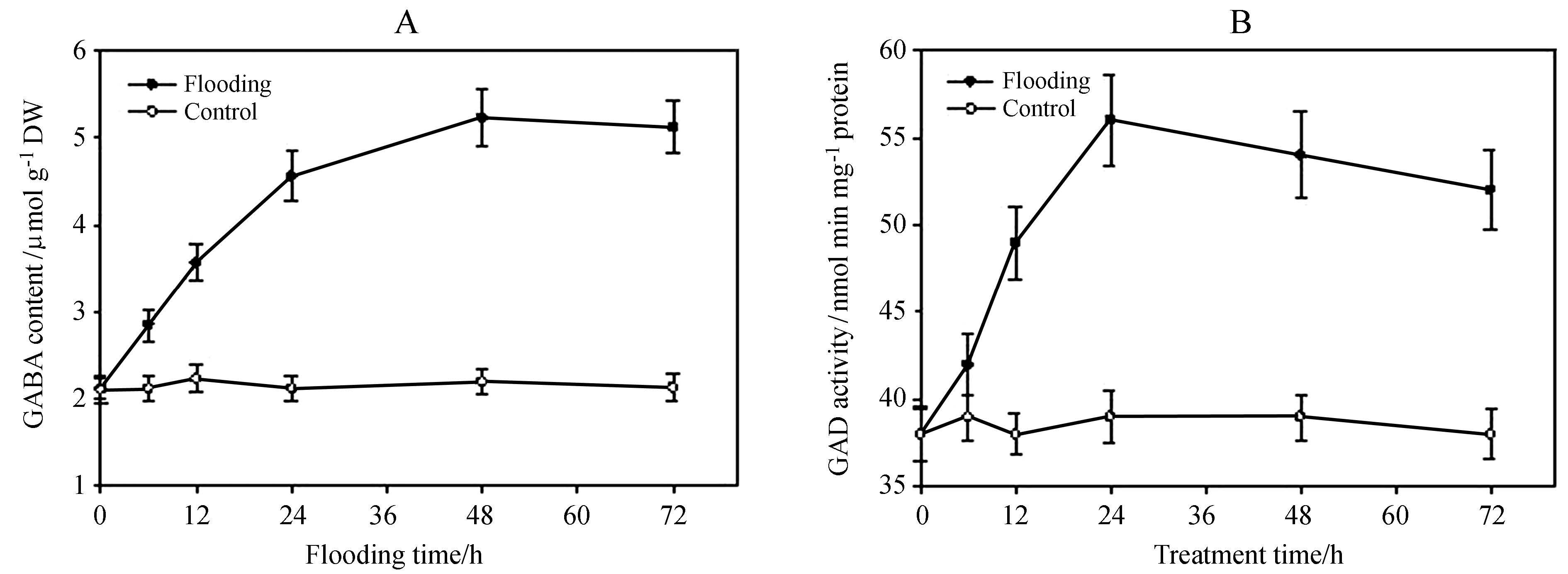 |
Fig.2 Flooding treatment promotes GABA accumulation and GAD enzyme activity. Two-week-old tobacco seedlings were exposed to flooding stress, and the GABA content (A) and GAD activity (B) were measured at the indicated time points. Data represent the means of three replicate experiments (± SE). |
Different treatments, such as wounding, can induce the biosynthesis of nicotine(Facchini, 2006). Here we found that flooding treatment also induced the biosynthesis of nicotine. As shown in Figure 3A, flooding gradually induced the accumulation of nicotine, while the control plant not subjected to flooding did not exhibit changes in nicotine content. NtADC, NtODC, NtPMT, and NtMPO are the main genes responsible for nicotine biosynthesis in tobacco. Here we found that flooding treatment could increase the transcription of these genes, with their levels peaking after 48 h or 72 h of flooding(Figure 3B). These results are in agreement with the observed increase in nicotine production following flooding, and suggest that these genes mediate the flooding-induced increase in nicotine biosynthesis.
 |
Fig.3 Flooding treatment induces the transcription of nicotine biosynthesis-related genes and promotes nicotine biosynthesis. Two-week-old tobacco seedlings were exposed to flooding stress, and the transcription of nicotine biosynthesis-related genes (NtADC, NtODC, NtPMT, and NtMPO; (A) and the levels of nicotine (B) were measured at the indicated time points. Data represent the means of three replicate experiments (± SE). |
Prompted by the findings that flooding promotes GABA accumulation and nicotine biosynthesis, we next examined the possible connection between GABA and nicotine biosynthesis under flooding. We treated tobacco seedlings with different GAD inhibitors, MPA(3-mercaptopropionate) and DTNB(dithio-bis-nitrobenzoic acid). We found that both of these inhibitor treatments markedly suppressed flooding-induced GABA accumulation(Figure 4). These data confirm that GABA accumulation depends on GAD activity during flooding. Furthermore, we found that MPA or DTNB treatment, respectively, also reduced the levels of NtADC, NtODC, NtPMT, and NtMPO transcript in tobacco seedlings subjected to floo-ding stress(Figure 5A). In agreement with this observation, we found that MPA or DTNB also suppressed flooding-induced nicotine biosynthesis(Figure 5B). These data indicate that GABA triggers the transcription of nicotine biosynthesis-related genes and nicotine biosynthesis under flooding stress.
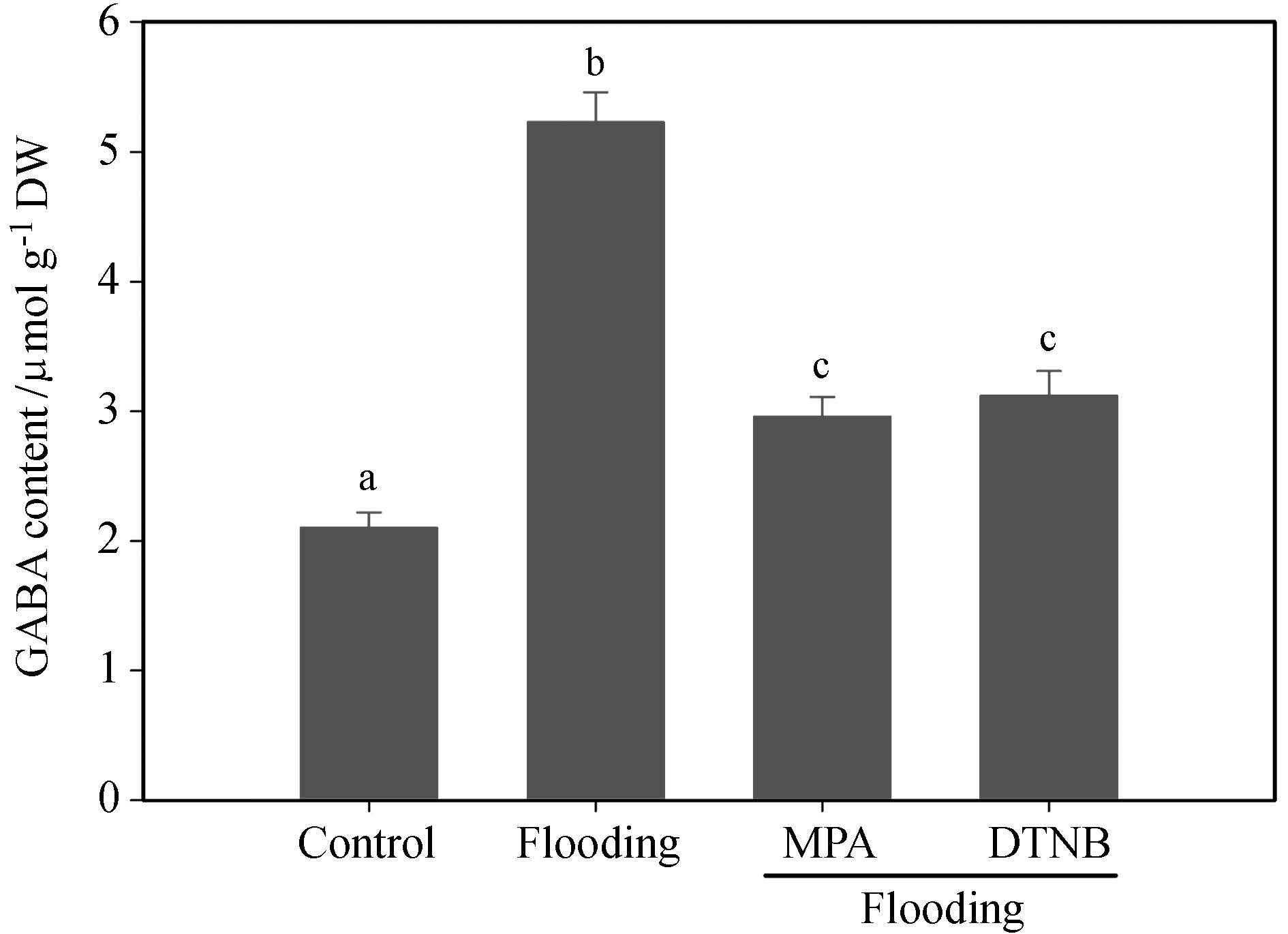 |
Fig.4 Suppressing GAD activity reduces nicotine biosynthesis. Two-week-old tobacco seedlings were exposed to flooding stress with or without 1 mmol·L-1 MPA or 10 μmol·L-1 DTNB, and the nicotine content was measured at the indicated time points. Data represent the means of three replicate experiments (± SE). Different letters indicate significant differences according to Tukey′s test (P < 0.05). |
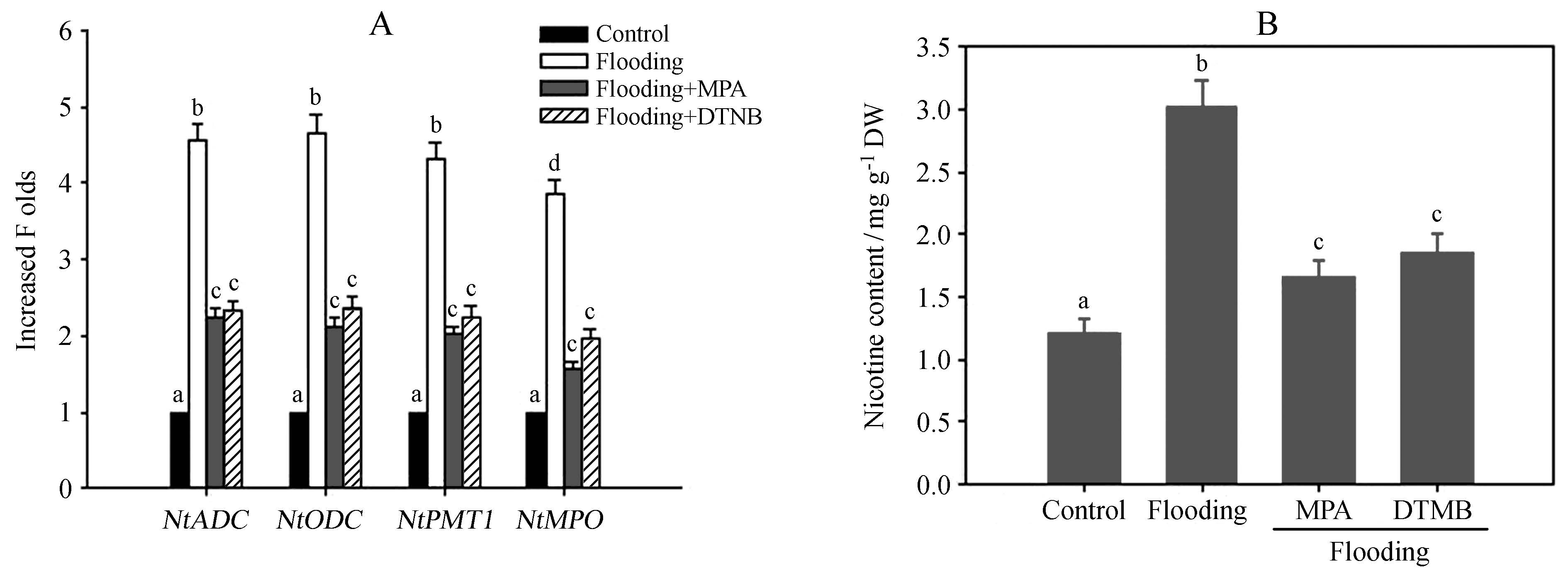 |
Fig.5 Suppressing GAD activity reduces the transcription of nicotine biosynthesis-related genes and nicotine biosynthesis. Two-week-old tobacco seedlings were exposed to flooding stress with or without 1 mmol·L-1 MPA or 10 μmol·L-1 DTNB, and the transcription of nicotine biosynthesis-related genes (NtADC, NtODC, NtPMT and NtMPO; (A) and nicotine content (B) were quantified. Data represent the means of three replicate experiments (± SE). Different letters indicate significant differences according to Tukey′s test (P < 0.05). |
To further confirm the potential role of GABA in mediating nicotine biosynthesis, we treated tobacco seedlings with different concentrations of GABA. As shown in Figure 6A, we found that concentrations of GABA ranging from 1 mmol·L-1 to 50 mmol·L-1 markedly induced the transcription of NtADC, NtODC, NtPMT, and NtMPO. Whereas treatment with 5 mmol·L-1 or 10 mmol·L-1 GABA markedly induced transcription of these genes, the effect was less striking when plants were treated with 50 mmol·L-1 GABA. Furthermore, GABA treatment also induced nicotine biosynthesis, with a 5 mmol·L-1 treatment having a greater effect on nicotine biosynthesis than a 10 mmol·L-1 or 50 mmol·L-1 treatment(Figure 6B). This finding is consistent with the observation that GABA promotes the transcription of nicotine biosynthesis-related genes.
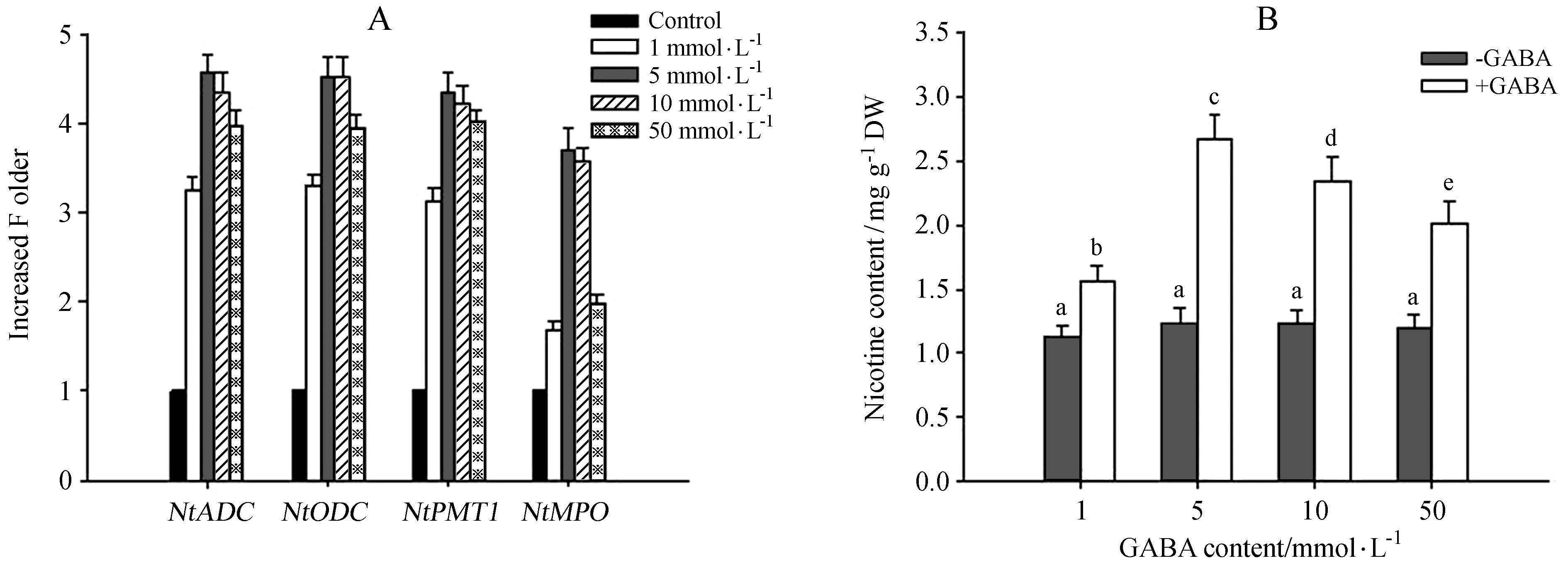 |
Fig.6 Exogenous GABA treatment induced the transcription of nicotine biosynthesis-related genes and nicotine biosynthesis. Two-week-old tobacco seedlings were treated with various concentrations of GABA, and the transcription of nicotine- related genes (NtADC, NtODC, NtPMT and NtMPO; A) and nicotine content (B) were quantified. Data represent the means of three replicate experiments (± SE). Different letters indicate significant differences according to Tukey′s test (P < 0.05). |
Increasing evidence suggests that GABA plays multiple physiological functions in plants, and is not merely a metabolite(Bouche and Fromm, 2004; Fait et al., 2008). Previous reports demonstrated that salt stress and insect attack rapidly induce GABA accumulation(Ramputh and Bown, 1996; Renault et al., 2010). In this study, we found that hypoxia of flooding stress also induces the accumulation of GABA in tobacco seedlings(Fan et al., 2015). Recently, comparative physiological and proteomic studies revealed that GABA enhances the tolerance of melon plants subjected to hypoxia stress(Fan et al., 2015). It is possible that GABA regulates cytosolic pH, sustains nitrogen/carbon fluxes or the TCA cycle, or increases antioxidant stress ability to improve tobacco tolerance to flooding stress(Bouche and Fromm, 2004; Vald-errama et al., 2006; Fait et al., 2008). Bouche and Fromm(2004)report that GABA catabolism prevents reactive oxygen species generations and suppresses cell death, indicating that GABA reduces ROS damage to enhance tobacco seedling survival during flooding. In plants and animals, GABA is mainly catabolized through a short pathway composed of three enzymes, i.e., glutamate decarboxylase(GAD), the mitochondrial enzyme GABA transaminase(GAD-T), and succinic semi aldehyde dehydrogenase(SSADH)(Fait et al., 2008). In Arabidopsis, the GAD gene family consists of five members(Renault et al., 2010). Here we found that the tobacco GAD enzyme is also the main enzyme responsible for GABA biosynthesis under flooding stress, because suppressing GAD enzyme activity by MPA or DTNB also markedly reduced GABA accumulation under flooding stress.
In tobacco, nicotine is the major economically important metabolite. Many agricultural practices, such as removing the shoot apex or applying nitrogen fertilizer, have been used to induce nicotine biosynthesis(Xi et al., 2005, 2008; Wang et al., 2008). We previously found that high temperatures also induce nicotine biosynthesis(unpublished data). Here, we found that flooding treatment significantly increases nicotine biosynthesis in tobacco seedlings primarily through activating the transcriptional levels of genes associated with nicotine biosynthesis, including NtADC, NtODC, NtPMT, and NtMPO (Wang and Bennetzen, 2015). We further presented the following three lines of evidence that GABA mediates flooding-induced nicotine biosynthesis: 1)flooding stress induced GABA biosynthesis in tobacco seedlings, in a GAD activity-dependent manner; 2)suppressing GAD activity with a GAD inhibitor(MPA or DTNB)also impaired flooding-induced nicotine biosynthesis in tobacco seedlings, and reduced the levels of NtADC, NtODC, NtPMT, and NtMPO transcript; and 3)direct application of exogenous GABA markedly induced the transcription of nicotine biosynthesis-related genes and promoted nicotine biogenesis. It is possible that GABA acts as the signal that enhances nicotine biosynthesisbiosynthesis under flooring stress through reactive oxygen species or intracellular redox status, since GABA can affect the intracellular redox status(Bouche and Fromm, 2004), while the intracellular redox status could affect multiple genes expression, thus we propose that GABA modulate nicotine biosynthesis under floor through changing the intracellular redox status, and finally triggering the expression of nicotine biosynthesis related genes, including NtADC, NtODC, NtPMT, and NtMPO.
In conclusion, we provide physiological and biochemical lines of evidence that GABA has novel roles in inducing nicotine biosynthesis in tobacco plants subjected to flooding. We found that flooding promoted the activity of GAD, which catalyzes the synthesis of GABA, and that the resulting GABA induced nicotine biosynthesis by increasing the transcription of a series of genes related to nicotine biosynthesis. Our results show that GABA mediates the increase in nicotine biosynthesis following exposure to environmental stress, and provide insight into the molecular mechanism underlying nicotine biosynthesis in plants subjected to environmental stress.
AcknowledgementsThis work was supported by major Science and Technology Program(110201101003-TS-03, TS-02-20110014, 2011YN02 and 2011YN03). The author wishes to thank Colleagues at Institute of Tibetan Plateau Research at Kunming Chinese Academy of Sciences. In addition, we thank Yang Qiuyun, Zhang Wei, Bai Xuegui, Liu Tianmeng, Yin Xianlun(Kunming Institute of Botany, Chinese Academy of Sciences) and Chen Xiaodong(Nanjing University of Science and Technology)for help with the field tobacco leaf collection in another tobacco project.
| Bailey-Serres, J., Voesenek, L.A., 2008. Flooding stress: acclimations and genetic diversity. Annu. Rev. Plant Biol. 59, 313-339. |
| Bouche, N., Fromm, H., 2004. GABA in plants: just a metabolite?. Trends. Plant Sci. 9, 110-115. |
| Chou, W.M., Kutchan, T.M., 1998. Enzymatic oxidations in the biosynthesis of complex alkaloids. Plant. J. 15, 289-300. |
| Facchini, P.J., 2006. Regulation of alkaloid biosynthesis in plants. Alkaloids Chem. Biol. 63, 1-44. |
| Fait, A., Fromm, H., Walter, D., et al., 2008. Highway or byway: the metabolic role of the GABA shunt in plants. Trends. Plant Sci. 13, 14-19. |
| Fan, L., Wu, X., Tian, Z., et al., 2015. Comparative proteomic analysis of gamma-aminobutyric acid responses in hypoxia-treated and untreated melon roots. Phytochemistry. |
| Ford, Y-Y., Ratcliffe, R.G., Robins, R.J., 1996. Phytohormone-induced GABA production in transformed root cultures of Datura stramonium: an in vivo 15N NMR study. J. Exp. Bot. 47(299), 811-818. |
| Hashimoto, T., Hibi, N., Yamada, Y., 1993. Subtractive hybridization. Plant Tissue. Culture. Lett. 10, 307-313. |
| Hibi, N., Fujita, T., Hatano, M., et al.,1992. Putrescine N-methyltransferase in cultured roots of Hyoscyamus albus. Plant Physiol. 100, 826-835. |
| Hu, X.Y., Kong, X.X., Wang, C.F., et al., 2014. Proteasome-mediated degradation of FRIGIDA modulates flowering time in Arabidopsis during verna lization. Plant Cell. 26(12), 4763-4781. |
| Kessler, A., Baldwin, I.T., 2002. Plant responses to insect herbivory: the emerging molecular analysis. Annu. Rev. Plant Biol. 53, 299-328. |
| Kinnersley, A.M., Turano, F.J., 2000. γ-Aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 19, 479-509. DOI: 10.1016/S0735-2689(01)80006-X. |
| Leete, E., 1980. Alkaloids derived from ornithine, lysine, and nicotinic acid. In Encyclopedia of Plant Physiology, New Series 8, Bell, E.A., Charlwood, B.V. (eds.) (Berlin: Springer- Verlag), 65-91. |
| Li, J., Sun, J., Yang, Y., et al., 2012. Identification of hypoxic-responsive proteins in cucumber roots using a proteomic approach. Plant Physiol. Biochem. 51, 74-80. |
| Morard, P., Silvestre, J., Lacoste, L., et al., 2004. Nitrate uptake and nitrite release by tomato roots in response to anoxia. J. Plant Physiol. 161, 855-865. |
| Niroula, R.K., Pucciariello, C., Ho, V.T., et al., 2012. SUB1A-dependent and -independent mechanisms are involved in the flooding tolerance of wild rice species. Plant J. 72, 282-293. |
| Oppenheim, R.W., Calderó, J., Cuitat, D., et al., 2003. Rescue of developing spinal motoneurons from programmed cell death by the GABAA agonist muscimol acts by blockade of neuromuscular activity and increased intramuscular nerve branching. Molecular and Cellular Neuroscience. 22, 331-343. |
| Paschold, A., Halitschke, R., Baldwin, I.T., 2007. Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J. 51, 79-91. |
| Ramputh, A.I., Bown, A.W., 1996. Rapid [gamma]-Aminobutyric Acid Synthesis and the Inhibition of the Growth and Development of Oblique-Banded Leaf-Roller Larvae. Plant Physiol. 111, 1349-1352. |
| Renault, H., Roussel, V., El Amrani, A., et al., 2010. The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol. 10, 20. |
| Roberts, M.R., 2007. Does GABA Act as a Signal in Plants?: Hints from Molecular Studies. Plant Signal. Behav. 2, 408-409. |
| Shoji, T., Ogawa, T., Hashimoto, T., 2008. Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant Cell Physiol. 49, 1003-1012. |
| Shoji, T., Yamada, Y., Hashimoto, T., 2000. Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant Cell Physiol. 41, 831-839. |
| Smith, T.A., 1981. Amines. In The Biochemistry of Plants. Vol. 7, Stumpf P.K., Conn, E.E. (eds.) (London: Academic Press), 249-268. |
| Steppuhn, A., Gase, K., Krock, B., et al., 2004. Nicotine′s defensive function in nature. PLoS Biol. 2, E217. |
| Valderrama, R., Corpas, F.J., Carreras, A., et al., 2006. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ. 29, 1449-1459. |
| Wang, C., Fan, L., Gao, H., et al., 2014. Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia-stressed melon roots. Plant Physiol. Biochem. 82, 17-26. |
| Wang, S.S., Shi, Q.M., Li, W.Q., et al., 2008. Nicotine concentration in leaves of flue-cured tobacco plants as affected by removal of the shoot apex and lateral buds. J. Integr. Plant Biol. 50, 958-964. |
| Wang, X., Bennetzen, J.L., 2015. Current status and prospects for the study of Nicotiana genomics, genetics, and nicotine biosynthesis genes. Mol. Genet. Genomics. 290, 11-21. |
| Xi, X., Li, C., Zhang, F., 2008. Tobacco plants can use nitrogen taken up before mechanical wounding to synthesize nicotine afterwards. Plant Signal. Behav. 3, 87-90. |
| Xi, X.Y., Li, C.J., Zhang, F.S., 2005. Nitrogen supply after removing the shoot apex increases the nicotine concentration and nitrogen content of tobacco plants. Ann. Bot. 96, 793-797. |
| Yang, R., Guo, Q., Gu, Z., 2013. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chemistry. 136(1), 152-159. |
| Zhu, P.J., Chiappinelli, V.A., 2002. Nicotinic receptors mediate increased GABA release in brain through a tetrodotoxin-insensitive mechanism during prolonged exposure to nicotine. Neuroscience. 115, 137-144. |



