b. College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
Tropical forest tree species are exposed to distinct environmental conditions, e.g., vertical variation in light availability (Whitmore, 1990; Turner, 2001; Deng et al., 2020). Forest canopy trees absorb strong solar irradiance and trap humidity, creating a favorable microclimate in the understory, which is characterized by lower light intensity, higher relative humidity and more stable temperatures (Lüttge, 2008). The dark, wet habitat of the understory harbors both adults and seedlings of understory tree species as well as the seedlings of canopy species. To adapt to these distinct habitats, tropical tree species use a variety of ecological strategies (e.g., seedling growth rate, recruitment rate) (Turner, 2001). Understanding the ecological strategies trees use to adapt to distinct tropical forest microhabitats may provide insights into regeneration niches of different tree species groups.
Understory tree species and canopy tree species use different ecological strategies. Understory tree species, which inhabit dark and stable microenvironments, invest in light acquisition traits, such as larger and thinner leaves, greater specific leaf area (SLA), and lower light compensation point of leaf photosynthesis (Deng et al., 2020; Schmitt et al., 2021). Understory tree species have also been shown to have relatively low maximal growth rates (Lieberman et al., 1985; Lieberman and Li 1992; Condit et al., 1995; Thomas, 1996). Canopy species, in contrast, use a more flexible strategy (van den Berg et al., 2012; Hubau et al., 2019). Regardless, tree species that can tolerate a wide range of environmental conditions at maturity (i.e., canopy species) may require narrower conditions for germination and establishment of its seeds and seedlings (Jin et al., 2018; Jiménez-Alfaro et al., 2019), which may alter the ecological strategy these species use for growth and survival. For instance, seedlings of canopy species (Parashorea chinensis) use a stress-tolerant strategy, whereas adults of this canopy species use a competitive strategy (Deng et al., 2020, 2022). In addition, understory microclimates in forests in monsoon climates are known to experience seasonal variations in both temperature and humidity (Deng et al., 2022). Although both the seedling of canopy and understory tree species grow on the forest floor (Song et al., 2016b), how each respond to seasonal changes in understory microclimate induced by monsoon climates remains unknown.
Regeneration of forest communities is largely achieved through seedling recruitment (Ashton et al., 2018; Canham and Murphy, 2016). Patterns of seedling recruitment are influenced by various factors. For instance, seedling recruitment varies inter-annually due to mast seeding of canopy tree species (Pesendorfer et al., 2021), which contribute to seedling banks (Wright et al., 2005; Norden et al., 2016; Pearse et al., 2016; Dou et al., 2018; Connell and Green, 2000). Furthermore, seedling recruitment is altered by changes in seasonal precipitation, which has been shown to influence community structure and dynamics in some tropical seasonal forests (Wang et al., 2018). Moreover, many tree species disperse seeds and recruit seedlings in the early rainy season to avoid the water stress, which may lead to recruitment failure in the dry season (Garwood, 1983; Lieberman and Li, 1992; Singh and Singh, 1993).
Tree seedling mortality and growth rate are also critical for the regeneration of forest communities. Both factors are highly influenced by abiotic factors, such as light, water and temperature (Mason et al., 2004; Song et al., 2016b; Xu et al., 2022; Mensah et al., 2023). Previous studies have shown that seedlings in forests with monsoon climates have higher mortality and lower growth rates in the dry season than in the rainy season (Turner, 1990; Delissio and Primack, 2003; Russell-Smith and Setterfield, 2006; Comita and Engelbrecht, 2009; Mensah et al., 2023). Studies have also shown that changes in water conditions can affect biotic process associated with seedling survival and growth, e.g., susceptibility to specialist pathogens (Milici et al., 2020) and herbivory (Zheng et al., 2001). In addition, drought events have been shown to increase mortality and decrease growth of canopy tree species seedlings in tropical forests (Delissio and Primack, 2003). Seasonal drought has also been shown to reduce the abundance and species richness of tree species seedlings (Song et al., 2016a). Research indicates that intense drought induced by climate change may alter the seasonal seedling survival in these forest (Uriarte et al., 2018; Nutiprapun et al., 2022), especially that of pioneer tree species (Sandoval-Martinez et al., 2022). Despite the importance of seedling recruitment, seedling mortality, and seedling growth rates, few studies have examined long-term seedling dynamics of canopy species and understory in tropical forests.
In this study, we used 11 years of census data to characterize seedling dynamics in a tropical seasonal rainforest in Xishuangbanna, southwestern China. Specifically, we examined whether seedling recruitment rate, relative growth rate, and mortality rate varied seasonally and whether these demographic measures differed between seedlings of canopy tree species and those of understory tree species. We hypothesized that seedlings of tree species in this forest have higher recruitment rate and relative growth rate in the rainy season than in the dry season. Seedlings of canopy tree species have higher seasonal recruitment rate, mortality rate and relative growth rate than those of understory tree species, performing more dynamic regeneration strategy.
2. Materials and methods 2.1. Study areaThis study was conducted in a 20-ha tropical seasonal rainforest dynamics plot in Xishuangbanna (XTRDP), southwestern China (101°34′E, 21°36′N) established in 2007 using the standards of the Center for Tropical Forest Science (CTFS) (Condit, 1998). The mean annual temperature in this region is 21.8 ℃ and the annual rainfall is around 1500 mm. This region is predominated by a monsoon climate that alternates between a rainy and dry season. Most rainfall (about 80%) occurs in the rainy season (May to October) (Cao et al., 2006). XTRDP (400 m × 500 m) was established in 2007 and contains a total of 95, 498 individuals of DBH ≥ 1 cm belonging to 468 morphospecies, in addition to another 336 individuals that have yet to be identified (Cao et al., 2008).
In 2007, 450 seedling quadrats of 1 m2 were established in the plot for seedling dynamics monitoring. All woody plants < 1 cm DBH (defined as seedlings) were tagged and identified. Heights of the seedlings were measured from the ground to the apical bud. The first seedling survey was carried out in November 2007 (Li et al., 2009). We conducted subsequent censuses in May (the end of the dry season and the beginning of the rainy season) and November (the end of the rainy season and the beginning of the dry season) in the following years, respectively. To ensure the stability of the field seedling census data, we used the seedling data collected from May 2009 to May 2019 (a total of 22 censuses) for this analysis, starting two years later than the initial seedling survey.
2.2. Data analysisFor community level analysis, we included all individuals from all tree species recorded in our seedling censuses. For analysis at the species level, we only used the tree species that had more than 30 individuals, to avoid the bias caused by small samples. After excluding the tree species that dominate evergreen broad-leaved forests on the ridges, 11 tree species were chosen for seedling dynamics analysis at the species level (Table S1). The importance values of these tree species at adult and seedling stages are provided in Table S1 based on the first seedling census of XTRDP in 2007 (Cao et al., 2008; Lan, 2009; Li et al., 2009). These tree species were selected because they were all typical species of the rainforest and were abundant and dominant in either canopy (C) or understory (U) layers respectively. Based on the stratification of this forest in Cao et al. (2008) and recent surveys on spatial structure of the canopy in the same forest (Deng et al., 2020; Deng, 2021), we defined tree species that occur in emergent and upper canopy (> 30 m in height) as canopy tree species, and defined those in lower layers (< 30 m in height) as understory tree species, because the latter are overshaded by the former.
The recruitment rate (RER) was defined as the proportion of recruited seedlings in all surviving seedlings at the t census (Condit et al., 1999):
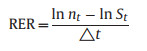
|
where RER is the recruitment rate at the t census; △t is the census interval (△t refers to 6 months for seasonal RER, 12 months for annual RER); nt is the number of all the seedlings (sum of the 450 seedling quadrats) recorded at census t; and St is the number of all the seedlings surviving until census t. We calculated the RER for each of the 11 tree species, all canopy tree species, all understory tree species, and all tree seedlings in the 450 quadrats as a whole community, respectively.
The seedling mortality rate (MR) (Comita and Engelbrecht, 2009) was calculated based on the following formula:
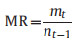
|
where MR is the mortality rate at the t census; mt is the total number of dead seedlings in the 450 seedling quadrats recorded at t census; and the nt is the total number of seedlings alive in all 450 seedling quadrats at the t−1 census. We calculated the MR for each of the 11 tree species, all canopy tree species, all understory tree species, and all tree seedlings in the 450 quadrats as a whole community, respectively.
The relative growth rate (RGR) at individual level (Seiwa and Kikuzawa, 2011) was calculated based on the following formula:

|
where RGR is the relative growth rate of each seedling at the t census; Ht is the height of the seedling at t census; Ht−1 is its height at t−1 census; and △t is the time interval between two contiguous censuses (6 months for seasonal RGR, and 12 months for annual RGR). For the RGR of the four categories (including each tree species, canopy tree species, understory tree species, and whole community), we first calculated the RGR of each individual respectively, then we calculated the mean RGR for each category based on the sum of the RGR of all seedlings divided by the total number of the seedlings in the category.
The RER, MR and RGR in the dry season refer to those from November to April of the next year (6 months), and the RER, MR and RGR in the rainy season refer to those from May to October (6 months). The annual RER, MR and RGR refer to those from October to November of the next year (12 months).
Non-parametric tests were utilized for data comparison, to 1) detect the seasonal differences in RER, MR and RGR at the community and species levels; 2) examine the differences in RER, MR and RGR between canopy and understory species in the dry and rainy seasons, respectively; and 3) determine the seasonal differences in RER, MR and RGR within each category (canopy tree species and understory tree species). Further, NMDS was used to test whether the seedling dynamics (RER, MR, and RGR) differs between canopy tree species and understory tree species. MANOVA (multivariate analysis of variance) was used to test if these seedling dynamics (ignoring category) in the rainy season were distinct from those in the dry season. All data analyses were performed using R software (R Core Team 2021).
3. Results 3.1. Seasonal differences in seedling dynamics at the community levelAt the community level, seedling RER and RGR were significantly higher in the rainy season than in the dry season, whereas MR showed no significant difference (Table 1). The dynamics of seedling RERs and RGRs over 11 years also showed that most of the RERs and RGRs were higher in the rainy seasons than in the dry seasons (Fig. S1).
| Dynamics | Rainy season | Dry season | p value | ||||||||
| Recruitment rate (RER) | 0.159 ± 0.133 | 0.068 ± 0.069 | 0.0066** | ||||||||
| Mortality rate (MR) | 0.129 ± 0.054 | 0.119 ± 0.035 | 1 | ||||||||
| Relative growth rate (RGR) | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.0052** | ||||||||
| Note: Significant level: *, p < 0.05; **, p < 0.01; ***, p < 0.001. | |||||||||||
RER for both canopy tree species (Parashorea chinensis and Pometia pinnata) was significantly higher in the rainy season than in the dry season, whereas MRs and RGRs of these species did not differ significantly between rainy and dry season (Table 2). Over the 11-year period, canopy tree species showed several peaks in recruitment, e.g., the rainy seasons in 2014 and 2017 for P. chinensis (C), and the rainy season in 2019 for P. pinnata (C) (Fig. S2). However, no dramatic peaks in canopy tree species MR or RGR were observed during the rainy or dry seasons over this same period.
| Species | Layer | Recruitment rate (RER) | p value | Mortality rate (MR) | p value | Relative growth rate (RGR) | p value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rainy season | Dry season | Rainy season | Dry season | Rainy season | Dry season | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parashorea chinensis | C | 0.419 ± 0.751 | 0.020 ± 0.026 | 0.0486* | 0.240 ± 0.209 | 0.253 ± 0.123 | 0.5190 | 0.003 ± 0.002 | 0.003 ± 0.002 | 0.8470 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pometia pinnata | C | 0.415 ± 0.619 | 0.042 ± 0.060 | 0.0030** | 0.137 ± 0.081 | 0.159 ± 0.054 | 0.6063 | 0.001 ± 0.002 | 0.003 ± 0.002 | 0.1513 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pittosporopsis kerrii | U | 0.116 ± 0.057 | 0.077 ± 0.035 | 0.08795 | 0.110 ± 0.028 | 0.103 ± 0.045 | 0.3316 | 0.003 ± 0.002 | 0.001 ± 0.001 | 0.0004*** | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Garcinia cowa | U | 0.054 ± 0.046 | 0.033 ± 0.043 | 0.3403 | 0.036 ± 0.032 | 0.036 ± 0.030 | 0.9734 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.2426 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mezzettiopsis creaghii | U | 0.109 ± 0.075 | 0.035 ± 0.030 | 0.0137* | 0.053 ± 0.031 | 0.069 ± 0.033 | 0.393 | 0.001 ± 0.003 | 0.002 ± 0.002 | 0.8977 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Knema furfuracea | U | 0.055 ± 0.080 | 0.008 ± 0.009 | 0.0128* | 0.023 ± 0.019 | 0.045 ± 0.018 | 0.0255* | 0.001 ± 0.001 | 0.001 ± 0.002 | 0.1513 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Saprosma ternatum | U | 0.057 ± 0.044 | 0.014 ± 0.014 | 0.0045** | 0.032 ± 0.015 | 0.028 ± 0.023 | 0.2498 | 0.006 ± 0.003 | 0.000 ± 0.002 | < 0.001*** | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cinnamomum bejolghota | U | 0.088 ± 0.182 | 0.023 ± 0.034 | 0.7898 | 0.057 ± 0.062 | 0.086 ± 0.048 | 0.2905 | 0.003 ± 0.002 | 0.001 ± 0.003 | 0.0473* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diospyros atrotricha | U | 0.079 ± 0.170 | 0.015 ± 0.019 | 0.5906 | 0.057 ± 0.039 | 0.040 ± 0.034 | 0.2371 | 0.002 ± 0.001 | 0.001 ± 0.001 | 0.2169 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dichapetalum gelonioides | U | 0.057 ± 0.045 | 0.028 ± 0.042 | 0.0214* | 0.042 ± 0.027 | 0.034 ± 0.025 | 0.2932 | 0.003 ± 0.002 | 0.001 ± 0.002 | 0.0066** | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pseuduvaria indochinensis | U | 0.128 ± 0.090 | 0.045 ± 0.021 | 0.0086** | 0.086 ± 0.020 | 0.082 ± 0.037 | 0.4779 | 0.003 ± 0.002 | 0.002 ± 0.002 | 0.1713 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Note: Significant level: *, p < 0.05; **, p < 0.01; ***, p < 0.001. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The seasonal dynamics of the nine understory tree species seedlings showed two distinct patterns. Two tree species (Diospyros atrotricha and Garcinia cowa) showed no significant seasonal differences in RER, MR or RGR. In contrast, seven tree species showed seasonal differences in at least one of these measures. No seasonal differences were observed in MR for any understory tree species except Knema furfuracea. RERs were higher in the rainy season than in the dry season for Mezzettiopsis creaghii, Knema furfuracea, Saprosma ternatum, Dichapetalum gelonioides and Pseuduvaria indochinensis. However, RGR was higher for several species (i.e., Pittosporopsis kerrii, S. ternatum, Cinnamomum bejolghota and D. gelonioides) in the rainy season (Table 2). The seedlings of these tree species also showed higher RER or RGR in the rainy season than in the dry season for most of the monitoring years (Figs. S2 and S4).
3.4. Differences in seedling dynamics: canopy tree species vs. understory tree speciesNMDS indicated that RER, MR, and RGR differed between canopy and understory tree species (Fig. 1; p < 0.01). Tree species seedling performance (combining RER, MR and RGR) also showed an overall significant seasonal difference (Fig. 1; p < 0.01).
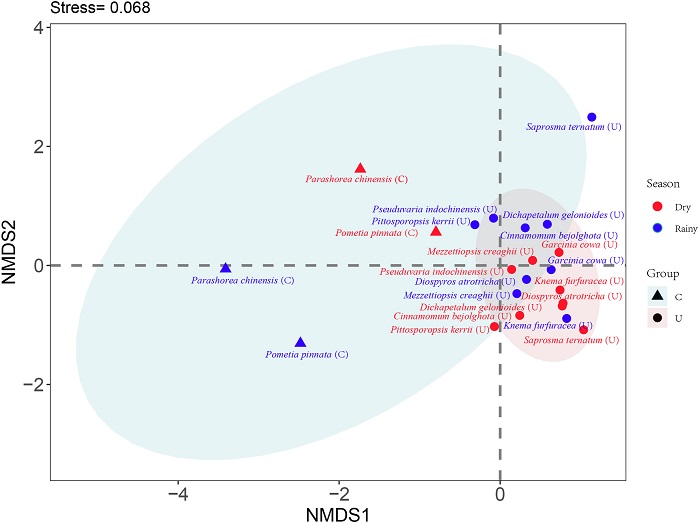
|
| Fig. 1 NMDS analysis of the seasonal performance of 11 tree species based on seedling RER, MR and RGR. Triangles indicate canopy tree species, circles indicate understory tree species. Blue refers to the value in the rainy season. Red refers to the value in the dry season. |
When we pooled data from canopy and understory tree species, the seasonal patterns of the two groups of tree species were different. Seedlings of canopy tree species showed higher RER in the rainy season than in the dry season, but no significant seasonal differences in MR and RGR were detected. However, both RER and RGR for understory tree species seedlings were higher in the rainy season than in the dry season, although no seasonal difference in mortality rate was detected either (Table 3).
| Dynamics | Season | Canopy | Understory | p value | ||||||||||||||||||||||||||||||||||
| Recruitment rate (RER) | Rainy season | 0.417 ± 0.672 | 0.082 ± 0.100 | 0.0111* | ||||||||||||||||||||||||||||||||||
| Dry season | 0.031 ± 0.046 | 0.031 ± 0.035 | 0.6178 | |||||||||||||||||||||||||||||||||||
| p value | < 0.001*** | < 0.001*** | ||||||||||||||||||||||||||||||||||||
| Mortality rate (MR) | Rainy season | 0.189 ± 0.163 | 0.055 ± 0.041 | < 0.001*** | ||||||||||||||||||||||||||||||||||
| Dry season | 0.206 ± 0.105 | 0.058 ± 0.042 | < 0.001*** | |||||||||||||||||||||||||||||||||||
| p value | 0.2549 | 0.6731 | ||||||||||||||||||||||||||||||||||||
| Relative growth rate (RGR) | Rainy season | 0.002 ± 0.002 | 0.003 ± 0.002 | 0.1694 | ||||||||||||||||||||||||||||||||||
| Dry season | 0.003 ± 0.002 | 0.001 ± 0.002 | 0.0025** | |||||||||||||||||||||||||||||||||||
| p value | 0.2292 | < 0.001*** | ||||||||||||||||||||||||||||||||||||
| Note: Significant level: *, p < 0.05; **, p < 0.01; ***, p < 0.001. The p value in standardized form shows the p value of the comparison between canopy and understory tree species in the same season; and the italic p values indicates p values of seasonal comparisons of seedling dynamics for each tree species, respectively. | ||||||||||||||||||||||||||||||||||||||
In the rainy season, RER was higher for canopy tree species than for understory tree species. MRs of canopy tree species, furthermore, were much higher than for understory tree species both in the rainy and dry seasons. The 11-year seasonal dynamics of seedling MR showed that the seedling MRs of the two canopy tree species fluctuated around or above 0.2, while those of most of the understory tree species were below 0.2 (Fig. S3). We also observed that the RGR of canopy species was significantly higher than that of understory species in the dry season (Table 3). That is to say, the seedlings of canopy tree species had higher MRs and higher RGRs than those of understory tree species in the dry season.
4. Discussion 4.1. Seasonality of seedling demography at community levelWe observed that at the community level most seedling recruitment and growth took place in the rainy season (Table 1; Fig. S1), suggesting that the onset of rainfall after a long spell of dry season triggers seedling emergence and growth. This finding is consistent with previous studies on the tropical seasonal forests of Central America and Thailand (Garwood, 1983; Lieberman and Li, 1992; Marod et al., 2002). We speculate that this is a common event in forests exposed to distinct alternating dry and rainy seasons.
We found that the MRs of tree species in this forest did not show significant seasonal variation at the community level (Table 1), nor were significant seasonal differences detected for either of the two species groups, i.e., canopy species or understory species (Table 3). This result was unexpected, as some studies on tropical seasonal forests have observed high seedling MRs in the dry season (Russell-Smith and Setterfield, 2006; Comita and Engelbrecht, 2009). In monsoonal regions, tree seedlings face biotic stress (e.g., pathogen and herbivory) in the rainy season and abiotic stress (e.g., drought) in the dry season (Aide, 1992; Comita and Engelbrecht, 2009; Lebrija-Trejos et al., 2023). These stresses can both lead to high seedling mortality, and generally, mortality of tree seedlings in tropical rainforests is high. The time interval (i.e., 6 months) we used to collect census data may have been too long to detect mortality dynamics. Garwood (1983) reported that the early rainy season is the optimal time for seedlings to emerge in understory. In a Costa Rican rain forest, the mean half-life of the multi-species seedling cohorts was 2.49 months (Li et al., 1996; Turner, 2001), indicating that more than half of the recruited seedlings were dead within 3 months from emergence. We recommend using a shorter interval and higher frequency protocol to monitor seedling mortality in the future.
The RGR of the community was higher in the rainy season than in the dry season (Table 1), which is consistent with results observed in BCI, Panama (Comita and Engelbrecht, 2009). A study on forest seedling dynamics on an elevational gradient ranging from 800 m to 1400 m in this region also reported a similar trend (Song et al., 2016a). Higher water availability promotes seedling growth in the rainy season, while drought stress in the dry season results in lower growth (Mensah et al., 2023). Therefore, seasonal drought could be one of the most important factors limiting seedling growth in forests predominated by monsoon climate.
4.2. Seedling dynamics of canopy tree species and understory tree speciesWe observed that canopy tree species had higher seedling RER than that of understory tree species in the rainy season, showing higher seasonal recruitment rates. Both Prashorea chinensis (C) and Pometia pinnata (C), on the other hand, showed mast seeding events for an interval of 3–4 years (P. chinensis in 2010, 2014 and 2017; P. pinnata in 2010, 2016 and 2019) within the 9-year seed rain monitoring from 2008 to 2017 (Dou et al., 2018). This would have contributed to the increase in seedling recruitment of the two canopy tree species. Furthermore, it has been reported that seeds of the two canopy species are desiccation-intolerant (recalcitrant) (Yan et al., 2007; Yu et al., 2008); these seeds either germinate or die within a short period after dispersal. Thus, the number of seeds transferring to seedlings on the forest floor may be higher during mast seeding years, resulting in the dramatic increase in seedling abundance that intensified variations between the two seasons. This was observed in the rainy seasons of 2014 and 2017 for P. chinensis (C), and in the rainy season of 2019 for P. pinnata (C) (Fig. S2). While six of the nine understory tree species in our study produce dormant seeds (Liu et al., 2021), they did not germinate immediately when they fell to the ground, maintaining relatively small variations in RER between the dry and rainy seasons respectively. Species with dormant seeds do not produce many mast seeding events in understory habitats (Dou et al., 2018). Garwood (1983) observed a similar trend in the understory species and shade-tolerant species at the BCI plot in Panama.
The MR of canopy tree seedlings was significantly higher than that of understory tree seedlings in both rainy and dry seasons (Table 3; Fig. S3). This result supports our hypothesis that seasonal mortality dynamics are stronger for seedlings of canopy tree species than for those of understory tree species. We speculate that understory tree species may have developed more stable drought-tolerant strategies than the canopy tree species, thus, regular seasonal drought has less effect on understory species seedlings demography. Therefore, the regeneration of canopy species may be more sensitive to seasonal drought events than that of understory species, and may be highly threatened by global drought scenarios. The difference in drought sensitivity, in the future, may deeply alter community composition (Engelbrecht et al., 2007).
The two canopy tree species did not show significant seasonal differences in RGR. This result contrasts with results in a simulated drought experiment of dipterocarp species, where seedlings grew faster in the wet period than in the dry period (O'Brien et al., 2017). Unlike in controlled greenhouse experiments, seedlings in the field face multiple stressors, e.g., higher herbivory than water limitation during the rainy season. Thus, patterns of RGR may differ between forest plots and greenhouse experiments. However, we found that four out of nine understory tree species showed higher RGRs in the rainy season than in the dry season (Table 2).
As predicted, seedling RGRs of canopy species were higher than those of understory species in the dry season (Table 3). We speculate that seedling growth of both canopy and understory species is inhibited by reduced solar radiation during the rainy season, whereas seedling growth of canopy species is induced by increased light availability in the dry season. Seedlings of understory tree species, which are adapted to shady habitats, undertake a more stable growth strategy. This is consistent with previous studies that indicate that the higher RGRs of canopy tree species allows for better access to light conditions (van den Berg et al., 2012). This finding also indicates that canopy tree species have a flexible growth strategy in part because these plants are acclimated to a wider range of light gradients (i.e., understory to canopy top). A study in a lowland evergreen forest in Malaysia also found that the growth rates of tree species were higher for canopy trees than for understory trees, which might be due to variations in allometry, photosynthetic capacity, and reproductive allocation (King et al., 2006). However, this high growth rate for canopy tree species may be at the cost of higher mortality (Table 3) because of the growth-mortality trade-off (Wright et al., 2010). This result also suggests that understory tree species show conservative adaption to seasonal drought. Understory tree species grow faster in the rainy season, taking advantage of better water conditions, but reduce their growth in the dry season to tolerate drought. The higher seedling mortality rate of canopy species also indicates that their shade-tolerant capacity was not as strong as that of understory species, although during the initial stages of development they undertake a low-light tolerant strategy (Yan and Cao, 2007a, 2007b, 2008; Deng et al., 2020).
Overall, our results provide empirical evidence that although living in the similar understory habitats, seedling demography of canopy tree species and understory tree species differ (Table 3; Fig. 1). This difference is likely the result of functional distinctions in ecological strategy at the level of seedling population. This differentiation in regeneration niche contributes to the coexistence of these tree species at their seedling stage, which helps maintain species diversity in this forest.
5. ConclusionsOur study shows that seedling recruitment and growth vary seasonally and distinctly in a tropical seasonal rainforest in southwestern China. Specifically, seedling recruitment rate and relative growth rate were higher in the rainy season than in the dry season. Although census data may need to be monitored over shorter and more frequent intervals, we also found that mortality rates remained relatively stable in the forest community. For canopy tree species, the seedling recruitment rate was higher in the rainy season than in the dry season, whereas mortality rate and relative growth rate did not differ seasonally. For understory tree species, relative growth rates were higher in the rainy season than in the dry season. Furthermore, in the rainy season, the seedling recruitment rate was higher in canopy tree species than in understory tree species; however, seedling recruitment rate was not significantly different between these two tree types in the dry season. In the dry season, relative growth rates were higher in canopy tree seedlings than in understory tree seedlings. Mortality rates did not differ seasonally in canopy trees species or understory tree species. However, mortality rates were higher in canopy tree seedlings than in understory tree seedlings in both rainy and dry seasons. Our study suggests that understory tree species utilize resources with a more conservative strategy, releasing seedlings in the rainy season and maintaining relatively stable populations with low mortality rates and seedling recruitment rates in both dry and rainy seasons.
AcknowledgementsThis research was supported by the NSFC China-US Dimensions of Biodiversity Grant (DEB: 32061123003), National Natural Science Foundation of China (31870410, 32171507), the Chinese Academy of Sciences Youth Innovation Promotion Association (Y202080), the Distinguished Youth Scholar of Yunnan (202001AV070016) and the West Light Foundation of the Chinese Academy of Sciences and the Ten Thousand Talent Plans for Young Top-notch Talents of Yunnan (YNWR-QNBJ-2018-309). We are grateful for the field assistance from Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies (XSTRES).
CRediT authorship contribution statement
Libing Pan: Writing – original draft, Methodology, Conceptualization. Xiaoyang Song: Writing – review & editing, Methodology, Formal analysis, Data curation, Conceptualization. Wenfu Zhang: Investigation, Data curation. Jie Yang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Min Cao: Writing – review & editing, Supervision, Project administration, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2024.04.010.
Aide, T.M., 1992. Dry season leaf production - an escape from herbivory. Biotropica, 24: 532-537. DOI:10.2307/2389016 |
Ashton, M.S., Hooper, E.R., Singhakumara, B., et al., 2018. Regeneration recruitment and survival in an Asian tropical rain forest: implications for sustainable management. Ecosphere, 9: e02098. DOI:10.1002/ecs2.2098 |
Canham, C.D., Murphy, L., 2016. The demography of tree species response to climate: seedling recruitment and survival. Ecosphere, 7: e01424. DOI:10.1002/ecs2.1424 |
Cao M., Zhu H., Wang H., et al, 2008. Xishuangbanna Tropical Seasonal Rainforest Dynamics Plot: Tree Distribution Maps, Diameter Tables and Species Documentation. Kunming, Yunnan, China: Yunnan Science and Technology Press.
|
Cao, M., Zou, X., Warren, M., et al., 2006. Tropical forests of Xishuangbanna, China. Biotropica, 38: 306-309. DOI:10.1111/j.1744-7429.2006.00146.x |
Comita, L.S., Engelbrecht, B.M.J., 2009. Seasonal and spatial variation in water availability drive habitat associations in a tropical forest. Ecology, 90: 2755-2765. DOI:10.1890/08-1482.1 |
Condit R., 1998. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots. Berlin: Springer-Verlag.
|
Condit, R., Hubbell, S.P., Foster, R.B., 1995. Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol. Monogr., 65: 419-439. DOI:10.2307/2963497 |
Condit, R., Ashton, P.S., Manokaran, N., et al., 1999. Dynamics of the forest communities at Pasoh and Barro Colorado: comparing two 50-ha plots. Philos. Trans. R. Soc. Lond. B-Biol. Sci., 354: 1739-1748. DOI:10.1098/rstb.1999.0517 |
Connell, J.H., Green, P.T., 2000. Seedling dynamics over thirty-two years in a tropical rain forest tree. Ecology, 81: 568-584. DOI:10.1890/0012-9658(2000)081[0568:SDOTTY]2.0.CO;2 |
Delissio, L.J., Primack, R.B., 2003. The impact of drought on the population dynamics of canopy-tree seedlings in an aseasonal Malaysian rain forest. J. Trop. Ecol., 19: 489-500. DOI:10.1017/S0266467403003547 |
Deng, Y., 2021. Canopy Spatial Structure and Species Adaptation in a Tropical Seasonal Rainforest. PhD Dissertation from the University of Chinese Academy of Sciences.
|
Deng, Y., Deng, X., Dong, J., et al., 2020. Detecting growth phase shifts based on leaf trait variation of a canopy Dipterocarp tree species (Parashorea chinensis). Forests, 11: 1145. DOI:10.3390/f11111145 |
Deng, Y., Dong, J.L., Zhang, W.F., et al., 2022. Quantifying the vertical microclimate profile within a tropical seasonal rainforest, based on both ground- and canopy-referenced approaches. iForest, 15: 24-32. DOI:10.3832/ifor3780-014 |
Dou, L., Zhang, W., Deng, X., et al., 2018. Nine-year seed rain dynamics in Parashorea chinensis forest in Xishuangbanna, Southwest China. Biodiv. Sci., 26: 919-930. DOI:10.17520/biods.2018101 |
Engelbrecht, B., Comita, L., Condit, R., et al., 2007. Drought sensitivity shapes species distribution patterns in tropical forests. Nature, 447: 80-82. DOI:10.1038/nature05747 |
Garwood, N.C., 1983. Seed germination in a seasonal tropical forest in Panama: a community study. Ecol. Monogr., 53: 159-181. DOI:10.2307/1942493 |
Hubau, W., De Mil, T., Van den Bulcke, J., et al., 2019. The persistence of carbon in the African forest understory. Nat. Plants, 5: 133-140. DOI:10.1038/s41477-018-0316-5 |
Jiménez-Alfaro, B., Fidelis, A., Commander, L., 2019. Seed germination niche across habitats: an introduction to this special issue. Folia Geobot., 54: 1-4. DOI:10.1007/s12224-019-09351-6 |
Jin, Y., Russo, S.E., Yu, M., 2018. Effects of light and topography on regeneration and coexistence of evergreen and deciduous tree species in a Chinese subtropical forest. J. Ecol., 106: 1634-1645. DOI:10.1111/1365-2745.12911 |
King, D.A., Davies, S.J., Noor, N.S.M., 2006. Growth and mortality are related to adult tree size in a Malaysian mixed dipterocarp forest. For. Ecol. Manage., 223: 152-158. DOI:10.1016/j.foreco.2005.10.066 |
Lan, G.Y., 2009. Tree Diversity of a 20-ha Plot in a Tropical Seasonal Rainforest in Xishuangbanna. PhD Dissertation from the University of Chinese Academy of Sciences.
|
Lebrija-Trejos, E., Hernandez, A., Wright, S.J., 2023. Effects of moisture and density-dependent interactions on tropical tree diversity. Nature, 615: 100-104. DOI:10.1038/s41586-023-05717-1 |
Li, M.G., Lieberman, M., Lieberman, D., 1996. Seedling demography in undisturbed tropical wet forest in Costa Rica. In: Swaine, M.D. (Ed.), The Ecology of Tropical Forest Tree Seedlings. UNESCO, Paris, pp. 285-314.
|
Li, X.L., Wang, H., Zheng, Z., et al., 2009. Composition, spatial distribution and survival during the dry season of tree seedlings in a tropical forest in Xishuangbanna, SW China. Chin. J. Plant Ecol., 33: 658-671. |
Lieberman, D., Li, M.G., 1992. Seedling recruitment patterns in a tropical dry forest in Ghana. J. Veg. Sci., 3: 375-382. DOI:10.2307/3235763 |
Lieberman, D., Lieberman, M., Hartshorn, G., et al., 1985. Growth rates and age–size relationships of tropical wet forest trees in Costa Rica. J. Trop. Ecol., 1: 97-109. DOI:10.1017/S026646740000016X |
Liu, Y., Baskin, C.C., Baskin, J.M., et al., 2021. Seed dormancy profiles for forest dynamics plot data: focusing on a tropical seasonal rainforest in Xishuangbanna, southwest China. Plant Biol., 23: 420-426. DOI:10.1111/plb.13232 |
Lüttge U., 2008. Physiological Ecology of Tropical Plants. Second ed. Berlin: Springer-Verlag.
|
Marod, D., Kutintara, U., Tanaka, H., et al., 2002. The effects of drought and fire on seed and seedling dynamics in a tropical seasonal forest in Thailand. Plant Ecol., 161: 41-57. DOI:10.1023/A:1020372401313 |
Mason, W.L., Edwards, C., Hale, S.E., 2004. Survival and early seedling growth of conifers with different shade tolerance in a Sitka spruce spacing trial and relationship to understorey light climate. Silva Fenn., 38: 357-370. |
Mensah, S., Lokossou, C.J.M., Assogbadjo, A.E., et al., 2023. Seasonal variation of environment and conspecific density-dependence effects on early seedling growth of a tropical tree in semi-arid savannahs. Glob. Ecol. Conserv., 43: e02455. |
Milici, V.R., Dalui, D., Mickley, J.G., et al., 2020. Responses of plant-pathogen interactions to precipitation: implications for tropical tree richness in a changing world. J. Ecol., 108: 1800-1809. DOI:10.1111/1365-2745.13373 |
Norden, N., Chave, J., Belbenoit, P., et al., 2016. Mast fruiting is a frequent strategy in woody species of Eastern South America. Ecology, 97: 2780-2790. DOI:10.1002/ecy.1519 |
Nutiprapun, P., Hermhuk, S., Nanami, S., et al., 2022. Effects of El Niño drought on seedling dynamics in a seasonally dry tropical forest in Northern Thailand. Glob. Change Biol., 29: 451-461. |
O'Brien, M.J., Ong, R., Reynolds, G., 2017. Intra-annual plasticity of growth mediates drought resilience over multiple years in tropical seedling communities. Glob. Change Biol., 23: 4235-4244. DOI:10.1111/gcb.13658 |
Pearse, I.S., Koenig, W.D., Kelly, D., 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol., 212: 546-562. DOI:10.1111/nph.14114 |
Pesendorfer, M.B., Ascoli, D., Bogdziewicz, M., et al., 2021. The ecology and evolution of synchronized reproduction in long-lived plants. Philos. Trans. R. Soc. Lond. B-Biol. Sci., 376: 1-8. |
R Core Team, 2021. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
|
Russell-Smith, J., Setterfield, S.A., 2006. Monsoon rain forest seedling dynamics, northern Australia: contrasts with regeneration in eucalypt-dominated savannas. J. Biogeogr., 33: 1597-1614. DOI:10.1111/j.1365-2699.2006.01527.x |
Sandoval-Martinez, J., Flores-Cano, J.A., Badano, E.I., 2022. Recruitment of pioneer trees with physically dormant seeds under climate change: the case of Vachellia pennatula (Fabaceae) in semiarid environments of Mexico. J. Plant Res., 135: 453-463. DOI:10.1007/s10265-022-01383-y |
Seiwa, K., Kikuzawa, K., 2011. Close relationship between leaf life span and seedling relative growth rate in temperate hardwood species. Ecol. Res., 26: 173-180. DOI:10.1007/s11284-010-0774-3 |
Schmitt, S., Raevel, V., Rejou-Mechain, M., et al., 2021. Canopy and understorey tree guilds respond differently to the environment in an Indian rain forest. J. Veg. Sci., 32. |
Singh, J.S., Singh, V.K., 1993. Phenology of seasonally dry tropical forest. Curr. Sci., 63: 684-689. |
Song, X., Li, J., Zhang, W., et al., 2016a. Variant responses of tree seedling to seasonal drought stress along an elevational transect in tropical montane forests. Sci. Rep., 6: 36438. DOI:10.1038/srep36438 |
Song, X., Nakamura, A., Sun, Z., et al., 2016b. Elevational distribution of adult trees and seedlings in a tropical montane transect, southwest China. Mt. Res. Dev., 36: 342-354. DOI:10.1659/MRD-JOURNAL-D-15-00109.1 |
Thomas, S.C., 1996. Asymptotic height as a predictor of growth and allometric characteristics in Malaysian rain forest trees. Am. J. Bot., 83: 556-566. DOI:10.1002/j.1537-2197.1996.tb12739.x |
Turner, I.M., 1990. The seedling survivorship and growth of three Shorea species in a Malaysian tropical rain forest. J. Trop. Ecol., 6: 469-478. DOI:10.1017/S0266467400004879 |
Turner I.M., 2001. The Ecology of Trees in the Tropical Rainforest. New York: Cambridge University Press.
|
Uriarte, M., Muscarella, R., Zimmerman, J.K., 2018. Environmental heterogeneity and biotic interactions mediate climate impacts on tropical forest regeneration. Glob. Change Biol., 24: e692-e704. |
van den Berg, E., Chazdon, R., Correa, B.S., 2012. Tree growth and death in a tropical gallery forest in Brazil: understanding the relationships among size, growth, and survivorship for understory and canopy dominant species. Plant Ecol., 213: 1081-1092. DOI:10.1007/s11258-012-0067-8 |
Wang, J., Sun, Z., Hui, D., et al., 2018. Responses of seedling performance to altered seasonal precipitation in a secondary tropical forest, southern China. For. Ecol. Manage., 410: 27-34. DOI:10.1117/12.2285344 |
Whitmore T.C., 1990. An Introduction to Tropical Rain Forests. Oxford: Clarendon Press.
|
Wright, S.J., Kitajima, K., Kraft, N.J.B., et al., 2010. Functional traits and the growth-mortality trade-off in tropical trees. Ecology, 91: 3664-3674. DOI:10.1890/09-2335.1 |
Wright, S.J., Muller-Landau, H., Calderón, O., et al., 2005. Annual and spatial variation in seedfall and seedling recruitment in a neotropical forest. Ecology, 86: 848-860. DOI:10.1890/03-0750 |
Xu, Z., Johnson, D.J., Zhu, K., et al., 2022. Interannual climate variability has predominant effects on seedling survival in a temperate forest. Ecology, 103: e3643. DOI:10.1002/ecy.3643 |
Yan, X., Cao, M., 2007a. Effects of light intensity on seed germination and seedling early growth of Shorea wantianshuea. Chin. J. Appl. Ecol., 18: 23-29. |
Yan, X., Cao, M., 2007b. Effects of shading treatments on the growth of Pometia tomentosa seedlings. J. Trop. Subtrop. Bot., 15: 465-472. |
Yan, X., Cao, M., Xu, H.L., 2007. Effects of desiccation and temperature on the germination of Shorea chinensis (Dipterocarpaceae) seeds. Seed Sci. Technol., 35: 232-236. DOI:10.15258/sst.2007.35.1.21 |
Yan, X., Cao, M., 2008. The endangered causes and protective strategies for Shorea wantianshuea, a tropical rain forest tree species in Xishuangbanna. J. Fujian For. Sci. Technol., 35: 187-191. DOI:10.1215/9780822388654-008 |
Yu, Y., Baskin, J.M., Baskin, C.C., et al., 2008. Ecology of seed germination of eight non-pioneer tree species from a tropical seasonal rain forest in southwest China. Plant Ecol., 197: 1-16. |
Zheng, Z., Chen, X.D., Mao, H.W., et al., 2001. Leaf growth and herbivory dynamics of saplings in tropical seasonal rainforest gaps in Xishuangbanna. Chin. J. Plant Ecol., 25: 679-686. DOI:10.21437/eurospeech.2001-192 |



