Plants have increasingly been introduced beyond their native ranges due to global trade and transportation (van Kleunen et al., 2015; Seebens et al., 2017; Wang et al., 2022). Nevertheless, few of these alien plant species successfully invade and threaten native community structure and function (Meyerson and Mooney, 2007; Caley et al., 2008; Chen et al., 2022). Several environmental conditions hinder invasion success, including resource availability, disturbance regimes, and environmental heterogeneity (Catford et al., 2012; Fristoe et al., 2021). The most common explanation for failed invasions, however, is biotic resistance (Elton, 1958; Levine et al., 2004; Guo et al., 2023), as invasive plants must interact with resident competitors (Chadwell and Engelhardt, 2008; te Beest et al., 2018) or enemies (Pearson et al., 2012; Ning et al., 2019), which may limit their establishment and spread. Although invasive plants should have escaped from their specialist natural enemies (Keane and Crawley, 2002), they will also encounter novel generalist enemies in the invaded habitats. As sharing no long-term coevolutionary history with native enemies, invasive plants may lack effective defences and cannot deter the native enemies effectively, i.e. the "increased susceptibility" hypothesis (Colautti et al., 2004), which may cause invaders to be under greater insect feeding pressure than native ones. However, invasive species may also be inadequate prey compared to native plants because of the envolutionarily novel of invaders for the native herbivores (Siemann and Rogers, 2003; Lankau et al., 2004; Xiong et al., 2008; Lucero et al., 2019). Therefore, the feeding preference of native generalist herbivores will determine the success or failure of alien plant invasion, but the conclusions are ambiguous.
Previous studies have proposed that alien plant invasion success is inhibited by diverse native communities due to niche complementarity and sample effects (Elton, 1958; Levine et al., 2004; Zhang et al., 2020). Niche complementarity posits that highly diverse native communities usually occupy limiting resources more completely and efficiently during aggravated resource pre-emption (Romanuk et al., 2009; van Elsas et al., 2012), leaving fewer ecological niches for arriving invaders (Jiang et al., 2011). Sampling effect holds that highly diverse communities are more likely to contain superior competitors able to resist exotic plant invasion (Wardle, 2001; Fargione and Tilman, 2005). Both niche complementarity and sampling effect focus on competition between a single trophic level (i.e. plant-plant interaction). However, it remains unclear how other extrinsic factors on other trophic levels that covary with diversity, such as herbivores, regulate competition and/or affect plant invasion success (Smith and Cote, 2019). On one hand, native generalist herbivores are more likely to shift from native plants to invasive plants in highly diverse plant communities. This shift in herbivore preference in highly diverse plant communities is mediated by the dilution of the physical and chemical cues that attract herbivores to native plants (Jactel et al., 2011; Castagneyrol et al., 2013; Hambäck et al., 2014; Underwood et al., 2014). On the other hand, diverse native communities are likely to have a higher tolerance to herbivores because of the higher complementary between members (Levine et al., 2004). Despite predictions that highly diverse native communities strengthen the efficacy of native generalist herbivores in reducing plant invasion success, experimental evidence is lacking. The synergies between native plant diversity and herbivory may be influenced by nutrient enrichment, which has long been suggested to promote plant invasion (Davis et al., 2000; Seabloom et al., 2015; Liu et al., 2017). Nutrient enrichment has been shown to increase plant biomass (Fay et al., 2015) and promote shifts in plant resource allocation to increase growth and decrease defense (Kessler and Baldwin, 2001; Shan et al., 2018; Descombes et al., 2020), subsequently affecting herbivory rates and herbivore feeding preferences (Descombes et al., 2020; Pellissier et al., 2018). However, these changes are not always isometric between invasive and native species that co-occur in one community (Descombes et al., 2020; Shan et al., 2023), resulting a different response to herbivory between invasive plants and native plants under similar nutrient conditions. In addition, nutrient enrichment alters the effects of native plant diversity on invasion (Mallon et al., 2015; Yang et al., 2017). It has been suggested that resource enrichment could reduce the competition effects between species by reducing niche pre-emption in diverse communities, and therefore relieve the negative diversity-invasibility relationship (Davis et al., 2000; Mallon et al., 2015). Moreover, nutrient availability is also considered a limiting resource for insects (Mattson Jr, 1980), and increased nutrient availability can prevent competitive exclusion by increasing herbivore consumption (Brose, 2008; Mortensen et al., 2018). At the same time, plants that grow in high nutrient conditions have higher compensatory abilities in response to herbivory (Gianoli and Salgado-Luarte, 2017; Hu and Dong, 2019). However, we know little about how nutrient enrichment affects the synergistic resistance of native plant diversity and herbivores to plant invasion.
In the present study, we tested how herbivory and native plant diversity synergistically influence alien plant invasion under different nutrient conditions. For this purpose, we grew invasive plants in a greenhouse in the presence of herbivores, in communities with high diversity and low diversity, and in different soil conditions. Specifically, we tested the following questions: (a) Will native generalist herbivore decrease invasion success of alien plant species? (b) Will the resistance of generalist herbivore to alien plant invasion be strengthened by higher native plant diversity? (c) Do nutrients play change the effect of herbivore and native plant diversity on plant invasion?
2. Materials and methods 2.1. Study speciesWe chose eight alien invasive plant species to be target species and 16 native plant species to construct the native communities. The classification of these species is based on The Checklist of the Naturalized Plants in China (Yan et al., 2019; Hao and Ma, 2023) and the database of flora of China (http://www.efloras.org). According to the occurrence records in the iPlant database (http://www.iplant.cn) and Global Biodiversity Information Facility (GBIF; http://www.gbif.org), all species are widely distributed in China, and may co-occur with each other in the wild. Seeds of these species were selected from wild populations in the grasslands of China or from The Germplasm Bank of Wild Species, China (Details in Table S1).
The insect herbivore used to assess the effect of herbivory on invasion success was Spodoptera litura (Noctuidae, Lepidoptera). S. litura is a generalist herbivore that feeds on more than 290 species of plants belonging to 99 families (Zou et al., 2016; Pham and Hwang, 2020). Larvae were obtained from a commercial insect company (KEYUN, Henan, China), and were fed with the same food before the experiment. The herbivore and all plant species used in this study are likely to co-occur in the wild, as their distribution overlaps (Fu et al., 2015).
2.2. Experimental set upTo test the effects of generalist herbivores on alien plant invasion success across different plant diversity and nutrient enrichment conditions, we performed a fully-crossed factorial experiment. Each factor had two levels: herbivory (with vs. without), diversity (4 vs. 8 species), and nutrient enrichment (low vs. high). Each treatment combination for each invasive plant species was replicated eight times, which resulted in a total of 512 experimental pots: 8 invasive plant species × 2 herbivory levels × 2 plant diversity levels × 2 nutrient treatment levels × 8 replicates (Fig. 1).
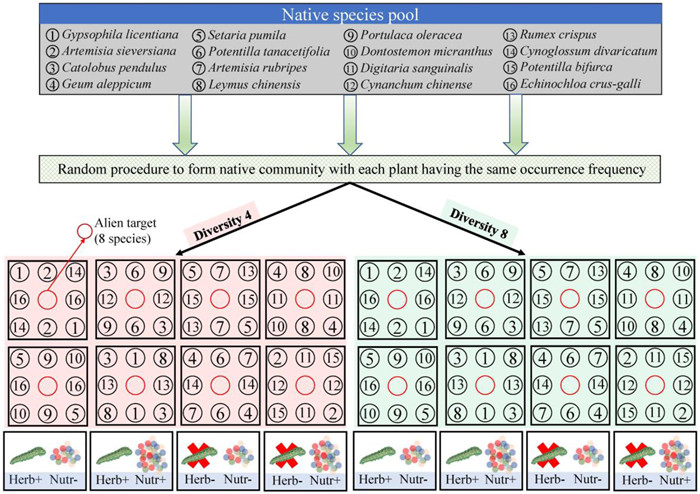
|
| Fig. 1 A schematic illustration of our experiment testing the effects of herbivory [with vs. without], native plant diversity [4 species vs. 8 species], and nutrient enrichment [high vs. low)] treatments on alien plant invasion. |
Seeds of each species were separately sowed into trays filled with potting soil. Because germination speed varies for each species, we sowed seeds at different times (from 31 March to 21 April 2021) to ensure all species were at a similar stage of development when the experiment began. We kept all trays under uniform conditions in a greenhouse (temperature: 22–28 ℃; natural lighting with an intensity of ca. 75% of outdoor light; and ca. 60% relative humidity).
On 22 April 2021, seedlings of similar size from the eight invasive alien species and 16 native species were transplanted into square plastic pots (bottom diameter: 15.5 cm, top diameter: 18.0 cm, height: 12.5 cm). Pots were filled with a 1:1 volume mixture of sand and fine vermiculite. For each of the eight invasive plant species, 64 seedlings (total 512 individuals) were transplanted into the centre of 512 pots (one individual per pot). In each pot, eight native plant individuals were transplanted, surrounding the alien plants to form the native community with two diversity levels (4 vs. 8 species). For low diversity native communities, we randomly selected four different plant species from the 16 native plant species, and each of these native species was planted twice per pot to ensure there were eight individuals in each pot. For high diversity native communities, we randomly selected eight different plant species from the 16 native plant species, so that each native species was only planted once per pot. During the sampling, each native species was selected twice and four times in the low and high plant diversity communities, respectively, resulting in eight different native communities in each diversity level as eight replicates (Fig. S1).
For nutrient enrichment treatments, we blended 11 g and 5.5 g slow-released fertilizer (Osmocote® Exact Standard; 15% N + 9.0% P2O5 + 12% K2O + 2.0% MgO + 0.02% B + 0.05% Cu + 0.45% Fe + 0.09% chelated by EDTA + 0.06% Mn + 0.02% Mo + 0.015% Zn, Everris International B.V., Geldermalsen, The Netherlands) in each pot to impose high and low nutrient conditions, respectively (Jin et al., 2022). Plastic dishes were placed under each pot to prevent nutrient solution and water from leaking during the experiment. All pots were randomly assigned and positioned in the greenhouse for seedlings rejuvenation, with temperatures ranging between 22 ℃ and 30 ℃ and a natural light/dark cycle. Two weeks after transplant, we randomly moved all pots into a cage outside (9.5 m × 4.5 m × 2.5 m), which protected all plants from rain but did not control the temperature. We watered each pot every day to ensure all pots had the same water availability conditions.
Three weeks after transplant, we started the above-ground herbivory treatments using Spodoptera litura as an insect herbivore. We firstly covered each pot with an insect net, which was lined with white nylon fabric that opened on the bottom. We then put S. litura into each pot that was assigned herbivore treatment, and observed the damage every day to determine if more herbivores should be added. During the herbivory treatment, we added S. litura three times (twelve 2nd instar individuals in total). The herbivory treatment lasted three weeks, during which ca. 30%–50% of the above-ground biomass was removed.
2.3. Herbivore feeding damage survey and plant harvestHerbivory damage on each plant in the herbivory treatment pots was measured from 22 to 24 June 2021. The severity of herbivory was quantified via visual inspection and classified into damage categories according to how much of the plant was consumed (Liu et al., 2023): 0 (0%); 1 (0–33%); 2 (33%–66%); 3 (66%–99%); 4 (100%). When plants achieved their largest biomass (25–27 June 2021), we harvested the aboveground biomass of alien and native species individually. Alien target plants died in two pots during the experiment, leaving 510 out of the original 512 pots to be harvested. The harvested aboveground biomass samples were dried to constant weight at 65 ℃ and then weighed. To calculate the proportion of aboveground biomass of alien target species in each pot, we divided the aboveground biomass of alien target species by the total aboveground biomass per pot.
Moreover, we further assessed the impact of plant diversity, nutrient enrichment and their interaction on the growth of Spodoptera litura larvae. After herbivory treatments, individual 4th instar larvae were taken from plants and starved for 24 h, allowing frass to pass, before being weighed. Because a few herbivores died during the treatment, we calculated the average weight of all herbivores in each pot. The larvae weights at 2nd instar were assumed to be equal within the species, and thus only final weights were recorded.
2.4. Statistical analysesWe performed all statistical analyses in R v.4.0.2 (R Core Team, 2020). Linear mixed-effects models were used to assess the individual and interactive effects of herbivory, native plant diversity, and nutrient enrichment on aboveground biomass of alien target species, aboveground biomass of native communities and aboveground biomass proportion of alien target species. In these models, aboveground biomass production of alien target species, native communities and aboveground biomass proportion of alien target species were specified as response variables. Herbivory (with vs. without), native plant diversity (4 vs. 8 species), nutrient availability (low vs. high) treatments, as well as all their three-way and two-way interactions, were specified as fixed-effect-independent variables. To meet the assumptions of normality of variance, aboveground biomass production of alien target species was nature-log-transformed, aboveground biomass production of native communities was square-root-transformed, and aboveground biomass proportion of alien target species was logit-transformed.
To account for the non-independence of replicates of the same species and for phylogenetic relatedness among target species, we included the identities of target species and their corresponding family as random factors in all models. In addition to accounting for variation among different communities, we also included the identities of the eight native communities as a random factor in all models. As the data did not fulfill the homoscedasticity assumption, for the analyses of aboveground biomass production and biomass proportion of alien target species, we included variance structures to allow for variance among species using the 'varIdent' function in the R package 'nlme' (Pinheiro et al., 2020). We assessed the significance of fixed-effect-independent variables (i.e., herbivory, plant diversity, nutrient enrichment, and interactions among them) using likelihood-ratio tests. The variance components were estimated using the restricted maximum-likelihood method of the full model (Zuur et al., 2009).
To test the effect of plant diversity and nutrients on herbivory preference for alien species, we first calculated the feeding preference index for alien species by dividing the damage level of invasive plants by the community weighted mean (CWM) damage level of each pot of the herbivory treatment (Hedges et al., 1999). The CWM damage level of each pot was calculated by averaging the damage level of all the species in each pot, weighted by their individual number. For the feeding preference index, values larger than 1 indicate a feeding preference for alien plants rather than native community species. Larger values indicate higher feeding preferences for alien plants. In the model, we set the feeding preference index as the response variable; plant diversity, nutrient enrichment, and their interaction were set as fixed effects; the identity of alien species and native communities were set as random effects.
We tested the effects of plant diversity, nutrient enrichment, and their interaction on insect weight using linear mixed models with the lme function in the "nlme" package (Pinheiro et al., 2020). In the model, insect weight was specified as a response variable. Native plant diversity (4 vs. 8 species) and nutrient availability (low vs. high) treatments, as well as all their two-way interactions, were specified as fixed-effect-independent variables. The identity of alien species and native communities were set as random effects. To meet the assumptions of normality of variance, insect weight was square-root-transformed.
3. ResultsHerbivore insects decreased the aboveground biomass of alien target species (−32.68%; Table 1; Fig. 2a) and native communities (−7.89%; Table 1; Fig. 2b). Native plant diversity did not affect the aboveground biomass of alien target species or native communities, nor did it affect the biomass proportion of alien target species (Table 1; Fig. 2). Nutrient enrichment significantly increased the aboveground biomass of alien target species (+30.89%; Table 1; Fig. 2a) and native communities (+45.03%; Table 1; Fig. 2b), but did not affect the aboveground biomass proportion of alien target species (Table 1; Fig. 2c). Effects of herbivory on biomass proportion of alien target species was altered by native plant diversity (Table 1; Fig. 2c). Specifically, the aboveground biomass proportion of alien target species was decreased greatly (30%) by herbivores in high diversity plant communities, but not in low diversity plant communities (Table 1; Fig. 2c).
| Fixed effects | Aboveground biomass of target plants (g) (ln-transformed) | Aboveground biomass of native communities (g) (square root transformed) | Aboveground biomass proportion of the target species (logit-transformed) | |||||
| χ2(df = 1) | p | χ2(df = 1) | p | χ2(df = 1) | p | |||
| Herbivory (Herb) | 58.007 | < 0.001 | 31.812 | < 0.001 | 26.225 | < 0.001 | ||
| Diversity (Dive) | 0.384 | 0.536 | 1.595 | 0.207 | 1.092 | 0.296 | ||
| Nutrient enrichment (Nutr) | 36.309 | < 0.001 | 183.703 | < 0.001 | 1.250 | 0.263 | ||
| Herb × Dive | 0.138 | 0.710 | 1.632 | 0.201 | 3.940 | 0.047 | ||
| Herb × Nutr | 0.223 | 0.637 | 0.399 | 0.528 | 0.084 | 0.772 | ||
| Dive × Nutr | 0.012 | 0.912 | 0.394 | 0.530 | 0.507 | 0.476 | ||
| Herb × Dive × Nutr | 1.181 | 0.277 | 1.554 | 0.212 | < 0.001 | 0.991 | ||
| Random effects | SD | SD | SD | |||||
| Species | 0.904 | 0.180 | 0.974 | |||||
| Family | 0.488 | 0.150 | 0.580 | |||||
| Native community | 0.304 | 1.325 | 0.984 | |||||
| Residual | 0.594 | 0.572 | 0.700 | |||||
| Rm2 | Rc2 | Rm2 | Rc2 | Rm2 | Rc2 | |||
| R2 of the model | 0.081 | 0.784 | 0.159 | 0.871 | 0.041 | 0.829 | ||
| Significant effects (P < 0.05) are in bold. Rm2: marginal R2, Rc2: conditional R2. | ||||||||
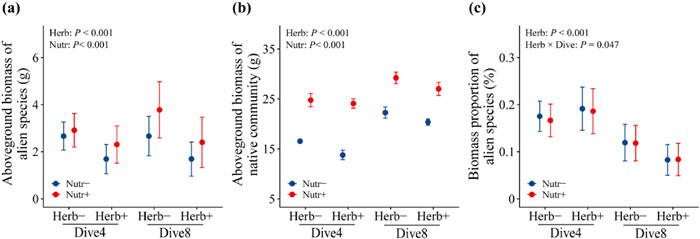
|
| Fig. 2 Mean values (1 ± SE) of aboveground biomass production of the alien target species (a), native communities (b), and biomass proportion of the alien target species (c) under herbivory [with (Herb+) vs. without (Herb-)], native plant diversity [4 species (Dive4) vs. 8 species (Dive8)], and nutrient enrichment [high (Nutr+) vs. low (Nutr-)] treatments. |
In all pots, herbivores preferred alien species over native species (Fig. 3; mean feeding preference index: 1.151 ± 0.039). Native plant diversity did not affect either herbivorous damage on alien plant species or the mean herbivorous damage on native communities (Fig. S2). High nutrient enrichment significantly decreased the mean herbivorous damage on the native community (−4.42%; Fig. S2b). As a result, feeding preference was not changed with plant diversity but increased with nutrient enrichment (Table 2; Fig. 3). In addition, the herbivore damage was different between native species (Fig. S3).
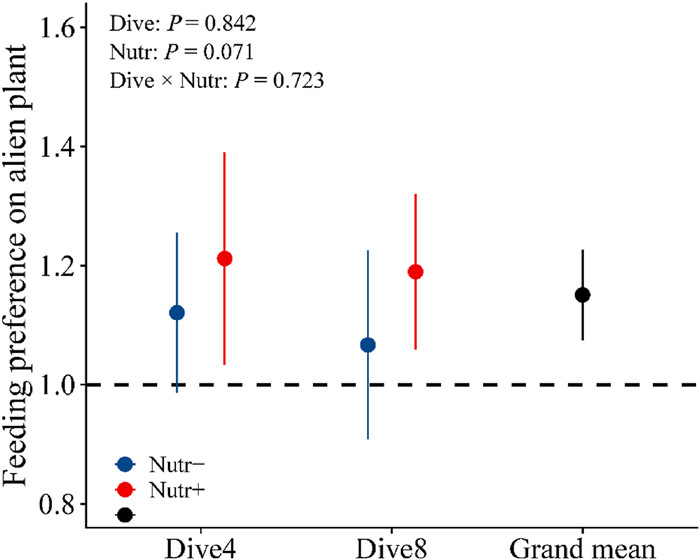
|
| Fig. 3 The effects of native community diversity [4 species (Dive4) vs. 8 species (Dive8)], nutrient enrichment [high (Nutr+) vs. low (Nutr-)] treatments and their interactions on the herbivore feeding preference on alien plants. The black solid line represents the grand mean preference across all pots regardless of treatments. |
| Fixed effects | Feeding preference on alien plant (square root transformed) | Herbivore weight (square root transformed) | |||
| χ2(df = 1) | p | χ2(df = 1) | p | ||
| Diversity (Dive) | 0.058 | 0.810 | 0.267 | 0.606 | |
| Nutrient enrichment (Nutr) | 4.175 | 0.041 | 10.649 | 0.001 | |
| Nutr × Dive | 0.209 | 0.647 | 3.339 | 0.068 | |
| Random effects | SD | SD | |||
| Species | < 0.001 | < 0.001 | |||
| Family | 0.202 | 0.027 | |||
| Native community | 0.085 | 0.103 | |||
| Residual | 0.132 | 0.158 | |||
| Rm2 | Rc2 | Rm2 | Rc2 | ||
| R2 of the model | 0.010 | 0.736 | 0.047 | 0.345 | |
| Significant effects (P < 0.05) are in bold. Rm2: marginal R2, Rc2: conditional R2. | |||||
Nutrient enrichment increased herbivore weight (+16.49%; Table 2; Fig. 4). This increase in herbivore weight was higher in native plant communities with low diversity (+25.83%) than in native plant communities with higher diversity (+7.66%) (Table 2; Fig. 4).
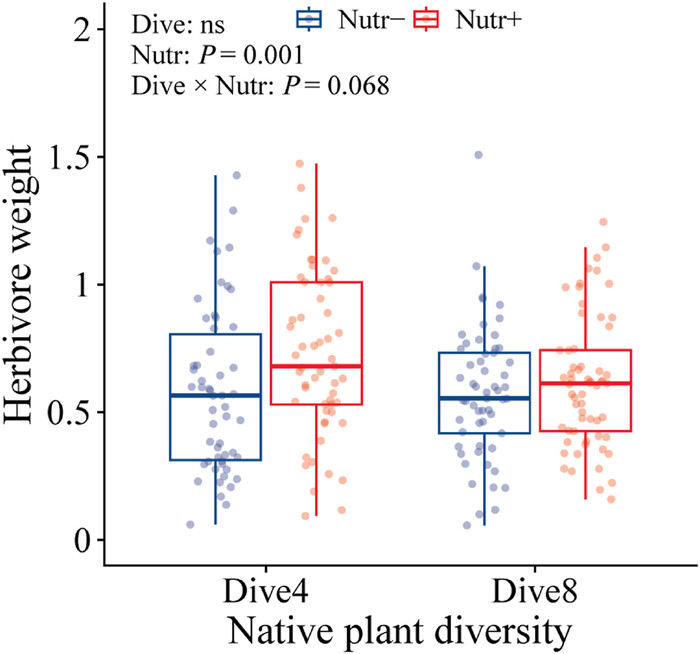
|
| Fig. 4 The herbivore weight under native plant diversity [4 species (Dive4) vs. 8 species (Dive8)], and nutrient enrichment [high (Nutr+) vs. low (Nutr-)] treatments. |
Our results show that a native generalist herbivory significantly decreased the absolute aboveground biomass of alien species and native communities, and also reduced the proportional biomass of alien species (Fig. 2), suggesting that the native generalist herbivore can reduce the invasion success of an alien species (Cushman et al., 2011; Pearson et al., 2012; Zhang et al., 2018; Ning et al., 2019). Alien plants species, which have not coevolved with native herbivores, are unable to effectively defend themselves from these native consumers. Consequently, generalist herbivores may transfer their feeding preference from native plants to alien species, and by doing so decrease the abundance of alien plants (i.e., the "increased susceptibility" hypothesis; Colautti et al., 2004; Müller-Schärer et al., 2004). Moreover, herbivore preference can also contribute to plant functional characteristics including low metabolic rate and conservative resource investment strategy, which can promote their range expansion but less vulnerable to herbivores (Liao et al., 2021). Consistent with this logic, our result showed that native generalist Spodoptera litura prefers alien target species over native species (Fig. 3), and thus limits alien plant species invasion.
Elton's hypothesis proposes that plant invasion can be more effectively prevented in diverse communities than in communities with limited species (Elton, 1958). However, empirical evidence does not consistently support this prediction (Fridley et al., 2007; Tomasetto et al., 2019). In our multispecies experiment, native plant diversity did not affect the aboveground biomass proportion of alien target species (Table 1; Fig. 2c). However, our results showed that plant diversity mediated the efficacy of a generalist herbivore in reducing plant invasion, i.e., communities with high native plant diversity and herbivores were less vulnerable to plant invasion (Fig. 2c). Studies with similar findings have attributed these results to the associational resistance hypothesis. This hypothesis posits that in communities with higher plant diversity, visual or chemical cues reduce the ability of herbivores to identify preferred native plant species, thus strengthening a feeding preference shift from native plant to invasive plants (Fagan et al., 2002; Hambäck et al., 2014; Underwood et al., 2014). However, in our study, the feeding preference for alien plants was not altered by plant diversity (Table 2; Fig. 3, Fig. 4 and S2).
Our findings may be explained by the complex species composition of communities with high plant diversity, which can provide more palatable plant species and prevent the preferred plant species from being overexploited (Yachi and Loreau, 1999; Callaway et al., 2005). For instance, previous studies have reported that grassland plots with high diversity are more tolerant to single grazing herds than are plots with low diversity (Duffy, 2002; Zhu et al., 2012). Similarly, higher diversity communities may be more likely to contain fewer damaged plants that could well complement an empty niche and/or resources left by heavily damaged plants, which may increase the compensatory growth and then hinder the invasion of alien species (Prieur-Richard et al., 2002; Fridley et al., 2007; Moreira et al., 2016). Our finding that herbivore damage differed between native species in our study supports these ideas (Fig. S3). Moreover, the "resource concentration hypothesis" suggests that high plant diversity may also indirectly constrain herbivore performance, as increased nutrient heterogeneity in high diversity communities dilutes high-quality resources (Root, 1973; Otway et al., 2005).
The effects of herbivory and plant diversity on alien plant invasion have been shown to be reduced in nutrient-rich environments (Davis et al., 2000; van Kleunen et al., 2010; Liu et al., 2017; Li et al., 2022). In this study, nutrient enrichment increased herbivore feeding preference for alien species (Fig. 3), although it did not change herbivore damage of alien species (Fig. S2). In contrast, native plants were damaged less by herbivores in enriched soil. Compensatory growth and resistance are two major defensive strategies that plants employ against herbivory and that influence plant growth and fitness (Strauss and Agrawal, 1999; Fornoni, 2011; Oduor, 2022). Plant compensatory growth following herbivory minimizes fitness losses through simultaneous shifts in physiology and resource allocation (Strauss and Agrawal, 1999). Reallocation of unused resources to growth or defense determines the effect of herbivory on plant invasion under different nutrient conditions (Pellissier et al., 2018; Shan et al., 2023). Our results suggest that alien and native species have different reallocation strategies for unused resources in enriched nutrient condition, that is, alien plants are more inclined to growth, whereas native plants are more inclined to defense (Heckman et al., 2019; Shan et al., 2023). These different strategies did not confer a competitive advantage for either native or alien plant species. Nor did these strategies influence the effect of generalist herbivory on alien plant invasion in nutrient enrichment conditions. Our findings are consistent with several studies that have found that high plant diversity can increase investment in anti-herbivore defense, independently of resource competition (Mraja et al., 2011; Moreira et al., 2014). These findings imply that a diverse native community can strengthen the invasion resistance in habitats with generalist herbivores, even when nutrient availability is enhanced, as predicted under future global climate change.
AcknowledgementWe thank the Professor Yanjie Liu for his assistance in the design of the experiment and in providing the glasshouse for the successful implementation of this study. We also thank Lichao Wang, Haoran Bai, Yan Zheng, and Luna Zhang for their practical assistance. This study is supported by Postdoctoral Funding from Jilin Province to Liping Shan (2020000147).
Author contributions
LS conceived the idea and designed the experiment. LS and MH performed the experiment. LS and MH analyzed the data. LS wrote the first draft of the manuscript, with further input from MH.
Data accessibility
Data supporting the results is archived in figshare: https://doi.org/10.6084/m9.figshare.22336540.v1.
Declaration of competing interest
The authors declare that they have no known competing financialinterestsor personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.09.002.
Brose, U., 2008. Complex food webs prevent competitive exclusion among producer species. Proc. Roy. Soc. B-Biol. Sci., 275: 2507-2514. DOI:10.1098/rspb.2008.0718 |
Caley, P., Groves, R.H., Barker, R., 2008. Estimating the invasion success of introduced plants. Divers. Distrib., 14: 196-203. DOI:10.1111/j.1472-4642.2007.00440.x |
Callaway, R.M., Kikodze, D., Chiboshvili, M., et al., 2005. Unpalatable plants protect neighbors from grazing and increase plant community diversity. Ecology, 86: 1856-1862. DOI:10.1890/04-0784 |
Castagneyrol, B., Giffard, B., Péré, C., et al., 2013. Plant apparency, an overlooked driver of associational resistance to insect herbivory. J. Ecol., 101: 418-429. DOI:10.1111/1365-2745.12055 |
Catford, J.A., Vesk, P.A., Richardson, D.M., et al., 2012. Quantifying levels of biological invasion: towards the objective classification of invaded and invasible ecosystems. Global Change Biol., 18: 44-62. DOI:10.1111/j.1365-2486.2011.02549.x |
Chadwell, T.B., Engelhardt, K.A., 2008. Effects of pre-existing submersed vegetation and propagule pressure on the invasion success of Hydrilla verticillata. J. Appl. Ecol., 45: 515-523. DOI:10.1111/j.1365-2664.2007.01384.x |
Chen, J., Zhang, H.Y., Liu, M.C., et al., 2022. Plant invasions facilitated by suppression of root nutrient acquisition rather than by disruption of mycorrhizal association in the native plant. Plant Divers., 44: 499-504. DOI:10.1016/j.pld.2021.12.004 |
Colautti, R.I., Ricciardi, A., Grigorovich, I.A., et al., 2004. Is invasion success explained by the enemy release hypothesis?. Ecol. Lett., 7: 721-733. DOI:10.1111/j.1461-0248.2004.00616.x |
Cushman, J.H., Lortie, C.J., Christian, C.E., 2011. Native herbivores and plant facilitation mediate the performance and distribution of an invasive exotic grass. J. Ecol., 99: 524-531. DOI:10.1111/j.1365-2745.2010.01776.x |
Davis, M.A., Grime, J.P., Thompson, K., 2000. Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol., 88: 528-534. DOI:10.1046/j.1365-2745.2000.00473.x |
Descombes, P., Kergunteuil, A., Glauser, G., et al., 2020. Plant physical and chemical traits associated with herbivory in situ and under a warming treatment. J. Ecol., 108: 733-749. DOI:10.1111/1365-2745.13286 |
Duffy, J.E., 2002. Biodiversity and ecosystem function: the consumer connection. Oikos, 99: 201-219. DOI:10.1034/j.1600-0706.2002.990201.x |
Elton C.S., 1958. Ecology of Invasions by Animals and Plants. London, UK: Methuen.
|
Fagan, W.F., Lewis, M.A., Neubert, M.G., et al., 2002. Invasion theory and biological control. Ecol. Lett., 5: 148-157. DOI:10.1046/j.1461-0248.2002.0_285.x |
Fargione, J.E., Tilman, D., 2005. Diversity decreases invasion via both sampling and complementarity effects. Ecol. Lett., 8: 604-611. DOI:10.1111/j.1461-0248.2005.00753.x |
Fay, P.A., Prober, S.M., Harpole, W.S., et al., 2015. Grassland productivity limited by multiple nutrients. Nat. Plants, 1: 1-5. DOI:10.1109/BMSB.2015.7177257 |
Fornoni, J., 2011. Ecological and evolutionary implications of plant tolerance to herbivory. Funct. Ecol., 25: 399-407. DOI:10.1111/j.1365-2435.2010.01805.x |
Fridley, J.D., Stachowicz, J.J., Naeem, S., et al., 2007. The invasion paradox: reconciling pattern and process in species invasions. Ecology, 88: 3-17. DOI:10.1890/0012-9658(2007)88[3:TIPRPA]2.0.CO;2 |
Fristoe, T.S., Chytrý, M., Dawson, W., et al., 2021. Dimensions of invasiveness: links between local abundance, geographic range size, and habitat breadth in Europe's alien and native floras. Proc. Natl. Acad. Sci. U.S.A., 118: e2021173118. DOI:10.1073/pnas.2021173118 |
Fu, X., Zhao, X., Xie, B., et al., 2015. Seasonal pattern of Spodoptera litura (Lepidoptera: Noctuidae) migration across the Bohai strait in northern China. J. Econ. Entomol., 108: 525-538. DOI:10.1093/jee/tov019 |
Gianoli, E., Salgado-Luarte, C., 2017. Tolerance to herbivory and the resource availability hypothesis. Biol. Lett., 13: 20170120. DOI:10.1098/rsbl.2017.0120 |
Guo, Q., Qian, H., Zhang, J., 2023. Does regional species diversity resist biotic invasions?. Plant Divers., 45: 353-357. DOI:10.1016/j.pld.2022.09.004 |
Hambäck, P.A., Inouye, B.D., Andersson, P., et al., 2014. Effects of plant neighborhoods on plant–herbivore interactions: resource dilution and associational effects. Ecology, 95: 1370-1383. DOI:10.1890/13-0793.1 |
Hao, Q., Ma, J.S., 2023. Invasive alien plants in China: an update. Plant Divers., 45: 117-121. DOI:10.1016/j.pld.2022.11.004 |
Heckman, R.W., Halliday, F.W., Mitchell, C.E., 2019. A growth–defense trade-off is general across native and exotic grasses. Oecologia, 191: 609-620. DOI:10.1007/s00442-019-04507-9 |
Hedges, L.V., Gurevitch, J., Curtis, P.S., 1999. The meta–analysis of response ratios in experimental ecology. Ecology, 80: 1150-1156. DOI:10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2 |
Hu, X.T., Dong, B.C., 2019. Herbivory and nitrogen availability affect performance of an invader Alternanthera philoxeroides and its native congener A. sessilis. Flora, 257: 151412. DOI:10.1016/j.flora.2019.05.011 |
Jactel, H., Birgersson, G., Andersson, S., et al., 2011. Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia, 166: 703-711. DOI:10.1007/s00442-011-1918-z |
Jiang, L., Brady, L., Tan, J., 2011. Species diversity, invasion, and alternative community states in sequentially assembled communities. Am. Nat., 178: 411-418. DOI:10.1086/661242 |
Jin, H., Chang, L., van Kleunen, M., et al., 2022. Soil mesofauna may buffer the negative effects of drought on alien plant invasion. J. Ecol., 110: 2332-2342. DOI:10.1111/1365-2745.13950 |
Keane, R.M., Crawley, M.J., 2002. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol., 17: 164-170. DOI:10.1016/S0169-5347(02)02499-0 |
Kessler, A., Baldwin, I.T., 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science, 291: 2141-2144. DOI:10.1126/science.291.5511.2141 |
Lankau, R.A., Rogers, W.E., Siemann, E., 2004. Constraints on the utilisation of the invasive Chinese tallow tree Sapium sebiferum by generalist native herbivores in coastal prairies. Ecol. Entomol., 29: 66-75. DOI:10.1111/j.0307-6946.2004.00575.x |
Levine, J.M., Adler, P.B., Yelenik, S.G., 2004. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett., 7: 975-989. DOI:10.1111/j.1461-0248.2004.00657.x |
Li, S., Jia, P., Fan, S., et al., 2022. Functional traits explain the consistent resistance of biodiversity to plant invasion under nitrogen enrichment. Ecol. Lett., 25: 778-789. DOI:10.1111/ele.13951 |
Liao, H., Pal, R.W., Niinemets, Ü., et al., 2021. Different functional characteristics can explain different dimensions of plant invasion success. J. Ecol., 109: 1524-1536. DOI:10.1111/1365-2745.13575 |
Liu, X., Lin, Z., Hu, K., et al., 2023. Geographical variation in community-wide herbivory matches patterns of intraspecific variation instead of species turnover. Global Ecol. Biogeogr., 32: 1140-1151. DOI:10.1111/geb.13690 |
Liu, Y., Oduor, A.M., Zhang, Z., et al., 2017. Do invasive alien plants benefit more from global environmental change than native plants?. Global Change Biol., 23: 3363-3370. DOI:10.1111/gcb.13579 |
Lucero, J.E., Schaffner, U., Asadi, G., et al., 2019. Enemy release from the effects of generalist granivores can facilitate Bromus tectorum invasion in the Great Basin Desert. Ecol. Evol., 9: 8490-8499. DOI:10.1002/ece3.5314 |
Mallon, C.A., Poly, F., Le Roux, X., et al., 2015. Resource pulses can alleviate the biodiversity–invasion relationship in soil microbial communities. Ecology, 96: 915-926. DOI:10.1890/14-1001.1 |
Mattson Jr., W.J., 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Evol. Syst., 11: 119-161. DOI:10.1146/annurev.es.11.110180.001003 |
Meyerson, L.A., Mooney, H.A., 2007. Invasive alien species in an era of globalization. Front. Ecol. Environ., 5: 199-208. DOI:10.1890/1540-9295(2007)5[199:IASIAE]2.0.CO;2 |
Moreira, X., Abdala-Roberts, L., Parra-Tabla, V., et al., 2014. Positive effects of plant genotypic and species diversity on anti–herbivore defenses in a tropical tree species. PLoS One, 9: e105438. DOI:10.1371/journal.pone.0105438 |
Moreira, X., Abdala-Roberts, L., Rasmann, S., et al., 2016. Plant diversity effects on insect herbivores and their natural enemies: current thinking, recent findings, and future directions. Curr. Opin. Ins. Sci., 14: 1-7. DOI:10.1016/j.cois.2015.10.003 |
Mortensen, B., Danielson, B., Harpole, W.S., et al., 2018. Herbivores safeguard plant diversity by reducing variability in dominance. J. Ecol., 106: 101-112. DOI:10.1111/1365-2745.12821 |
Mraja, A., Unsicker, S.B., Reichelt, M., et al., 2011. Plant community diversity influences allocation to direct chemical defence in Plantago lanceolata. PLoS One, 6: e28055. DOI:10.1371/journal.pone.0028055 |
Müller-Schärer, H., Schaffner, U., Steinger, T., 2004. Evolution in invasive plants: implications for biological control. Trends Ecol. Evol., 19: 417-422. DOI:10.1016/j.tree.2004.05.010 |
Ning, Z., Xie, T., Liu, Z., et al., 2019. Native herbivores enhance the resistance of an anthropogenically disturbed salt marsh to Spartina alterniflora invasion. Ecosphere, 10: e02565. DOI:10.1002/ecs2.2565 |
Oduor, A.M., 2022. Invasive plant species that experience lower herbivory pressure may evolve lower diversities of chemical defense compounds in the exotic range. Am. J. Bot., 109: 1382-1393. DOI:10.1002/ajb2.16053 |
Otway, S.J., Hector, A., Lawton, J.H., 2005. Resource dilution effects on specialist insect herbivores in a grassland biodiversity experiment. J. Anim. Ecol., 74: 234-240. DOI:10.1111/j.1365-2656.2005.00913.x |
Pearson, D.E., Potter, T., Maron, J.L., 2012. Biotic resistance: exclusion of native rodent consumers releases populations of a weak invader. J. Ecol., 100: 1383-1390. DOI:10.1111/j.1365-2745.2012.02025.x |
Pellissier, L., Descombes, P., Hagen, O., et al., 2018. Growth–competition–herbivore resistance trade–offs and the responses of alpine plant communities to climate change. Funct. Ecol., 32: 1693-1703. DOI:10.1111/1365-2435.13075 |
Pham, T.A., Hwang, S.-Y., 2020. High temperatures reduce nutrients and defense compounds against generalist Spodoptera litura F. in Rorippa dubia. Arthropod-plant inte., 14: 333-344. DOI:10.1007/s11829-020-09750-z |
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., R Core Team, 2020. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-148 [WWW document] URL. https://CRAN.R-project.org/package=nlme. (Accessed 13 May 2020).
|
Prieur-Richard, A.H., Lavorel, S., Linhart, Y.B., et al., 2002. Plant diversity, herbivory and resistance of a plant community to invasion in Mediterranean annual communities. Oecologia, 130: 96-104. DOI:10.1007/s004420100774 |
R Core Team, 2020. R: A Language and Environment for Statisticalcomputing [WWW document] URL. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. (Accessed 22 June 2020).
|
Romanuk, T.N., Zhou, Y., Brose, U., et al., 2009. Predicting invasion success in complex ecological networks. Philos. Trans. R. Soc. B-Biol. Sci., 364: 1743-1754. DOI:10.1098/rstb.2008.0286 |
Root, R.B., 1973. Organization of a plant–arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol. Monogr., 43: 95-124. DOI:10.2307/1942161 |
Seabloom, E.W., Borer, E.T., Buckley, Y.M., et al., 2015. Plant species' origin predicts dominance and response to nutrient enrichment and herbivores in global grasslands. Nat. Commun., 6: 7710. DOI:10.1038/ncomms8710 |
Seebens, H., Blackburn, T.M., Dyer, E.E., et al., 2017. No saturation in the accumulation of alien species worldwide. Nat. Commun., 8: 1-9. DOI:10.1038/s41467-016-0009-6 |
Shan, L., Oduor, A.M., Huang, W., et al., 2023. Nutrient enrichment promotes invasion success of alien plants via increased growth and suppression of chemical defense. Ecol. Appl.: e2791. |
Shan, L., Song, C., Zhang, X., et al., 2018. Effects of long–term nitrogen and phosphorus addition on plant defence compounds in a freshwater wetland. Ecol. Indicat., 94: 1-6. DOI:10.1109/ei2.2018.8582309 |
Siemann, E., Rogers, W.E., 2003. Herbivory, disease, recruitment limitation, and success of alien and native tree species. Ecology, 84: 1489-1505. DOI:10.1890/0012-9658(2003)084[1489:HDRLAS]2.0.CO;2 |
Smith, N.S., Cote, I.M., 2019. Multiple drivers of contrasting diversity–invasibility relationships at fine spatial grains. Ecology, 100: e02573. DOI:10.1002/ecy.2573 |
Strauss, S.Y., Agrawal, A.A., 1999. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol., 14: 179-185. DOI:10.1016/S0169-5347(98)01576-6 |
te Beest, M., Mpandza, N.J., Olff, H., 2018. Biotic resistance affects growth and reproduction, but not survival of a high-impact woody invader in African savannas. J. Veg. Sci., 29: 532-540. DOI:10.1111/jvs.12633 |
Tomasetto, F., Duncan, R.P., Hulme, P.E., 2019. Resolving the invasion paradox: pervasive scale and study dependence in the native–alien species richness relationship. Ecol. Lett., 22: 1038-1046. DOI:10.1111/ele.13261 |
Underwood, N., Inouye, B.D., Hambäck, P.A., 2014. A conceptual framework for associational effects: when do neighbors matter and how would we know?. Q. Rev. Biol., 89: 1-19. DOI:10.1086/674991 |
van Elsas, J.D., Chiurazzi, M., Mallon, C.A., et al., 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. U.S.A., 109: 1159-1164. DOI:10.1073/pnas.1109326109 |
van Kleunen, M., Dawson, W., Essl, F., et al., 2015. Global exchange and accumulation of non-native plants. Nature, 525: 100-103. DOI:10.1038/nature14910 |
van Kleunen, M., Weber, E., Fischer, M., 2010. A meta–analysis of trait differences between invasive and non–invasive plant species. Ecol. Lett., 13: 235-245. DOI:10.1111/j.1461-0248.2009.01418.x |
Wang, A., Melton, A.E., Soltis, D.E., et al., 2022. Potential distributional shifts in North America of allelopathic invasive plant species under climate change models. Plant Divers., 44: 11-19. DOI:10.1016/j.pld.2021.06.010 |
Wardle, D.A., 2001. Experimental demonstration that plant diversity reduces invasibility–evidence of a biological mechanism or a consequence of sampling effect?. Oikos, 95: 161-170. DOI:10.1034/j.1600-0706.2001.950119.x |
Xiong, W., Yu, D., Wang, Q., et al., 2008. A snail prefers native over exotic freshwater plants: implications for the enemy release hypotheses. Freshw. Biol., 53: 2256-2263. DOI:10.1111/j.1365-2427.2008.02058.x |
Yachi, S., Loreau, M., 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl. Acad. Sci. U.S.A., 96: 1463-1468. DOI:10.1073/pnas.96.4.1463 |
Yan X., Wang Z., Ma J., 2019. The Checklist of the Naturalized Plants in China. Shanghai, China: Shanghai Science and Technology Press.
|
Yang, T., Wei, Z., Friman, V.P., et al., 2017. Resource availability modulates biodiversity–invasion relationships by altering competitive interactions. Environ. Microbiol., 19: 2984-2991. DOI:10.1111/1462-2920.13708 |
Zhang, Y., Meng, H., Wang, Y., et al., 2018. Herbivory enhances the resistance of mangrove forest to cordgrass invasion. Ecology, 99: 1382-1390. DOI:10.1002/ecy.2233 |
Zhang, Z., Liu, Y., Brunel, C., et al., 2020. Evidence for Elton's diversity–invasibility hypothesis from belowground. Ecology, 101: e03187. DOI:10.1002/ecy.3187 |
Zhu, H., Wang, D., Wang, L., et al., 2012. The effects of large herbivore grazing on meadow steppe plant and insect diversity. J. Appl. Ecol., 49: 1075-1083. DOI:10.1111/j.1365-2664.2012.02195.x |
Zou, X., Xu, Z., Zou, H., et al., 2016. Glutathione S-transferase SlGSTE1 in Spodoptera litura may be associated with feeding adaptation of host plants. Insect Biochem. Mol. Biol., 70: 32-43. DOI:10.1016/j.ibmb.2015.10.005 |
Zuur A., Ieno E.N., Walker N., et al, 2009. Mixed Effects Models and Extensions in Ecology with R. New York: Springer.
|



