b. Bryology Laboratory, School of Life Sciences, East China Normal University, Shanghai 200241, China;
c. Shanghai Institute of Eco-Chongming (SIEC), 3663 Northern Zhongshan Road, Shanghai 200062, China
Species richness (i.e., the number of species) is not uniformly distributed across the surface of the Earth. In general, species richness decreases with increasing latitude (Rosenzweig, 1995). This trend, known as the latitudinal diversity gradient, has long been considered as one of the few laws in ecology (Lomolino et al., 2010), and is the most famous diversity pattern described in ecology (Rosenzweig, 1995). For terrestrial species at a global scale, the latitudinal diversity gradient has been confirmed in many major groups of organisms (e.g., vascular plants, Kreft et al., 2007; angiosperms, Qian et al., 2023b; ferns, Qian et al., 2023a; amphibians, Buckley et al., 2007; birds, Davies et al., 2007; mammals, Buckley et al., 2010), although the latitudinal diversity gradient may not be observed in some groups of organisms at a smaller taxonomic scale (Cerezer et al., 2022).
Bryophytes, which include liverworts, mosses and hornworts, originated about 500 million years ago (Morris et al., 2018). Studies on the latitudinal diversity gradient for bryophytes as a whole at a global scale is lacking. In liverworts at a global scale, Wang et al. (2017) documented liverwort species richness in each latitudinal band with 20° within each of three longitudinal segments, and found that liverwort species richness generally decreases with increasing latitude. In mosses at a global scale, Geffert et al. (2013) analyzed a set of regional moss floras and Sanbonmatsu and Spalink (2022) analyzed a different set of moss assemblages; however, neither Geffert et al. (2013) nor Sanbonmatsu and Spalink (2022) investigated moss species richness in latitudinal bands. Thus, the results of these two studies cannot be used to determine whether the latitudinal diversity gradient that has been commonly found in other major groups of organisms can be found in mosses in latitudinal bands across the world.
Here, we integrate moss distributional data from different sources and take an approach similar to Wang et al. (2017) to analyze the data based on latitudinal band. Our results show that moss species richness decreases strongly with increasing latitude, regardless of whether the globe is considered as a whole or different longitudinal segments (e.g., Old World versus New World) are considered separately.
2. Materials and methodsLatitudinally, we divided the globe into the tropics (between 23.4° N and 23.4° S) and extra-tropics; we also divided the globe into the Northern and Southern Hemispheres (i.e., two broad latitudinal sections). Longitudinally, we divided the globe into the New World and the Old World, and further divided the latter into two parts at 75° E longitude, as in Qian (2008), which resulted in three broad longitudinal segments (i.e., New World, western Old World, and eastern Old World). The combination of the two broad latitudinal sections (i.e., Northern and Southern Hemispheres) and three longitudinal segments (New World, western Old World, and eastern Old World) resulted in six geographic regions. Finally, we divided the globe into latitudinal bands starting at the equator, each band having 20° in latitude, as in Wang et al. (2017).
The primary data sources used to generate moss species lists for each latitudinal band in each of the six geographic regions are Geffert et al. (2013) and Sanbonmatsu and Spalink (2022). We assigned species in each of the operational geographic units in Geffert et al. (2013) to a latitudinal band in a geographic region based on the latitude and longitude of the centroid of the operational geographic unit. Similarly, we assigned each of the species occurrences used in Sanbonmatsu and Spalink (2022) to a latitudinal band in a geographic region based on the latitude and longitude of the species occurrence. Species lists generated based on these two data sources were supplemented with additional sources (e.g. Andean Bryophytes, http://legacy.tropicos.org/ProjectAdvSearch.aspx?projectid=21; Checklist of the bryophytes of India, Dandotiya et al., 2011; The Species Catalogue of China: Bryophytes, Jia and He, 2013). We used the package U.Taxonstand (Zhang and Qian, 2023) to standardize botanical nomenclature of mosses based on the database Bryophyte Nomenclator (Brinda and Atwood, 2023). All infra-specific names were collapsed to species. As a result, 9164 moss species were included in this study.
Area varies among the latitudinal bands at a global scale and within each of the six geographic regions, which could affect species richness. Considering that species richness is often linearly correlated with log-transformed sampling area (Rosenzweig, 1995), following previous studies (e.g., Fridley et al., 2006; Guo et al., 2021; Qian et al., 2019), we accounted for the effect of variation in sampling area on species richness by calculating species density (i.e., species richness after accounting for sampling area) for each latitudinal band. Specifically, we divided the number of species in each latitudinal band by the log10-transformed area (in square kilometer) of the latitudinal band.
3. Results 3.1. Species richness in the tropical versus extra-tropical latitudesFor the globe as a whole, species richness of mosses in the tropical latitudes (between 23.4° N and 23.4° S) was substantially higher than that in the extra-tropical latitudes (6673 versus 5479 species). When the Old and New Worlds were considered separately, moss species richness in the tropical latitudes was substantially higher than that in the extra-tropical latitudes (4655 versus 3895 species) in the Old World, but was lower in the tropics than in the extra-tropics (2725 versus 3060 species) in the New World.
3.2. Species richness and density across latitudinal gradientsMoss species richness decreased with increasing latitude in both the Northern Hemisphere and the Southern Hemisphere, regardless of whether the globe was considered as a whole or the Old and New Worlds were considered separately (Fig. 1a−c; Table 1). For example, in the Northern Hemisphere, moss species richness in the latitudinal band located at 0−20° N was about four times that in the latitudinal band located at 60−80° N (4653 versus 1228 species). When the eastern and western segments of the Old World were considered separately, the aforementioned trends held except that species richness in the latitudinal band at 40−60° N in the western Old World was slightly higher than that in the two latitudinal bands located between 0° and 40° N (Fig. 1d). When species density, which accounted for variation in area among latitudinal bands, was considered, the above-described trends held (Fig. 2; Table 1). Pearson's correlation coefficient between latitude and species richness or density is −0.99 for both the Northern and Southern Hemispheres (Table 1), indicating strong latitudinal diversity gradient in mosses across the world.
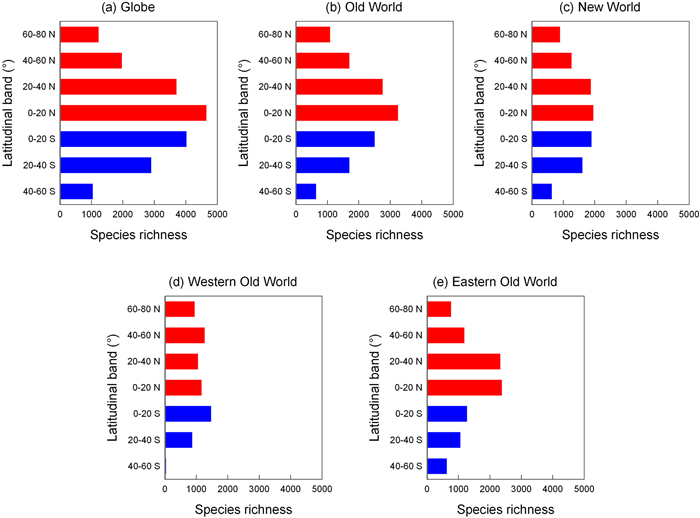
|
| Fig. 1 Species richness of mosses in each of the seven latitudinal bands in the globe (a), the Old World (b), the New World (c), the western Old World (d), and the eastern Old World (e). Each latitudinal band is 20° in width. Red and blue colors indicated latitudinal bands located in the Northern and Southern Hemispheres, respectively. |
| Geographic region | Northern Hemisphere | Southern Hemisphere | |||
| SR | SD | SR | SD | ||
| Old and New Worlds | −0.988 | −0.988 | −0.990 | −0.991 | |
| Old World | −0.990 | −0.992 | −0.997 | −1.000 | |
| New World | −0.967 | −0.972 | −0.955 | −0.940 | |
| Western Old World | −0.403 | −0.143 | −0.996 | −0.993 | |
| Eastern Old World | −0.949 | −0.967 | −0.981 | −1.000 | |
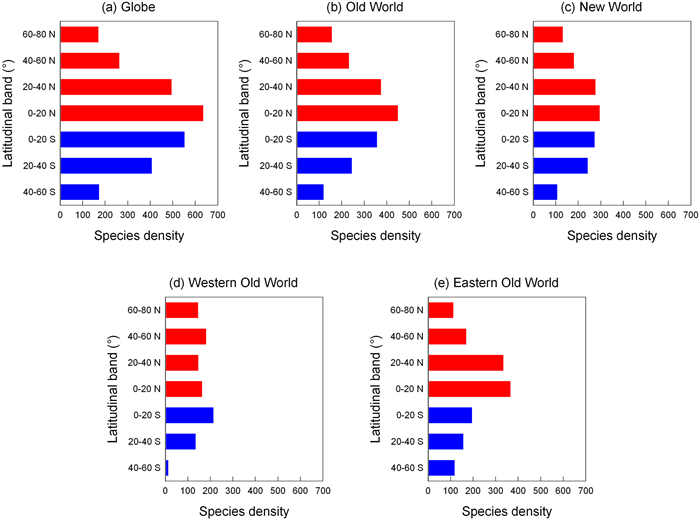
|
| Fig. 2 Species density of mosses in each of the seven latitudinal bands in the globe (a), the Old World (b), the New World (c), the western Old World (d), and the eastern Old World (e). Each latitudinal band is 20° in width. Red and blue colors indicated latitudinal bands located in the Northern and Southern Hemispheres, respectively. |
At a global scale, several studies have investigated geographic patterns of species richness of mosses (e.g., Shaw et al., 2005; Geffert et al., 2013; Möls et al., 2013; Sanbonmatsu and Spalink, 2022). While these studies have substantially helped understand geographic distributions in mosses worldwide, all of these studies analyzed species richness in regional floras, rather than species richness in latitudinal bands, and none of these studies have shown support for a clear latitudinal diversity gradient in mosses. For example, Shaw et al. (2005) showed that, when variation in size among areas is accounted for, areas in the tropics are generally not richer in moss species than areas at higher latitudes. Geffert et al. (2013) stated that they did not find a general latitudinal diversity gradient of increasing moss species with decreasing latitude. Similarly, Sanbonmatsu and Spalink (2022) stated that they were not able to find any significant relationship between moss species richness and latitude.
At a continental or semi-continental scale, several studies have reported geographic patterns of species richness in mosses, again in regional floras rather than in latitudinal bands, but a clear latitudinal diversity gradient of decreasing species richness with increasing latitude has not been found. For example, Mateo et al. (2016) found that an inverse latitudinal diversity gradient in European mosses (i.e., moss species richness is higher, rather than lower, in high latitudes than in low latitudes). Carter et al. (2022) showed that in North America, areas with the highest species richness of mosses are located in mid latitudes, not low latitudes. Chen et al. (2015) concluded that moss species richness does not change along the latitudinal gradient in China.
The present study is the first to compare moss species richness between the tropics and extra-tropics and among different latitudinal bands across the globe, within the Old World and New World separately, and within either the eastern or the western Old World. Compared with previous studies, our study used the most complete and accurate species lists. For example, at a global scale, Geffert et al.'s (2013) study used 8349 moss species based on the botanical nomenclature of Brinda and Atwood (2023), and Sanbonmatsu and Spalink's (2022) study used 3093 moss species based on the botanical nomenclature of Brinda and Atwood (2023); in contrast, the present study included 9164 moss species, which accounted for about three quarters of the total moss flora of the world (~12,000 species; Goffinet and Buck, 2004). Some of the moss species that were not included in this study are only distributed in oceanic islands, for which species distributions were not documented in the data sources used in this study.
Our study demonstrated that moss species richness is higher in the tropics (in latitudes between 23.4° N and 23.4° S) than in the extra-tropics at the global scale, even though land area in the latter (excluding the Antarctica and Greenland under the ice sheets) is 1.705 times that in the former. This result holds for the Old World, in which land area in the extra-tropics is 1.696 times as large as that in the tropics. In the New World, moss species richness in the extra-tropics is 1.123 times that in the tropics, but land area in the extra-tropics is 1.724 times as large as that in the tropics. If the land area in the tropics were as large as that in the extra-tropics in the New World, moss species richness in the tropics would undoubtedly exceed that in the extra-tropics, due to the species–area relationship (Rosenzweig, 1995).
Our study showed that in either the Northern Hemisphere or the Southern Hemisphere, moss species richness clearly decreases monotonically with increasing latitude, regardless of whether the globe is considered as a whole, or the Old and New Worlds are considered separately (Fig. 1). Although the decline of land area towards high latitudes in the Southern Hemisphere would likely have caused, to some degree, the decline of moss species richness towards high latitudes in this hemisphere, the decline of moss species richness towards high latitudes in the Northern Hemisphere cannot be attributed to the variation in land area among latitudinal bands, because a latitudinal band located at a higher latitude often has a larger land area. For example, in the Northern Hemisphere, land area in either of the latitudinal bands at 20−40° N and 40−60° N is about 1.44 times that of the latitudinal band located at 0−20° N. When species density, which accounts for the effect of variation in area size on species richness, is considered, the patterns of species density across latitudinal bands are similar to those of raw species richness, suggesting that the latitudinal diversity gradients observed in this study were not driven by variation in land area among latitudinal bands.
Of the ten latitudinal gradients shown in Fig. 1 (i.e., five panels by two hemispheres), the latitudinal gradient in the western Old World in the Northern Hemisphere is the only one that does not show a monotonic trend of declining moss species richness with increasing latitude, because species richness in the latitudinal band at 40−60° N is higher than that in the latitudinal band at either 0−20° N or 20−40° N. Lower moss species richness in these two latitudinal bands is likely because much of these two bands is located in the Sahara Desert, in which precipitation is scarce. Because mosses are poikilohydric, and their water content is directly regulated by ambient humidity (Patiño and Vanderpoorten, 2018), water availability in an area is a key factor influencing moss species richness in the area. In addition, the lack of a latitudinal diversity gradient in mosses within the western Old World in the Northern Hemisphere, and the higher moss species richness in the extra-tropics than in the tropics in the New World, may result from a skewed distribution of sampling efforts. This is due to the fact that the botanical inventorying of temperate regions, such as Europe and North America, has been carried out in a more comprehensive manner compared to that of tropical regions (Gefert et al., 2013; Patiño and Vanderpoorten, 2018).
Future work will undoubtedly increase our knowledge on species distributions. Considering that species at higher latitudes tend to have larger distributional ranges (Stevens, 1989) and thus have a greater chance to have already been documented and that moss floras in temperate latitudes have been better studied than those in tropical latitudes (Gefert et al., 2013; Patiño and Vanderpoorten, 2018), it is likely that species composition at higher latitudes has been more completely documented than that at lower latitudes in this study. Future work will likely lead to finding more new species occurrences at lower, particularly tropical, latitudes. This would enhance the steepness of the latitudinal diversity gradients that the present study has found for mosses.
In conclusion, this study shows, for the first time, strong evidence for latitudinal diversity gradients in mosses both at a global scale and at a hemisphere scale. Our results are consistent with those for liverworts (Wang et al., 2017), which are the sister lineage of mosses (Puttick et al., 2018). Considering that latitudinal diversity gradients in vascular plants in general, and angiosperms and ferns in particular, have been previously reported (Kreft et al., 2007; Qian et al., 2023a, 2023b), and that evidence for latitudinal diversity gradient for mosses is lacking until now, our study fills a critical knowledge gap.
AcknowledgementsWe thank two anonymous reviewers for their helpful comments.
Data availability statement
Moss species distribution data were obtained from the publications that were cited in this study (including https://doi.org/10.1179/1743282012Y.0000000038 and https://doi.org/10.1111/jbi.14333).
CRediT authorship contribution statement
Hong Qian: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Zun Dai: Writing – review & editing, Data curation. Jian Wang: Writing – review & editing, Data curation.
Declaration of competing interest
The authors have no competing interest to declare.
Brinda, J.C., Atwood, J.J., 2023. Bryophyte nomenclator. In: Bánki, O., Roskov, Y., Döring, M., et al. (Eds. ), Catalogue of Life Checklist (Jan 2023). https://doi.org/10.48580/dfqt-8zm.
|
Buckley, L.B., Jetz, W., 2007. Environmental and historical constraints on global patterns of amphibian richness. Proc. Roy. Soc. B-Biol. Sci., 274: 1167-1173. DOI:10.1098/rspb.2006.0436 |
Buckley, L.B., Davies, T.J., Ackerly, D.D., et al., 2010. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. Roy. Soc. B-Biol. Sci., 277: 2131-2138. DOI:10.1098/rspb.2010.0179 |
Carter, B.E., Misiewicz, T.M., Mishler, B.D., 2022. Spatial phylogenetic patterns in the North American moss flora are shaped by history and climate. J. Biogeogr., 49: 1327-1338. DOI:10.1111/jbi.14385 |
Cerezer, F.O., Machac, A., Rangel, T.F., et al., 2022. Exceptions to the rule, relative roles of time, diversification rates and regional energy in shaping the inverse latitudinal diversity gradient. Global Ecol. Biogeogr., 31: 1794-1809. DOI:10.1111/geb.13559 |
Chen, S.-B., Slik, J.W.F., Mao, L.-F., et al., 2015. Latitudinal diversity gradients in bryophytes and woody plants: the roles of temperature and water availability. J. Syst. Evol., 53: 535-545. DOI:10.1111/jse.12158 |
Dandotiya, D., Govindapyari, H., Shantanu, K., et al., 2011. Checklist of the bryophytes of India. Arch. Bryol., 88: 1-126. |
Davies, R.G., Orme, C.D.L., Storch, D., et al., 2007. Topography, energy and the global distribution of bird species richness. Proc. Roy. Soc. B-Biol. Sci., 274: 1189-1197. DOI:10.1098/rspb.2006.0061 |
Fridley, J.D., Qian, H., White, P.S., et al., 2006. Plant species invasions along the latitudinal gradient in the United States: comment. Ecology, 87: 3209-3213. DOI:10.1890/0012-9658(2006)87[3209:PSIATL]2.0.CO;2 |
Geffert, J.L., Frahm, J.P., Barthlott, W., et al., 2013. Global moss diversity: spatial and taxonomic patterns of species richness. J. Bryolog., 35: 1-11. DOI:10.1179/1743282012Y.0000000038 |
Goffinet, B., Buck, W.R., 2004. Systematics of the bryophyta (mosses): from molecules to a revised classification. Mol. Syst. Bryophyt., 98: 205-239. |
Guo, Q., Cade, B.S., Dawson, W., et al., 2021. Latitudinal patterns of alien plant invasions. J. Biogeogr., 48: 253-262. DOI:10.1111/jbi.13943 |
Jia, Y., He, S., 2013. The Species Catalogue of China: Bryophytes. Beijing: Science Press.
|
Kreft, H., Jetz, W., 2007. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. U.S.A., 104: 5925-5930. DOI:10.1073/pnas.0608361104 |
Lomolino, M.V., Riddle, B.R., Whittaker, R.J., et al., 2010. Biogeography, fourth ed. Sinauer Associates, Sunderland.
|
Mateo, R.G., Broennimann, O., Normand, S., et al., 2016. The mossy north: an inverse latitudinal diversity gradient in European bryophytes. Sci. Rep., 6: 25546. DOI:10.1038/srep25546 |
Morris, J.L., Puttick, M.N., Clark, J.W., et al., 2018. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. U.S.A., 115: E2274-E2283. |
Möls, T., Vellak, K., Vellak, A., et al., 2013. Global gradients in moss and vascular plant diversity. Biodivers. Conserv., 22: 1537-1551. DOI:10.1007/s10531-013-0492-6 |
Patiño, J., Vanderpoorten, A., 2018. Bryophyte biogeography. Crit. Rev. Plant Sci., 37: 175-209. DOI:10.1080/07352689.2018.1482444 |
Puttick, M.N., Morris, J.L., Williams, T.A., et al., 2018. The interrelationships of land plants and the nature of the ancestral embryophyte. Curr. Biol., 28: 733-745. |
Qian, H., 2008. Effects of historical and contemporary factors on global patterns in avian species richness. J. Biogeogr., 35: 1362-1373. DOI:10.1111/j.1365-2699.2008.01901.x |
Qian, H., Deng, T., Jin, Y., et al., 2019. Phylogenetic dispersion and diversity in regional assemblages of seed plants in China. Proc. Natl. Acad. Sci. U.S.A., 116: 23192-23201. DOI:10.1073/pnas.1822153116 |
Qian, H., Kessler, M., Zhang, J., et al., 2023a. Global patterns and climatic determinants of phylogenetic structure of regional fern floras. New Phytol., 239: 415-428. DOI:10.1111/nph.18920 |
Qian, H., Zhang, J., Jiang, M.-C., 2023b. Global patterns of taxonomic and phylogenetic diversity of flowering plants: biodiversity hotspots and coldspots. Plant Divers, 45: 265-271. |
Rosenzweig, M.L., 1995. Species Diversity in Space and Time. Cambridge: Cambridge University Press.
|
Sanbonmatsu, K.K., Spalink, D., 2022. A global analysis of mosses reveals low phylogenetic endemism and highlights the importance of long-distance dispersal. J. Biogeogr., 49: 654-667. DOI:10.1111/jbi.14333 |
Shaw, A.J., Cox, C.J., Goffinet, B., 2005. Global patterns of moss diversity: taxonomic and molecular inferences. Taxon, 54: 337-352. DOI:10.2307/25065362 |
Stevens, G.C., 1989. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat., 133: 240-256. |
Wang, J., Vanderpoorten, A., Hagborg, et al., 2017. Evidence for a latitudinal diversity gradient in liverworts and hornworts. J. Biogeogr., 44: 487-488. DOI:10.1111/jbi.12909 |
Zhang, J., Qian, H., 2023. U.Taxonstand: an R package for standardizing scientific names of plants and animals. Plant Divers., 45: 1-5. |



