b. Guangxi Key Laboratory of Forest Ecology and Conservation, College of Forestry, Guangxi University, Nanning 530004, China;
c. Key Laboratory of National Forestry and Grassland Administration on Biodiversity Conservation in Karst Mountainous Areas of Southwestern China, School of Life Sciences, Guizhou Normal University, Huaxi College Town, Gui'an District, Guiyang 550025, China
Forest carbon sequestration, a process critical to mitigating climate change, refers to the fixation of carbon dioxide from the atmosphere into glucose (via photosynthesis), which is then converted into biomass for long-term carbon storage in forests (Hyvönen et al., 2007; Pan et al., 2013; Zeng et al., 2021). Several recent studies aimed at elucidating the mechanisms underlying carbon sequestration and storage in forest ecosystems have focused on forest aboveground biomass (AGB) stocks. However, these studies mainly focused on typical tropical and temperate forests (Zheng et al., 2006; Hao et al., 2018; Aguirre Gutiérrez et al., 2019). Although some studies have been conducted on AGB stocks in subtropical forests, these studies were based on small plots or division of a large plot into many small plots in a single site (Ali et al., 2017; Li et al., 2019; Rodríguez-Hernández et al., 2021). Southern China includes both subtropical and marginal tropical regions. The forests in this region are characterized by high diversity, owing to their varied topography and climatic conditions. Despite substantial research efforts, knowledge of the specific factors that impact AGB in these forests remains limited.
Exploring the effects of environmental factors and forest attributes on AGB is essential because of their roles in regulating carbon sequestration and storage. Across a regional gradient, climate and topography strongly shape species distribution (Toledo et al., 2012; Gonmadje et al., 2017; Cai et al., 2023), and influence plant growth and AGB stocks (Chu et al., 2016; Ali et al., 2019a; Linger et al., 2020). For example, studies have shown that high annual precipitation drives AGB accumulation in Neotropical forests (Poorter et al., 2015, 2017). AGB accumulation has also been shown to be affected by elevation, although this effect varies between forests (Culmsee et al., 2010; Ensslin et al., 2015; Venter et al., 2017). Additionally, species diversity has a considerable positive effect on the productivity and AGB stocks of forests, although, the strength of this relationship varies among ecosystems (Fridley et al., 2012; Liang et al., 2016). For example, in plantation forests, species diversity was shown to be significantly positively correlated with AGB stocks (Huang et al., 2018; Feng et al., 2022), whereas in natural forests, both positive and negative relationships between these factors have been reported (Chisholm et al., 2013; Poorter et al., 2015; Ali et al., 2016; Fotis et al., 2018). In addition to taxonomic diversity, stand structure has been reported as another important factor affecting AGB (Zhang et al., 2015; Ali et al., 2016; Ullah et al., 2021). For example, Zhang and Chen (2015) found that the AGB of Canadian boreal forests increased with tree size heterogeneity through the complementarity effect. A similar result was observed in secondary subtropical forests in eastern China (Ali et al., 2016). Thus, the promotion of resource utilization through tree size differentiation in forest communities may be an important mechanism regulating AGB accumulation. Forest age also plays a vital role in forest carbon storage and sequestration (Pregitzer et al., 2004; Liu et al., 2014; Poorter et al., 2016). Notably, the size of the sample forest plot may influence the estimation of AGB, with an overestimation of AGB in small plots (Hernández-Stefanoni et al., 2018). The scale of the sample plots is another factor that may influence the relationship between species diversity and the AGB of forests (Chisholm et al., 2013). For example, Poorter et al. (2015) found that in Neotropical forests, species diversity was most strongly correlated with AGB at a small spatial scale (0.1 ha), whereas no clear relationship was found at a larger spatial scale (1 ha).
In this study, we used data from 30 forest plots (1 ha each) in seven nature reserves to characterize the effects of environmental factors, forest structure, and species diversity on aboveground biomass stocks of natural forests in the subtropical and marginal tropical zones of the Guangxi Zhuang Autonomous Region in southern China. In addition, we evaluated the potential capacity of natural forests in the study region to sequester carbon.
2. Materials and methods 2.1. Study sites and forest plotsThe research was carried out in the Guangxi Zhuang Autonomous Region (20°54'–26°23'N, 104°26'–112°03'E; hereafter, Guangxi, ) in southern China. The region lies at the southeastern edge of the Yunnan-Guizhou Plateau. The topography of the region is characterized by a high elevation in the northwest, a low elevation in the southeast, and a general inclination from southeast to northwest. The region is mountainous (62.05% of the total area), and 35% of the land is covered by karst landforms, mainly distributed in western, central and northeastern Guangxi (Fig. 1). This region lies in the transition from the middle subtropical to northern tropical zones and has a typical monsoon climate. Across the region, the mean annual temperature ranges from 17.6 ℃ to 23.8 ℃ and the mean annual precipitation (MAP) ranges from 723.9 mm to 2983.8 mm, but rainfall distribution varies seasonally and gradually decreases from east to west. The complex climatic and geological conditions of Guangxi contribute to the abundance, diversity, and spatial distribution patterns of vegetation. Natural forests in this region include tropical monsoon rainforests, evergreen broadleaf forests, mixed evergreen and deciduous broadleaf forests and karst mountain forests. Moving from east to west, moisture-loving species are gradually replaced by drought-tolerant species.

|
| Fig. 1 Distribution map of the 30 forest plots in Guangxi, China. ML, Mulun Nature Reserve (karst); SWDS, Shiwandashan Nature Reserve; DMS, Damingshan Nature Reserve; CWLS, Cenwanglaoshan Nature Reserve; JWS, Jiuwanshan Nature Reserve; DYS, Dayaoshan Nature Reserve; and HP, Huaping Nature Reserve. The numbers on the map indicate the number of plots. |
According to the methodology developed by the Center for Tropical Forest Science, Smithsonian Tropical Research Institute, 30 dynamic forest plots of 1 ha each (100 m × 100 m) were established in seven well-separated national nature reserves across Guangxi, with 3–5 plots at each site (Fig. 1). The surveyed plots spanned from northern tropical to middle subtropical climates, ranging from 106°21′ to 110°15′E and 21°50′ to 25°37′N, and from 340.0 to 1842.84 m in elevation (Table S1). Trees in each plot with a diameter at breast height (DBH) ≥ 5 cm were included in this study.
2.2. Species diversitySpecies diversity metrics included species richness and species evenness. We used the species richness (S) based on the total number of tree species per forest plot and species evenness based on Pielou evenness index (E) to quantify tree species diversity, according to the methods described by Zhang et al. (2012). The Pielou evenness index was calculated using the following equations:

|
(1) |
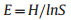
|
(2) |
where Pi is the proportional basal area of ith species in each plot. Calculations of species diversity were performed using the "vegan" R package (Oksanen et al., 2022).
2.3. Stand structural attributes and ageThe stand structural attributes used here include variations in tree size and stand basal area. Tree size variation was quantified using the coefficient of variation of tree DBH (DBH CoV), which was calculated as the ratio of the standard deviation of all DBH measurements to the mean DBH of each plot (Brassard et al., 2008; Varga et al., 2005). Stand age reflects the developmental stage of a tree community and is an important driver of biomass. In this study, the average DBH of the five largest trees in each plot was used as a proxy for stand age.
2.4. Forest aboveground biomass estimationThe AGB (Mg) of individual trees with DBH ≥ 5 cm was calculated using the allometric equation developed by Chave et al. (2015), which has been widely used in AGB estimation for tropical and subtropical forests (Ali et al., 2016; Poorter et al., 2017; Rodríguez-Hernández et al., 2021).
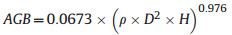
|
(3) |
where D is the stem diameter (cm), ρ is the wood density (g cm−3), and H is the height (m) of trees in a given plot. The wood densities of tree species from Shiwandashan (SWDS), Damingshan (DMS), and Mulun (ML) were determined using the water displacement method, and those of the remaining species were collected from an existing database (Chave et al., 2009; Zhang et al., 2011). In cases where the wood density of a particular species was unavailable, the genus-, family-, or plot-level mean wood density value was used instead, according to protocols established in previous studies (Jucker et al., 2018; Ali et al., 2019a). For a more accurate estimate of tree height, we measured the DBH and tree height at four sites: SWDS, DMS, ML, and Cenwanglaoshan (CWLS). Approximately 90 trees were measured according to the DBH gradient in each site. These data were used to establish a height (H)-diameter (D) model for each site (Table S2) and to estimate the height for all trees in each plot at each site. The tree heights at the remaining three sites, Dayaoshan (DYS), Huaping (HP), and Jiuwanshan (JWS), were estimated using the CWLS height (H)-diameter (D) model because all four sites have similar climatic conditions. The height (H)-diameter (D) model and AGB calculations were performed using the "BIOMASS" package in R v.4.1.3 (Réjou-Méchain et al., 2017). For comparison, the AGB data of tropical, subtropical, and Neotropical forest plots were collected from previous studies (Zheng et al., 2006; Lü et al., 2010; Lin et al., 2012; Poorter et al., 2017).
2.5. Environmental variablesDue to the great difference in precipitation between the eastern and western parts of this region, we evaluated the impact of MAP on forest AGB. Climatic data from 74 meteorological stations in Guangxi were obtained from the China Meteorological Data Service Center (http://data.cma.cn) and included daily meteorological records from 1981 to 2010. The MAP at each station was calculated and then used to estimate the MAP of each plot. The MAP of each plot was estimated based on the MAP of the 74 stations using the Kriging spatial mapping method in the "mgcv" (Wood, 2017) and "gstat" (Gräler et al., 2016) R packages. Because Guangxi is a mountainous region, elevation was also factored into the analysis.
2.6. Statistical analysesAll statistical analyses were conducted using the R software (v.4.3.2, R Core Team). Linear regression analyses were performed to examine the relationship between variables (DBH CoV and AGB, stand basal area and AGB; age proxy and DBH CoV). The multiple regression model was used to determine the relative effects of abiotic and biotic factors on AGB. All variables were standardized to a mean of 0 and standard deviation of 1 prior to multiple regression analysis. The collinearity of variables was determined using the "vif" function in the "car" R package (Fox et al., 2019). After removing the strongly multicollinear variables, the stand age proxy, MAP, elevation, species richness, and species evenness index (VIF < 2) were selected as independent variables in the multiple regression model. Finally, the relative importance of the stand age proxy, MAP, elevation, species richness, and species evenness in influencing AGB was assessed by comparing the standardized regression coefficients of each independent variable.
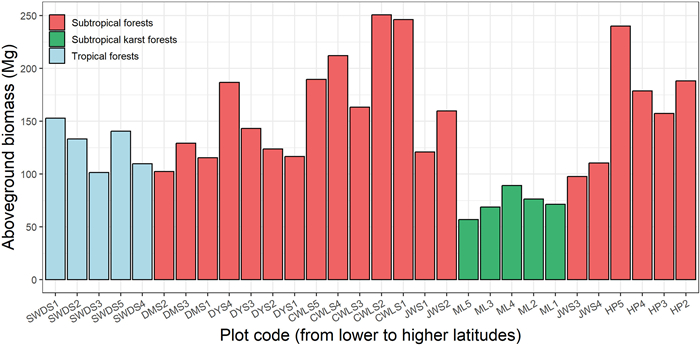
3. ResultsAcross our seven study sites, the mean AGB was the highest in the CWLS Nature Reserve (212.37 ± 16.63 Mg ha−1), followed by that in the HP Nature Reserve (191.09 ± 17.54 Mg ha−1). The mean AGB of the five plots in the SWDS Nature Reserve, which is located in the tropical–subtropical transitional zone, was 127.57 ± 9.58 Mg ha−1. The lowest mean AGB was found in the ML karst forests (72.59 ± 5.26 Mg ha−1) (Fig. 2).
|
| Fig. 2 Aboveground biomass of the 30 forest plots (1 ha each) in the seven sites. |
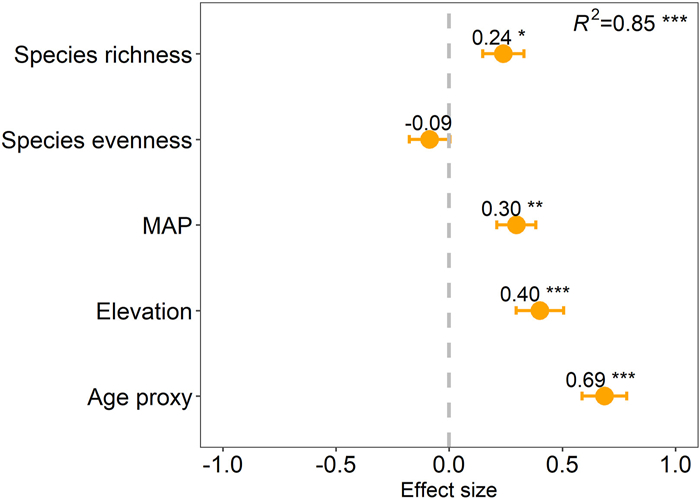
Multiple regression analysis revealed that AGB was positively correlated with the stand age proxy, elevation, MAP, and species richness (P < 0.001, P < 0.001, P < 0.01 and P < 0.05, respectively), with the stand age proxy having a relatively greater effect. However, AGB and species evenness were not correlated (P = 0.35) (Fig. 3).
|
| Fig. 3 Effect size resulting from a multiple regression among aboveground biomass, stand age proxy (mean diameter at breast height of the 5 largest trees), mean annual precipitation (MAP), elevation, species richness and species evenness for the 30 forest plots. Each variable was standardized before comparing the effect sizes of independent variables. *P < 0.05; **P < 0.01; ***P < 0.001. |
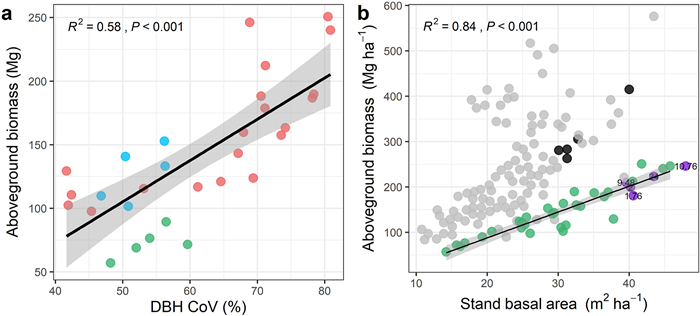
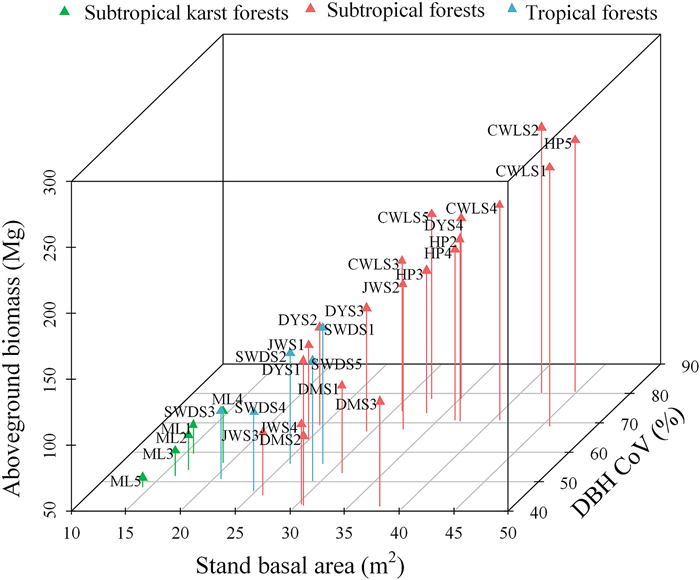
Our result showed that DBH CoV and stand basal area had a strong and significant (R2 = 0.58, P < 0.001; R2 = 0.84, P < 0.001, respectively) positive effect on AGB (Fig. 4a and b). We also found that in stands with a similar basal area, communities with a lower DBH CoV also had a lower AGB. For example, the AGB of DMS1, DMS2, DMS3, JWS3, and JWS4 was lower than that of other plots with similar basal areas (Fig. 5).
|
| Fig. 4 Relationship between aboveground biomass, (a) coefficient of variation of tree diameter at breast height (DBH CoV) (red dots indicate subtropical forests, green dots indicate subtropical karst forests, and blue dots indicate tropical forests) and (b) stand basal area across sites from different continents (the regression line is only fitted for the data of the present study; green dots). The plots from the literature that were larger than 1 ha have been marked with the plot area, and the rest are 1 ha plots; grey dots indicate Neotropical forests; purple dots indicate subtropical forests in China; and black dots indicate tropical forests in China. |
|
| Fig. 5 Three-dimensional scatter plot of stand basal area, coefficient of variation of tree diameter at breast height (DBH CoV), and aboveground biomass of the 30 forest plots. |
Our results showed that the AGB of the forest plots in this study was lower than that of some mature tropical and subtropical forests in other regions of China and Neotropical forests in the Americas. We found that AGB was positively correlated with elevation, MAP, stand structural attributes, and species richness. Overall, the old-growth forests had relatively higher AGB, whereas the karst forests had the lowest AGB, likely due to growth restriction resulting from limited soil water and nutrients. Our study addresses a gap in the existing knowledge regarding the AGB stocks of natural forests in a species-rich region of southern China.
4.1. Aboveground biomass is positively correlated with precipitation and elevationWater is required for plant metabolic processes such as photosynthesis and respiration. Therefore, water availability is an important driver of AGB (O'Brien, 2006). Our results show that MAP positively affected AGB stocks, which is consistent with the results of previous studies on tropical, subtropical, and temperate forests in China (Ali et al., 2019a; Chen et al., 2023). A large amount of annual rainfall can extend the growing season, thereby improving the tree biomass accumulation (Poorter et al., 2017). Wang et al. (2022) reported that water is the main factor driving canopy height in subtropical and tropical forests. Consequently, forests in humid climates can accumulate higher amounts of biomass. Conversely, extreme drought is likely to reduce forest biomass accumulation. Numerous studies have reported increased tree mortality owing to an increase in drought events (Brodribb et al., 2020; González et al., 2021; Bauman et al., 2022; Wang et al., 2023). The positive correlation between MAP and AGB stocks indicates that increased water availability can increase AGB in the subtropical and northern tropical regions of China. Therefore, changes in rainfall patterns resulting from climate warming are likely to have considerable impacts on forest ecosystem services in these regions.
Our results also reveal that elevation positively affects AGB stocks, i.e., the AGB of high-elevation forests tended to be higher than that of low-elevation forests, as observed in tropical forests (Alves et al., 2010). This may in part be due to the lower degree of disturbance in high-elevation forests, although it was different from the unimodal pattern observed across the elevation gradient in other studies (Ensslin et al., 2015; Phillips et al., 2019). However, in all these studies, the highest biomass was found in the middle of the elevation (around 2000 m). Therefore, the increasing pattern prior to reaching the peak in these unimodal patterns was the same as in the present study. The elevation gradient of our study plots ranged from 340.0 m to 1842.84 m. These results suggest the important role of the elevation gradient in AGB stocks and that conservation of high-elevation forests is beneficial for carbon accumulation and mitigating climate change.
4.2. Stand structural attributes, species richness, and age influence aboveground biomassOur results indicate that AGB was positively correlated with the proxy used for stand age. Stand age reflects the duration of stand biomass accumulation (Pregitzer et al., 2004), which is associated with stand structure, productivity, and biomass stocks (Michaletz et al., 2014; Matsuo et al., 2021). Our results are consistent with those of other studies, showing that mature forests store more biomass than young forests (Xu et al., 2010; Becknell et al., 2012; Liu et al., 2018). We found that AGB was relatively high in the forests of the CWLS Nature Reserve, which are well protected, primary, and mature. This finding is likely because compared to young forests, mature forests usually have larger trees and greater structural diversity (Fig. S1), resulting in greater forest biomass storage (Slik et al., 2013; Lutz et al., 2018; Ali et al., 2019b; Poulsen et al., 2020). These results suggest that stand age may be an important factor that determines the relationship between structural diversity and AGB.
A growing body of research have demonstrated that forests with higher species diversity tend to have higher productivity or AGB stocks (Poorter et al., 2015; Zhang et al., 2017; Liu et al., 2018). Consistent with these studies, we found that AGB was positively correlated with species richness. High species richness can improve ecosystem productivity or AGB stocks through niche complementarity (enhancing facilitation) or selection effects (highly productive species and high biomass species were included) (Poorter et al., 2015). However, we did not observe a significant relationship between species evenness and AGB. This could be related to the humid environment of our study region. Similarly, Chen et al. (2023) found species evenness was not correlated with AGB in subtropical humid forests. The lack of a statistically significant relationship between species evenness and AGB may be related to the limited plot data of our study. This issue should be clarified in future studies by including more forest plots.
Positive effects of stand structural diversity on AGB have been universally reported for boreal forests (Zhang and Chen, 2015), temperate forests (Aponte et al., 2020), subtropical secondary forests (Ali et al., 2016), and tropical forests (Ali et al., 2019c). Higher structural diversity enables coexisting species from different ecological niches to make full use of different resources, such as light, water, and mineral nutrients, which may promote AGB accumulation (Zhang et al., 2015). Our results also showed that the AGB increased significantly with increasing stand basal area. In addition, our results showed that in forests with the same stand basal area, those with a larger DBH CoV had larger AGB stocks. A high stand basal area can be caused by many small trees of similar size or trees of different sizes (including large and small trees) (Poorter et al., 2015). It is also possible that mature forests had higher tree stature than secondary forests (Nyirambangutse et al., 2017). This may explain why the AGB of our plots was lower than that of other forest sites with a similar stand basal area.
4.3. Natural forests in southern China exhibit a large potential carbon sequestration capacityOur results showed that the average AGB in Guangxi was 141.11 Mg ha−1. Specifically, the AGB of subtropical forests ranged from 97.60 Mg ha−1 to 250.63 Mg ha−1, with an average of 161.62 Mg ha−1. This is lower than that of some mature subtropical forests in other regions of China (Lin et al., 2012; Shen et al., 2016; Rodríguez-Hernández et al., 2021). Notably, those forests are old-growth forests, with some as old as 400 years. The average AGB of the SWDS National Nature Reserve in the tropical zone was 127.57 Mg ha−1, which is lower than those in other tropical forests in China and Neotropical forests in the Americas (Fig. 4b). The average AGB of the karst forest plots in the ML National Nature Reserve was 72.59 Mg ha−1. This karst forest has a lower AGB than the subtropical and tropical forests in this study, which may be closely related to the unique karst habitat. Trees in karst forests mostly grow on rocks or in barren soil, resulting in lower tree stature. This, in turn, affects biomass accumulation. AGB was similar to that of secondary karst forests (mean value was 80.1 Mg ha−1) in Guizhou Province (Liu et al., 2009) but much lower than that of a well-protected tropical karst forest (mean value was 247.57 Mg ha−1) in Xishuangbanna, Yunnan Province (Tang et al., 2012).
Taken together, these results indicate that the AGB of forests in Guangxi is lower than that of mature subtropical and tropical forests at other sites. One possible explanation for these results is that most of the forests examined in Guangxi were historically logged, with many large trees being felled, lowering forest structural diversity. Compared to mature forests (such as CWLS), plots of SWDS have more individuals, but fewer big trees. In addition, canopy height is also lower. Accordingly, the forests examined in this study had relatively lower biomass stocks. In a previous study, Yu et al. (2014) reported that stand age is a key factor accounting for the high carbon dioxide uptake capacity of East Asian subtropical forests because of the high level of photosynthesis required by young forests to produce biomass. When we monitored five year-interval tree growth in the SWDS plots of the present study, we found this tropical forest had higher carbon sequestration capacity (mean value was 5 Mg ha−1 year−1; Zeng et al., unpublished data) than other primarily tropical forests (1.68 Mg ha−1 year−1) (van der Sande et al., 2017). Therefore, as forests reach an old-growth age, they potentially have remarkable carbon sequestration capacity.
5. ConclusionsIn summary, our results showed that forest AGB stocks in the study region of southern China were lower than those in other tropical and subtropical forests in China and Neotropical forests in the Americas. Furthermore, we found that AGB accumulation is promoted by stand age, tree size heterogeneity, and species richness. Moreover, our study highlights that AGB stocks in this region are promoted by higher precipitation and elevation. Climate warming, accompanied by changes in rainfall patterns, is likely to have a substantial impact on forest ecosystem services in the subtropical and northern tropical regions of China. Old-growth forests exhibit higher structural diversity and biomass stocks than secondary forests. Nevertheless, many natural forests in southern China are not fully stocked, and their continued growth will increase carbon storage. Therefore, the protection of natural forests is important for mitigating climate change through carbon sequestration.
AcknowledgmentsThe establishment of the 30 1-ha forest dynamics plots was supported by the Guangxi Key R & D Program (project No. AB16380254) and a research project of Guangxi Forestry Department (Guilinkezi [2015] No.5). This study was also supported a grant for Bagui Senior Fellow (C33600992001).
CRediT authorship contribution statement
Wen-Hao Zeng: Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Shi-Dan Zhu: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Data curation, Conceptualization. Ying-Hua Luo: Supervision, Resources, Project administration, Investigation, Data curation. Wei Shi: Software, Methodology, Formal analysis, Data curation. Yong-Qiang Wang: Methodology, Investigation, Formal analysis, Data curation. Kun-Fang Cao: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2024.04.012.
Aguirre-Gutiérrez, J., Oliveras, I., Rifai, S., et al., 2019. Drier tropical forests are susceptible to functional changes in response to a long-term drought. Ecol. Lett., 22: 855-865. DOI:10.1111/ele.13243 |
Ali, A., Yan, E.R., 2017. The forest strata-dependent relationship between biodiversity and aboveground biomass within a subtropical forest. For. Ecol. Manag., 401: 125-134. DOI:10.1016/j.foreco.2017.06.056 |
Ali, A., Yan, E.R., Chen, H.Y.H., et al., 2016. Stand structural diversity rather than species diversity enhances aboveground carbon storage in secondary subtropical forests in Eastern China. Biogeosciences, 13: 4627-4635. DOI:10.5194/bg-13-4627-2016 |
Ali, A., Lin, S.L., He, J.K., et al., 2019a. Climatic water availability is the main limiting factor of biotic attributes across large-scale elevational gradients in tropical forests. Sci. Total Environ., 647: 1211-1221. DOI:10.1016/j.scitotenv.2018.08.072 |
Ali, A., Lin, S.L., He, J.K., et al., 2019b. Big-sized trees overrule remaining trees' attributes and species richness as determinants of aboveground biomass in tropical forests. Global Ecol. Biogeogr., 25: 2810-2824. DOI:10.1111/gcb.14707 |
Ali, A., Lin, S.L., He, J.K., et al., 2019c. Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. For. Ecol. Manag., 432: 823-831. DOI:10.1016/j.foreco.2018.10.024 |
Aponte, C., Kasel, S., Nitschke, C.R., et al., 2020. Structural diversity underpins carbon storage in Australian temperate forests. Global Ecol. Biogeogr., 29: 789-802. DOI:10.1111/geb.13038 |
Alves, L., Vieira, S.A., Scaranello, M.A., et al., 2010. Forest structure and live aboveground biomass variation along an elevational gradient of tropical Atlantic moist forest (Brazil). For. Ecol. Manag., 260: 679-691. DOI:10.1016/j.foreco.2010.05.023 |
Bauman, D., Fortunel, C., Delhaye, G., et al., 2022. Tropical tree mortality has increased with rising atmospheric water stress. Nature, 608: 528-533. DOI:10.1038/s41586-022-04737-7 |
Becknell, J.M., Kissing Kucek, L., Powers, J.S., 2012. Aboveground biomass in mature and secondary seasonally dry tropical forests: a literature review and global synthesis. For. Ecol. Manag., 276: 88-95. DOI:10.1016/j.foreco.2012.03.033 |
Brassard, B.W., Chen, H.Y.H., Wang, J.R., et al., 2008. Effects of time since stand-replacing fire and overstory composition on live-tree structural diversity in the boreal forest of central Canada. Can. J. For. Res., 38: 52-62. DOI:10.1139/X07-125 |
Brodribb, T.J., Powers, J., Cochard, H., et al., 2020. Hanging by a thread? Forests and drought. Science, 368: 261-266. DOI:10.1126/science.aat7631 |
Chave, J., Coomes, D., Jansen, S., et al., 2009. Towards a worldwide wood economics spectrum. Ecol. Lett., 12: 351-366. DOI:10.1111/j.1461-0248.2009.01285.x |
Chisholm, R.A., Muller-Landau, H.C., Rahman, K.A., et al., 2013. Scale-dependent relationships between tree species richness and ecosystem function in forests. J. Ecol., 101: 1214-1224. DOI:10.1111/1365-2745.12132 |
Chave, J., Réjou-Méchain, M., Búrquez, A., et al., 2015. Improved allometric models to estimate the aboveground biomass of tropical trees. Global Change Biol., 20: 3177-3190. |
Chu, C.J., Bartlett, M., Wang, Y.S., et al., 2016. Does climate directly influence NPP globally?. Global Change Biol., 22: 12-24. DOI:10.1111/gcb.13079 |
Chen, G.P., Cai, Q., Ma, S.H., et al., 2023. Climate and forest attributes influence above-ground biomass of deciduous broadleaf forests in China. J. Ecol., 111: 495-508. DOI:10.1111/1365-2745.14042 |
Culsee, H., Leuschner, Moser, G., et al., 2010. Forest aboveground biomass along an elevational transect in Sulawesi, Indonesia, and the role of Fagaceae in tropical montane rain forests. J. Biogeogr., 37: 960-974. DOI:10.1111/j.1365-2699.2009.02269.x |
Cai, Q., Ma, S.H., Sun, L.J., et al., 2023. Elevational patterns of tree species richness and forest biomass on two subtropical mountains in China. Forests, 14: 1337. DOI:10.3390/f14071337 |
Ensslin, A., Rutten, G., Pommer, U., et al., 2015. Effects of elevation and land use on the biomass of trees, shrubs and herbs at Mount Kilimanjaro. Ecosphere, 6: 45. |
Feng, Y.H., Schmid, B., Loreau, M., et al., 2022. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science, 376: 865-868. DOI:10.1126/science.abm6363 |
Fotis, A.T., Murphy, S.J., Ricart, R.D., et al., 2018. Above-ground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol., 106: 561-570. DOI:10.1111/1365-2745.12847 |
Fridley, J.D., Grime, J.P., Huston, M.A., et al., 2012. Comment on "productivity is a poor predictor of plant species richness". Science, 335: 1441. DOI:10.1126/science.1215042 |
Fox, J., Weisberg, S., 2019. An {R} Companion to Applied Regression, third ed. Sage, Thousand Oaks CA. URL: https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
|
González, M., R., Posada, J.M., Carmona, C.P., et al., 2021. Diverging functional strategies but high sensitivity to an extreme drought in tropical dry forests. Ecol. Lett., 24: 451-463. DOI:10.1111/ele.13659 |
Gräler, B., Pebesma, E., Heuvelink, G., 2016. Spatio-Temporal Interpolation using gstat. R. J., 8: 204-218. DOI:10.32614/rj-2016-014 |
Gonmadje, C., Picard, N., Gourlet-Fleury, S., et al., 2017. Altitudinal filtering of large-tree species explains above-ground biomass variation in an Atlantic Central African rain forest. J. Trop. Ecol., 33: 143-154. DOI:10.1017/S0266467416000602 |
Hao, M.H., Zhang, C.Y., Zhao, X.H., et al., 2018. Functional and phylogenetic diversity determine woody productivity in a temperate forest. Ecol. Evol., 8: 2395-2406. DOI:10.1002/ece3.3857 |
Huang, Y.Y., Chen, Y.X., Castro-Izaguirre, N., et al., 2018. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science, 362: 80-83. DOI:10.1126/science.aat6405 |
Hernández-Stefanoni, J.L., Reyes-Palomeque, R., Castillo-Santiago, M.J., et al., 2018. Effects of sample plot size and GPS location errors on aboveground biomass estimates from LiDAR in tropical dry forests. Rem. Sens., 10: 1586. DOI:10.3390/rs10101586 |
Hyvönen, R., Ågren, G.I., Linder, S., et al., 2007. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol., 173: 463-480. DOI:10.1111/j.1469-8137.2007.01967.x |
Jucker, T., Bongalov, B., Burslem, D.F.R.P., et al., 2018. Topography shapes the structure, composition and function of tropical forest landscapes. Ecol. Lett., 21: 989-1000. DOI:10.1111/ele.12964 |
Lü, X.T., Yin, J.X., Jepsen, M.R., et al., 2010. Ecosystem carbon storage and partitioning in a tropical seasonal forest in Southwestern China. For. Ecol. Manag., 260: 1798-1803. DOI:10.1016/j.foreco.2010.08.024 |
Liang, J.J., Crowther, T.W., Picard, N., et al., 2016. Positive biodiversity-productivity relationship predominant in global forests. Science, 354: aaf8957. DOI:10.1126/science.aaf8957 |
Lin, D.M., Lai, J.S., Muller-Landau, H.C., et al., 2012. Topographic variation in aboveground biomass in a subtropical evergreen Broad-Leaved Forest in China. PLoS One, 7: e48244. DOI:10.1371/journal.pone.0048244 |
Linger, E., Hogan, J.A., Cao, M., et al., 2020. Precipitation influences on the net primary productivity of a tropical seasonal rainforest in Southwest China: a 9-year case study. For. Ecol. Manag., 467: 118153. DOI:10.1016/j.foreco.2020.118153 |
Liu, Y.C., Yu, G.R., Wang, Q.F., et al., 2014. How temperature, precipitation and stand age control the biomass carbon density of global mature forests. Global Ecol. Biogeogr., 23: 323-333. DOI:10.1111/geb.12113 |
Liu, X.J., Trogisch, S., He, J.S., et al., 2018. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. R. Soc. B-Biol. Sci., 285: 20181240. DOI:10.1098/rspb.2018.1240 |
Liu, C.C., Fen, W.Y., Liu, Y.G., et al., 2009. Biomass of canopy and shrub layers of karst forests in Puding, Guizhou, China. Chin. J. Plant Ecol., 33: 698-705. |
Li, Y., Bao, W.K., Bongeres, F., et al., 2019. Drivers of tree carbon storage in subtropical forests. Sci. Total Environ., 654: 684-693. DOI:10.1016/j.scitotenv.2018.11.024 |
Lutz, J.A., Furniss, T.J., Johnson, D.J., et al., 2018. Global importance of large-diameter trees. Global Ecol. Biogeogr., 27: 849-864. DOI:10.1111/geb.12747 |
Matsuo, T., Martínez-Ramos, M., Bongers, F., et al., 2021. Forest structure drives changes in light heterogeneity during tropical secondary forest succession. J. Ecol., 109: 2871-2884. DOI:10.1111/1365-2745.13680 |
Michaletz, S.T., Cheng, D.L., Kerkhoff, A.J., et al., 2014. Convergence of terrestrial plant production across global climate gradients. Nature, 512: 39-43. DOI:10.1038/nature13470 |
Nyirambangust, B., Zibera, E., Uwizeye, F.K., et al., 2017. Carbon stocks and dynamics at different successional stages in an Afromontane tropical forest. Biogeosciences, 14: 1285-1303. DOI:10.5194/bg-14-1285-2017 |
O'Brien, E.M., 2006. Biological relativity to water-energy dynamics. J. Biogeogr., 33: 1868-1888. DOI:10.1111/j.1365-2699.2006.01534.x |
Oksanen, J., Simpson, G.L., Blanchet, F.G., et al., 2022. Vegan: community ecology package. R package version 2.6. https://CRAN.R-project.org/package=vegan.
|
Pan, Y., Birdsey, R.A., Phillips, O.L., Jackson, R.B., 2013. The structure, distribution, and biomass of the world's forests. Annu. Rev. Ecol. Evol. Syst., 44: 593-622. DOI:10.1146/annurev-ecolsys-110512-135914 |
Poorter, L., Bongers, F., Aide, T.M., et al., 2016. Biomass resilience of Neotropical secondary forests. Nature, 530: 211-214. DOI:10.1038/nature16512 |
Poorter, L., Van der Sande, M.T., Arets, E.J.M.M., et al., 2017. Biodiversity and climate determine the functioning of Neotropical forests. Global Ecol. Biogeogr., 26: 1423-1434. DOI:10.1111/geb.12668 |
Poorter, L., Sande, M.T., Thompson, J., et al., 2015. Diversity enhances carbon storage in tropical forests. Global Ecol. Biogeogr., 24: 1314-1328. DOI:10.1111/geb.12364 |
Poulsen, J.R., Medjibe, V.P., White, L.J.T., et al., 2020. Old growth Afrotropical forests critical for maintaining forest Carbon. Global Ecol. Biogeogr., 29: 1785-1798. DOI:10.1111/geb.13150 |
Pregitzer, K.S., Euskirchen, E.S., 2004. Carbon cycling and storage in world forests: biome patterns related to forest age. Global Change Biol., 10: 2052-2077. DOI:10.1111/j.1365-2486.2004.00866.x |
Phillips, J., Ramirez, S., Wayson, C., 2019. Differences in carbon stocks along an elevational gradient in tropical mountain forests of Colombia. Biotropica, 51: 490-499. DOI:10.1111/btp.12675 |
Réjou-Méchain, M., Tanguy, A., Piponiot, C., et al., 2017. Biomass: anr package for estimating above-ground biomass and its uncertainty in tropical forests. Methods Ecol. Evol., 8: 1163-1167. DOI:10.1111/2041-210X.12753 |
Rodríguez-Hernández, D.I., Deane, D.C., Wang, W.T., et al., 2021. Direct effects of selection on aboveground biomass contrast with indirect structure-mediated effects of complementarity in a subtropical forest. Oecologia, 196: 249-261. DOI:10.1007/s00442-021-04915-w |
Shen, Y., Yu, S.X., Lian, J.Y., et al., 2016. Tree aboveground carbon storage correlates with environmental gradients and functional diversity in a tropical forest. Sci. Rep., 6: 25304. DOI:10.1038/srep25304 |
Slik, J.W.F., Paoli, G., McGuire, K., et al., 2013. Large trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Global Ecol. Biogeogr., 22: 1261-1271. DOI:10.1111/geb.12092 |
Toledo, M., Peña-Claros, M., Bongers, F., et al., 2012. Distribution patterns of tropical woody species in response to climatic and edaphic gradients. J. Ecol., 100: 253-263. DOI:10.1111/j.1365-2745.2011.01890.x |
Tang, J.W., Yin, J.X., Qi, J.F., et al., 2012. Ecosystem carbon storage of tropical forests over limestone in Xishuangbannan, SW China. J. Trop. For. Sci., 24: 399-407. |
Ullah, F., Gilani, H., Sanaei, A., et al., 2021. Stand structure determines aboveground biomass across temperate forest types and species mixture along a local-scale elevational gradient. For. Ecol. Manag., 486: 118984. DOI:10.1016/j.foreco.2021.118984 |
Varga, P., Chen, H.Y., Klinka, K., 2005. Tree-size diversity between single - and mixed - species stands in three forest types in western Canada. Can. J. For. Res., 35: 593-601. DOI:10.1139/x04-193 |
Venter, M., Dwyer, J., Dieleman, W., et al., 2017. Optimal climate for large trees at high elevations drives patterns of biomass in remote forests of Papua New Guinea. Global Change Biol., 23: 4873-4883. DOI:10.1111/gcb.13741 |
Van der Sande, M.T., Pena-Claros, M., Ascarrunz, N., et al., 2017. Abiotic and biotic drivers of biomass change in a Neotropical forest. J. Ecol., 105: 1223-1234. DOI:10.1111/1365-2745.12756 |
Wood, S.N., 2017. Generalized Additive Models: an Introduction with R, Second ed. Chapman and Hall/CRC.
|
Wang, B.J., Fang, S.A., Wang, Y.Y., et al., 2022. The shift from energy to water limitation in local canopy height from temperate to tropical forests in China. Forests, 13: 639. DOI:10.3390/f13050639 |
Wang, Y.Q., Song, H.Q., Chen, Y.J., et al., 2023. Hydraulic determinants of drought-induced tree mortality and changes in tree abundance between two tropical forests with different water availability. Agric. For. Meteorol., 331: 109329. DOI:10.1016/j.agrformet.2023.109329 |
Xu, B., Guo, Z.D., Piao, S.L., et al., 2010. Biomass carbon stocks in China's forests between 2000 and 2050: a prediction based on forest biomass-age relationships. Sci. China Life Sci., 53: 776-783. DOI:10.1007/s11427-010-4030-4 |
Yu, G.R., Chen, Z., Piao, S.L., et al., 2014. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. U.S.A., 111: 4910-4915. DOI:10.1073/pnas.1317065111 |
Zeng, Z.Q., Tang, H., Hu, Q., Wang, S.L., et al., 2021. Tree biomass distribution patterns with a forest succession in subtropical China. Agron. J., 113: 706-710. DOI:10.1002/agj2.20406 |
Zhang, S.B., Slik, J.W.F., Zhang, J.L., et al., 2011. Spatial patterns of wood traits in China are controlled by phylogeny and the environment. Global Ecol. Biogeogr., 20: 241-250. DOI:10.1111/j.1466-8238.2010.00582.x |
Zhang, Y., Chen, H.Y.H., Reich, P.B., 2012. Forest productivity increases with evenness, species richness and trait variation: a global meta-analysis. J. Ecol., 100: 742-749. DOI:10.1111/j.1365-2745.2011.01944.x |
Zhang, Y., Chen, H.Y.H., 2015. Individual size inequality links forest diversity and above-ground biomass. J. Ecol., 103: 1245-1252. DOI:10.1111/1365-2745.12425 |
Zhang, Y., Chen, H.Y.H., Taylor, A.R., 2017. Positive species diversity and above-ground biomass relationships are ubiquitous across forest strata despite interference from overstorey trees. Funct. Ecol., 31: 419-426. DOI:10.1111/1365-2435.12699 |
Zheng, Z., Feng, Z.L., Cao, M., et al., 2006. Forest structure and biomass of a tropical seasonal rain forest in Xishuangbanna, southwest China. Biotropica, 38: 318-327. DOI:10.1111/j.1744-7429.2006.00148.x |



