b. CAS Center for Excellence in Biotic Interactions, University of Chinese Academy of Sciences, Beijing 100049, China;
c. State Key Laboratory of Plant Diversity and Specialty Crops, Beijing 100093, China
Maize (Zea mays L.) is one of the most important cereal crops and is widely cultivated worldwide. However, the production of maize is threatened by insect pests, causing substantial annual yield losses (16–25%) (Deutsch et al., 2018). During the coevolution with insects, maize evolved to have various specialized metabolites as defenses (Erb and Kliebenstein, 2020), including benzoxazinoids (Bxs) and phenolic compounds as direct defenses (Wouters et al., 2016; Gesteiro et al., 2021). Maize also produces many volatile compounds that function as indirect defenses, recruiting the predators or parasitoids to prey on or parasitize the larvae of insects (Kessler and Baldwin, 2001).
Accumulation of anti-insect metabolites is intricately regulated by a series of signaling pathways, including those controlled by phytohormones, such as jasmonates (JA), ethylene (ET), and salicylic acid (SA) (Howe and Jander, 2008; Erb and Reymond, 2019). The JA pathway plays a key role in regulating insect resistance across all plants, including maize. Silencing the JA signaling receptor COI1 (coronatine-insensitive 1) in the wild tobacco Nicotiana attenuata resulted in highly decreased contents of defensive metabolites and these plants were very susceptible to the lepidopteran insect Manduca sexta (Paschold et al., 2007). The Arabidopsis myc2 myc3 myc4 mutants, lacking three important basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4, which are required for the JA signaling, contained very little glucosinolates, and Spodoptera littoralis grew much larger on myc2 myc3 myc4 than on the wild-type (WT) Arabidopsis (Schweizer et al., 2013). The double mutants of the maize oxo-phytodienoate reductase genes OPR7 and OPR8, which have highly reduced JA levels, showed compromised resistance to Spodoptera exigua (Yan et al., 2012). Lipoxygenase 10 (LOX10) is one of the JA biosynthetic genes, and the maize lox10 mutants exhibited increased susceptibility to S. exigua, along with decreased release of green leaf volatiles and volatile terpenes, resulting in reduced attractiveness to the parasitoid wasp Cotesia marginiventris (Christensen et al., 2013). The maize myc2ab double mutants were highly susceptible to the insects Mythimna separata and Spodoptera frugiperda, and consistently, highly decreased levels of Bxs and volatile terpenes were detected in these maize mutants (Ma et al., 2023). In contrast, the ET pathway has contrasting effects on plant resistance to insects in different plant species (Broekgaarden et al., 2015). Knocking out ACS2 in rice, an ET biosynthetic gene, resulted in low ET levels and reduced trypsin protease inhibitor contents and thus compromised resistance to the striped stem borer (Chilo suppressalis); however, these plants were resistant to the brown planthopper (Nilaparvata lugens) due to increased volatile emission (Lu et al., 2014). In maize, ET signaling is a negative regulator of the anti-insect Bxs 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) and DIMBOA-glucoside (DIMBOA-Glc) (Zhang et al., 2021).
Multiple lines of evidence revealed that SA is also involved in plant resistance to herbivorous insects, and the SA pathway may crosstalk with the JA signaling (Wu and Baldwin, 2010; Aerts et al., 2021). Potato aphids (Macrosiphum euphorbiae) survived longer on transgenic tomato (Solanum lycopersicum) plants, which ectopically expressed the NahG (salicylate hydroxylase) gene, than on the WT plants (Li et al., 2006). NPR1 is the SA receptor (Fu et al., 2012; Wu et al., 2012; Ding et al., 2018). However, the aphids Myzus persicae and Brevicoryne brassicae grew better on the WT Arabidopsis plants than on the npr1 mutants (Mewis et al., 2006). Costarelli et al. (2020) revealed that in the SA-deficient Arabidopsis NahG plants, the JA levels were higher than in the WT plants after 6 h of Eurydema oleracea feeding, indicating that SA negatively regulates the accumulation of JA in Arabidopsis. Exogenously applying SA to maize roots could prime leaves for enhanced insect elicitor-induced JA levels and increased defensive volatile emission in leaves (Engelberth et al., 2011). Much research is still needed to understand the roles of SA in plant defense against insects.
Among JA, ET, and SA, although the defensive roles of JA and ET have been studied (Yan et al., 2012; Christensen et al., 2013; Zhou et al., 2015; Zhang et al., 2021; Ma et al., 2023), yet very little is known about whether and how SA regulates maize resistance to insects. In this study, we expressed the NahG gene in maize and it was found that these NahG-expressing maize plants exhibited compromised insect resistance compared with that of the WT maize plants. Furthermore, we show that after simulated insect herbivory treatment, the NahG maize lines had normal levels of JA and JA-Ile but decreased contents of SA and ET, and the NahG plants exhibited decreased levels of defensive metabolites Bxs and the phenolic compound chlorogenic acid (CA). These results provide new insight into the function of SA signaling pathway in regulating maize direct defenses.
2. Materials and methods 2.1. Plant materialsMaize (Zea mays L.) inbred line KN5585 was used in all experiments. To create the NahG transgenic maize, the salicylate hydroxylase gene (NahG) from the bacterium Pseudomonas putida was cloned and inserted into pCAMBIA3301, under the constitutive promoter cauliflower mosaic virus CaMV 35S, forming pCAMBIA1301-NahG. The plasmid was subsequently transformed into maize using an Agrobacterium tumefaciens-mediated method by the Weimi Biotechnology Company (http://www.wimibio.com/). Basta-resistant positive transformants were screened and further confirmed by sequencing the PCR products of the Basta-resistance gene and NahG gene in the T-DNA region (all primers used are listed in Table S1). Finally, two independent T3 homozygous lines (L4 and L8) were selected and used for all experiments.
2.2. Plant growth and treatmentMaize seeds were germinated directly in 18-cm diameter plastic pots filled with commercial potting soil (Pindstrup, http://www.pindstrup.com). Seedling growth was maintained in a greenhouse under natural light conditions at 25 ± 4 ℃ during the day and 20 ± 4 ℃ at night. Plants at the V3 stage (the third leaves fully expanded from the whorl) were used for the experiments.
Oral secretions (OS) were collected from the third-to fifth-instar Spodoptera litura larvae reared on maize leaves. The larvae were gently squeezed to provoke regurgitation, and the OS were collected with a pipette. The OS were then centrifuged at 10,000 g at 4 ℃ for 10 min and the supernatants were aliquoted and stored at -80 ℃ for future use. To simulate insect feeding on plants, the third leaves were wounded with a pattern wheel along the midrib and immediately 20 μl of S. litura OS were gently rubbed to wounds (WOS treatment). For comparisons, 20 μl of water was applied to the puncture wounds (WW treatment). The treated local leaves were harvested at the indicated times, and the untreated plants served as controls.
For SA, MeJA (methyl jasmonate), or ET treatment, plants were placed in 102-l plastic boxes and thoroughly sprayed with 1 mM SA, 1 mM MeJA, or 1 mM ethephon (all chemicals were dissolved in water containing 0.015% (v/v) Silwet-L77), and the boxes were immediately closed. Water containing 0.015% (v/v) Silwet-L77 was used for mock treatments. Samples were harvested after 48 h.
2.3. Phytohormone extraction and quantificationLeaf samples were harvested in liquid nitrogen and pulverized in liquid nitrogen. Each sample (~150 mg) was mixed with 1 ml of pre-cooled ethyl acetate supplemented with 20 ng of D6-JA (HPC Standards GmbH, Germany), 5 ng of 13C6-JA-Ile (HPC Standards GmbH, Germany), and 5 ng of D4-SA (Olchemim, Czech Republic) as the internal standards. After vortexing for 10 min, the samples were centrifuged at 13,000 g for 15 min at 4 ℃. The supernatants were carefully transferred to new 2 ml microfuge tubes and then completely dried at 30 ℃ in a vacuum concentrator (Eppendorf). Each of the dried pellet was suspended in 0.2 ml of 50% methanol by vigorous vortexing (2000 rpm) for 10 min. After centrifugation at 13,000 g for 15 min at 4 ℃, 0.1 ml of the supernatants were transferred to glass vials with inserts, which were analyzed on an HPLC-MS/MS system (LCMS-8040 system, Shimadzu) installed with a Shim-pack XR-ODS III column (2.0 mm I.D. × 75 mm L., 1.6 μm, Shim-pack). A gradient mode was employed: mobile phase consisted of solvent A (0.05% formic acid and 5 mM ammonium formate in water) and solvent B (methanol). Gradient elution was carried out at 20%–95% B for over 6.5 min, keeping at 95% B for 1.5 min, and then re-equilibration at 80% A for 2 min. Detection was under a negative electrospray ionization mode. For each endogenous phytohormone or internal standard, a specific parent ion with a unique mass-to-charge (m/z) ratio was selected and fragmented, generating daughter ions. The chromatogram for each compound was obtained based on the specific daughter ions. The peak areas of the internal standards and target compounds were measured, and the concentration of each phytohormone was determined by comparing its peak area with that of its respective internal standard.
For ET measurement, two treated or untreated maize leaves were immediately enclosed in 20-ml glass vials. After 24 h, 0.3 ml of the air in each vial was sampled and injected into a gas chromatograph system (GC-2014, Shimadzu) installed with a flame ionization detector and an HP-AL/S column (30.0 m long, 0.53 mm diameter, 15.0 μm film thickness, Agilent Technologies). The carrier gas was nitrogen at a flow rate of 7 ml/min. The injector temperature was set to 180 ℃, and the column temperature was constantly at 40 ℃. ET at different concentrations was used to create a standard curve, which was used to calculate the concentrations of ET in the samples.
2.4. Quantification of secondary metabolitesLeaf samples were pulverized with liquid nitrogen. For extraction of Bxs and CA, approximately 100 mg of each ground tissue sample was mixed with 1 ml of extraction solution (70% methanol, 0.5% formic acid, 1 μg of internal standard 4-methylumbelliferone). After vortexing for 10 min and sonicated for 10 min, samples were centrifuged at 15,000 g for 10 min, and 400 μl of the supernatants were transferred to 2-ml HPLC glass vials for analysis on an HPLC-MS/MS system (LCMS8040, Shimazu). The HPLC column was a Shim-pack XR-ODS column (2.0 mm I.D. × 75 mm L., 1.6 μm). Gradient elution was carried out at 5%–20% B (0.05% formic acid in acetonitrile (v/v)) over 3.0 min, followed by 20%–98% B over 3.0 min, keeping at 98% B for 1 min, and then re-equilibration at 95% A (0.05% formic acid in water (v/v)) for 3.0 min. The flow rate was set at 0.3 ml/min. The column temperature was maintained at 40 ℃. The injection volume was 2 μl. The triple quadrupole mass spectrometer was equipped with an electrospray ionization (ESI) source in the positive mode and was configured to operate in Q3 SIM or MRM mode. The identities of DIMBOA-Glc, HDMBOA-Glc, HDM2BOA-Glc, DIM2BOA-Glc, and MBOA were confirmed using purified standards provided by Prof. Matthias Erb (University of Bern). The other Bxs were putatively identified based on their molecular masses and by comparing the chromatographic profiles of Bxs between the inbred line W22 maize (the WT background of the bx2: : Ds mutant) and the bx2: : Ds mutant (Zm00001d048710), which showed significantly reduced levels of Bxs. Each Bx's relative content was determined by quantifying its peak area and normalizing it with the peak area of internal standard. To quantify CA, CA at different concentrations was used to create a standard curve, which was used to extrapolate the concentrations of CA in the samples. The detailed mass spectrometry conditions can be found in Table S2.
To analyze the volatile terpenes, leaf samples were ground in liquid nitrogen, and 200 mg of each sample was quickly transferred to a 2 ml glass vial and 5 μl of n-hexane containing the internal standard 1-undecanol (100 ng) was added to each sample. A solid-phase microextraction (SPME) fiber coated with 100 μm of polydimethylsiloxane (Supleco/57342-U) was inserted through the septum of the sample vials, which was heated at 60 ℃ for 50 min for absorption of volatiles, and then the SPME fiber was inserted into the injector of a gas chromatograph (GC) (GC-2014, Shimadzu). The GC carrier gas was nitrogen at a flow rate of 1 ml/min. The injector temperature was set to 250 ℃, and the column temperature was raised from 45 ℃ to 250 ℃ at a rate of 3 ℃/min. An SH-Rtx-5 column (30.0 m long, 0.25 mm diameter, 0.25 μm film thickness, Shimadzu) was used as the GC column. The compounds were identified according to commercially available authentic compounds.
2.5. BioassaySpodoptera litura, S. frugiperda, and M. separata eggs were purchased from Henan Jiyuan Baiyun Industry Company (http://www.keyuannpv.cn/). The eggs were kept at 25 ℃ to hatch the larvae. One clip cage enclosing 10 larvae was fixed to the third leaves of a pair of plants. For each group, 10 clip cages (from 20 replicated plants) were used. After 3, 5, and 7 days, the masses of all larvae were recorded.
To analyze the anti-insect effect of CA, 50 s-instar Spodoptera litura larvae were reared at 28 ℃ individually on an artificial diet or on the same artificial diet containing 0.25 mg/g CA, following a previously published method (Kundu et al., 2018). The diets were renewed every 2 days. Larval masses were recorded before being fed with artificial diet and each day after feeding on artificial diet consecutively for five days.
2.6. RNA extraction and RT-qPCR analysisTotal RNA was extracted using the TRIzol reagent (Invitrogen). The purity and concentrations of the RNA samples were determined on a spectrophotometer (NanoDrop2000, ThermoFisher Scientific). The integrity of the RNA samples was confirmed by gel electrophoresis. RT-qPCR analysis followed a previously published method (Zhang et al., 2021). The primers used are listed in Table S1.
2.7. RNA-seq and analysis of differentially expressed genesThree biological replicates, each of which was a pooled sample of three individual plants, were used for RNA-seq analysis. RNA sequencing was performed on a DNBSEQ-T7 platform (MGI) at a depth of 5 Gb of raw data. The clean reads were aligned to the maize KN5585 reference genome (Liu et al., 2020) using HISAT2 (Kim et al., 2015). The bam files were sorted using the SAMtools (Kaisers et al., 2015) and the transcript abundance was calculated. Differentially expressed genes (DEGs) were identified using the threshold value of Padj < 0.05 and |log2FC| ≥ 1 (FC = fold change). The Venn 2.1 (https://bioinfogp.cnb.csic.es/tools/venny) was applied to identify the overlapping DEGs between WOS-responsive and SA-dependent. To draw heatmaps, TBtools (Chen et al., 2020) was utilized. Gene Ontology (GO) enrichment analysis was conducted using online tools (https://david.ncifcrf.gov/).
2.8. Protein extraction and quantificationLeaf samples were pulverized in liquid nitrogen. For every 100 mg of leaf sample, 200 μl of ice-cold protein extraction buffer (5 mM EDTA, 5 mM EGTA, 100 mM HEPES pH 7.7, 1 mM phenylmethylsulfonyl fluoride, 10 mM DTT, 10% glycerol, proteinase inhibitor cocktail (Roche)) was added, and then mixed thoroughly by vortexing. After centrifugation twice at 13,000 g for 15 min at 4 ℃, the supernatants were transferred to fresh tubes, and the protein concentrations were determined using a Bradford assay kit (Bio-Rad) with bovine serum albumin as the standard.
2.9. Quantification of total carbon and nitrogenTo quantify the total carbon and nitrogen content, leaves were dried for two days in an oven at 100 ℃. Subsequently, the dried tissue samples were pulverized by grinding in mortars and then analyzed for total carbon and nitrogen content using an Elemental Combustion System (Elementar, vario MICRO).
2.10. Waldbauer nutritional assayAnalysis of nutritional indices was carried out using the detached third leaves following the previously published methods (Waldbauer, 1968; Rayapuram and Baldwin, 2006). The bases of the excised leaves were immediately wrapped with water-soaked cotton balls to prevent water loss, and the leaves were scanned on a scanner. Subsequently, four leaves from each genotype were placed into an individual transparent plastic box, and ten of the first instar of Spodoptera litura larvae were allowed to feed on them. When nearly half of the leaves were consumed by the insects, fresh leaves were used to replace the insect-damaged leaves. The removed old leaves were scanned, and the leaf areas were determined using ImageJ. The feces were collected. The experiment lasted for 11 days. Nutritional indices were calculated: consumption index (CI) = mc/(md × d); approximate digestibility (AD) = (mc-me)/mc; efficiency of conversion of ingested food (ECI) = md/mc; efficiency of conversion of digested food (ECD) = md/(mc-me); mc = dry mass of leaf consumed; d = days of feeding; me = dry mass of feces; and md = larval dry mass.
3. Results 3.1. NahG maize plants have increased internode lengths and leaf sizesTo investigate the function of SA in maize defense against herbivory, we developed two transgenic lines L4 and L8, in which the NahG gene was ectopically expressed under the control of a CaMV (cauliflower mosaic virus) 35S promoter. The expression of NahG gene in the L4 and L8 plants was confirmed by using semi-quantitative RT-PCR to amplify a partial NahG sequence from the cDNA samples prepared from leaves of WT, L4, and L8 plants (Fig. S1). Under our glasshouse conditions, compared to the WT plants, the second internode lengths of the NahG plants increased by 16.8%–37% (Fig. 1A and B), and the second leaf widths and lengths of the NahG plants were respectively 32.3%–41.9% (Fig. 1C) and 14.6%–23.5% increased (Fig. 1D). However, the morphological differences were no longer observed in adult plants and there were no significant differences between the NahG and WT plants in terms of agronomic traits, including the number of rows per cob (Fig. S2A), number of kernels per cob (Fig. S2B), grain weight per cob (Fig. S2C), and weight per 100 grains (Fig. S2D). These results indicate that SA plays a role in regulating maize morphological characteristics in the seedling stage.
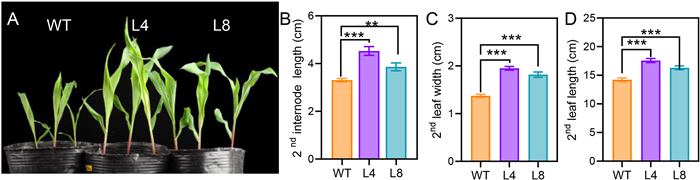
|
| Fig. 1 Morphological phenotypes of the NahG maize seedlings. (A) A photograph showing two-week-old seedlings of wild-type (WT) and NahG plants (L4 and L8). The lengths of the second internodes (B) and the widths (C) and lengths (D) of the second fully expanded leaves were measured in 15-day old seedlings. Data are means ± SE (n = 19–22). Asterisks indicate significant differences between WT and NahG lines (Student's t-test; **, P < 0.01; ***, P < 0.001). |
The generalist insect Spodoptera litura is major pest for maize in China. Next, we sought to determine whether SA is involved in regulating simulated S. litura feeding-induced phytohormones. We measured JA, JA-Ile, SA, and ET levels after WW or WOS treatments in WT, L4, and L8 plants. JA and JA-Ile contents did not show significant differences among the genotypes after any treatment (Fig. 2A and B). After WW or WOS treatment, SA levels were slightly decreased in the WT maize; importantly, compared with the WT plants, we found that the NahG maize exhibited a reduction in SA levels of 28%–80% when plants were untreated; and after the WW and WOS treatments, compared with those in the WT plants, the SA levels in the NahG maize decreased by 21% and 56% (L4 and L8, respectively) and 39% and 68% (L4 and L8, respectively) (Fig. 2C), indicating the effectiveness of NahG in degrading SA. Unexpectedly, after WOS treatment, the ET level was downregulated by 56% and 59% in the L4 and L8 lines, respectively (Fig. 2D), suggesting that the SA pathway regulates S. litura herbivory-induced ET biosynthesis.
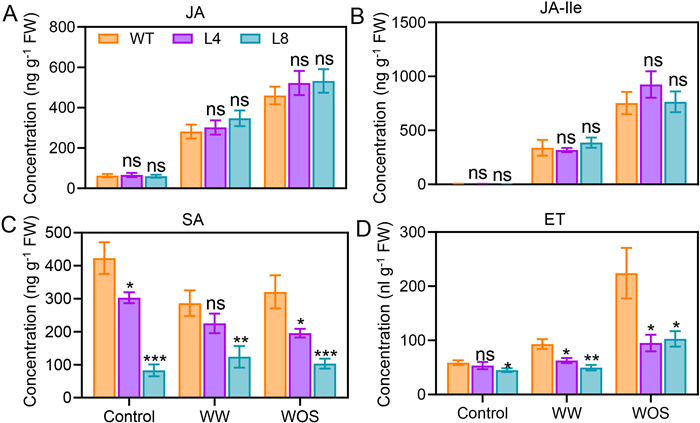
|
| Fig. 2 Contents of JA, JA-Ile, SA, and ET in WT and NahG maize plants under different conditions. WT and NahG (L4 and L8) plants were treated with mechanical wounding (WW), Spodoptera litura oral secretions (WOS), or untreated (control). After 1.5 h, leaf samples were collected for quantification of JA (A), JA-Ile (B), and SA (C). To quantify ET, leaf samples were collected and enclosed in vials for 24 h, and ET was quantified by gas chromatography (D). Data are means ± SE (n = 6–8 for (A) to (C); n = 4–6 for (D)). Asterisks indicate significant differences between the WT and transgenic NahG lines (Student's t-test; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant). |
To determine whether the SA pathway is involved in maize defense against Spodoptera litura, newly hatched larvae of S. litura were infested on the WT, L4, and L8 plants, and larval masses were recorded after feeding for 3, 5, and 7 days. On as early as on day 3, S. litura larvae that fed on the L4 plants were 35% heavier than those that fed on the WT plants. After infestation for 5 and 7 days, S. litura larvae on L4 and L8 plants were 24% and 35% (day 5) and 25% and 34% (day 7) heavier than those on the WT plants. Thus, SA positively regulates the resistance of maize to the generalist herbivore S. litura (Fig. 3A).
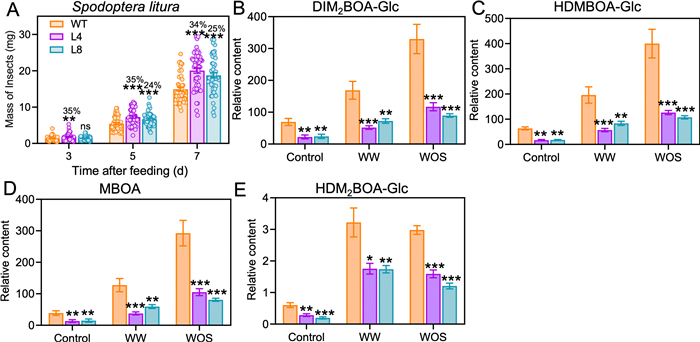
|
| Fig. 3 Spodoptera litura growth bioassay and contents of benzoxazinoids in WT and NahG maize. (A) Larval weights of S. litura on WT and NahG (L4 and L8) maize. Weights of larvae were recorded after 3, 5, and 7 days (d) of feeding. Data are means ± SE (n = 47–50). (B–E) Relative contents of major benzoxazinoids (Bxs) in the WT, L4, and L8 maize plants. Plant samples were harvested after plants were treated with mechanical wounding (WW) or S. litura oral secretions (WOS) for 48 h, and untreated plants served as controls. Data are means ± SE (n = 8). Asterisks indicate significant differences between the WT and NahG lines (Student's t-test, *, P < 0.05; **, P < 0.01; ***, P < 0.001). |
Another two insect species, the generalist Spodoptera frugiperda and the specialist Mythimna separata, were used to examine whether SA is also important for maize resistance to these insect species. We found that both insects grew larger on the NahG plants than on the WT plants (~30%) (Fig. S3). Thus, SA seems to be important in maize resistance to both generalist and specialist lepidopteran herbivores.
3.4. Maize SA pathway positively controls herbivory-induced BxsTo analyze whether the increased susceptibility of the SA-deficient lines to insects was associated with decreased levels of anti-insect metabolites, Bxs and volatile terpenes which are known to be important defense metabolites in maize were quantified. The third leaves of the WT and both NahG lines (L4 and L8) were treated with WW or WOS or untreated (as controls), and the Bx levels were measured 48 h after the treatments. In the WT maize, after WW treatment the contents of the major Bxs, DIM2BOA-Glc, HDMBOA-Glc, MBOA, and HDM2BOA-Glc, increased 1.4-, 2.1-, 2.3-, and 4.3-fold, respectively; after WOS treatment, these metabolites increased 3.7-, 5.3-, 6.5-, and 4.0-fold, respectively (Fig. 3B–E). Importantly, after WW treatment, the contents of these Bxs in the L4 and L8 were about half (46%–71%) of those in the WT plants (Fig. 3B–E), and after WOS treatment, the contents of these four Bxs in the NahG plants were also decreased (In L4 and L8 respectively, 64% and 73% for DIM2BOA-Glc, 68% and 73% for HDMBOA-Glc, 64% and 72% for MBOA, and 47%–57% for HDM2BOA-Glc) (Fig. 3B–E). The other Bxs in the L4 and L8 plants showed similar levels to those in the WT plants under all conditions (Fig. S4). Next, indirect defenses, the volatile sesquiterpenoids, including (E)-α-bergamotene, (E)-β-farnesene, and (E, E)-4,8, 12-trimethyltrideca-1,3, 7,11-tetraene (TMTT), were measured in the WT, L4, and L8 plants. Under either control or WOS conditions, sesquiterpenoids in the NahG maize were the same as those in the WT maize plants (Fig. S5). Therefore, we inferred that SA plays an important role in regulating herbivory-induced Bxs, and the compromised resistance NahG plants to S. litura was a result of decreased Bxs.
To further corroborate the role of SA in regulation of Bxs, we applied SA to the WT maize plants and determined whether SA treatment could change the accumulation of Bxs. Indeed, compared with those in the mock treatment group, foliar application of 1 mM SA upregulated most of Bxs (Fig. S6), confirming that SA positively regulates the levels of the Bxs.
3.5. NahG maize exhibits decreased herbivory-induced chlorogenic acidExcept for Bxs and volatile sesquiterpenoids, many phenolic compounds, including chlorogenic acid (CA), may have broad-spectrum effects against insect herbivores (Naveed et al., 2018). The CA levels in the WT, L4, and L8 maize were measured in samples collected 48 h after WW and WOS treatment. Under the control conditions, the levels in the L4 and L8 plants were similar to those in the WT plants. CA levels were induced 1.7- and 3.0-fold in the WT maize plants after WW and WOS treatment, respectively (Fig. 4A), while the levels of CA in L4 and L8 were 49% and 32% (WW) and 58% and 23% (WOS) less than those in the WT plants, respectively (Fig. 4A).
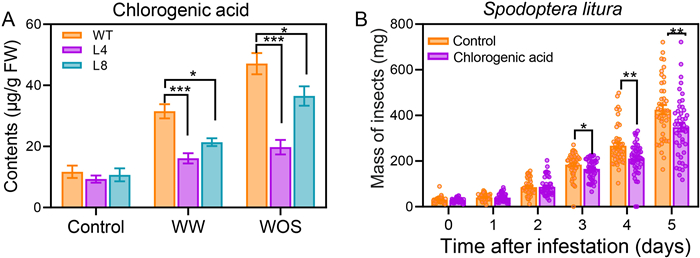
|
| Fig. 4 Contents of chlorogenic acid in WT and NahG maize and effect of chlorogenic acid on growth of Spodoptera litura. (A) Chlorogenic acid (CA) contents in WT and NahG (L4 and L8) maize leaves after WW or WOS treatment. The concentrations of CA were determined in samples harvested 48 h after treatment. (B) Larval masses of S. litura grown on normal and CA-containing artificial diet. A total of 50 neonates were infested on normal (control) artificial diet or artificial diet containing 0.25 mg/g CA. Larval masses were recorded for five consecutive days. The values are means ± SE. Asterisks indicate significant differences (Student's t-test; n = 10 for (A); n = 50 for (B); *, P < 0.05; **, P < 0.01; ***, P < 0.001). |
Whether CA is anti-Spodoptera litura has not been tested. To this end, we sought to examine if CA is toxic to S. litura. Indeed, our artificial feeding experiment showed that S. litura larvae fed on the artificial diet supplemented with 0.25 mg/g CA were respectively 10%, 18%, and 18% smaller than those fed on normal artificial diet on day 3, 4, and 5, respectively, even though no significant differences were observed on the first two days (Fig. 4B). Thus, CA is an anti-S. litura metabolite. The above results suggest that SA positively regulates maize resistance to insects at least partly by regulating the levels of CA.
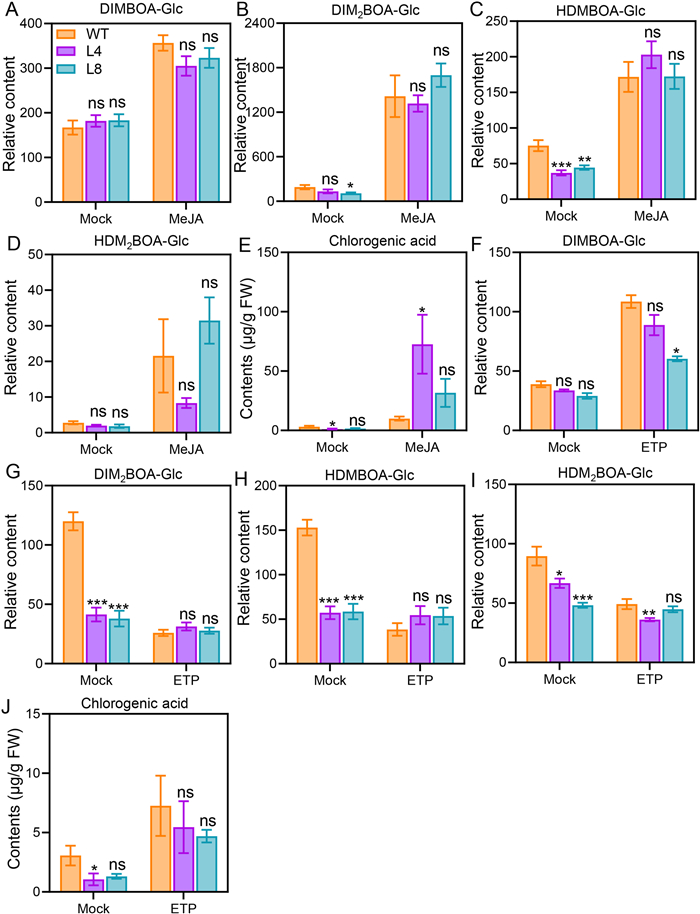
3.6. NahG plants accumulate normal levels of Bxs and CA in response to MeJA or ETIn Arabidopsis, the key transcriptional coactivator of SA signaling NPR1 binds to the important transcription factor MYC2 of the JA pathway, and thus inhibits the activity of MYC2 (Nomoto et al., 2021). Although the JA and JA-Ile levels in the NahG maize were comparable to those in the WT maize (Fig. 2A and B), SA may suppress JA signaling. To study whether the SA pathway interferes with the JA and ET pathways in regulating Bxs and CA accumulation, we applied MeJA and ET on the WT and NahG maize plants. After foliar treatment with 1 mΜ MeJA or 1 mΜ ethephon (a donor of ET) for 48 h, the contents of Bxs and CA in the WT and NahG maize were measured. It was found that most of the Bxs and CA increased in the MeJA-treated WT maize plants, but there were no differences between the NahG maize plants and the WT plants (Fig. 5A–E). In response to ET treatment, the levels of Bxs and CA in the NahG plants were similar to those in the WT plants as well (Fig. 5F–J). Collectively, our results suggested that neither the JA nor ET pathway requires SA signaling to regulate the accumulation of Bxs and CA, arguing against that the SA pathway interacts with the JA or ET pathway in maize defense against insects.
|
| Fig. 5 Relative levels of benzoxazinoids and chlorogenic acid in WT and NahG plants after MeJA or ET treatment. Relative contents of benzoxazinoids (Bxs) (A–D) and concentrations of chlorogenic acid (E) in WT and NahG maize plants (L4 and L8) after MeJA treatment. Effect of exogenously applied ethephon (ETP) on relative contents of Bxs (F–I) and concentrations of chlorogenic acid (J) in WT and NahG plants. WT and NahG maize plants were enclosed in 102-l plastic boxes and 1 mM MeJA or 1 mM ETP was sprayed on the leaves. Samples were harvested 48 h after treatment. Data are means ± SE (n = 10 for (A–D) and (F–I); n = 6 for (E) and (J). Asterisks indicate significant differences between the WT and transgenic NahG lines (Student's t-test; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant). |
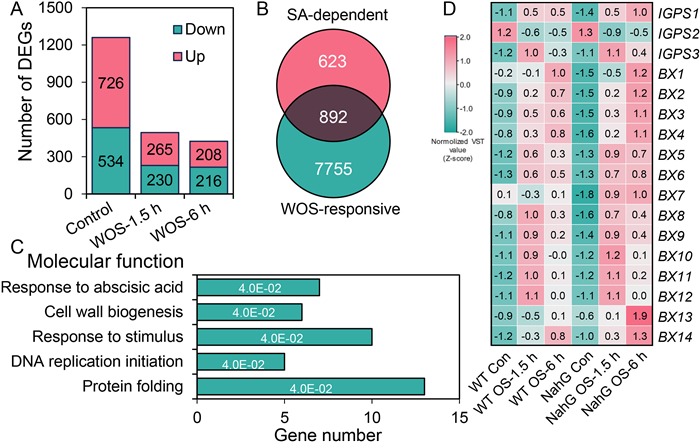
To gain further insight into the function of SA in regulating herbivory-induced responses, WT maize plants and one of the NahG lines (L4) were treated with WOS. After 1.5 and 6 h, the treated leaves, including control samples, were collected and used for RNA sequencing (three biological replicates for each group of samples). Comparing the transcriptomes of L4 maize with those of WT maize plants under control and WOS conditions, 1260 (726 up- and 534 down-regulated) differentially expressed genes (DEGs) were identified between L4 and WT plants under the control conditions, and the numbers of DEGs decreased to 495 (265 up- and 230 down-regulated) and 424 (208 up- and 216 down-regulated) after 1 and 6 h of WOS treatment, respectively (Fig. 6A and Table S3). In the WT plants, a total of 8647 genes exhibited significantly different expression levels at 1.5 and 6 h in response to WOS treatment; these DEGs were designated as WOS-regulated genes (Fig. S7A and Table S4). The DEGs identified by WT and NahG plants under control and WOS treatment (1.5 and 6 h) were combined, resulting in 1515 DEGs, which were designated as the SA-dependent genes (Fig. S7B and Table S3). Venn diagram analyses of these two datasets indicated that only 10.3% (892 of 8647) of the WOS-responsive genes were regulated by SA (Fig. 6B). Gene Ontology (GO) functional analysis revealed that the 892 genes, which were SA-regulated herbivory-induced genes, were enriched in the GO terms "response to abscisic acid", "cell wall biogenesis", "response to stimulus", "DNA replication initiation", and "protein folding" (Fig. 6C and Table S5).
|
| Fig. 6 Genome-wide transcriptome analysis of WT and NahG maize plants under different treatments. The WT and NahG (L4) maize were untreated (control) or wounded with a pattern wheel and immediately 20 μl of Spodoptera litura oral secretions were applied to wounds. The samples were collected at 1.5 and 6 h for RNA-seq analysis. Transcriptome sequencing was conducted using three biological replicates, with each replicate consisting of a pool of three individual plants. (A) Overview of the numbers of DEGs between WT and L4 plants under control and WOS treatment. (B) Venn diagram showing the overlap of WOS-responsive and SA-dependent genes. The WOS-responsive genes (8647 genes) were identified by comparing WOS-treated WT plants with untreated WT plants at 1.5 and 6 h (Fig. S7A). The SA-dependent genes (1515 genes) were identified by comparing L4 and WT plants at 0, 1.5, and 6 h after WOS treatment (Fig. S7B). (C) Gene Ontology (GO) pathway analysis of 892 WOS-responsive and SA-dependent genes with FDR from the statistical overrepresentation test are shown. (D) Heatmap indicating the relative expression levels of Bx biosynthesis genes. Con, control; the variance-stabilized transformation (VST) of read counts of the genes were used for determining the relative gene expression levels. The VST values were normalized by Z-scores. |
Specifically, we inspected the DEGs in the NahG plants resulted from WOS treatment, and in total 7894 DEGs (1.5 and 6 h) were found (Fig. S8A and Table S6). Next, these genes were subjected to Venn diagram analysis with the 8647 DEGs (Fig. S6A and Table S4) regulated by WOS treatment in WT plants (Fig. S8B and Table S7): 1) 6289 genes were commonly regulated by WOS in WT and NahG plants; 2) 2358 genes were exclusively regulated in the WT plants, and the regulation of these genes by WOS are dependent on the SA pathway; 3) 1605 genes were exclusively regulated in the NahG plants, whose regulation by WOS is normally inhibited by the SA pathway. Further GO enrichment analysis of these specific DEGs in WT (2358) and NahG (1605) plants did not result in any significantly enriched pathways.
Considering the decreased levels of Bxs in the NahG plants, we also retrieved the relative expression levels of Bx biosynthesis genes from our transcriptome data. Unexpectedly, we found that most of the Bx genes were upregulated in L4 plants compared to those in the WT plants, especially at 6 h (Fig. 6D). Then, we confirmed the expression patterns of these genes using real-time quantitative PCR (RT-qPCR): the expression levels of IGPS1, IGPS3, BX1 to BX3, and BX7 to BX9 were more highly induced in L4 plants than in the WT plants at 6 h after the WOS treatment (Fig. S9). The key genes involved in CA biosynthesis were all WOS-responsive, but there were no obvious differences between the WT and L4 plants (Fig. S10). These results suggest that SA may regulate certain Bx and CA biosynthetic genes posttranscriptionally.
Given that herbivory-induced ET levels were about 54%–57% lower in the NahG plants than in the WT plants (Fig. 2D), we specifically inspected the relative expression levels of 1-aminocyclopropane-1-carboxylic acid synthases (ACSs) and 1-aminocyclopropane-1-carboxylic acid oxidases (ACOs), which encode the key enzymes of ET biosynthesis. A previous study revealed that there are three ACS and four ACO genes in the inbred maize B73 (Gallie and Young, 2004). Consistent with the ET phenotype, most of the ET biosynthesis genes were downregulated at 6 h post treatment in our RNA-seq experiments (Fig. S11). These findings suggest that the SA pathway may play a role in regulating herbivory-induced ET biosynthesis via modulation of ACS and ACO genes expression.
To investigate the downstream network of SA, we performed a further analysis on these 892 genes (Fig. 6B) to identify the transcription factors (TFs) that may influence the accumulation of Bxs and CA. Finally, 81 TFs belonging to 24 transcription factor families were identified to be WOS-responsive and SA-dependent (Fig. S12 and Table S8). Most of the TFs belonged to the ERF (16 genes), WRKY (11 genes), and bHLH (9 genes) families. Presumably, these TFs might act downstream of SA to regulate the accumulation of Bxs and CA.
3.8. NahG plants have similar nutrients as the WT plantsIn addition to anti-insect metabolites, the nutrient composition of plants, including the protein content, total N content, and C: N ratio, are crucial for larval growth (Mattson, 1980). However, the total N content or C: N ratio in the L4 and L8 plants comparable with those in the WT maize (Fig. S13A and B). Quantification of total protein contents indicated no significant differences between the WT and NahG plants in any conditions (Fig. S13C).
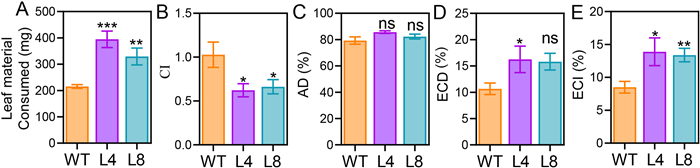
Next, a Waldbauer nutritional assay (Waldbauer, 1968; Lu et al., 2018) was conducted to analyze the effect of plant nutrients and defensive metabolites on insect growth (Table S9). In comparison to S. litura fed on the WT plants, those fed on NahG plants consumed 83% (L4) and 53% (L8) more leaf tissues (Fig. 7A). Consistently, the consumption index was about 26%–38% smaller in the L8 and L4 group than in the WT group (Fig. 7B). The approximate digestibility values were similar among the WT, L4, and L8 groups, suggesting that NahG plants had similar nutritional values as the WT plants. The efficiency of conversion of digested food and the efficiency of conversion of ingested food were higher in the NahG plant group than in the WT plant group (Fig. 7C–E), supporting that maize Bxs and CA are toxic to S. litura larvae and that these metabolites inhibited insect feeding.
|
| Fig. 7 Waldbauer nutritional assay of WT and NahG maize plants. WT and NahG (L4 and L8) maize leaves were detached and placed in plastic boxes for Spodoptera litura feeding. The trial lasted for 11 days to determine the leaf materials consumed (A), consumption index (B), approximate digestibility (C), efficiency of conversion of digested food (D), and efficiency of conversion of ingested food (E). Data are means ± SE. Asterisks indicate significant difference between WT and transgenic NahG lines (Student's t-test; n = 5–6; *, P < 0.05; **, P < 0.01; ***, P < 0.001). |
Unlike JA, which has been intensively studied for its role in plant resistance to insects (Wu and Baldwin, 2010; Erb and Reymond, 2019), the functions of SA in plant–insect interactions remain to be studied. Here, we show that in addition to being involved in maize growth and development during the seedling stage, the SA pathway also positively regulates maize resistance to caterpillars by positively modulating the accumulation of the defensive metabolites Bxs and CA.
SA may have a negative effect on plant stature. For example, the Arabidopsis mpk4 mutants, which have very high SA contents, are dwarf, and expressing NahG in the mpk4 mutants to reduce the SA levels resulted in somewhat increased plant sizes (Petersen et al., 2000). Constitutively increasing SA in Arabidopsis by expressing the two Pseudomonas aeruginosa genes pchA and pchB, which encode an isochorismate synthase and an isochorismate pyruvate-lyase, respectively, led to stunted growth and reduced seed production (Mauch et al., 2001). In another study, low SA content enhanced vegetative growth and seed yield in the SA-deficient NahG transgenic Arabidopsis plants (Abreu and Munne-Bosch, 2009). However, in some species, SA may not be involved in plant growth and development: in the wild tobacco Nicotiana attenuata, silencing MPK4 resulted in augmented SA levels and decreased rosette size and stalk length; however, crossing these MPK4-silenced plants with N. attenuata expressing NahG to minimize SA levels did not change the morphology of these MPK4-silenced plants (Hettenhausen et al., 2012), suggesting that SA may not affect the growth of N. attenuata. In the present study, the NahG maize exhibited largely increased vegetative growth during the early growth stage (Fig. 1); however, expression of NahG in maize did not affect the major yield parameters (Fig. S2). It appears that the basal level of SA in maize seedlings suppresses plant growth, but not during the later stages of maize growth and development. The underlying mechanisms require further study.
Bxs are vital metabolites required for maize defense against multiple feeding guilds (Niemeyer, 2009; Ahmad et al., 2011). We found that the SA-treated maize plants accumulated greater contents of Bxs in comparison to mock-treated plants (Fig. S6), and importantly, in response to simulated herbivory, the NahG maize lines had lower levels of Bxs than did the WT maize plants (Fig. 3B–E). These data indicate that SA is a positive regulator of Bxs in maize defense against insects. In contrast, compared with WT plants, Arabidopsis and tobacco plants expressing NahG were shown to be more resistant to the piercing-sucking insect E. oleracea (Costarelli et al., 2020; Ederli et al., 2020). When NahG was expressed in N. attenuata, no differences of insect (M. sexta) growth were detected between the WT and NahG plants (Gilardoni et al., 2011). Thus, the role of SA in plant resistance to insects is very likely plant- and insect species-specific.
However, how SA regulates maize defenses remains unclear. In Arabidopsis, SA was found to suppress JA-mediated resistance against Spodoptera exigua (Cipollini et al., 2004). Exogenous application of SA suppressed JA-induced volatiles in lima bean (Phaseolus lunatus) plants, which are repellent to spider mites Tetranychus urticae (Wei et al., 2014). Recent evidence suggests that the SA pathway does not mediate rice resistance to the piercing-sucking herbivore brown planthopper Nilaparavata lugens (Stål) (Xu et al., 2021). We found that in maize, after WOS treatment, although the SA-deficient NahG plants exhibited lower SA contents but still had normal JA and JA-Ile levels (Fig. 2A and B), indicating that SA does not change JA biosynthesis. Applying MeJA to WT and NahG maize induced similar levels of Bxs (Fig. 5A–D) and CA (Fig. 5E). Thus, SA also does not seem to interfere with the JA signaling. These data support the notion that the interaction between the SA and JA is dependent on the species of plants and the interacting biotic factors (insects/pathogens) (Wu and Baldwin, 2010; Robert-Seilaniantz et al., 2011). It is noteworthy that the Bx biosynthesis genes were more highly induced by WOS in the NahG maize than in the WT maize, and the levels of CA biosynthesis genes were similar between the WT and NahG maize. Hence, it is possible that SA signaling regulates the accumulation of Bxs and CA in a post-transcriptional manner, a hypothesis that requires further study. It would be also necessary to study whether and how NPR1, the receptor of SA, functions in maize resistance to insects.
Expressing NahG in Arabidopsis also led to decreased ET levels in response to Pseudomonas syringae pv. tomato infection (Heck et al., 2003). Similarly, our phytohormone analysis indicated that SA is required for herbivory-induced ET production, as the NahG maize produced 56%–59% less ET than did the WT maize. These data suggest that the SA and ET pathway likely interact during maize response to insect herbivory: transcriptionally the SA pathway regulates herbivory-induced expression of ACSs and ACOs (Fig. S11). However, our previous study indicated that ET signaling plays a negative role in regulating Bxs (Zhang et al., 2021); moreover, after applying ethephon to WT and NahG maize, these maize plants showed similar levels of Bxs and CA (Fig. 5F–J). Thus, it is likely that the regulatory effect of SA on Bxs is not dependent on the ET pathway.
The Waldbauer nutritional assay has been used to demonstrate the contribution of plant nutrients and defensive metabolite composition to larval growth (Waldbauer, 1968; Rayapuram and Baldwin, 2006). Even though compared with the WT plants, the NahG maize plants showed no significant differences in total protein contents, Spodoptera litura exhibited higher leaf material consumption, AD, ECD, and ECI (Fig. 7). However, the larvae showed a lower CI on NahG maize plants, which was presumably caused by a compensatory mechanism to detoxify defensive metabolites (Giertych et al., 2005; Rayapuram and Baldwin, 2006). Hence, in the NahG maize, defensive secondary metabolites rather than nitrogen or nutrients play a pivotal role in affecting larval growth. Similarly, our previous study found that in rice and tobacco plants consumed respectively by S. litura and Mythimna separata, the main defense-related metabolites are the key determining factors for the growth of these insects (Lu et al., 2018).
In addition to the JA and ET signaling (Zhang et al., 2021; Ma et al., 2023), by using NahG to manipulate SA levels in maize, this study demonstrates that the SA pathway is also involved in maize defense against lepidopteran insects, in a fashion that is independent of the JA and ET pathway. Future studies should reveal the mechanisms by which SA signaling regulates the biosynthesis of Bxs and CA, including whether NPR1 is key to SA signaling in maize and what transcription factors function downstream of the SA signaling to regulate maize defenses. If SA signaling plays a role in maize resistance to pierce-sucking insects, e.g., aphids, is also worth studying.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (U23A20199 (J.W.), 32302464 (C.M.)), the Postdoctoral Fellowship Program of CPSF (GZC20232764 (C. M.)), the Yunnan Revitalization Talent Support Program "Yunling Scholar" Project (J.W.), Yunnan Innovation Team Project (202105AE160013 (J.W.)), the Strategic Priority Research Program of Chinese Academy of Sciences (XDPB16 (J.W.)), the CAS "Light of West China" Program (J.Q.), the General and Key Project of Applied Basic Research Program of Yunnan (202201AS070053 (J.Q.)), the Special Research Assistant of Chinese Academy of Sciences (C.M.), and the Postdoctoral Directional Training Foundation of Yunnan Province (M.Z.).
Data availability
The transcriptome data can be accessed from the Beijing Institute of Genomics under the BioProject: PRJCA023481.
CRediT authorship contribution statement
Yohannes Besufekad Setotaw: Writing – original draft, Performed experiments, Formal analysis. Jing Li: Writing – original draft, Formal analysis, Performed experiments, Conceptualization, Project administration. Jinfeng Qi: Formal analysis, Funding acquisition, Formal analysis. Canrong Ma: Funding acquisition, Formal analysis. Mou Zhang: Funding acquisition, Formal analysis. Cuilian Huang: Formal analysis. Lei Wang: Formal analysis. Jianqiang Wu: Project administration, Funding acquisition, Writing – original draft, Formal analysis, Conceptualization.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2024.03.004.
Abreu, M.E., Munne-Bosch, S., 2009. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot., 60: 1261-1271. DOI:10.1093/jxb/ern363 |
Aerts, N., Pereira Mendes, M., Van Wees, S.C.M., 2021. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J., 105: 489-504. DOI:10.1111/tpj.15124 |
Ahmad, S., Veyrat, N., Gordon-Weeks, R., et al., 2011. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol., 157: 317-327. DOI:10.1104/pp.111.180224 |
Broekgaarden, C., Caarls, L., Vos, I.A., et al., 2015. Ethylene: traffic controller on hormonal crossroads to defense. Plant Physiol., 169: 2371-2379. |
Chen, C., Chen, H., Zhang, Y., et al., 2020. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant, 13: 1194-1202. DOI:10.1016/j.molp.2020.06.009 |
Christensen, S.A., Nemchenko, A., Borrego, E., et al., 2013. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J., 74: 59-73. DOI:10.1111/tpj.12101 |
Cipollini, D., Enright, S., Traw, M.B., et al., 2004. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol., 13: 1643-1653. DOI:10.1111/j.1365-294X.2004.02161.x |
Costarelli, A., Bianchet, C., Ederli, L., et al., 2020. Salicylic acid induced by herbivore feeding antagonizes jasmonic acid mediated plant defenses against insect attack. Plant Signal. Behav., 15: 1704517. DOI:10.1080/15592324.2019.1704517 |
Deutsch, C.A., Tewksbury, J.J., Tigchelaar, M., et al., 2018. Increase in crop losses to insect pests in a warming climate. Science, 361: 916-919. DOI:10.1126/science.aat3466 |
Ding, Y., Sun, T., Ao, K., et al., 2018. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell, 173: 1454-1467. DOI:10.1016/j.cell.2018.03.044 |
Ederli, L., Salerno, G., Bianchet, C., et al., 2020. Eurydema oleracea negatively affects defenses in Arabidopsis by inducing salicylic acid-mediated signaling pathway. Arthropod Plant Interact., 14: 139-148. DOI:10.1007/s11829-019-09728-6 |
Engelberth, J., Viswanathan, S., Engelberth, M.J., 2011. Low concentrations of salicylic acid stimulate insect elicitor responses in Zea mays seedlings. J. Chem. Ecol., 37: 263-266. DOI:10.1007/s10886-011-9926-3 |
Erb, M., Kliebenstein, D.J., 2020. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol., 184: 39-52. DOI:10.1104/pp.20.00433 |
Erb, M., Reymond, P., 2019. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol., 70: 527-557. DOI:10.1146/annurev-arplant-050718-095910 |
Fu, Z.Q., Yan, S., Saleh, A., et al., 2012. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature, 486: 228-232. DOI:10.1038/nature11162 |
Gallie, D.R., Young, T.E., 2004. The ethylene biosynthetic and perception machinery is differentially expressed during endosperm and embryo development in maize. Mol. Genet. Genom., 271: 267-281. DOI:10.1007/s00438-004-0977-9 |
Gesteiro, N., Butron, A., Estevez, S., et al., 2021. Unraveling the role of maize (Zea mays L.) cell-wall phenylpropanoids in stem-borer resistance. Phytochemistry, 185: 112683. DOI:10.1016/j.phytochem.2021.112683 |
Giertych, M.J., Bakowski, M., Karolewski, P., et al., 2005. Influence of mineral fertilization on food quality of oak leaves and utilization efficiency of food components by the gypsy moth. Entomol. Exp. Appl., 117: 59-69. DOI:10.1111/j.1570-7458.2005.00332.x |
Gilardoni, P.A., Hettenhausen, C., Baldwin, I.T., et al., 2011. Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell, 23: 3512-3532. DOI:10.1105/tpc.111.088229 |
Heck, S., Grau, T., Buchala, A., et al., 2003. Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J., 36: 342-352. DOI:10.1046/j.1365-313X.2003.01881.x |
Hettenhausen, C., Baldwin, I.T., Wu, J., 2012. Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol., 158: 759-776. DOI:10.1104/pp.111.190074 |
Howe, G.A., Jander, G., 2008. Plant immunity to insect herbivores. Annu. Rev. Plant Biol., 59: 41-66. DOI:10.1146/annurev.arplant.59.032607.092825 |
Kaisers, W., Schaal, H., Schwender, H., 2015. rbamtools: an R interface to samtools enabling fast accumulative tabulation of splicing events over multiple RNA-seq samples. Bioinformatics, 31: 1663-1664. DOI:10.1093/bioinformatics/btu846 |
Kessler, A., Baldwin, I.T., 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science, 291: 2141-2144. DOI:10.1126/science.291.5511.2141 |
Kim, D., Langmead, B., Salzberg, S.L., 2015. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods, 12: 357-360. DOI:10.1038/nmeth.3317 |
Kundu, A., Mishra, S., Vadassery, J., 2018. Spodoptera litura-mediated chemical defense is differentially modulated in older and younger systemic leaves of Solanum lycopersicum. Planta, 248: 981-997. DOI:10.1007/s00425-018-2953-3 |
Li, Q., Xie, Q.G., Smith-Becker, J., et al., 2006. Mi-1-Mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol. Plant Microbe Interact., 19: 655-664. DOI:10.1094/MPMI-19-0655 |
Liu, H.J., Jian, L., Xu, J., et al., 2020. High-throughput CRISPR/Cas9 mutagenesis streamlines trait gene identification in maize. Plant Cell, 32: 1397-1413. DOI:10.1105/tpc.19.00934 |
Lu, C., Qi, J., Hettenhausen, C., et al., 2018. Elevated CO2 differentially affects tobacco and rice defense against lepidopteran larvae via the jasmonic acid signaling pathway. J. Integr. Plant Biol., 60: 412-431. DOI:10.1111/jipb.12633 |
Lu, J., Li, J., Ju, H., et al., 2014. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol. Plant, 7: 1670-1682. DOI:10.1093/mp/ssu085 |
Ma, C., Li, R., Sun, Y., et al., 2023. ZmMYC2s play important roles in maize responses to simulated herbivory and jasmonate. J. Integr. Plant Biol., 65: 1041-1058. DOI:10.1111/jipb.13404 |
Mattson, W.J., 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Systemat., 11: 119161. |
Mauch, F., Mauch-Mani, B., Gaille, C., et al., 2001. Manipulation of salicylate content in Arabidopsis thaliana by the expression of an engineered bacterial salicylate synthase. Plant J., 25: 67-77. DOI:10.1111/j.1365-313X.2001.00940.x |
Mewis, I., Tokuhisa, J.G., Schultz, J.C., et al., 2006. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry, 67: 2450-2462. DOI:10.1016/j.phytochem.2006.09.004 |
Naveed, M., Hejazi, V., Abbas, M., et al., 2018. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother., 97: 67-74. DOI:10.1016/j.biopha.2017.10.064 |
Niemeyer, H.M., 2009. Hydroxamic acids derived from 2-hydroxy-2-1,4-benzoxazin-3(4)-one: key defense chemicals of cereals. J. Agric. Food Chem., 57: 1677-1696. DOI:10.1021/jf8034034 |
Nomoto, M., Skelly, M.J., Itaya, T., et al., 2021. Suppression of MYC transcription activators by the immune cofactor NPR1 fine-tunes plant immune responses. Cell Rep., 37: 110125. DOI:10.1016/j.celrep.2021.110125 |
Paschold, A., Halitschke, R., Baldwin, I.T., 2007. Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J., 51: 79-91. DOI:10.1111/j.1365-313X.2007.03119.x |
Petersen, M., Brodersen, P., Naested, H., et al., 2000. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell, 103: 1111-1120. DOI:10.1016/S0092-8674(00)00213-0 |
Rayapuram, C., Baldwin, I.T., 2006. Using nutritional indices to study LOX3-dependent insect resistance. Plant Cell Environ., 29: 1585-1594. DOI:10.1111/j.1365-3040.2006.01534.x |
Robert-Seilaniantz, A., Grant, M., Jones, J.D.G., 2011. Hormone crosstalk in plant disease and defense: more than just JASMONATE-SALICYLATE antagonism. Annu. Rev. Phytopathol., 49: 317-343. DOI:10.1146/annurev-phyto-073009-114447 |
Schweizer, F., Fernandez-Calvo, P., Zander, M., et al., 2013. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell, 25: 3117-3132. DOI:10.1105/tpc.113.115139 |
Waldbauer, G.P., 1968. The consumption and utilization of food by insects. Adv. Insect Physiol, 5: 229-288. |
Wei, J., van Loon, J.J., Gols, R., et al., 2014. Reciprocal crosstalk between jasmonate and salicylate defence-signalling pathways modulates plant volatile emission and herbivore host-selection behaviour. J. Exp. Bot., 65: 3289-3298. DOI:10.1093/jxb/eru181 |
Wouters, F.C., Blanchette, B., Gershenzon, J., et al., 2016. Plant defense and herbivore counter-defense: benzoxazinoids and insect herbivores. Phytochemistry Rev., 15: 1127-1151. DOI:10.1007/s11101-016-9481-1 |
Wu, J.Q., Baldwin, I.T., 2010. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet., 44: 1-24. DOI:10.1146/annurev-genet-102209-163500 |
Wu, Y., Zhang, D., Chu, J.Y., et al., 2012. The NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep., 1: 639-647. DOI:10.1016/j.celrep.2012.05.008 |
Xu, J., Wang, X., Zu, H., et al., 2021. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol., 230: 1639-1652. DOI:10.1111/nph.17251 |
Yan, Y., Christensen, S., Isakeit, T., et al., 2012. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell, 24: 1420-1436. DOI:10.1105/tpc.111.094151 |
Zhang, C., Li, J., Li, S., et al., 2021. ZmMPK6 and ethylene signalling negatively regulate the accumulation of anti-insect metabolites DIMBOA and DIMBOA-Glc in maize inbred line A188. New Phytol., 229: 2273-2287. DOI:10.1111/nph.16974 |
Zhou, X., Yan, S., Sun, C., et al., 2015. A maize jasmonate Zim-domain protein, ZmJAZ14, associates with the JA, ABA, and GA signaling pathways in transgenic Arabidopsis. PLoS One, 10: e0121824. DOI:10.1371/journal.pone.0121824 |



