b. College of Life Science, Hebei University, Baoding 071002, China;
c. Liaoning Key Laboratory for Biological Invasions and Global Changes, College of Bioscience and Biotechnology, Shenyang Agricultural University, Shenyang, Liaoning 110866, China;
d. College of Horticulture, China Agricultural University, Beijing 100193, China;
e. State Key Laboratory of Efficient Utilization of Arid and Semi-arid Arable Land in Northern China, Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing 100081, China;
f. Key Laboratory of Arable Land Quality Monitoring and Evaluation, Ministry of Agriculture and Rural Affairs, Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Invasive plants have greatly reduced global biodiversity and sustainability (Bajwa et al., 2019; Hudgins et al., 2023). This is particularly true in agroecosystems with monoculture planting and excessive fertilization, which incur a high risk of alien plant invasion (Chen et al., 2013). Mounting evidence has shown that invasive plants can gain a competitive advantage over native plants by suppressing nutrient acquisition of native plants from symbiotic microbes such as mycorrhizal fungi and/or rhizobia (Grove et al., 2017; Birnbaum et al., 2018; Liu et al., 2022). Additionally, invasive plant roots have been observed to exhibit increased mycorrhizal associations, further contributing to their invasiveness (Tian et al., 2021; Sun et al., 2022; Yu et al., 2022).
Legume plants produce abundant nitrogen (N) through symbiotic nitrogen fixation (SNF) in their nodules (Zhong et al., 2023). Studies on plant intercropping have found that SNF-derived N can be utilized by mycorrhizal fungi associated with gramineous plants. These fungi can colonize legume nodules and transfer N to gramineous plants (He et al., 2003; Ingraffia et al., 2019). On the other hand, SNF-derived N can be released as root exudates from legume roots or during the decomposition of nodules after their death. This N is then taken up by the roots of gramineous plants (Thilakarathna et al., 2016; Zhang et al., 2020). Therefore, it is reasonable to speculate that invasive plants may also benefit from the SNF-derived N of native legumes through these mechanisms, although SNF processes can be inhibited by invasive plants. However, few studies have explored its potential contribution to the invasiveness of alien plants. Legume plants associate with both rhizobia and mycorrhizal fungi, and both associations are energy costly (Wang et al., 2021; Li et al., 2022). However, little is known about how these two symbiotic associations respond to alien plant invasion. For example, it is unclear whether both associations in native legumes are reduced or whether one symbiotic association is reduced and the other is facilitated by competition with invasive plants. Clarifying this issue would provide valuable insights for optimizing symbiotic associations (such as rhizobia, mycorrhizal fungi, or both) of crops in response to alien plant invasion.
The ability of roots to acquire nutrients depends on both the quantity and activity of the roots involved in the acquisition process (Zhang et al., 2023, 2024). Root quantity refers to the total surface area of the roots involved in foraging for soil nutrients. Root activity is often represented by nutrient content, such as N, or by enzymes activity (e.g., root acid phosphatase, Apase) in the roots, as these factors are closely related to nutrient uptake by roots (Bardgett et al., 2014; Han and Zhu 2021; Han et al., 2022). Recent studies have demonstrated that there can be a decoupling of the quantity and activity of roots during nutrient acquisition (Bergmann et al., 2020; Zhang et al., 2024). Therefore, to gain a comprehensive understanding of how native and invasive plants compete for soil nutrients via their roots, it is necessary to consider both the quantity and activity of roots. Furthermore, the N-fixing ability of legumes depends on the quantity (number or biomass of nodules) and activity (N-fixing rate by nodules) of nodules. Increased N-fixation activity has frequently been observed with fewer nodules in legumes, given the high energy demands of the N-fixation process in the nodules (McCulloch and Porder 2020; Ke et al., 2022). Therefore, both the quantity and activity of the nodules should be considered when assessing the amount of SNF-derived N. However, to date, few studies have explored the responses of both roots and N-fixation nodules in native legumes to invasive plants within the framework of quantity–activity for nutrient acquisition. It remains unclear how the quantity and activity of roots vary in coordination or independently of the nodules.
In this study, we aim to test two hypotheses by conducting monoculture and mixed planting experiments with a noxious invasive plant, Xanthium strumarium, and an important native legume crop in China, Glycine max. The hypotheses are as follows: (1) X. strumarium can compete with G. max for nodule-fixed N by increasing the quantity and activity of its roots, as well as enhancing its mycorrhizal association. (2) when in competition with X. strumarium, both the quantity of the roots and nodules in G. max will decrease, whereas the activities of these agents for nutrient acquisition will increase, aiming to alleviate growth suppression of the legume caused by the invasive plants.
2. Materials and methods 2.1. Plant materials and experimental designThe study was conducted at the research station at Shenyang Agricultural University (123°33′E, 41°48′N) where the elevation is 59 m. Mean annual temperature is 8.1 ℃, and mean annual precipitation is 722 mm. Here, Xanthium strumarium was selected as the invasive plant and Glycine max as the native plant. X. strumarium is an annual herb, native to North America, and has become a harmful invasive plant in northeast China (Iqbal et al., 2020, 2021; Chen et al., 2022). This invasive plant becomes a significant threat to agriculture field crops, particularly G. max, which is widely planted in northeast China (Iqbal et al., 2020, 2021).
In mid-October 2017, we collected seeds from mature individual plants of X. strumarium near Qipanshan, Shenyang. Seeds of G. max were bought from Dafeng Seed Industry Development Co., Ltd, Shenyang China. In late April 2018, both seeds of the invasive and native plants were disinfected with 0.5% potassium permanganate solution, washed with distilled water, and then sown in pots (25 cm × 16 cm × 14 cm) filled with 4 kg soil. The soils samples were collected from a forest stand near Dongling Park, Shenyang. Briefly, the top soil was sampled, sieved, air-dried and subsequently mixed with sterilized river sands (see Chen et al., 2022 for details). Initial soil physical and chemical characters were as follows: soil pH, 7.34; organic matter content, 44 g kg−1; total N content, 0.22 g kg−1; total phosphorus content, 0.06 g kg−1; total potassium content, 1.48 g kg−1; available N content, 125.67 mg kg−1; available phosphorus content, 3.10 mg kg−1; available potassium content, 55 mg kg−1.
In mid-May, seedlings were thinned to one individual plant per pot for the monoculture plantings, and one G. max individual and one X. strumarium individual per pot for the mixture. Only one plant individual was set in the monoculture plantings, a little different from studies with two individuals in this planting usually aiming to account for intraspecific competition (McNickle 2020). However, we aimed to explore how the invasive plants compete with the native legume plant. That is, we focus mainly on the interspecific competition of the soil resource between the invasive and the native plants rather than the intraspecific competition within each species. Therefore, the set of one individual in the monoculture plantings can fulfill such purpose on interspecific competition, as has been adopted in previous studies (McNickle 2020; Chen et al., 2022). Five replicates were established for each planting types. In each pot, root physiological traits (acid phosphatase), mycorrhizal colonization rate and activity of biological N-fixation were measured. However, due to the destructive sampling nature of these measurements, the root systems could not be kept intact to obtain data on root biomass. Therefore, an additional five replicates were set up for both monoculture and mixed plantings to measure plant biomass and leaf morphological traits.
2.2. Plant sampling and trait measurementsPlants were harvested in late July 2018. Shoot biomass was measured after the samples were dried at 60 ℃ for 48 h. Plant roots were separated into coarse (diameter > 2 mm) and fine (diameter < 2 mm) roots, and their biomass was measured separately.
Total leaf area of an individual plant was measured by Li-3000C (Li-COR, Lincoln, NE, USA), and then the leaf biomass was measured. Randomly selected sub-samples of fine roots were scanned, and the images were used to calculate fine root surface using WinRhizo Pro (2016 software, Regent Instruments, Canada). The scanned roots were then dried and weighed. Total surface area of fine roots of an individual plant was calculated according to the portion of the fine root sub-sample to total fine root biomass of the individual plant.
Root acid phosphatase activity (Apase) was determined following Png et al. (2017), using para-nitrophenyl phosphate (pNPP) as the substrate (Png et al., 2017; Kavka et al., 2021). Briefly, fresh root samples were ground, shaken and centrifuged. The supernatant was then terminated with sodium hydroxide, and the concentration of para-nitrophenol (pNP) was calculated by UV–VIS spectrophotometer (UV–Vis Spectrophotometer, Shimadzu, Japan). Root acid phosphatase activity (Apase) was calculated as: Apase = pNP/(reaction time × root weight) (see Method S1 for details).
Mycorrhizal colonization rate was measured following previous studies (Trouvelot et al., 1986; Chen et al., 2022; Bi et al., 2023). Briefly, 50 root segments (~1 cm in length) were randomly selected including terminal two root branch orders usually with the highest mycorrhizal colonization rate in the root system. The root segments were consecutively soaked into KOH solution, acetic acid solution, and then dyed in acetic acid ink observation of mycorrhizal colonization using a microscope (Nikon MODEL ECLIPSE Ni-U, Japan). Mycorrhizal colonization rate was divided into five categories, each with a weight of 0.95, 0.70, 0.30, 0.05 and 0.01, respectively. Mycorrhizal colonization rate was then calculated as follows:
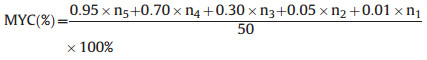
|
n1–n5 is the number of root segments examined in each above mycorrhizal colonization category.
The quantity aspect of the N-fixing strategy for the soybean was assessed by the nodule number and nodule biomass per plant as well as the biomass of a single nodule. The activity aspect of the N-fixing strategy was indicated by the nitrogenase iron protein (nifH) gene copies in the nodule where higher nifH gene copies indicate higher N-fixation rate of the nodule (Yang et al., 2020, 2023; Libourel et al., 2023). The nifH gene copies were determined by the following steps: 1) Nodule DNA extraction; 2) Quantitative real-time PCR assay; 3) Quantify the nifH gene present in the samples (see Method S2 for details).
We also collected bulk soil and rhizosphere soil in each pot to measure soil total C and N contents as well as δ15N content. Briefly, the pots were destroyed to expose plant roots. The soil easily shaken off the roots was treated as bulk soil and the soil adhering to roots and not easily shaken off was carefully collected as rhizosphere soil. The soil samples were then ground using GT200 to determine soil C and N content using Elementary analytical instrument (Elementary analytical instrument, Germany). Soil δ15N content was measured using SerCon Integra 2 Integrated EA-IRMS isotope mass spectrometer (SeCron, Cheshire Crewe, UK). The C, N and δ15N contents for leaves and roots were measured in a similar procedure. The contribution of total leaf N by the N-fixation (%Ndfa) was calculated following previous studies (Balboa and Ciampitti 2020; Cox et al., 2022):
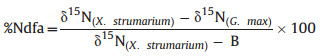
|
X. strumarium was used as the reference plant. Leaf δ15N of the reference plant (δ15N () is calculated as the average leaf δ15N of this plant across the monoculture and mixed plantings (see Fig. 2). δ15N ( is the leaf δ15N in each pot with G. max.
B is the leaf δ15N value of legume plants depending solely on atmospheric N for their N source. We used B value was −2.7‰ across legume species (Balboa and Ciampitti 2020). B values for soybean from all previous glasshouse studies ranged from −2.7‰ to −1.98‰ (Balboa and Ciampitti 2020) with an average of −1.97‰. In our study, the lowest leaf δ15N of the soybean was −2.67‰, and therefore we used the lowest B value of previous studies (i.e., −2.7‰) to calculate %Ndfa in our study. The absolute amount of N in G. max leaves contributed by N-fixation of nodules (i.e., Ndfa amount) was calculated as:
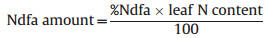
|
In addition, we used leaf δ15N of X. strumarium in monoculture plantings as leaf δ15N of reference plants (see Fig. S8). The results of %Ndfa in G. max in the monoculture and mixed plantings using this new leaf δ15N of the reference plant (Fig. S8) were very similar to that based on the above reference plants. This suggests that the selection of reference plant leaf δ15N did not alter the result of leaf %Ndfa in the native legume plant. Therefore, we only presented the result based on the average leaf δ15N of the reference plant across the monoculture and mixed plantings.
2.3. Data analysisThe difference in leaf and root traits of the native or invasive plant between monoculture and mixture was analyzed using independent sample t test. Linear regressions were employed to explore trait relationships among roots, leaves, nodules and soil nutrients. Significant level was set at 0.05, and 0.05 < p < 0.1 was considered as marginal significance. All the analyses were performed in R 4.2.0 (R core team).
3. Results 3.1. Responses of above- and belowground plant biomass to competitionComparing with Glycine max in monoculture plantings, biomass of G. max in the mixture decreased by 41% for total (p < 0.01), 43% for shoot (p < 0.01), 36% for root compartment (p < 0.01), respectively (Fig. S1A–C). In contrast, biomass of Xanthium strumarium showed no significant difference between monocultures and mixtures (all p values > 0.05) (Fig. S1A–C).
3.2. Responses of leaf and root traits to competitionCompared with monoculture, values of G. max in the mixture decreased by 39% for leaf biomass (p < 0.01) (Fig. S2A), 38% for total leaf area (p < 0.01) (Fig. S2B), and 42% for total leaf N content (p < 0.01) (Fig. S2D). No difference was observed for leaf N concentration (p = 0.62) between monoculture and mixture (Fig. S2C). By contrast, only leaf biomass (−16%, p = 0.02) but not leaf area, leaf N concentration and total leaf N content was significantly reduced when growing together with G. max compared with values from monoculture (Fig. S2B–D).
Compared with monoculture, fine root biomass of G. max in the mixtures decreased by 36% (p < 0.01), total fine root surface area decreased by 45% (p < 0.01), fine root nitrogen (RN) concentration decreased by 17% (p = 0.02), and acid phosphatase activity decreased by 33% (p < 0.01) (Fig. 1A–D). By contrast, X. strumarium in mixture increased fine root N concentration by 40% (p = 0.016) while showing no significant differences for fine root biomass, total fine root surface area per plant and acid phosphatase activity compared with X. strumarium in the monoculture (Fig. 1).
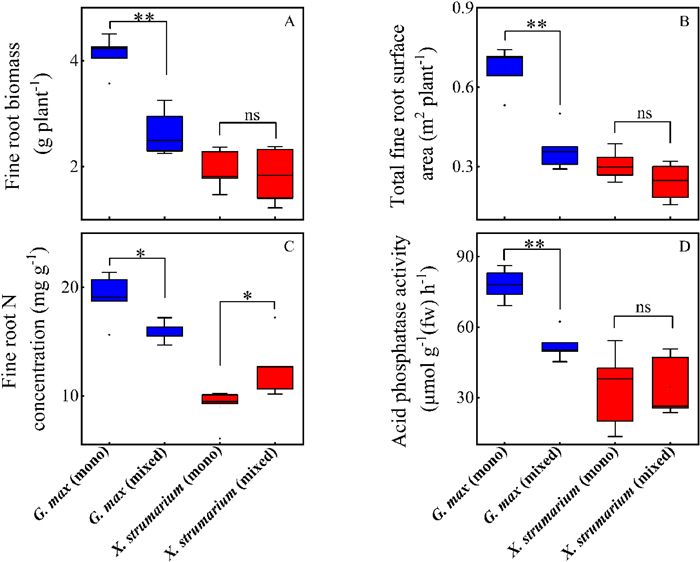
|
| Fig. 1 Fine root traits of Glycine max and Xanthium strumarium in monoculture (mono) and mixture (mixed). (A) Fine root biomass, (B) Total fine root surface area, (C) Fine root N concentration, (D) Acid phosphatase activity. **, p < 0.01; *, p < 0.05; ns, non-significant. |
Compared with monoculture plantings, nodule number per plant of Glycine max in mixed plantings decreased by 49% (p < 0.01), nodules biomass per plant decreased by 45% (p < 0.01), single nodule biomass remained unchanged (p = 0.94), while nifH gene copies increased by 106% (p < 0.01) (Fig. 2A–C). Compared with G. max in monoculture plantings, proportion of total leaf N derived from atmospheric N-fixation (%Ndfa) increased in mixed plantings of G. max (p = 0.002, Fig. 2D), while absolute amount of Ndfa remained the same (p = 0.41, Fig. 2E). In addition, nifH gene copies in nodules of the native legume were positively correlated with %Ndfa (R2 = 0.51, p = 0.02, Fig. 2F).
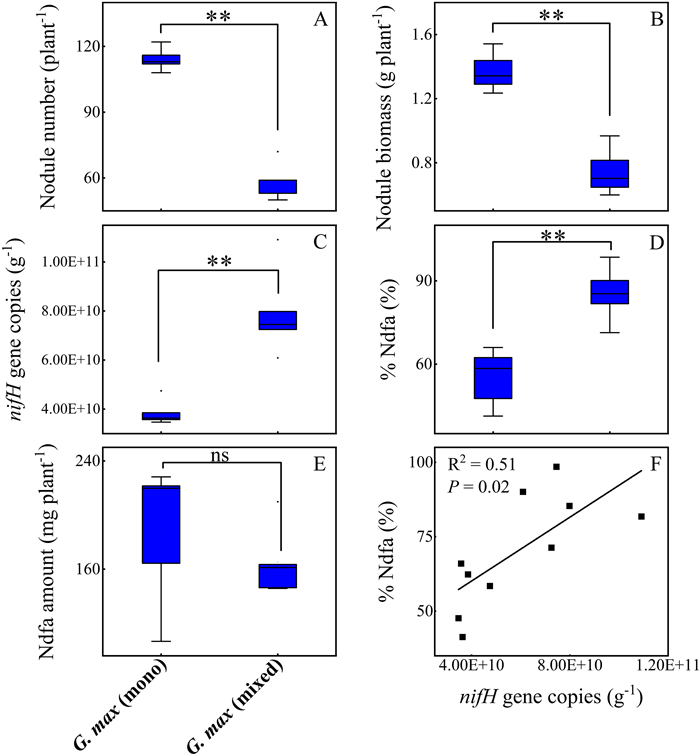
|
| Fig. 2 N-fixing related traits of Glycine max in monoculture (mono) and mixture (mixed). (A) Nodule number per plant, (B) Nodule biomass per plant, (C) nifH gene copy number per unit nodule weight, (D) proportion of total leaf N contributed by N-fixation of nodules (%Ndfa), (E) absolute leaf N content per plant contributed by N-fixation of nodules (Ndfa amount). (F) Relationships between nifH gene copies and %Ndfa. %Ndfa: proportion of total leaf N contributed by N-fixation of nodules. **, p < 0.01; ns, non-significant. |
The isotope measurements indicated that leaf δ15N of G. max decreased in the mixed plantings compared to the monoculture, whereas leaf δ15N of Xanthium strumarium increased in the mixed plantings compared to the monoculture (Fig. S3). In addition, mixed plantings, compared with monoculture, had no effect on mycorrhizal colonization rate of G. max (p = 0.86), whereas significantly increased mycorrhizal colonization rate of X. strumarium by 42% (p < 0.001) (Fig. 3).
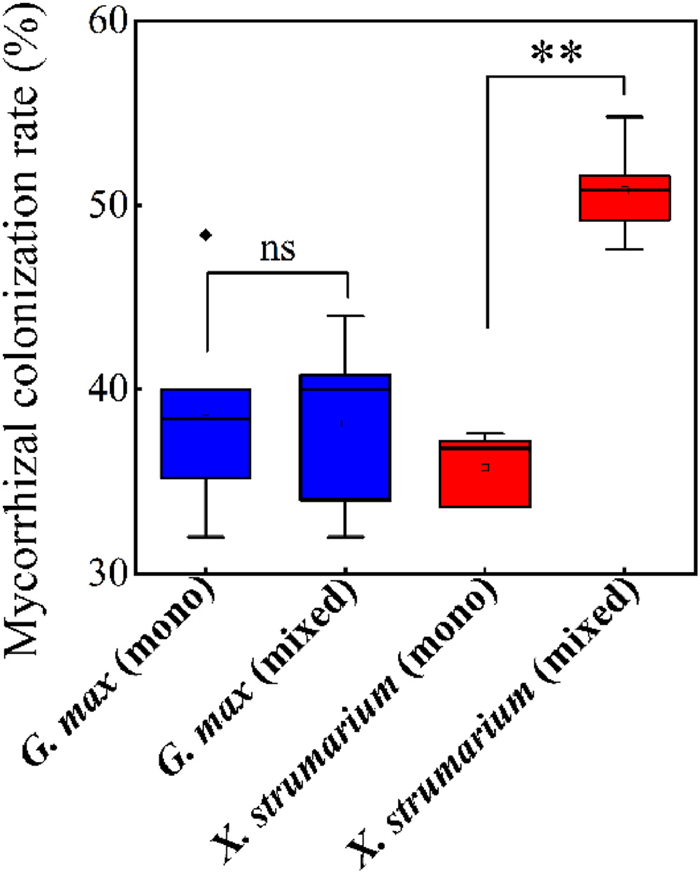
|
| Fig. 3 Mycorrhizal colonization rate of Glycine max and Xanthium strumarium in monoculture (mono) and mixture (mixed). **, p < 0.01; ns, non-significant. |
Compared with monoculture plantings, rhizosphere soil N of G. max in mixed plantings decreased by 7% (p = 0.038) (Fig. 4A). Such effect of mixed plantings on rhizosphere soil N were not significant for X. strumarium (p = 0.64) (Fig. S4). In addition, rhizosphere soil N of G. max was positively correlated with nodule biomass (Fig. 4B) and negatively correlated with nifH gene copies (Fig. 4C).
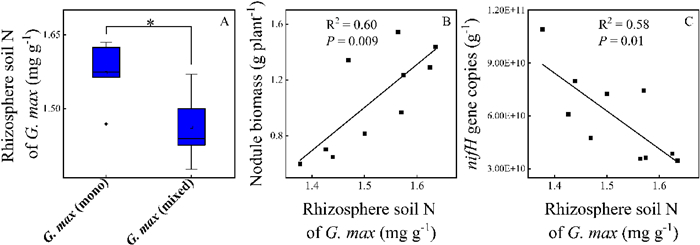
|
| Fig. 4 Rhizosphere soil N of Glycine max in different plantation (A) and its relationship with nodule biomass (B) and nifH gene copies (C). mono: monoculture, mixed: mixture. *, p < 0.05. |
Typically, legume plants produce abundant N resources from the N fixation of their nodules, which can be utilized by the roots and/or mycorrhizal fungi of plants coexisting with legume plants (Zhang et al., 2020). If this facilitation effect is present in the Glycine max-Xanthium strumarium system, we would expect to observe depleted leaf δ15N values of X. strumarium given that the nodule-fixed N is depleted in δ15N. Surprisingly, our results showed that leaf δ15N of X. strumarium increased rather than decreased in mixed plantings compared to monoculture plantings (Fig. S3). This suggests that X. strumarium may not directly utilize biologically fixed N from G. max, contradicting hypothesis 1.
We speculate that the lack of utilization of nodule-fixed N by the invasive plant could be attributed to several factors. First, the nodules facing with X. strumarium may exhibit greater resistance, potentially through the production of defensive chemicals, against infection by the mycorrhizal fungi of the invasive plant (Weston and Mathesius 2013; Semchenko et al., 2014; Kong et al., 2018). Second, reduced root activity owing to interspecific competition could lead to a significant decrease in root exudates from the legume (Fig. 1C) (Sun et al., 2020), thereby reducing the availability of nodule-fixed N. Given that N fixation is an energy-demanding process, legume roots in competition with invasive plant may reduce exudates containing nodule-fixed N. Third, nodule numbers were greatly reduced in the mixed planting (Fig. 2A), which could limit the colonization of these nodules by the mycorrhizal fungi of the invasive plants. Although we currently lack direct evidence for these mechanisms, investigating them would greatly enhance our understanding of how legumes respond and adapt to the invasion of alien plants. Considering the typically strong competitive ability of invasive plants for soil resources, it is possible that the conservation of the nodule-fixed N against exploitation by X. strumarium could be applicable to other legumes as well. However, this speculation warrants further investigation.
4.2. Belowground interactions between the native legume and the invasive plantWhile X. strumarium may not have access to the nodule-fixed N, this invasive plant can still suppress G. max by outcompeting the native plant for soil resources (see Method S3 in the supporting information). Contrary to our hypothesis 2, in the presence of competition from X. strumarium, both the quantity and activity of the native legume's roots were reduced (Fig. 1). This could be due to the greatly enhanced belowground competitive ability of the invasive plant, including higher root quantity and activity and higher mycorrhizal colonization rate of this plant in mixed plantings compared to monoculture plantings (Fig. 1, Fig. 3). Therefore, intensive competition from the invasive plant could reduce soil resources to a low level (Fig. 4), thereby suppressing the compensatory increase in the root activity of the native legume.
In contrast to legume roots, our results showed a decrease in nodule quantity and an increase in nodule activity in mixed plantings compared to monoculture plantings. This response is due to the intense competition for soil resources by the invasive plant X. strumarium (Fig. 4). For example, the reduction in rhizosphere soil N content of the native legume caused by X. strumarium (Fig. S5A) can limit plant growth, and hence reduce carbon allocation to nodule production (Fig. S6). Meanwhile, the lower rhizosphere soil N content of the legume can downregulate genes that inhibit N-fixing and/or upregulate genes that stimulate N-fixing, as reported in previous studies, leading to higher nodule activity (Salvagiotti et al., 2008; Li et al., 2009, 2022; Lin et al., 2018).
Considering the responses of nutrient acquisition strategies by legume roots, nodules, and mycorrhizal fungi (which remain unchanged, see Fig. 3) to the invasive plant X. strumarium, we anticipated an increased contribution by the nodule-fixed N to the legume N acquisition. This expectation was supported by the isotopic evidence of a higher percentage of %Ndfa for G. max in mixed plantings than in monoculture plantings (Fig. 2D), as well as a positive relationship between %Ndfa and nodule activity (Fig. 2F). Together, our results demonstrate that the invasive plant increase nodule activity in the native legume through strong competition for soil nutrients, thereby enhancing the dependence of the native legume on nodule-fixed N (Fig. 2D).
4.3. Competition of soil resources by mycorrhizal fungi of the invasive plantConsistent with our expectation, the invasive plant, X. strumarium, had higher mycorrhizal association in the mixture plantings than that in the monoculture plantings. If the increased mycorrhizal association in the invasive plant is used to acquire the rhizosphere soil N of the native legume, we would expect a negative relationship between the two variables. However, our result revealed a positive relationship between mycorrhizal association of X. strumarium and the rhizosphere soil N content of G. max (Fig. S5B). We also noted a higher root P acquisition ability in the mixed compared to monoculture plantings, as indicated by an increased proportion of total root Apase accounted for by X. strumarium in the mixed plantings (Fig. S7). This indirectly suggests that growth of X. strumarium in the mixed planting may be limited by P availability. Therefore, the increased mycorrhizal association of the invasive plant is likely used to acquire P rather than N in the rhizosphere soil of the native legume. Taken together, our findings suggest that roots and mycorrhizal fungi of X. strumarium may synergistically work to promote P acquisition in competition with the native legume.
Interestingly, the mycorrhizal association with G. max roots was unaffected in the mixed plantings (Fig. 3). Previous evidence has shown that G. max, in competition with the invasive plant, increases its dependence on biologically fixed N, which could entail an increased need for P for stoichiometric homeostasis of the N: P ratio (Zhong et al., 2023). As aforementioned, increased mycorrhizal association of X. strumarium in the mixture plantings can enhance its competition for soil P. Meanwhile, root Apase was reduced in the mixed planting. Therefore, keeping the mycorrhizal association of G. max unchanged in mixed plantings could be an effective way to partially meet the increased P demand of the native legume in mixed plantings.
5. ConclusionAlthough the native legume plant can produce plenty of N through N-fixing by nodules, the invasive plant, Xanthium strumarium, does not appear to scavenge this fixed N from nodules. Instead, the invasive plant, with higher root activity and quantity, suppressed N acquisition by the roots of the native legume, while increasing its dependence on legume N-fixation by stimulating nodule activity in N-fixing (Fig. 5). Meanwhile, increased mycorrhizal association of X. strumarium seems to facilitate exploitation of rhizosphere soil P from the native legume. Therefore, the activity–quantity framework shows promise for enhancing our understanding of how alien plants successfully invade native legumes.
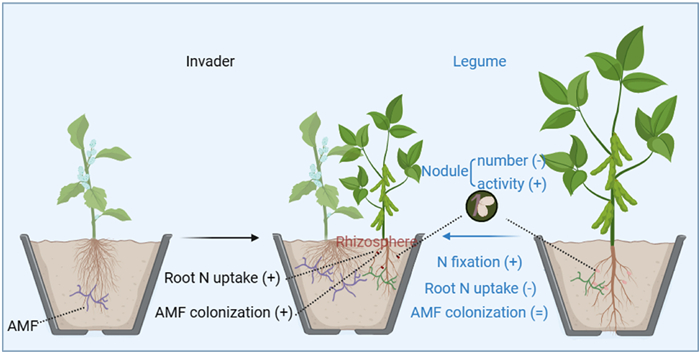
|
| Fig. 5 Conceptual model for belowground resource competition between the invader (Xanthium strumarium) and the native legume (Glycine max). When in mixed plantings, the invader, X. strumarium, increases nutrient acquisition through its higher root nitrogen (N) uptake from the rhizosphere soil of the native legume. Meanwhile the invasive plant shifts to be with higher arbuscular mycorrhizal colonization (AMF) in the mixed plantings likely facilitating phosphorus acquisition from rhizosphere soil of the native legume. Depletion of rhizosphere soil N of the native legume by the invasive plant reduces native root N uptake and nodule quantity while greatly stimulates nodule activity for N-fixation, consequently resulting a higher contribution of N-fixing to the native plant N acquisition. '+', '–' and '=' indicate increase, decrease and no change of belowground nutrient acquisition in the mixed relative to the monoculture plantings, respectively. |
This study had certain limitations that should be addressed in future studies. First, it is important to note that we only examined one invasive plant species and one native legume species, which may restrict the generalizability of our findings. However, our study highlights the significant roles of invasive plant roots and mycorrhizal fungi in resource competition, specifically for N and P, which are common traits of invasive plants. Additionally, the mechanism underlying the increased dependence of the native legume on nodule-fixed N is commonly observed in legume plants, i.e., stimulating nodule activity through the depletion of rhizosphere soil N in legumes by an invasive competitor. Therefore, despite the limitations mentioned above, our conclusions based on a few species may have broader applicability to other invasive plants and native legumes. Second, future studies could consider incorporating native weedy species, such as gramineous or herbaceous species, as a comparison to invasive plants to elucidate the differences between invasive plants and weedy species in driving belowground interspecific competition. Third, although our study focused on examining belowground resource competition as a contributing factor to invasive success, it is crucial to consider the role of allelochemicals released by invasive plants in suppressing the growth of native plants. Therefore, future studies should consider both the allelopathy and resource competition of invasive plants relative to native legumes. Finally, future studies should concentrate on the composition of mycorrhizal fungi and the rhizobium community, which are important for nutrient acquisition from the soil or atmosphere.
AcknowledgementsWe thank Haiyan Zhang, Xinyu Lu and Qingjie Pang for their assistance in the pot experiment and Prof. Yanjie Liu for his valuable guidance in writing the draft. We much appreciate four referees and the handling editor for their nice comments and suggestions for improvement of this manuscript. This study was funded by the National Natural Science Foundation of China (32171746, 31870522, 42077450, 32371786), the leading talents of basic research in Henan Province, Funding for Characteristic and Backbone Forestry Discipline Group of Henan Province, the Scientific Research Foundation of Henan Agricultural University (30500854), and Research Funds for overseas returnee in Henan Province, China. This work also was supported by National Key Research and Development Program of China (2019YFE0117000).
Data availability statement
Data are available in Dryad Digital Repository, a publicly available database, or acquirable on request of the corresponding author.
CRediT authorship contribution statement
Meixu Han: Writing – review & editing, Writing – original draft. Haiyang Zhang: Writing – review & editing, Formal analysis. Mingchao Liu: Supervision, Resources. Jinqi Tang: Investigation, Data curation. Xiaocheng Guo: Investigation. Weizheng Ren: Validation. Yong Zhao: Funding acquisition. Qingpei Yang: Software. Binglin Guo: Software. Qinwen Han: Data curation. Yulong Feng: Supervision, Project administration. Zhipei Feng: Methodology. Honghui Wu: Writing – review & editing. Xitian Yang: Funding acquisition. Deliang Kong: Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2024.04.003.
Bajwa, A.A., Farooq, M., Nawaz, A., et al., 2019. Impact of invasive plant species on the livelihoods of farming households: evidence from Parthenium hysterophorus invasion in rural Punjab, Pakistan. Biol. Invasions, 21: 3285-3304. DOI:10.1007/s10530-019-02047-0 |
Balboa, G.R., Ciampitti, I.A., 2020. Estimating biological nitrogen fixation in field-grown soybeans: impact of B value. Plant Soil, 446: 195-210. DOI:10.1007/s11104-019-04317-1 |
Bardgett, R.D., Mommer, L., De Vries, F.T., 2014. Going underground: root traits as drivers of ecosystem processes. Trends Ecol. Evol., 29: 692-699. DOI:10.1016/j.tree.2014.10.006 |
Bergmann, J., Weigelt, A., van der Plas, F., et al., 2020. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv., 6. DOI:10.1126/sciadv.aba3756 |
Bi, B., Yin, Q., Hao, Z., 2023. Root phosphatase activity is a competitive trait affiliated with the conservation gradient in root economic space. For. Ecosyst., 10: 100111. DOI:10.1016/j.fecs.2023.100111 |
Birnbaum, C., Morald, T.K., Tibbett, M., et al., 2018. Effect of plant root symbionts on performance of native woody species in competition with an invasive grass in multispecies microcosms. Ecol. Evol., 8: 8652-8664. DOI:10.1002/ece3.4397 |
Chen, G.Q., He, Y.H., Qiang, S., 2013. Increasing seriousness of plant invasions in croplands of eastern China in relation to changing farming practices: a case study. PLoS One, 8: e74136. DOI:10.1371/journal.pone.0074136 |
Chen, J., Zhang, H.Y., Liu, M.C., et al., 2022. Plant invasions facilitated by suppression of root nutrient acquisition rather than by disruption of mycorrhizal association in the native plant. Plant Divers., 44: 499-504. DOI:10.1016/j.pld.2021.12.004 |
Cox, A., Boots-Haupt, L., Brasier, K., et al., 2022. Using δ15N to screen for nitrogen fixation: reference plant position and species. Agron. J., 114: 1842-1850. DOI:10.1002/agj2.21032 |
Grove, S., Haubensak, K.A., Gehring, C., et al., 2017. Mycorrhizae, invasions, and the temporal dynamics of mutualism disruption. J. Ecol., 105: 1496-1508. DOI:10.1111/1365-2745.12853 |
Han, M., Chen, Y., Li, R., et al., 2022. Root phosphatase activity aligns with the collaboration gradient of the root economics space. New Phytol., 234: 837-849. DOI:10.1111/nph.17906 |
Han, M., Zhu, B., 2021. Linking root respiration to chemistry and morphology across species. Global Change Biol., 27: 190-201. DOI:10.1111/gcb.15391 |
He, X., Critchley, C.N.R., Bledsoe, C.S., 2003. Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit. Rev. Plant Sci., 22: 531-567. DOI:10.1080/713608315 |
Hudgins, E.J., Cuthbert, R.N., Haubrock, P.J., et al., 2023. Unevenly distributed biological invasion costs among origin and recipient regions. Nat. Sustain.. DOI:10.1038/s41893-023-01124-6 |
Ingraffia, R., Amato, G., Frenda, A., et al., 2019. Impacts of arbuscular mycorrhizal fungi on nutrient uptake, N2 fixation, N transfer, and growth in a wheat/faba bean intercropping system. PLoS One, 14: e0213672. DOI:10.1371/journal.pone.0213672 |
Iqbal, M.F., Feng, Y.L., Feng, W.W., et al., 2021. Ecological impacts of the invasive plant Xanthium strumarium and the impacts of three aboveground herbivores on the invader. Ecol. Indicat., 131: 108140. DOI:10.1016/j.ecolind.2021.108140 |
Iqbal, M.F., Liu, M.C., Iram, A., et al., 2020. Effects of the invasive plant Xanthium strumarium on diversity of native plant species: a competitive analysis approach in North and Northeast China. PLoS One, 15: e0228476. DOI:10.1371/journal.pone.0228476 |
Kavka, M., Korn, K., Hazarika, M., et al., 2021. Potato root and leaf phosphatase activity in response to P deprivation. J. Plant Nutr. Soil Sci., 184: 668-677. DOI:10.1002/jpln.202100112 |
Ke, X., Xiao, H., Peng, Y., et al., 2022. Phosphoenolpyruvate reallocation links nitrogen fixation rates to root nodule energy state. Science, 378: 971-977. DOI:10.1126/science.abq8591 |
Kong, C.H., Zhang, S.Z., Li, Y.H., et al., 2018. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun., 9: 3867. DOI:10.1038/s41467-018-06429-1 |
Li, Q., Zhang, H., Song, Y., et al., 2022. Alanine synthesized by alanine dehydrogenase enables ammonium-tolerant nitrogen fixation in Paenibacillus sabinae T27. Proc. Natl. Acad. Sci. U.S.A., 119: e2215855119. DOI:10.1073/pnas.2215855119 |
Li, Y.Y., Yu, C.B., Cheng, X., et al., 2009. Intercropping alleviates the inhibitory effect of N fertilization on nodulation and symbiotic N2 fixation of faba bean. Plant Soil, 323: 295-308. DOI:10.1007/s11104-009-9938-8 |
Libourel, C., Keller, J., Brichet, L., et al., 2023. Comparative phylotranscriptomics reveals ancestral and derived root nodule symbiosis programmes. Nat. Plants, 9: 1067-1080. DOI:10.1038/s41477-023-01441-w |
Lin, J.S., Li, X., Luo, Z., et al., 2018. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants, 4: 942-952. DOI:10.1038/s41477-018-0261-3 |
Liu, Y., Huang, W., Yang, Q., et al., 2022. Research advances of plant invasion ecology over past 10 years. Biodivers. Sci., 30: 23438. DOI:10.17520/biods.2022438 |
McCulloch, L., Porder, S., 2020. Lower nodule biomass with increased nitrogenase efficiency in Robinia pseudoacacia seedlings when grown under low soil phosphorus conditions. SN Appl. Sci., 2. DOI:10.1007/s42452-020-03518-z |
McNickle, G.G., 2020. Interpreting plant root responses to nutrients, neighbours and pot volume depends on researchers' assumptions. Funct. Ecol., 34: 2199-2209. DOI:10.1111/1365-2435.13517 |
Png, G.K., Turner, B.L., Albornoz, F.E., et al., 2017. Greater root phosphatase activity in nitrogen-fixing rhizobial but not actinorhizal plants with declining phosphorus availability. J. Ecol., 105: 1246-1255. DOI:10.1111/1365-2745.12758 |
Salvagiotti, F., Cassman, K.G., Specht, J.E., et al., 2008. Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crops Res., 108: 1-13. DOI:10.1016/j.fcr.2008.03.001 |
Semchenko, M., Saar, S., Lepik, A., 2014. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol., 204: 631-637. DOI:10.1111/nph.12930 |
Sun, D., Yang, X., Wang, Y., et al., 2022. Stronger mutualistic interactions with arbuscular mycorrhizal fungi help Asteraceae invaders outcompete the phylogenetically related natives. New Phytol., 236: 1487-1496. DOI:10.1111/nph.18435 |
Sun, L., Ataka, M., Han, M., et al., 2020. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol., 229. DOI:10.1111/nph.16865 |
Thilakarathna, M.S., McElroy, M.S., Chapagain, T., et al., 2016. Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. A review. Agron. Sustain. Dev., 36: 58. DOI:10.1007/s13593-016-0396-4 |
Tian, B., Pei, Y., Huang, W., et al., 2021. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J., 15: 1919-1930. DOI:10.1038/s41396-021-00894-1 |
Trouvelot, A., Kough, J.L., Gianinazzi-Pearson, V., 1986. Physiological and genetical
aspects of mycorrhizae: proceedings of the 1st european symposium on
mycorrhizae. In: Gianinazzi, S., Gianinazzi-Pearson, V. (Eds. ), European Symposium on Mycorrhizae. INRA Press, Paris, pp. 217e221.
|
Wang, T., Guo, J., Peng, Y., et al., 2021. Light-induced mobile factors from shoots regulate rhizobium-triggered soybean root nodulation. Science, 374: 65-71. DOI:10.1126/science.abh2890 |
Weston, L.A., Mathesius, U., 2013. Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol., 39: 283-297. DOI:10.1007/s10886-013-0248-5 |
Yang, J., Xiang, N., Liu, Y., et al., 2023. Organelle-dependent polyprotein designs enable stoichiometric expression of nitrogen fixation components targeted to mitochondria. Proc. Natl. Acad. Sci. U.S.A., 120: e2305142120. DOI:10.1073/pnas.2305142120 |
Yang, Y., Liu, L., Singh, R.P., et al., 2020. Nodule and root zone microbiota of salt-tolerant wild soybean in coastal sand and saline-alkali soil. Front. Microbiol., 11: 2178. DOI:10.3389/fmicb.2020.523142 |
Yu, H., He, Y., Zhang, W., et al., 2022. Greater chemical signaling in root exudates enhances soil mutualistic associations in invasive plants compared to natives. New Phytol., 236: 1140-1153. DOI:10.1111/nph.18289 |
Zhang, H., Wang, X., Gao, Y., et al., 2020. Short-term N transfer from alfalfa to maize is dependent more on arbuscular mycorrhizal fungi than root exudates in N deficient soil. Plant Soil, 446: 23-41. DOI:10.1007/s11104-019-04333-1 |
Zhang, Y., Cao, J.J., Yang, Q.P., et al., 2023. The worldwide allometric relationship in anatomical structures for plant roots. Plant Divers., 46: 621-629. DOI:10.1016/j.pld.2023.05.002 |
Zhang, Y., Cao, J.J., Lu, M.Z., et al., 2024. The origin of bi-dimensionality in plant root traits. Trends Ecol. Evol., 39: 78-88. DOI:10.1016/j.tree.2023.09.002 |
Zhong, Y., Tian, J., Li, X., et al., 2023. Cooperative interactions between nitrogen fixation and phosphorus nutrition in legumes. New Phytol., 237: 734-745. DOI:10.1111/nph.18593 |



