b. College of Life Sciences, Chongqing Normal University, Chongqing 401331, China;
c. Wolong National Natural Reserve Administration Bureau, Wenchuan 623006, Sichuan, China;
d. University of Chinese Academy of Sciences, Beijing 100049, China
Gastrochilus D. Don is an orchid genus that comprises 71 epiphytic species mainly distributed in southern China and the Southeast Asian Archipelago (Don, 1825; Tsi, 1996; Chen et al., 2009; Kumar et al., 2014; Pridgeon et al., 2014; Liu et al., 2016, 2019a; Raskoti, 2016; Liu, 2017; Govaerts et al., 2021; Li et al., 2022). China is the diversity center of the genus and has two-thirds of its species with over half of them being narrowly endemic to the country (Li et al., 2021; Zhou et al., 2021a, 2021b; Chen et al., 2022; Liao et al., 2022; Zhang et al., 2022a). It is also a genus of potentially high economic and medicinal values due to its morphological diversity, colorful labellum, and phytochemical production such as bioactive alkaloids (Luning, 1967; Pridgeon et al., 2014).
Lindley synonymized Gastrochilus with Saccolabium Blume, and this treatment had been followed by many orchid taxonomists for nearly 80 years (Lindley, 1833; Bentham and Hooker, 1883; Hooker, 1890; King and Pantling, 1898; Smith, 1905). When they are united, the name Gastrochilus is rejected in favour of Saccolabium. Later, Schlechter (1913) reinstated Gastrochilus as a distinct genus for its unique labellum being distinctively differentiated into an epichile and a hypochile (vs. absent in Saccolabium) among other morphological straits and his treatment was now widely adopted (e.g., Tsi, 1996; Liu, 2017; Liu et al., 2019a; Li et al., 2022).
Various systematic and phylogenetic hypotheses for infrageneric relationships of Gastrochilus have been proposed based on morphological and/or molecular data. Based on the morphological characters of then known 43 species, Tsi (1996) divided the genus into three sections, i.e., G. sect. Caespitosi Z.H. Tsi, G. sect. Gastrochilus, and G. sect. Microphylli (Bentham & Hook. f.) Seidenf. Zou et al. (2015) firstly analyzed phylogenetic relationships among nine species based on nuclear and plastid markers (nrITS, atpI-atpH, matK, psbA-trnH and trnL). As part of his doctoral thesis, Liu et al. (2019a) revised Gastrochilus taxonomy in China based on a combined data set consisting of one nuclear (nrITS) and four plastid markers (matK, psbA-trnH, psbM-trnD, trnL-F). His study concluded that the genus was monophyletic and resolved it into five clades, which were incongruent with the subdivisions of Tsi (1996). In his thesis, Liu (2017) proposed a new infrageneric classification of the genus, and established three new sections "G. sect. Acinacifolius Q. Liu & J.Y. Gao", "G. sect. Brachycaulis Q. Liu & J.Y. Gao", and "G. sect. Viscosae Q. Liu & J.Y. Gao". These three new sectional names were not validly published under Art. 30.9 of International Code of Nomenclature for algae, fungi and plants (Turland et al., 2018). However, in the most recent phylogenetic studies, based on data of eight molecular markers (nrITS, atpH-I, matK, psbA-trnH, psbM-trnD, rbcL, trnL-F and rps19-rpl22) of 34 species, Li et al. (2022) found different infrageneric relationships from those of Liu et al. (2019a).
In addition to the incongruent results of these major studies above, a number of studies drew different or conflicting conclusions in terms of infrageneric relationships and taxonomic treatment in Gastrochilus (e.g., Tsi, 1996; Zou et al., 2015; Liu, 2017; Liu et al., 2019a, 2020; Chen et al., 2022; Li et al., 2022; Liao et al., 2022; Zhang et al., 2022a). An updated phylogeny of the genus with a large taxon sampling is desperately needed for three reasons. Firstly, some species with similar morphological characters are difficult to distinguish from one another (Seidenfaden, 1988; Chen et al., 2009), such as G. somai (Hayata) Hayata and G. japonicus (Makino) Schltr (Tsi, 1996; Jin et al., 2010). Secondly, some recently described species of Gastrochilus have a quite restricted distribution (Tsi, 1996; Xu et al., 2010; Liu et al., 2016; Zhou et al., 2021a, 2021b) and have been poorly known other than their type materials, making it difficult to assess their true distributions, e.g., G. intermedius (Griff. ex Lindl.) Kuntze, G. xuanenensis Z.H. Tsi, and G. nanchuanensis Z.H. Tsi. Thirdly, quite a few species have been described in the past five years, and their systematic relationships remain unclear (Liu and Gao, 2018; Liu et al. 2019b, 2023; Wu et al., 2019; Rao et al., 2019; Liao et al., 2022; Nguyen et al., 2022; Zhang et al., 2022a).
In this study, we aim to elucidate the infrageneric classification of Gastrochilus. For this purpose, we reconstructed the phylogenetic relationships of 52 species in Gastrochilus using maximum likelihood (ML) and Bayesian inference (BI) methods, and analyzed the morphological characters in the context of these new evolutionary relationships. Our results represent the most comprehensively sampled molecular phylogeny of Gastrochilus to date, with increased sampling in southeast China and the tropical Asia. In addition, we also describe and illustrate two newly discovered species of this genus collected during our recent field surveys in southwest China from 2021 to 2022.
2. Materials and methods 2.1. Morphological observationsFresh material was collected from the field (Table S1). Voucher specimens have been deposited at CDBI (the acronyms of herbaria follow Thiers (2021)). Morphological information was obtained from observations and measurements of living plants in the field and dried herbarium specimens deposited mainly at CDBI, IBSC, IBK, K, KUN and PE. Detailed morphological characteristics (including floral color) of the two new species were observed and photographed based on living material and the terminology following Beentje (2012).
2.2. DNA extraction and sequencingNew sequences in the present study were obtained by the following protocols. Total DNA was extracted exclusively from silica-gel dried leaves via a Plant DNA Isolation Kit (Cat.No.DE-06111, Foregene, Chengdu, China). The sequences were amplified using the same primers (Table S2) as previous studies of Gastrochilus (Liu et al., 2019a). The PCR program consisted of an initial 4 min preheating stage at 98 ℃, followed by 35 cycles of 30 s at 98 ℃ (denaturation), 30 s at 48–56 ℃ (annealing) and 60–100 s at 68 ℃ (extension), followed by a final 8 min extension at 68 ℃. PCR products were sent to TSINGKE Biotech (Chengdu, China) for sequencing. The nrITS, matK, psbA, psbM and trnL-F sequences of G. sororius Schltr. were extracted from the genomic raw reads (unspecified paired, accession: SRR12339718), which were submitted to Royal Botanical Gardens, Kew by Johnson et al. (2019), using Easy353 v.1.5.0 (Zhang et al., 2022b) with default parameters. Detailed information concerning the sampled taxa, voucher collections and GenBank accession numbers (including the sequences retrieved from GenBank) are summarized in Table S1.
2.3. Phylogenetic analysesAll sequences were edited via Sequencher v.4.1.4 (Gene Codes, Ann Arbor, Michigan, USA) and aligned using MAFFT v.7.475 (Katoh and Standley, 2013) with default parameters. The incongruence length difference test (ILD) was used to quantify the conflicts between nuclear DNA and plastid DNA data in PAUP v.4.0a169 (Darlu and Lecointre, 2002; Swofford, 2002). The nucleotide substitution models for these data matrices were estimated using jModeltest v.2.1.6 (Posada, 2008) software and the best fit models were selected using the corrected Akaike Information Criterion (AICc). Bayesian inference (BI) and Maximum likelihood (ML) analyses were performed to infer the phylogenetic relationships within this genus based on a data set of combined nrITS and plastid sequences (matK, psbA-trnH, psbM-trnD, trnL-F). BI analysis was conducted using MrBayes v.3.2.7a (Ronquist and Huelsenbeck, 2003), with two separate Markov-chain Monte Carlo (MCMC) Chains (1, 000, 000 generations and sampled every 1000 generations). The first 25% of the trees were discarded as burn-in, and the remaining trees were used to generate a majority-rule consensus tree. ML analyses were performed using IQ-TREE v.1.4.2 (Nguyen et al., 2014) with branch support estimated using 2000 replicates of both SH-like approximate likelihood-ratio test (SH-aLRT) (Guindon et al., 2010) and the ultrafast bootstrapping algorithm (UFboot) (Minh et al., 2013).
2.4. Character evolutionFour morphological characteristics were selected to study character evolution in Gastrochilus. The one vegetative trait was leaf shape and length and was divided into four states: < 5 cm, long elliptical/5–15 cm, falcate oblong/ > 15 cm, and long strips. The three reproductive characteristics included epichile hairs (papillate hairs/fimbriate hairs/glabrous epichile), marginal shape of epichile (fringed/entire or slightly erose), and epichile surface being smooth or not (a smooth epichile/an epichile with central thickened cushion or irregular projection). Patterns of character states are presented in the ML tree without outgroups under the maximum parsimony criterion using the Mesquite v.3.70 software package (Maddison and Maddison, 2011) with default parameters. All the morphological characteristics were coded for further analysis (see Table 1).
| Character | Character states |
| Epichile hairs | 0: papillate hairs; 1: fimbriate hairs; 2: glabrous epichile |
| Leaf shape and length | 0: < 5 cm, long elliptical; 1: 5–15 cm, falcate oblong; 2: > 15 cm, long strips |
| Margin shape of epichile | 0: fringed; 1: entire or slightly erose |
| Epichile smooth or not | 0: smooth epichile; 1: epichile with central thickened cushion or irregular projection |
The aligned nrITS matrix was 685 nucleotides in length with 195 variable sites and the combined four plastid markers matrix included 3601 nucleotides in length with 200 variable sites, consisting of 815 bp for matK, 709 bp for psbA-trnH, 1078 bp for psbM-trnD, and 999 bp for trnL-F, respectively. The attributes of five plastid markers are summarized in Table S2. The ILD test (P = 0.19) showed that the plastid DNA and nrITS were suitable for combined analysis, which resulted in consistent phylogenetic topologies (Fig. 1-Ⅰ) by applying the two algorithmic methods. Our data reinforced the monophyly of Gastrochilus as a whole (BI/ML = 0.99/91%), and its members were further resolved into six monophyletic clades. Clade A (BI/ML = 1/100%) and Clade B (BI/ML = 1/100%) were resolved as sister to the rest of Gastrochilus with strong support (BI/ML = 0.99/91%), followed by Clade C (BI/ML = 1/97%), Clade D (BI/ML = 1/91%) and a clade including Clade E (BI/ML = 0.88/91%) and Clade F (BI/ML = 1/99%), which were sister to each other (BI/ML = 1/92%). It is noted that the generic type, G. calceolaris (Buch.-Ham. ex Sm.) D. Don, was resolved in Clade C. In addition, G. tianbaoensis Q. Liu & Y.H. Tan, G. linearifolius Z.H. Tsi and G. intermedius were clustered into Clade C, while G. pseudodistichus (King & Pantl.) Schltr. was resolved in a new clade including three species described quite recently, G. kadooriei Kumar et al., G. wenshanensis Q. Liu et al. and G. tsii Q. Liu et al.
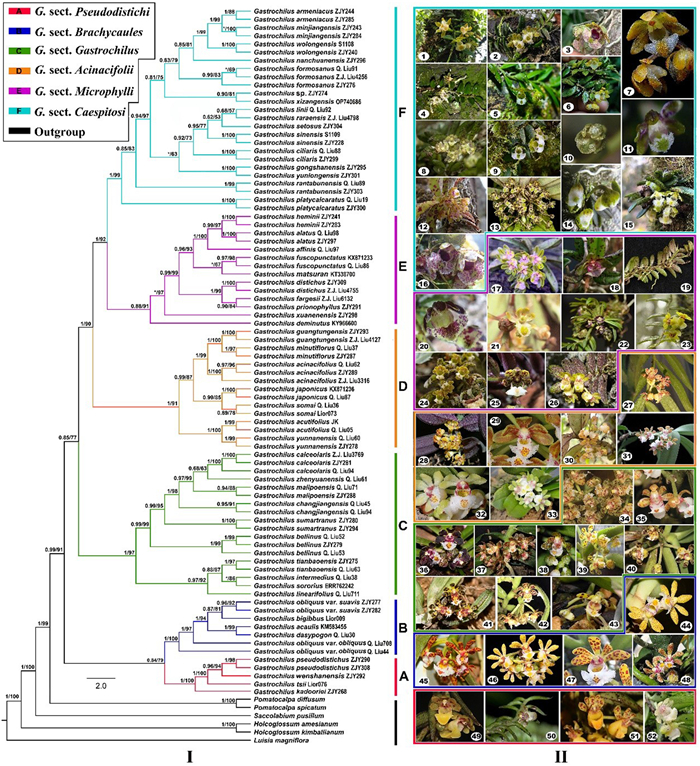
|
| Fig. 1 Ⅰ. Phylogenetic tree of Gastrochilus. Numbers before slash indicate Bayesian posterior probabilities and numbers after slash indicate ML bootstrap supports for major lineages. Asterisk (*) indicates that a node is not supported in the analysis. Ⅱ. Morphological characteristics of 52 sampled Gastrochilus species. 1. G. armeniacus. 2. G. minjiangensis. 3. G. gongshanensis. 4. G. wolongensis. 5. G. formosanus. 6. G. sp. 7. G. linii. 8. G. ciliaris. 9. G. sinensis. 10. G. xizangensis. 11. G. setosus. 12. G. raraensis. 13. G. platycalcaratus. 14. G. nanchuanensis. 15. G. rantabunensis. 16. G. yunlongensis. 17. G. xuanenensis. 18. G. matsuran. 19. G. fargesii. 20. G. affinis. 21. G. deminutus. 22. G. heminii. 23. G. alatus. 24. G. distichus. 25. G. prionophyllus. 26. G. fuscopunctatus. 27. G. guangtungensis. 28. G. minutiflorus. 29. G. acutifolius. 30. G. acinacifolius. 31. G. yunnanensis. 32. G. japonicus. 33. G. somai. 34. G. changjiangensis. 35. G. tianbaoensis. 36. G. bellinus. 37. G. sororius. 38. G. sumatranus. 39. G. malipoensis. 40. G. zhengyuanensis. 41. G. calceolaris. 42. G. linearifolius. 43. G. intermedius. 44. G. obliquus var. obliquus. 45. G. obliquus var. suavis. 46. G. dasypogon. 47. G. acaulis. 48. G. bigibbus. 49. G. tsii. 50. G. kadooriei. 51. G. pseudodistichus. 52. G. wenshanensis. Photo credits: Yue-Hong Cheng (1, 2); Ming-Feng Long (3, 24, 30); Min Liao (4, 36, 41); Jodyhsieh (5, 15); Jun-Yi Zhang (6, 16, 17, 22, 25, 27, 39); http://www.lhbkw.com/(7, 8, 31, 51); Feng Li (9); Chen et al. (2022) (10); Li et al. (2022) (11); Chen Shu (12); http://www.orchidspecies.com/(13, 18, 28, 29, 32); Jin et al. (2019) (14); Xiao-Hua Jin (19, 23); Su-Dung Sheng (26); http://orchids.la.coocan.jp/(33); Liu et al. (2019a) (34, 40); Liu et al. (2016) (35, 47); Philipp Wojtas (37); http://www.epharmacognosy.com/(38); Bhattacharjee et al. (2021) (42); Zi Wang (43); Xin-Xin Zhu (44, 45); Lynn O'Shaughnessy (46); http://www.bloggang.com/ (48); Liu et al. (2023) (49, 52); Kumar et al. (2014) (50). |
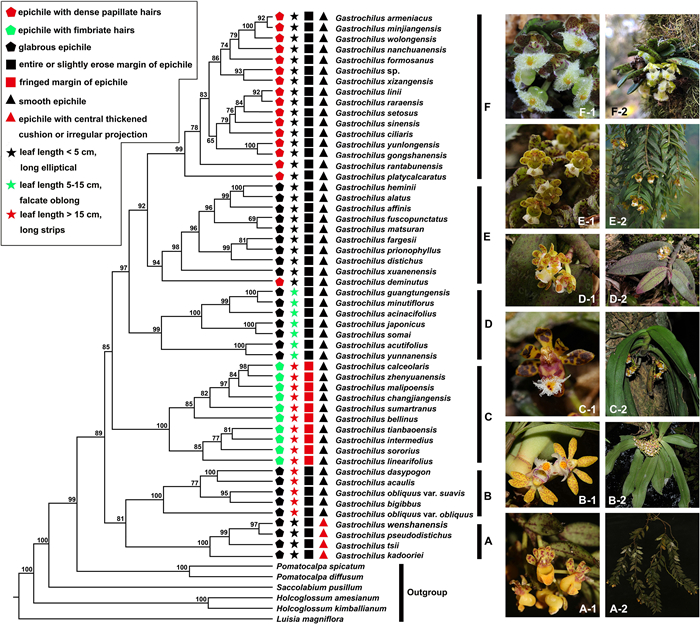
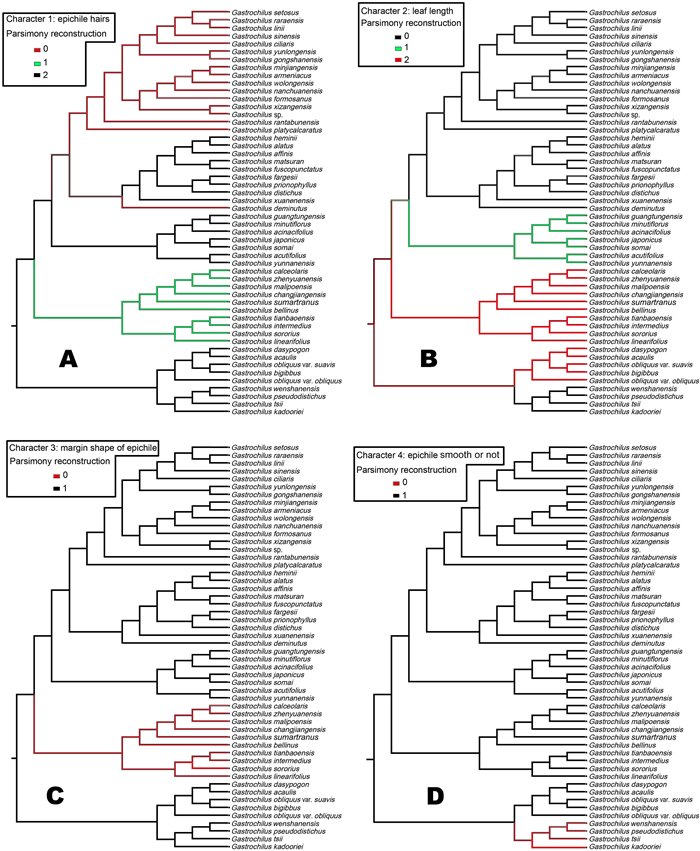
Morphologically, labellum structure and leaf size of Gastrochilus species exhibited the most diversity (Fig. 1-Ⅱ: 1–52). As shown in Fig. 2 and illustrated in Fig. 3A–D distinguishable patterns of character states across the phylogeny were revealed for our four chosen key morphological traits (epichile hairs, leaf length, margin shape of epichile, and epichile smooth or not). Our analyses indicate that glabrous epichile hairs (character 1) are the ancestral in Gastrochilus, epichiles with fimbriate hairs were derived once and epichiles with dense papillate hairs were derived twice independently, respectively (Fig. 3A). The ancestral traits of Clades B and C include leaf length > 15 cm and long stripes, whereas those of Clades A, D and F are long elliptical leaves with a length < 5 cm (Fig. 3B). In addition, we found that the entire or slightly erose marginal shape of epichiles is ancestral state in Gastrochilus, while the fringed marginal shape of epichiles was derived one time independently in Clade C (Fig. 3C). In Clades B–F, smooth epichiles are reconstructed as the ancestral state in Gastrochilus, whereas an epichile with central thickened cushion or irregular projection derived one time independently in Clade A (Fig. 3D).
|
| Fig. 2 Morphological character reconstruction of Gastrochilus on the ML tree. A. G. sect. Pseudodistichi (A-1, 2, type: G. pseudodistichus). B. G. sect. Brachycaules (B-1, 2, type: G. obliquus). C. G. sect. Gastrochilus (C-1, 2, type: G. calceolaris). D. G. sect. Acinacifolii (D-1, 2, type: G. acinacifolius). E. G. sect. Microphylli (E-1, 2, type: G. distichus). F. G. sect. Caespitosi (F-1, 2, type: G. rantabunensis). Photo credits: Liu et al. (2023) (A-1, 2); Xin-Xin Zhu (B-1); Bing Liu (B-2); Jun-Yi Zhang (C-1, E-1, F-1); Jun Liu (C-2); Yan Liu (D-1, 2); Hai-Ning Li (E-2); Jody Hsieh (F-2). |
|
| Fig. 3 Maximum parsimony character optimization on the ML tree of Gastrochilus using the software package Mesquite. A. character 1: epichile hairs (0: papillate hairs, 1: fimbriate hairs, 2: glabrous). B. character 2: leaf length (0: < 5 cm, 1: 5–15 cm, 2: > 15 cm). C. character 3: margin shape of epichile (0: fringed, 1: entire or slightly erose). D. character 4: epichile smooth or not (0: smooth epichile, 1: epichile with central thickened cushion or irregular projection). |
The identities of two newly discovered species, Gastrochilus armeniacus and G. minjiangensis, are supported by this study. They are closely related species (BI/ML = 1/99%) and form a clade with G. wolongensis Jun Y. Zhang, B. Xu & Yue H. Cheng (BI/ML = 1/99%). Moreover, G. nanchuanensis is further supported to be sister to a clade containing these three species (BI/ML = 0.85/81%) within Clade F.
4. Discussion 4.1. Phylogeny of GastrochilusBased on our combined plastid and nuclear markers and the largest sampling (ca. 71% of all species) of Gastrochilus so far, our phylogenetic results further support the monophyly of Gastrochilus (Fig. 1-I), which is in agreement with previous studies (Zou et al., 2015; Liu, 2017; Liu et al., 2019a, 2020; Chen et al., 2022; Li et al., 2022; Liao et al., 2022; Zhang et al., 2022a). Within Gastrochilus, our data resolved the genus into six well-supported clades, a phylogeny different from what was reported by either Tsi (1999)with 43 species, or Liu et al. (2019a) with 28 species, or Li et al. (2022) with 34 species. Our phylogenetic analysis supports most species in G. sects. Brachycaules sensu Q. Liu & J.Y. Gao (2017), Gastrochilus sensu Q. Liu & J.Y. Gao (2017), Acinacifolii sensu Q. Liu & J.Y. Gao (2017), Microphylli sensu Q. Liu & J.Y. Gao (2017) and Caespitosi sensu Tsi (1996), although a few species are incongruent with their results, such as G. tianbaoensis, G. intermedius, G. pseudodistichus and G. linearifolius. This result shows that the infrageneric taxonomy of this genus can be improved by increasing representative species in phylogenetic analysis.
4.2. Infrageneric relationships of GastrochilusIn our study, phylogenetic and molecular evidence were used to divide the genus Gastrochilus into six clades.
Of the four species in Clade A (Fig. 1-I-A and 2A), three of them, Gastrochilus kadooriei, G. wenshanensis and G. tsii, were described quite recently (Kumar et al., 2014; Liu et al., 2023), which explains why this monophyletic lineage was not detected in previous studies (e.g., Liu, 2017). Morphologically, this lineage shares short leaves (< 5 cm in length) and labellum with a semilunar epichile being smooth, glabrous and entire in margin (Figs. 1-3). Although G. pseudodistichus had been placed in G. sect. Microphylli (corresponding to our Clade E) by Tsi (1996) on the basis of its distichous leaves, its sister relationship with G. wenshanensis in Clade A is highly supported in addition to their floral similarity. Phylogenetically, Clade A is more closely related to Clade B than to any other clades in the genus, while these two groups of species are clearly differentiated from each other by the size and arrangement of leaves and the shape and decoration of the epichile, among other characters. Therefore, this group merits being treated as a separate section, which we name G. sect. Pseudodistichi.
The species composition of Clade B (Figs. 1-I-B and 2B) partially agrees with that of Clade I in Liu et al. (2019a), with the exception of Gastrochilus acaulis (Hook. f.) Kuntze, G. bigibbus (Rchb. f. ex Hook. f.) Kuntze and G. obliquus var. suavis (Seidenf.) Z.H. Tsi. Species of Clade B share short and stout stems (to 2 cm in length and ca. 6 mm in diameter), relatively longer leaves (more than 15 cm in length), and an epichile with an apical central thickened cushion that is mostly glabrous and erose on the margin (Figs. 1-3). Both are obviously distinguishable from species in G. sect. Gastrochilus (most species of this section corresponding to Tsi, 1996). Thus, it is more reasonable to treat this group of species as a separate section as suggested by Liu (2017). It is notable that G. suavis Seidenf. was treated as a variety of G. obliquus (Lindl.) Kuntze on the basis of their floral resemblance and then only the color and spot pattern on sepals and petals being different (Tsi, 1996). However, our molecular data indicate that it is more closely related to G. bigibbus than to G. obliquus var. obliquus (Fig. 1-I-A). All species in this group are actually superficially similar to each other and their morphological demarcations need further clarification. It is therefore here we maintain the species G. suavis as cited in the following G. sect. Brachycaules.
Our Clade C (Figs. 1-I-C and 2C) consists of 10 species. In general, these species have long (more than 15 cm in length) and stout (6–7 mm in diameter) stems, relatively larger leaves (more than 15 cm in length) with a mostly asymmetrically bilobed apex, and an epichile in suborbicular-triangular outline, with central thickened cushion and are elongated fimbriate lacerate (or hairy) on the margin. The more recently published additional accounts, such as Gastrochilus tianbaoensis, G. changjiangensi Q. Liu & M.Z. Huang and G. zhenyuanensis Q. Liu & D.P. Ye, correspond to G. sect. Gastrochilus as defined by Tsi (1996). It is worth mentioning that G. tianbaoensis was resolved in Clade III in Liu et al. (2019a), similar to our G. sect. Aacinacifolii. However, our analysis strongly suggests that G. tianbaoensis should be placed in G. sect. Gastrochilus, and it forms a sister relationship with a clade containing G. intermedius and G. sororius.
The seven species in our clade D (Figs. 1-I-D and 2D) mostly have stem 10–15 cm in length and 4–6 mm in diameter, medium-sized leaves of 5–15 cm in length, and a subtriangular epichile with a central thickened cushion. These combined characters can differentiate the species ascribed to Gastrochilus sect. Gastrochilus, and they generally also agree with a separate sectional demarcation proposed in Liu (2017). G. somai is here considered a different species and is resolved as sister to G. japonicus, in contrast to Tsi (1996), where G. somai was synonymized with G. japonicus on the basis of its superficial vegetative and floral characters. Its identity has also been supported by both Jin et al. (2010), based on detailed perusal of inflorescence and the morphology of labellum (i.e., its umbel like inflorescence and different width between a hypochile and an epichile, among others) and Liu (2017) based on molecular data.
Clade E (Figs. 1-I-E and 2E) is mostly in accordance to Gastrochilus sect. Microphylli sensu Tsi (1996). The clade is characterized by floral traits such as small flowers (petals and sepals < 6 mm in length), nearly reniform glabrous epichiles with central irregular projections, and in some species, hypochiles split into two conical protrusions (Figs. 1-3). This clade contains 10 species and most of them are separated from G. sect. Microphylli sensu Tsi (1996). The exception is G. xuanenensis, which was placed in G. sect Caespitosi (referring to our Clade F) by Tsi (1996) based on its short and erect stems and tufted or closely spaced distichous leaves. As shown in Fig. 1, this species is resolved as sister to the other eight species, and they together are sister to G. deminutus J.M.H. Shaw. Moreover, extensive field observations in its type locality have revealed that its glabrous epichile is quite stable and agrees with the following morphological range of G. sect. Microphylli.
Most of the 16 species in our Clade F (Figs. 1-I-F and 2F) are narrowly endemic to specific locations in south China, Japan, and Vietnam. They are characterized by short stems, alternate or basal leaves, an epichile with a central thickened cushion and densely covered with papillate hairs. This group corresponds to the sectional definition of Gastrochilus sect. Caespitosi by Tsi (1996). Liu (2017) rejected this section and combined some species that were ascribed to G. sect. Microphylli to make an unpublished section "as G. sect. Viscosae" on the basis of the hairy epichile of those species. However, Liu (2017) did not include some of the key species in their phylogenetic analysis, especially G. xuanenensis. Our analysis, which has increased sampling, strongly supports the monophyly of Clade F (Fig. 1-I-F), within which the sectional type species of G. sect. Caespitosi designated by Tsi (1996), G. rantabunensis C. Chow ex T.P. Lin, is nested. It is reasonable to adopt this section. This section, which includes our new species G. armeniacus and G. minjiangensis (described in the following) (Fig. 1-Ⅱ: 1–16), requires updated morphological analysis.
4.3. Morphological character evolutionEvolutionary changes in reproductive structures are of fundamental importance to theories about the early evolution and subsequent diversification of flowering plants (Kim and Choi, 2011). This study provides a robust and well-resolved framework to test infrageneric classification and to understand morphological character evolution of Gastrochilus. Thus, we employed four morphological characters (epichile hairs, leaf length, margin shape of epichile, and epichile smooth or not) to indicate the evolutionary pattens and trends of Gastrochilus clades.
Three types of epichile hairs (Character 1 in Fig. 3A) occur in Gastrochilus: epichiles with dense papillate, fimbriate hairs and glabrous epichile. This character was the basis for the infrageneric classification proposed by Liu et al. (2017) that distinguishes G. sect. Caespitosi (Clade F) from other sections. In the present study, the most likely ancestral state was determined to be the labellum that has an epichile with a glabrous adaxial surface, which then evolved one time into fimbriate hairs in Clade C and following two independent evolutionary changes into dense papillate hairs in Clades E (only G. deminutus) and F. Notably, the epichile of G. deminutus is morphologically similar to Clade F, with a central thickened cushion that is sparsely covered with short papillate hairs adaxially. However, similar to species in Clade E, the hypochile of species in Clade F splits into two conical protrusions at the apex. Therefore, our results suggest this species represents a transitional state between the two clades.
Three type of leaf length (Character 2 in Fig. 3B) were used to reconstruct the evolutionary patterns and trends of the Gastrochilus clades. In the current study, long elliptical leaves shorter than 5 cm (Clades A, E and F) and falcate oblong leaves between 5 and 10 cm (Clade D) seem to have evolved from long strips of leaves longer than 15 cm in length (Clades B and C). Our phylogenetic analysis indicated that vegetative morphological characters (leaf length) are homoplasious states, as detected for G. wenshanensis, G. tsii, G. pseudodistichus and G. kadooriei, where contradictory results were inferred among different traits.
Margin shape of the epichile (Character 3 in Fig. 3C) has been used to distinguish G. sect. Gastrochilus (Clade C) from other sections of Gastrochilus. Our results relating to the evolutionary trend for margin shape of epichile support the view of Liu et al. (2017) who separate species from G. sect. Gastrochilus into Clades B and D.
Our ancestral state reconstruction revealed that epichiles with central thickened cushions or irregular projections are probably a primitive character state (Character 4 in Fig. 3D). In contrast, the smooth epichile of G. sect. Pseudodistichi (Clade A) was the most evolved character state, and is present on plants distributed in S and SW China, northeastern India, Myanmar, Vietnam, and Thailand.
In conclusion, our molecular phylogeny strongly indicates that a labellum with an adaxially epichile entirely or slightly erose at the margin is the ancestral states in Gastrochilus (Figs. 2, 3A and 3C). Vegetative ancestral traits of the genus include having strips of leaves longer than 15 cm in length (Figs. 2 and 3B). We also detected homoplasies between G. wenshanensis, G. tsii, G. pseudodistichus and G. kadooriei, where contradictory results were inferred among different straits. The well-resolved phylogeny of the entire genus presented here, together with more detailed phylogenies of particular clades, will permit the reconstruction of a new infrageneric classification.
5. Taxonomic treatment 5.1. Gastrochilus D. Don in Prodr. Fl. Nepal: 32. 1825Type: Gastrochilus calceolaris (Buch.-Ham. ex Sm.) D. Don ≡ Aerides calceolaris Buch.-Ham. ex Sm.
Herb epiphytic, prostrate or drooping. Roots long and curved, vermiform. Stems thick and short or slender, with few to many nodes. Leaves numerous, slightly fleshy or leathery, usually biseriate and alternate, flattened, apex unlobed or 2–3-lobed, base with jointed and clasping sheaths. Inflorescences lateral, unbranched or rarely branched, with stout or slender inflorescence stalk and inflorescence axis; racemes or umbels, with few to many flowers. Flowers small to moderately large, ± fleshy; sepals and petals mostly similar, ± extending and usually obovate; labellum divided into an epichile and a hypochile, epichile extending forward perpendicular to hypochile; hypochile firmly adnate to both sides of and nearly parallel to the column, galeate, cup-shaped, hemispherical or subconical; column short and thick, footless; rostellum short, 2-lobed; anther nearly globose, apex narrowed; pollinia waxy, 2, subglobose, porate or rarely cleft, attached by a common narrow stipe to a bilobed viscidium.
The genus comprises 73 species distributed in southern China and the Southeast Asian Archipelago and can be divided into six sections.
5.2. Key to sections of Gastrochilus1a Epichile smooth, without thickened central cushion or projection; leaf apex usually 3-split with a central long awn … … … … … … … … … …. … … … … … 1. Sect. Pseudodistichi
1b Epichile with central thickened cushion or irregular projection; leaf apex undivided or otherwise split without central long awn … … … … … … … … … … … … …… … … …... 2
2a Plants erect, stem thick and short; leaves clustered at the base of stem; epichile margin slightly erose … … … … … … … … … … … … … … … … …… … … 2. Sect. Brachycaules
2b Plant usually prostrate or pendulous, or rarely erect, but generally elongated; leaves alternate along stem; epichile lacerate to fringe on margin, or glabrous … … … … …… … 3
3a Leaves to 5 cm in length, usually oblong or elliptic; epichile either glabrous or with papillate hairs … … … … … … … … … … … … … … … … … … … … … … …..… … … … … … 4
4a Leaves arranged in distinctly distichous plane; epichile glabrous … … … … … … … … … … … 3. Sect. Microphylli
4b Leaves radially alternate, sometimes rather densely spaced; epichile densely coved with papillate hairs … … … … … … … … … … … … … … … … … … 4. Sect. Caespitosi
3b Leaves 5–15 cm, or much longer and strip-shaped; epichile with lacerate fringe on margin, on some species erose … … … … … … … … … … …. …. …. … … … …… … … … … … 5
5a Stem thick and elongated; leaves without awned tip at apex; epichile with lacerate fringe on margin … … … … … …. … … … … … …. …. …. …. …… … … … … 5. Sect. Gastrochilus
5b Stem slender, elongated or rarely short; leaves usually with 2–3 awned tips; epichile lacerate or erose on margin … … … … … … … … … … … …. …… … … … 6. Sect. Acinacifolii
5.3. Gastrochilus sect. Gastrochilus. 盆距兰组Plants pendulous or erect. Stem stout and elongated. Leaves long strips, more than 15 cm in length, apex laterally 2-lobed into distinctly unequal parts. Epichile suborbicular-triangular, with a central thickened cushion, adaxially along margin with long fimbriate hairs, margin itself irregularly fimbriate or erose. Species assigned to this section clustered together in our Clade C (Fig. 1).
Eleven species are known in this section and are mainly distributed in S and SW China (Hainan, Xizang and Yunnan), Nepal, Bhutan, India, Myanmar, Laos, Vietnam, Thailand, Malaysia, the Philippines, and Indonesia. These species are Gastrochilus bellinus (Rchb. f.) Kuntze, G. calceolaris (Buch.-Ham. ex J.E. Smith) D. Don, G. changjiangensis Q. Liu & M.Z. Huang, G. intermedius (Griff. ex Lindl.) Kuntze, G. linearifolius Z.H. Tsi, G. malipoensis X.H. Jin & S.C. Chen, G. pseudocalceolaris S. Dey et al., G. sororius Schltr., G. sumatranus J.J. Sm., G. tianbaoensis Q. Liu & Y.H. Tan, and G. zhenyuanensis Q. Liu & D.P. Ye.
5.4. Gastrochilus sect. Pseudodistichi Jun Y. Zhang & H. He, sect. nov. 小唇组Type: Gastrochilus pseudodistichus (King & Pantl.) Schltr. ≡ Saccolabium pseudodistichum King & Pantl.
Plants pendulous. Stems elongated and slender, often about 2 mm thick. Leaves distichous and alternate, ovate-lanceolate, to 5 cm in length, apex acute and usually 2–3-split into short awns with the central one longest. Flowers small, sepals to 6 mm long, epichile semilunar and entire, thickly fleshy, smooth and glabrous. This section corresponds to our Clade A (Fig. 1).
This section consists of six species mainly distributed in S and SW China (Hong Kong, Xizang and Yunnan), northeastern India, Myanmar, Vietnam, and Thailand. Four species are sampled in the present study. These species are Gastrochilus dulongjiangensis Q. Liu & J.Y. Gao, G. kadooriei Kumar et al., G. pankajkumarii Vuong et al., G. pseudodistichus (King et Pantl.) Schltr., G. tsii Q. Liu et al., and G. wenshanensis Q. Liu et al.
5.5. Gastrochilus sect. Brachycaules Q. Liu & J.Y. Gao ex Jun Y. Zhang & H. He, sect. nov. 短茎组.Type: Gastrochilus obliquus (Lindl.) Kuntze ≡ Saccolabium obliquum Lindl.
Plants erect. Stem thick and short. Leaves borne at the base of stem, broad, long strips, usually more than 15 cm in length, undivided or equilaterally bicircularly divided in the apex. Epichile subtriangular, with a central thickened cushion, adaxially glabrous, margin lacerate or erose.
This section includes ten species distributed mainly in S and SW China (Hainan, Sichuan and Yunnan), Nepal, Bhutan, India, Myanmar, Laos, Vietnam, and Thailand. These species are Gastrochilus acaulis (Hook. f.) Kuntze, G. bigibbus (Rchb. f. ex Hook. f.) Kuntze, G. dasypogon (Lindl.) Kuntze, G. flabelliformis (Blatt. & McCann) C.J. Saldanha, G. hainanensis Z.H. Tsi, G. obliquus (Lindl.) Kuntze, G. patinatus (Ridl.) Schltr., G. pechei (Rchb. f.) Kuntze, G. suavis Seidenf., and G. subpapillosus Z.H. Tsi.
5.6. Gastrochilus sect. Acinacifolii Q. Liu & J.Y. Gao ex Jun Y. Zhang & H. He, sect. nov. 镰叶组.Type: Gastrochilus acinacifolius Z.H. Tsi.
Plants pendulous or erect. Stems elongated. Leaves falcate oblong, 5–15 cm in length, apex acute and sometimes awned. Epichile subtriangular, with a central thickened cushion, adaxially glabrous and lacerate or erose in margin.
This section consists of 12 species widely distributed in S China (Chongqing, Guangdong, Hong Kong, Hainan, Sichuan, Taiwan, Yunnan and Xizang), Nepal, Bhutan, India, Myanmar, Laos, Vietnam, and Thailand. These species are Gastrochilus acinacifolius Z.H. Tsi, G. acutifolius (Lindl.) Kuntze, G. arunachalensis A.N. Rao, G. fargesii (Kraenzl.) Schltr., G. guangtungensis Z.H. Tsi, G. japonicus (Makino) Schltr., G. labrosus Q. Liu & X.H. Jin, G. menglaensis Q. Liu, Jian W. Li & X.H. Jin, G. minutiflorus Aver., G. rutilans Seidenf., G. somai (Hayata) Hayata, and G. yunnanensis Schltr.
5.7. Gastrochilus sect. Microphylli (Benth. & Hook. f.) Seidenf. in Opera Bot. 95: 285. 1988, "Microphyllae" ≡ Saccolabium sect. Microphylla Benth. & Hook. f., Gen. Pl. 3: 579. 1883, "Microphyllae". ≡ Saccolabium sect. Distichophylla Hook. f., Fl. Ind. Brit. 6: 55. 1890, "Distichophyllae", nom. illeg. (Art. 52). 列叶组.Type: Gastrochilus distichus (Lindl.) Kuntze.
Plants prostrate, rarely pendulous. Stems elongated and slender, often about 2 mm thick. Leaves distichous and alternate, blade narrow, < 5 cm in length, the apex usually acute with 2–3 awns. Flowers small, sepals to 6 mm long, epichile subreniform, glabrous, with central irregular projection, and hypochile sometimes splits into two conical protrusions at apex.
When the section is transferred from Saccolabium to Gastrochilus, the epithet might be corrected as "Microphylli" under Art. 21.2 of Shenzhen Code (Turland et al., 2018).
This section is composed of 12 species distributed mainly in S and SW China (Taiwan, Sichuan, Xizang and Yunnan), Nepal, Bhutan, and India. These species are Gastrochilus distichus (Lindl.) Kuntze, G. heminii M. Liao, B. Xu & Yue H. Cheng, G. affinis (King & Pantl.) Schltr., G. alatus X.H. Jin & S.C. Chen, G. fuscopunctatus (Hayata) Hayata, G. matsuran (Makino) Schltr., G. prionophyllus H. Jiang, D.P. Ye & Q. Liu, G. xuanenensis Z.H. Tsi, G. deminutus J.M.H. Shaw, G. minimus Jian W. Li, D.P. Ye & X.H. Jin, G. yei Jian W. Li & X. H. Jin, and G. nepalensis Raskoti.
5.8. Gastrochilus sect. Caespitosi Z. H. Tsi in Guihaia 16(2): 143. 1996. 毛唇组.Type: Gastrochilus rantabunensis C. Chow ex T.P. Lin.
Plants prostrate or pendulous. Stems elongated or short. Leaves radially alternate or quite densely spaced from each other, to 5 cm in length, apex acute. Epichile with a central thickened cushion, and adaxially densely covered with papillate hairs.
This section comprises 22 species distributed widely in S and SW China (Chongqing, Fujian, Guangdong, Guangxi, Guizhou, Hubei, Jiangsu, Sichuan, Taiwan, Yunnan, Xizang, and Zhejiang), Japan, and Vietnam. These species are Gastrochilus armeniacus Jun Y. Zhang, B. Xu & Yue H. Cheng, G. ciliaris F. Maek., G. deltoglossus T.C. Hsu et al., G. dresslerii Vuong, Aver. & V.S. Dang, G. formosanus (Hayata) Hayata, G. gongshanensis Z.H. Tsi, G. hoi T.P. Lin, G. linii Ormerod, G. matsudae Hayata, G. minjiangensis Jun Y. Zhang, B. Xu & Yue H. Cheng, G. nanchuanensis Z.H. Tsi, G. nanus Z.H. Tsi, G. platycalcaratus (Rolfe) Schltr., G. rantabunensis C. Chow ex T.P. Lin, G. raraensis Fukuyama, G. saccatus Z.H. Tsi, G. setosus Aver. & Vuong, G. sinensis Z.H. Tsi, G. toramanus (Makino) Schltr., G. wolongensis Jun Y. Zhang, B. Xu & Yue H. Cheng, G. xizangensis W.S. Chen, M. Lei & X.L. Wang, G. yehii S.I. Hsieh, C.T. Lee & J.H. Wu, and G. yunlongense W.H. Rao, L.J. Chen & Z.J. Liu.
5.9. Gastrochilus armeniacus Jun Y. Zhang, B. Xu & Yue H. Cheng, sp. nov. (杏黄盆距兰xing huang pen ju lan). Figs. 1-Ⅱ-1 and 4Type: CHINA. Sichuan Province: Wenchuan County, Sanjiang Town, deciduous broadleaf forest, on tree trunk, elev. ca. 1380 m, in flowering, 30 April 2022, Jun-Yi Zhang, Min Liao & Yue-Hong Cheng ZJY147 (Holotype CDBI!).
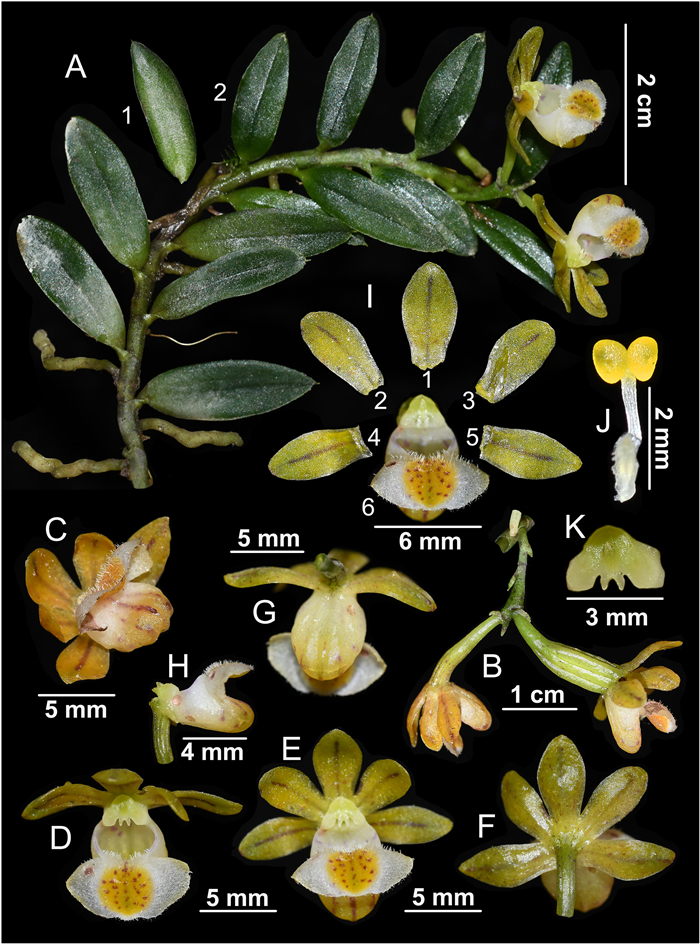
|
| Fig. 4 Gastrochilus armeniacus A. Flowering plant (A1: leaf reverse, A2: leaf front). B. Inflorescence. C-G. Flowers. H. Labellum. I. Dissection of a flower (I1: dorsal sepal; I2–I3: petals; I4–I5: sepals; I6: labellum). J. Stamen. K. Stigma. |
Description. Epiphyte on tree trunk. Roots vermiform. Stems prostrate and pendulous, 7–15 cm long, ca. 2 mm thick, unbranched, internodes 4–6 mm long. Leaves dichotomously alternate, green, unspotted, slightly leathery, elongate-elliptic, 1.2–1.8 × 0.4–0.8 cm, apex acute and 3-lobed. Inflorescence shortly umbellate-like, with 1–2 flowers; peduncle slender, 6–9 mm long, slightly enlarged distally, with 2 alternate involucres; floral bracts small, ovate-triangular, ca. 1 mm long, apex acute; pedicel and ovary ca. 1.2 cm long; flowers apricot-yellow, later turning golden yellow, ca. 1.4 × 1.2 cm; dorsal sepal elliptic, with one red-purple stripe, 4–6 × 3–4 mm, apex obtuse, with 1 vein; lateral sepals obliquely oblong, with one red-purple stripe, 5–7 × 3–4 mm, apex subacute, with 1 vein; petals obovate, with one red-purple stripe, 4–6 × 2–3 mm, apex rounded, with 1 vein; epichile reniform or reniform-triangular, recurved outward, 3–4 × 6–9 mm, apex obtuse, margin slightly undulate, sparsely covered with short papillate hairs, central golden yellow thickened tissue with purple-red spots; hypochile helmet-shaped, elongated, 4–5 × 6–7 mm, apex rounded, with 3–5 ridges on the outside surface, upper side margins almost at the same level as epichile; stamen column cylindrical, ca. 1.5 mm long; anther cap almost hemispheric, with two chambers, 1 × 1.2 mm; pollinia 2, 0.7 × 0.6 mm, yellow, full and nearly spherical, with a depression in the central; stigma deeply sunken, inverted V-shaped, ca. 1 mm long, yellow-green, apically forked into a subtriangular outline.
Distribution and habitat. The new species is currently known only from Wenchuan County, Sichuan Province, Southwest China. It is epiphytic, growing on tree trunks at an elevation about 1380 m.
Phenology. It flowers from April to May.
Etymology. The specific epithet 'armeniacus' refers to the apricot-yellow color of its flowers.
Relationships. This new species is similar to Gastrochilus saccatus and G. wolongensis (Fig. 1-Ⅱ-4), but it can be easily distinguished from the latter two species by its longer unbranched stems, lightly lathery leaves, apricot-yellow flowers, 1-veined sepals and petals with one red-purple stripe, and epichile with purple-red spotted golden yellow center of thickened tissue. A more detailed comparison of the three species is presented in Table 2.
| Character | G. armeniacus | G. minjiangensis | G. wolongensis | G. saccatus | G. formosanus |
| Steams | Unbranched, 7–15 cm | Branched, 10–16 cm | Unbranched, 4–9 cm | Branched, 3–9 cm | Branched, 10–40 cm |
| Leaves | Slightly leathery, green unspotted | Fleshy, green unspotted | Fleshy, green with purplish red spots | Fleshy, green unspotted | Fleshy, green with purplish red spots |
| Peduncle length | 0.6–0.9 cm | 0.6–0.9 cm | 0.4–0.7 cm | 0.5–0.7 cm | 1.0–1.5 cm |
| Flowers | Apricot-yellow; without spots; ca. 1.4 × 1.2 cm | Yellowish, with purplish spots; 1.3 × 1.2 cm | Yellowish green, with purplish red stripes; 1.2 × 1.3 cm | Unknown | Yellowish, with purplish spots; 0.8 × 0.9 cm |
| Dorsal sepal | Elliptic, with one red-purple stripe, 4–6 × 3–4 mm, apex obtuse, with 1 vein | Elliptic, with purplish red spots, 5.4–6.8 × 3.5–4.5 mm, apex obtuse, with 1 vein | Elliptic-oblong, with three red-purple stripes, 6.8–8.9 × 4.2–5.8 mm, apex slightly pointed, with 1 vein | Elliptic, with purplish red spots, 4.8 × 2.8 mm, apex obtuse, with 1 vein | Elliptic-oblong, with purplish red spots, 4.2–5.6 × 2.5–3.5 mm, apex obtuse, with 3 veins |
| Lateral sepals | Obliquely oblong, with one red-purple stripe, apex subacute, with 1 vein | Obliquely oblong, with purplish red spots, apex obtuse, with 1 vein | Obliquely oblong, with three red-purple stripes, apex obtuse, with 3 veins | Obliquely oblong, with purplish red spots, apex obtuse, with 1 vein | Obliquely oblong, with purplish red spots, apex obtuse, with 3 veins |
| Petals | Obovate, with one red-purple stripe, 4–6 × 2–3 mm, apex rounded, with 1 vein | Obovate, with purplish red spots, 4.5–6 × 2.8–3.6 mm, apex rounded, with 1 vein | Subobovate, with three red-purple stripes, 7.1–8.5 × 4.5–6 mm, apex subrounded, with 3 veins | Obovate, with purplish red spots, 4 × 2.6 mm, apex rounded, with 1 vein | Oblanceolate, with purplish red spots, 4–5 × 2.8–3 mm, apex rounded, with 3 veins |
| Hypochile | Helmet-shaped, elongated, 4–5 × 6–7 mm, apex rounded, with 4–5 ridges on the outside | Subcupular, ca. 6 × 5 mm, apex rounded, with sparse purplish-red spots on the outside | Subcupular, 3.7 × 5.8 mm, apex rounded, with conspicuous purplish-red stripes on the outside | Subhelmet-shaped or cup-shaped, 4 × 3 mm, apex rounded, with 4 ridges on the outside | Subcupular, ca. 3 × 4 mm, apex rounded, with sparse purplish-red spots on the outside |
| Epichile | Reniform or reniform-triangular, recurved outward, 3–4 × 6–9 mm, apex obtuse, margin slightly undulate, sparsely covered with short papillate hairs, central golden yellow thickened tissue with purple-red spots | Reniform, spreading, 7–11 × 4–6 mm, apex broadly notched, margin entire or slightly undulate, sparsely covered with short papillate hairs central yellow-green thickened tissue with conspicuous purplish-red spots | Reniform, spreading, 4.5–5.2 × 10–12.3 mm, apex broadly emarginate, margin entire, densely covered with short papillate hairs central yellow-green thickened tissue with purple-red spots | Reniform or reniform-triangular, recurved outward, 2.7 × 9 mm, apex obtuse, margin entire, densely covered with short papillate hairs, central yellow thickened tissue with purplish-red spots | Triangular or suborbicular, recurved outward, 2.2–3.2 × 7–9 mm, apex obtuse or subtruncate, margin entire, densely covered with short papillate hairs, central yellow thickened tissue with black spots |
| Flowering period | April and May | April and May | March and April | Unknown | Throughout year |
Conservation status. From available field observation, its habitat is remotely located and rarely susceptible to anthropogenic disturbance. However, owing to the difficulty in accessing similar habitats, we provisionally assess it as Data Deficient (DD) according to the categories and criteria of IUCN (2022) pending further investigation of more extensive inventories.
5.10. Gastrochilus minjiangensis Jun Y. Zhang, B. Xu & Yue H. Cheng, sp. nov. (岷江盆距兰min jiang pen ju lan). Figs. 1-Ⅱ-2 and 5Type. CHINA. Sichuan Province: Wenchuan County, Sanjiang Town, Xihe, deciduous broadleaf forest, on tree trunk, elev. ca. 1460 m, in flowering, 30 April 2022, Jun-Yi Zhang, Min Liao & Yue-Hong Cheng ZJY146 (Holotype CDBI!; Isotype CDBI!).

|
| Fig. 5 Gastrochilus minjiangensis A. Flowering plant. B. Leaves (B1: front, B2: reverse). C. Inflorescence. D-E. Flowers. F. Dissection of a flower (F1: dorsal sepal; F2–F3: petals; F4–F5: sepals; F6: labellum). G-H. Labellum. I. Stamen. |
Description. Epiphyte on tree trunks. Roots vermiform. Stems prostrate, slender, 10–16 cm long, ca. 2 mm thick, often branched, internodes 4–6 mm. Leaves green, unspotted, distichously alternate, slightly fleshy, oblong or elliptic, 1–2 × 4–6 mm, apex acute and 3–lobed. Inflorescence shortly umbellate, with 1–2 flowers; peduncle slender, smooth and fleshy, 6–9 mm long, slightly inflated distally; floral bracts reduced and usually inconspicuous, ovate-triangular, ca. 1 mm, apex acute; pedicel and ovary yellowish with purple-red spots, ca. 8 mm; flowers yellowish with conspicuous purple-red spots, ca. 1.3 × 1.2 cm; dorsal sepal elliptic, with purplish red spots, 5.4–6.8 × 3.5–4.5 mm, apex obtuse, with 1 vein; lateral sepals as large as the dorsal sepal, obliquely oblong, with purplish red spots, apex obtuse, with 1 vein; petals obovate, with purplish red spots, 4.5–6 × 2.8–3.6 mm, apex rounded, with 1 vein; epichile reniform, spreading, 7–11 × 4–6 mm, apex broadly notched, margin entire or slightly undulate, sparsely covered with short papillate hairs, central yellow-green thickened tissue with conspicuous purplish-red spots; hypochile nearly cupular, ca. 6 × 5 mm, apex rounded, with sparse purplish-red spots, upper oral margin almost at the same level as the epichile; stamen column cylindrical, ca. 2 mm long; anther cap almost hemispheric, with two chambers, 0.8 × 1 mm; pollinia 2, 0.8 × 0.6 mm, yellow, full and nearly spherical, with a depression in the central; stigma deeply sunken, ca. 0.8 mm long, white, apically forked into nearly ellipsoidal outline.
Distribution and habitat. This new species is only known from the type locality in Southwest China, and detailed information concerning the location and habitat is the same as the above cited type specimen.
Phenology. It flowers from April to May.
Etymology. Its specific epithet refers to the Minjiang River, of which the watershed includes the area of the type locality of this new orchid.
Relationships. It morphologically most resembles Gastrochilus formosanus (Fig. 1-Ⅱ-5), but can be clearly discerned from the latter in having green unspotted leaves (vs. purplish red spots on both sides), shorter inflorescence stalk (0.6–0.9 cm vs. 1–1.5 cm), reduced to inconspicuous floral bracts (vs. 2–3 mm), 1-veined petals and sepals (vs. 3-veined), and larger flowers (1.4 × 1.2 cm vs. 0.8 × 0.9 cm). More detailed comparison including other species in the same section is summarized in Table 2.
Conservation status. Similar to that of Gastrochilus armeniacus, its present conservation status is not clear. Because habitats that we assume are suitable for the optimal growth of this new species are quite widespread in the Wolong Nature Reserve, we tentatively assess it as Data Deficient (DD) based on the Categories and Criteria IUCN (2022), at least until more thorough investigations have been completed.
AcknowledgmentsWe thank Hong-Bo Ding, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, for access to literature. We are especially indebted to Prof. Yun-Fei Deng (South China Botanical Garden, CAS) for his critical suggestions and comments on previous versions of the manuscript. We are also indebted to Feng Chen (Chongqing Museum of Natural History) for rechecking accepted species of Gastrochilus. We would like to thank Ming-Feng Long, Jody Hsieh, Feng Li, Chen Shu, Xiao-Hua Jin, Su-Dung Sheng, Philipp Wojtas, Zi Wang, Xin-Xin Zhu, Hai-Ning Li, Yan Liu, Bing Liu and Lynn O'Shaughnessy for providing plant photos. We would like to give special thanks to Dr. Li-Bing Zhang for his guidance on the manuscript. This study was supported by the National Key Research and Development Program of China (Grant No. 2020YFE0203200), the Second Tibetan Plateau Scientific Expedition and Research (STEP) program (Grant Nos. 2019QZKK0301 & 2019QZKK0502), 2022 Central Finance Forestry Grassland Ecological Protection and Restoration National Park Subsidy Project, 2022–2023 Subsidy Projects of Prohibited Developmental Areas from the Transfer Payment of the National Key Ecological Functional Areas, 2023 Central financial protection and restoration funds for forestry and grassland ecology, and Wild Plants Sharing and Service Platform of Sichuan Province.
Author contributions
BX, HH and JYZ designed the study. YHC, SLJ, and TMH assisted in extensive field investigation and sample collection. JYZ and ML performed the experiments and analyzed the data. JYZ wrote the manuscript. ML, YF, HH and BX revised the manuscript. All authors read and approved the final manuscript.
Data accessibility statement
The newly sequenced data reported in this article are available in the GenBank Nucleotide Database (https://www.ncbi.nlm.nih.gov/) with accession numbers OP348887–OP348896, OP373112–OP373133, OQ566796–OQ566821.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.08.001.
Beentje, H., 2012. The Kew Plant Glossary, an illustrated dictionary of plant terms. Kew Publishing, London p. 164.
|
Bentham, G., Hooker, J.D., 1883. Genera Platarum. London, vol. 3, p. 579.
|
Bhattacharjee, A., Agrawala, D.K., Jalal, J.S., et al., 2021. Three new synonyms in Gastrochilus (Orchidaceae) with notes on typification of Gastrochilus calceolaris and misreport of Gastrochilus changjiangensis from India. Bot. Lett., 169: 106-118. DOI:10.1080/23818107.2021.2000889 |
Chen, S.C., Tsi, Z.H., Wood, J.J., 2009. Gastrochilus D. Don. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds). Flora of China vol. 25 (Orchidaceae). Missouri Botanical Garden Press, Science Press and St. Louis, Beijing, pp. 491-498.
|
Chen, W.S., Lei, M., Ma, C.B., et al., 2022. Gastrochilus xizangensis (Aeridinae, Vandeae, Orchidaceae), a new species from Xizang, China. Phytotaxa, 566: 219-226. DOI:10.11646/phytotaxa.566.2.6 |
Darlu, P., Lecointre, G., 2002. When does the incongruence length difference test fail?. Mol. Biol. Evol., 19: 432-437. DOI:10.1093/oxfordjournals.molbev.a004098 |
Don, D., 1825. Prodromus Florae Nepalensis. J. Gale, London p. 256.
|
Govaerts, R., Campacci, M.A., Baptista, D.H., et al., 2021. World Checklist of Orchidaceae. The board of trustees of the Royal Botanic Gardens, Kew. http://www.kew.org/wcsp/monocots/.
|
Guindon, S., Dufayard, J.F., Lefort, V., et al., 2010. New algorithms and methods to estimate Maximum-Likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol., 59: 307-321. DOI:10.1093/sysbio/syq010 |
Hooker, J.D., 1890. Flora of British India, vol. 6. L. Reeve, London.
|
IUCN., 2022. Guidelines for using the IUCN Red List Categories and Criteria, prepared by the Standards and Petitions Committee, version 15. http://www.iucnredlist.org/documents/RedListGuidelines.pdf.
|
Jin, X.H., Dai, Z.Q., Liu, Q.Y., et al., 2010. Miscellaneous taxonomic notes on Orchidaceae from China. Acta Botanica Yunnan., 32: 331-333. |
Jin, X.H., Ye, D.P., Li, J.W., 2019. Atlas of native orchids in China. Henan Science and Technology Press, Zhengzhou, China p. 1080.
|
Johnson, M.G., Pokorny, L., Dodsworth, S., et al., 2019. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-Medoids Clustering. Syst. Biol., 68: 594-606. DOI:10.1093/sysbio/syy086 |
Katoh, K., Standley, D.M., 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol., 30: 772-780. DOI:10.1093/molbev/mst010 |
Kim, C., Choi, H.K., 2011. Molecular systematics and character evolution of Typha (Typhaceae) inferred from nuclear and plastid DNA sequence data. Taxon, 60: 1417-1428. DOI:10.1002/tax.605017 |
King, G., Pantling, R., 1898. The orchids of Sikkim himalaya. Annal Royal Botanical Garden Calcutta, 8: 217-229. |
Kumar, P., Gale, S.W., Kocyan, A., et al., 2014. Gastrochilus kadooriei (Orchidaceae), a new species from Hong Kong, with notes on allied taxa in section Microphyllae found in the region. Phytotaxa, 164: 91-103. DOI:10.11646/phytotaxa.164.2.3 |
Li, J.W., Ya, J.D., Ye, D.P., et al., 2021. Taxonomy notes on Vandeae (Orchidaceae) from China: five new species and two new records. Plant Divers., 43: 379-389. DOI:10.1016/j.pld.2021.01.009 |
Li, Y., Jin, W.T., Zhang, L.G., et al., 2022. Biogeography and diversification of the tropical and subtropical asian genus Gastrochilus (Orchidaceae, Aeridinae). Diversity, 14: 396. DOI:10.3390/d14050396 |
Liao, M., Cheng, Y.H., Zhang, J.Y., et al., 2022. Gastrochilus heminii (Orchidaceae, Epidendroideae), a new species from Sichuan, China, based on molecular and morphological data. PhytoKeys, 215: 95-106. DOI:10.3897/phytokeys.215.91061 |
Lindley, J., 1833. The genera and species of Orchidaceous plants. Ridgeways, London pp. 220-225.
|
Liu, D.K., Tu, X.D., Zhao, Z., et al., 2020. Plastid phylogenomic data yield new and robust insights into the phylogeny of Cleisostoma – Gastrochilus clades (Orchidaceae, Aeridinae). Mol. Phylogenet. Evol., 145: 106729. DOI:10.1016/j.ympev.2019.106729 |
Liu, Q., 2017. Taxonomic revision study of Gastrochilus D. Don (Orchidaceae) in China. Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Yunnan, China pp. 1-145.
|
Liu, Q., Gao, J.Y., 2018. Gastrochilus dulongjiangensis (Aeridinae, Vandeae, Epidendroideae, Orchidaceae), a new species from Yunnan Province, China. Phytotaxa, 340: 293-296. DOI:10.11646/phytotaxa.340.3.11 |
Liu, Q., Kumar, P., Gao, J.Y., 2019b. Note on Gastrochilus gongshanensis (Orchidaceae). Kew Bull., 74: 21. DOI:10.1007/s12225-019-9808-3 |
Liu, Q., Song, Y., Jin, X.H., et al., 2019a. Phylogenetic relationships of Gastrochilus (Orchidaceae) based on nuclear and plastid DNA data. Bot. J. Linn. Soc., 189: 228-243. DOI:10.1093/botlinnean/boy084 |
Liu, Q., Tan, Y.H., Gao, J.Y., 2016. A new species of Gastrochilus (Aeridinae, Vandeae, Orchidaceae) and a new record species from Yunnan, China. Phytotaxa, 282: 66-70. DOI:10.11646/phytotaxa.282.1.8 |
Liu, Q., Wu, X.F., Zhou, S.S., et al., 2023. New species and record of Gastrochilus (Orchidaceae, Aeridinae) from China and Laos. Phytotaxa, 585: 210-224. DOI:10.11646/PHYTOTAXA.585.3.3 |
Luning, B., 1967. Studies on Orchidaceae alkaloids IV. Screening of species for alkaloids 2. Phytochemistry, 6: 857-861. DOI:10.1016/S0031-9422(00)86032-X |
Maddison, W.P., Maddison, D.R., 2011. Mesquite: A Modular System for Evolutionary Analysis, version 2.75. https://mesquite.project.org.
|
Minh, B.Q., Nguyen, M.A.T., von-Haeseler, A., 2013. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol., 30: 1188-1195. DOI:10.1093/molbev/mst024 |
Nguyen, L.T., Schmidt, H.A., von-Haeseler, A., et al., 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating Maximum-Likelihood phylogenies. Mol. Biol. Evol., 32: 268-274. DOI:10.1093/molbev/msu300 |
Nguyen, V.C., Averyanov, L.V., Maisak, T.V., et al., 2022. Gastrochilus pankajkumarii, (Aeridinae, Epidendroideae, Orchidaceae) a new lithophytic orchid from southern Vietnam. Taiwania, 67: 35-39. DOI:10.6165/tai.2022.67.35 |
Posada, D., 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol., 25: 1253-1256. DOI:10.1093/molbev/msn083 |
Pridgeon, A.M., Cribb, P.J., Chase, M.W., et al., 2014. Genera orchidacearum: Epidendroideae, volume 6, Part 3. Oxford University Press, Oxford p. 544.
|
Rao, W.H., Liu, Z.J., Zhang, G.Q., et al., 2019. A new epiphytic species of Gastrochilus (Orchidaceae: Epidendroideae) from Yunnan, China. Phytotaxa, 413: 296-300. DOI:10.11646/phytotaxa.413.4.5 |
Raskoti, B.B., 2016. A new species of Gastrochilus and new records for the orchids of Nepal. Phytotaxa, 233: 179-184. DOI:10.11646/phytotaxa.233.2.5 |
Ronquist, F., Huelsenbeck, J.P., 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572-1574. DOI:10.1093/bioinformatics/btg180 |
Schlechter, F.R.R., 1913. Die Gattungen Gastrochilus Don. Und Gastrochilus Wall. Repertorium specierum novarum regni vegetabilis. Selbstverlag des Herausgebers, Berlin pp. 313-315.
|
Seidenfaden, G., 1988. Orchid genera in Thailand XIV. Fifty–nine vandoid genera. Opera Bot., 95: 1-398. |
Smith, J.J., 1905. Die orchideen von Java. Flora von Buitenzorg, 6: 632. |
Swofford, D.L., 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0 a169. Sinauer Associates, Sunderland.
|
Thiers, B., 2021. Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/science/ih.
|
Tsi, Z.H., 1996. A preliminary revision of Gastrochilus (Orchidaceae). Guihaia, 16: 123-154. |
Turland, N.J., Wiersema, J.H., Barrie, F.R., et al., (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159, Koeltz Botanical Books, Glashutten. https://doi.org/10.12705/Code.2018.
|
Wu, X.F., Ye, D.P., Pan, B., et al., 2019. Validation of Gastrochilus prionophyllus (Vandeae, Orchidaceae), a new species from Yunnan Province, China. In: Cai, J., Yu, W.B., Zhang, T., Li, D.Z. (Eds). Revealing of the plant diversity in China's biodiversity hotspots. PhytoKeys 130, 161-169. https://doi.org/10.3897/phytokeys.130.34555.
|
Xu, Z.H., Jiang, H., Ye, D.P., et al., 2010. The wild Orchids in Yunnan. Yunnan Science and Technology Press, Kunming p. 496.
|
Zhang, J.Y., Cheng, Y.H., Liao, M., et al., 2022a. Gastrochilus wolongensis (Orchidaceae): a new species from Sichuan, China, based on molecular and morphological data. Ecosyst. Health Sust., 8: 2101546. DOI:10.1080/20964129.2022.2101546 |
Zhang, Z., Xie, P.L., Guo, Y.L., et al., 2022b. Easy353: a tool to get angiosperms353 genes for phylogenomic research. Mol. Biol. Evol., 39: msac261. DOI:10.1093/molbev/msac261 |
Zhou, Z.H., Shi, R.H., Zhang, Y., et al., 2021a. Orchid diversity in China: recent discoveries. Plant Divers., 43: 341-342. DOI:10.1016/j.pld.2021.07.004 |
Zhou, Z.H., Shi, R.H., Zhang, Y., et al., 2021b. Orchid conservation in China from 2000 to 2020: achievements and perspectives. Plant Divers., 43: 343-349. DOI:10.1016/j.pld.2021.06.003 |
Zou, L.H., Huang, J.X., Zhang, G.Q., et al., 2015. A molecular phylogeny of Aeridinae (Orchidaceae: Epidendroideae) inferred from multiple nuclear and chloroplast regions. Mol. Phylogenet. Evol., 85: 247-254. DOI:10.1016/j.ympev.2015.02.014 |



