b. University of the Chinese Academy of Sciences, Beijing 100049, China;
c. T-STAR Core Team, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Yunnan 666303, China;
d. School of Biology and Ecology, University of Maine, Orono, ME, USA;
e. Climate Change Institute, University of Maine, Orono, ME 04469, USA;
f. School of Biological and Chemical Science, Pu'er University, Xueyuan Road, Yunnan 665000, China;
g. Institute of Ecology and Geobotany, School of Ecology and Environmental Sciences, Yunnan University, Kunming, Yunnan 650091, China;
h. School of Ecology and Environment, Southwest Forestry University, Kunming, Yunnan 650224, China;
i. Yuanjiang Savanna Ecosystem Research Station, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Yuanjiang, Yunnan 653300, China
Over the past century, droughts have led to extensive forest mortality across biomes worldwide (Allen et al., 2010; Hammond et al., 2022). An increasing body of research suggests that many tropical regions are experiencing a decrease in annual precipitation and an increase in maximum temperatures, rendering them highly vulnerable to drought (Dai, 2011; Yuan et al., 2019; Tao et al., 2022; Doughty et al., 2023). Other research has demonstrated that the failure of the xylem water transport system was the primary mechanism triggering tree mortality during drought events (Anderegg et al., 2016; Adams et al., 2017; Choat et al., 2018). Under normal conditions, water evaporation inside the leaves creates a negative pressure (tension), which draws water from the soil through the roots and the stem to the leaves (Dixon and Joly, 1895; Tyree, 1997; Tyree and Zimmermann, 2002). However, elevated xylem tension under drought conditions potentially increases the risk of hydraulic failure and may permanently damage plant tissues (Brodribb et al., 2021; Terra et al., 2021). Plants employ various strategies to prevent drought-induced hydraulic failure, including increasing the resistance to embolism (blockage of xylem conduits by air bubbles), which may favor plant survival during drought (Bartlett et al., 2014; Anderegg et al., 2016; Brodribb et al., 2016), adopting hydraulic vulnerability segmentation mechanisms to protect vital organs (Tyree and Zimmermann, 2002; Pivovaroff et al., 2014), and extracting water from deep soil strata (Ennajeh et al., 2008). Nevertheless, drought response strategies vary across different taxa and habitats (Ennajeh et al., 2008; Choat et al., 2012; Aritsara et al., 2022; Chen et al., 2021a).
Bamboo plants, a diverse group of woody grasses belonging to the subfamily of Bambusoideae, are highly prevalent in tropical areas (Vorontsova et al., 2016) with high value in ecosystem services, biodiversity and economics (Akinlabi et al., 2017; Zhou et al., 2017; Enarth Maviton and Sankar, 2023). Bamboos are an essential component of tropical ecosystems; for instance, bamboo forests were reported to be highly efficient carbon sinks due to their fast-growing habit and can store up to 392 Mg C ha−1 (Yuen et al., 2017). The extensive root systems of bamboo plants can also help mitigate soil erosion caused by heavy rainfall or land degradation (Liese and Köhl, 2015). Furthermore, bamboo species are extensively utilized in human diets, construction, furniture, and paper production (Yang et al., 2004; Enarth Maviton and Sankar, 2023). However, many bamboo species exhibit extensive defoliation and high mortality rates during drought events (Zhang et al., 2019; Fadrique et al., 2021), while the physiological mechanism accounting for this high mortality rate is poorly understood until now.
Hydraulic properties, such as embolism resistance (xylem tension causing 50% loss of hydraulic conductance), are closely linked to plant drought resistance and survival rate during the drought (Choat et al., 2012, 2018; Anderegg et al., 2016; Chen et al., 2021a; McDowell et al., 2022). However, our understanding of the hydraulic properties of bamboo species is limited due to the tubular structure of their stems, which complicates the application of conventional measurement methods. Fortunately, recent advances in techniques, such as optical observation and X-ray computed microtomography (MicroCT), have provided new opportunities for studying plant hydraulics, enabling non-invasive visualization of embolism occurrence in structures such as bamboo stems (Brodersen et al., 2013; Brodribb et al., 2016; Chen et al., 2021b). Unlike trees, bamboo species lack secondary growth (Clark et al., 2015; Liese and Köhl, 2015), which suggests they cannot replace dysfunctional vascular tissues caused by drought-induced embolism. Bamboos can develop high root pressure at night (Yang et al., 2023), potentially enabling the refilling of embolized vessels after mild to moderate droughts (Cao et al., 2012). Nevertheless, the low soil water availability during extreme droughts may overwhelm the efficiency of this refilling mechanism. Meanwhile, it has been reported that repeated embolism and refilling cycles can fragilize the resistance of the vessel network to drought, a phenomenon known as "embolism fatigue" (Hacke et al., 2001).
The concept of hydraulic vulnerability segmentation (HVS) suggests that the terminal organs of a plant, such as leaves, are more susceptible to embolism than the stem (Zhu et al., 2016). By shedding less costly leaves, plants can reduce their transpiration rates, conserve water, and thereby protect "more expensive" organs, such as the stem and coarse roots, against drought-induced damages (Pivovaroff et al., 2014; Tyree and Zimmermann, 2002). This strategy is commonly observed in deciduous species and regions with high seasonal variations in water availability (Johnson et al., 2016). Indeed, deciduous plant leaves are generally more susceptible to hydraulic failure than stems (Zhu et al., 2016). Structural and hydraulic evidence has demonstrated the effectiveness of this mechanism in many woody species (Johnson et al., 2016; Levionnois et al., 2020).
Moreover, drawing water from deep soil layers and large soil volumes is another effective strategy for plants to cope with moderate and severe drought episodes (Watson, 1968; West et al., 2012). This strategy is particularly important for species in Mediterranean regions (Ennajeh et al., 2008). Despite the high evaporation demand, a sufficient water supply can prevent hydraulic dysfunctions in the plant (West et al., 2012). However, it is important to note that Gramineae plants, such as bamboos, generally have shallow roots (Zhang et al., 2019; Schenk and Jackson, 2002). Therefore, water uptake becomes challenging during drought due to their shallow-rooting habit, as water from surface soil layers is quickly depleted or evaporated.
During the dry seasons of 2019 and 2020, a historically unprecedented drought event occurred in regions from southwest China to the Indo-China Peninsula (Ding and Gao, 2020), resulting in large-scale tree mortality, particularly bamboo species. A survey on bamboo collections within the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, indicated that some bamboo species experienced significantly higher mortality rates than their coexisting plant species. Cephalostachyum pergracile is a well-known bamboo species, widely used in traditional bamboo fragrant rice in Southwest China and Southeast Asian countries (Yang et al., 2004). It naturally distributes in lowland valleys and on the lower slopes of tropical regions (Enarth Maviton and Sankar, 2023). During the extreme drought period of 2019–2020, this species exhibited a remarkably higher mortality rate than other coexisting bamboo species (Fig. 1).

|
| Fig. 1 Images of dying Cephalostachyum pergracile in 2019 (a, b & c) and dynamics of the climatic conditions at the study site from January 2017 to June 2023. The climatic data include six months-timescale Standardized Precipitation Evapotranspiration Index (SPEI_6), which was downloaded from www.spei.csic.es (a, consulted on 2023-11-08), the Photosynthetically Active Radiation (PAR, d), the daily precipitation (e) and the 20 cm topsoil humidity (f, brown lines) and average daytime atmospheric humidity (f, blue lines). Subpanel (a) shows the bamboo plantations in XTBG. The blue window in (a) highlight two dying C. pergracile clumps; (b) and (c) show terminal branches of the dying culms in the middle of the drought event in 2019 (April). The gray shadows in (d, e, f & g) highlight the extreme drought period of 2019–2020. |
In this study, we selected Cephalostachyum pergracile as an example to investigate the hydraulic properties and the drought response strategies adopted by bamboo species. We evaluated the drought resistance and safety margins of its stems and leaves, the degree of hydraulic vulnerability segmentation, and the soil water source partitioning. Additionally, we monitored its seasonal dynamics in water potential and xylem native embolism level. Due to the higher mortality rate of C. pergracile compared to the coexisting bamboo species, we hypothesized that: 1) C. pergracile has low drought resistance, hydraulic safety and narrow safety margins, which may explain its high drought-induced mortality rate; 2) C. pergracile exhibits weak hydraulic vulnerability segmentation due to its humid-tropical origins, thus showing a limited ability to protect expensive organ such as stems from drought-induced damage; 3) C. pergracile primarily relies on water from shallow soil layers, exacerbating water stress under drought conditions due to its limited capacity to access deep soil water when soil water in shallow layer is depleted.
2. Materials and methods 2.1. Study site and speciesThe study was conducted in a bamboo plantation within the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (XTBG, 101°46′E, 21°54′N, at an elevation of 540 m) in Mengla County, Xishuangbanna prefecture, Yunnan province, Southwest China. The mean annual temperature in the area is 21.7 ℃, with temperatures ranging from 15.1 ℃ in January to 21.7 ℃ in June, and the mean annual rainfall is 1493 mm. This region experiences a distinct dry season and wet season, with the dry season lasting from November to April and consisting of a cool-dry period (from November to February) and a hot-dry period (from March to April), while the wet season (rainy season) occurs between May and October. In 2019 and 2020, this region experienced historical unprecedented drought events caused by strong El Niño effects (Ding and Gao, 2020; Feng et al., 2022; Shen et al., 2022). During this period, the lack of precipitation, high temperature, and low Standardized Precipitation Evapotranspiration Index (SPEI) substantially reduced the atmosphere humidity and soil water content, especially in the upper 20-cm-deep soil stratum (Fig. 1d, e, f & g).
Cephalostachyum pergracile is one of the most abundant species in the bamboo garden of XTBG. This species belongs to the subfamily of Bambusea and is native to the Southeast Asian region, typically growing at elevations between 50 and 1200 m. It thrives on lower slopes and in well-drained valleys and grows preferably on well-hydrated loamy soil (Enarth Maviton and Sankar, 2023; Yang et al., 2004). C. pergracile exhibits sympodial growth (clumping bamboos) with a culm diameter ranging from 5 to 7 cm and a maximal height of 20 m (Enarth Maviton and Sankar, 2023). Six distinct healthy clumps were selected for this study. Based on our observations, C. pergracile displayed severe terminal branch die-back and whole-individual death during the extreme drought of 2019–2020 (Fig. 1a–c).
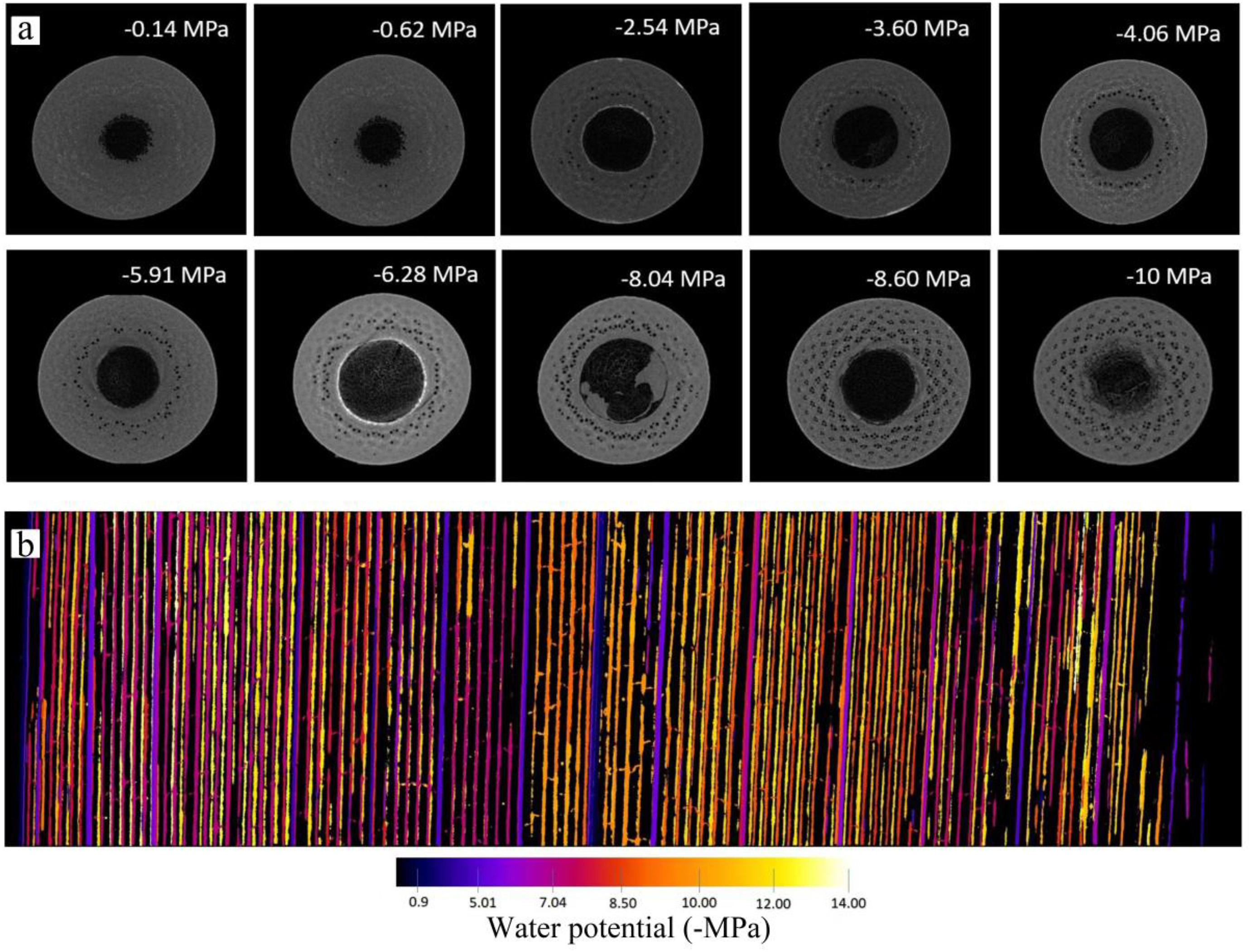
2.2. Stem vulnerability curveThe stem vulnerability curves (VCs) were determined using the MicroCT scanning method (Chen et al., 2021b) during the wet season (July to October) in 2022 and 2023. Twenty-five 2–3 m long healthy branches were randomly harvested from the six clumps before sunrise to measure stem VCs. All leaves were sprayed with water, sealed in black plastic bags, and promptly transported to the laboratory. All samples were cut under water, and the cut ends were submerged to rehydrate for more than 2 h before the measurement. Subsequently, the basal cut-end was sealed with fast-drying glue and parafilm, and the branches were left to dehydrate in an air-conditioned laboratory to achieve different levels of dehydration. The water potential was measured using a pressure chamber (PMS1505D-EXP, Corvallis, OR, USA). When a specific water potential was reached, the leaf and xylem water potentials were equilibrated by sealing the entire branch in a black plastic bag for at least 30 min until water potentials at different positions were similar. Then, the branch was cut twice under water to release the xylem tension and obtain an 8–10 cm long segment at the end of the cutting series. The obtained segment was sealed with parafilm and scanned twice with the MicroCT apparatus (SkyScan1275; Bruker Corporation, Billerica, MA, USA). The first scan highlighted the initially embolized vessels (Fig. 2a, Video S1). The second scan was conducted after the segment had been flushed with high-pressure air (100 kPa) for 3–5 min to embolize all the vessels (Chen et al., 2021b). The diameter (Di) of each embolized vessel i in the scanned images was measured using ImageJ (Schindelin et al., 2012). In this approach, the percent loss of conductivity (PLC) was calculated as the ratio between the conductivity of the air-filled vessels before and after flushing (Kt0 and Ktmax, respectively). Based on the Hagen–Poiseuille equation Eq. (1), the PLC of each segment was calculated using Eq. (2). The vulnerability curve was constructed by fitting the percent loss of conductivity (PLC) and water potentials to Eq. (3) (Pammenter and Vander Willigen, 1998) using the fitplc package (Duursma and Choat, 2017) to estimate the xylem water potential at 50% loss of conductivity (P50stem).
|
| Fig. 2 Embolism propagation in the stem (a) and leaf (b) of Cephalostachyum pergracile during dehydration. The water potentials were labeled in the image for stems (a), and indicated by different colors in the leaf (b). |
Supplementary video related to this article can be found at https://doi.org/10.1016/j.pld.2023.12.003
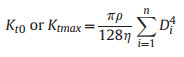
|
(1) |
With η is water viscosity, and ρ is water's volumetric weight.
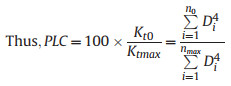
|
(2) |
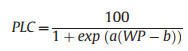
|
(3) |
With a and b are fitted equation parameters.
2.3. Leaf hydraulic traitsOne leafy branch was collected from each of the six selected clumps in the early morning during the wet season (July–October) between 2019 and 2022. Leaves were covered with black plastic bags, and the base of the branch was recut under water and left to rehydrate for more than 3 h.
2.3.1. Leaf pressure–volume curveSix mature leaves were taken from the six rehydrated branches. The initial water potential was initially checked to be close to -0.1 MPa. Each leaf was preliminary scanned to determine the leaf area (LA). Then, the leaf fresh weight (FW) and the water potential Ψleaf (MPa) were repeatedly measured using a 0.0001 g precision digital scale (Mettler-Toledo ME204E, Shanghai, China) and the pressure chamber, respectively. A total of 12–15 measurements were performed until the leaf water potentials stopped decreasing or began to rise. Finally, the leaf dry mass was determined after oven-drying at 70 ℃ for more than 72 h (Perez-Harguindeguy et al., 2013). The pressure–volume curve of each leaf was fitted using a publicly available Excel spreadsheet (Sack et al., 2011) to determine the turgor loss point (πTLP).
2.3.2. Leaf water-releasing curvesSix mature leaves were selected from six rehydrated branches to determine the leaf water-releasing curves. The leaf sheath was removed, and the saturated weight (SW) of each leaf was first measured using the 0.0001 g resolution digital scale. Then, each leaf was dehydrated in a growth chamber set at 26 ℃ and 60% relative humidity. Selected leaves were repeatedly weighted to obtain the fresh weight (FW) until no substantial change in water content was observed. Due to the non-linear dehydration rate of the leaf, the measurement frequency was gradually decreased as the leaf sample dried. Finally, each leaf was dried at 70 ℃ for more than 48 h, and the dry weight (DW) was measured.
At each step, the relative water content (RWC) was calculated as per Eq. (4):
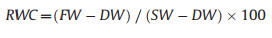
|
(4) |
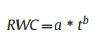
|
(5) |
RWC was fitted to a power function (Eq. (5)) against the time interval t (min) during the measurements using the sma function from the smatr R-package (Warton et al., 2012). Then, the fitted parameters were used to estimate the time needed to dehydrate the leaf to 70% SWC (T70).
2.3.3. Leaf vulnerability curveWe constructed leaf vulnerability curves (VCs) using an optical method (Brodribb et al., 2016). Three rehydrated terminal branches (1.5–2 m) were randomly selected to measure leaf VCs. The selected leaf was placed on the scanner bed (Epson V850 pro, USA) and was automatically grayscale-scanned at 4800 dpi at 10-min intervals for 3–5 days (Fig. 2b, Video S2) until it was fully dehydrated (Chen et al., 2021b). The water potentials were periodically measured using the pressure chamber from leaves attached to the twig to predict the water potentials during dehydration. Because the bamboo leaves dehydrated faster than other woody species, such as angiosperm woody trees, the room temperature and relative humidity were strictly maintained at approximately 65% and 24 ℃, and the branch was enclosed in black plastic bags to slow the dehydration. The water potentials were measured using the pressure chamber to construct the Ψleaf–time relationships. Afterward, the 10-min time resolution Ψleaf values were predicted from the Ψleaf–time relationships. Using ImageJ (ImageJ 1.53c, National Institutes of Health, USA), we used the image subtraction method (Brodribb et al., 2016b) to estimate the cumulative embolism area in the leaf veins over time. The percentage of the embolized area (PEA) was quantified as the ratio of embolized pixels at a given water potential to the number of embolized pixels at the fully embolized stage. One vulnerability curve per leaf was constructed by fitting PEA against the leaf water potential using a Weibull model with the fitplc R-package (Duursma and Choat, 2017) to estimate the leaf water potential at 50% PEA (P50leaf).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.pld.2023.12.003
2.4. Diurnal water potential and native embolismWe monitored predawn (Ψpre) and midday (Ψmid) water potentials during different seasons. The predawn samples were collected between 05:30 and 06:30 a.m., and midday samples were collected from 13:00 to 15:00 p.m. Canopy twigs from each individual (n = 6) were cut and immediately sealed in vapor-saturated bags. The water potential of each twig was measured using a pressure chamber after equilibrium for about 10 min inside the bag.
The native embolism during the wet and dry seasons was measured in five branches collected from five clumps at midday. To avoid 'cutting-under tension' artifacts (Wheeler et al., 2013), the targeted branches were sprayed with water and bagged for over 30 min to release the tension before harvest. A truck crane with a 25-m arm was used to assist the whole operation. The collected samples were transported to the laboratory, where they were cut to the desired length under water and submerged for about 30 min to release the remaining tension. The protocol for sample collection, treatment and MicroCT measurements was the same as for the stem vulnerability curves.
2.5. Xylem tissue samples and soil hydrogen–oxygen isotope compositionTo quantify the contribution of different soil depth profiles to bamboo water use in the dry season, we collected soil and bamboo tissue samples in April 2023 for hydrogen–oxygen isotope composition analysis. Two healthy, sun-exposed, mature clumps were selected, and soil samples were collected at three horizontal positions nearby. A terminal branch was cut from the top of each individual with a telescopic pruner. Then, a 10-cm-long segment was excised from the branch and immediately placed in glass containers, sealed with parafilm, and stored at 4 ℃.
Soil samples were collected using an auger at eight different depths (0–2, 2–5, 5–10, 10–20, 20–50, 50–80, 80–120, and 120–160 cm) for soil water potential and isotope composition measurements. The water from the xylem and the soil samples were extracted using a vacuum condensation extraction system (Li-2100, Lica United, Beijing, China). Subsequently, the isotope composition of the extracted water was analyzed using a liquid water isotope analyzer (GLA431-TLWIA, ABB, Canada). The isotope ratios R for 2H and 18O were calculated using Eq. (6), and the isotopic values δ2H and δ18O were determined using Eq. (7).
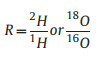
|
(6) |
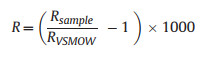
|
(7) |
With RVSMOW is the Vienna Standard Mean Ocean Water isotope ratio.
2.6. Data analysisAll the data analyses and illustrations were performed using R v.4.3.1 (R Core Team, 2023). To analyze the water source partitioning, the isotopic values of all the soil strata were collected, and their relative contributions to the xylem water were analyzed using Bayesian mixing models with the MixSIAR R-package (Stock and Semmens, 2016). The models mixed values based on two biotracers (2H and 18O) to differentiate the contribution of various sources (soil strata) to the isotopic composition of the xylem water. The hydraulic safety traits were compared by assessing the overlap between their confidence intervals.
3. ResultsBoth stems and leaves exhibited 'S-shape' vulnerability curves (Fig. 3a and b). Cephalostachyum pergracile showed high drought resistance in both the leaf and stem. In particular, the estimated P50stem and P50leaf were -6.07 ± 0.48 MPa and -8.36 ± 0.12 MPa, respectively. The water potential at leaf turgor loss was -4.14 ± 0.4 MPa (Fig. 4a). Unexpectedly, P50stem was 2.29 MPa less negative than P50leaf, indicating a lack of effective hydraulic vulnerability segmentation (Fig. 3a and b). In contrast, πTLP was 1.93 MPa less negative than P50stem (Fig. 4a). There was no overlap between the 95% confidence interval of the three traits. The leaves of C. pergracile had low water-retention capacity and took only 80.14 ± 5.22 min to dehydrate the fully hydrated leaves to 70% relative water content at 26 ℃ and 60% relative humidity (Fig. 5).
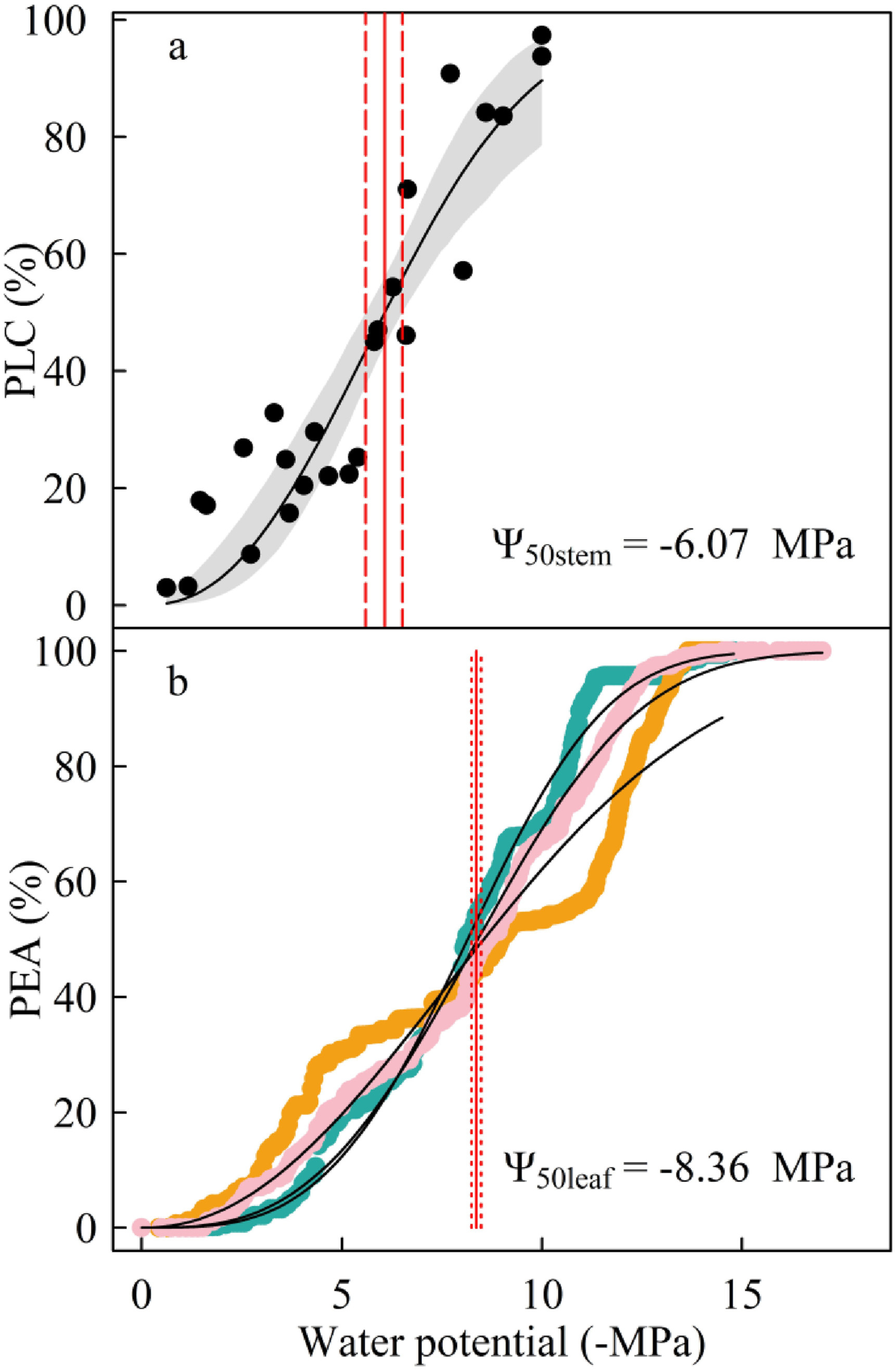
|
| Fig. 3 Stem (a) and leaf (b) vulnerability curves of Cephalostachyum pergracile. PLC, percent loss of conductivity; PEA, percent of the embolized area. The solid and dashed vertical lines in are the mean and bootstrap 95% confidence interval of the water potential at 50% loss of conductivity of the stem (a, P50stem) or at 50% embolized vein area (b, P50leaf). |
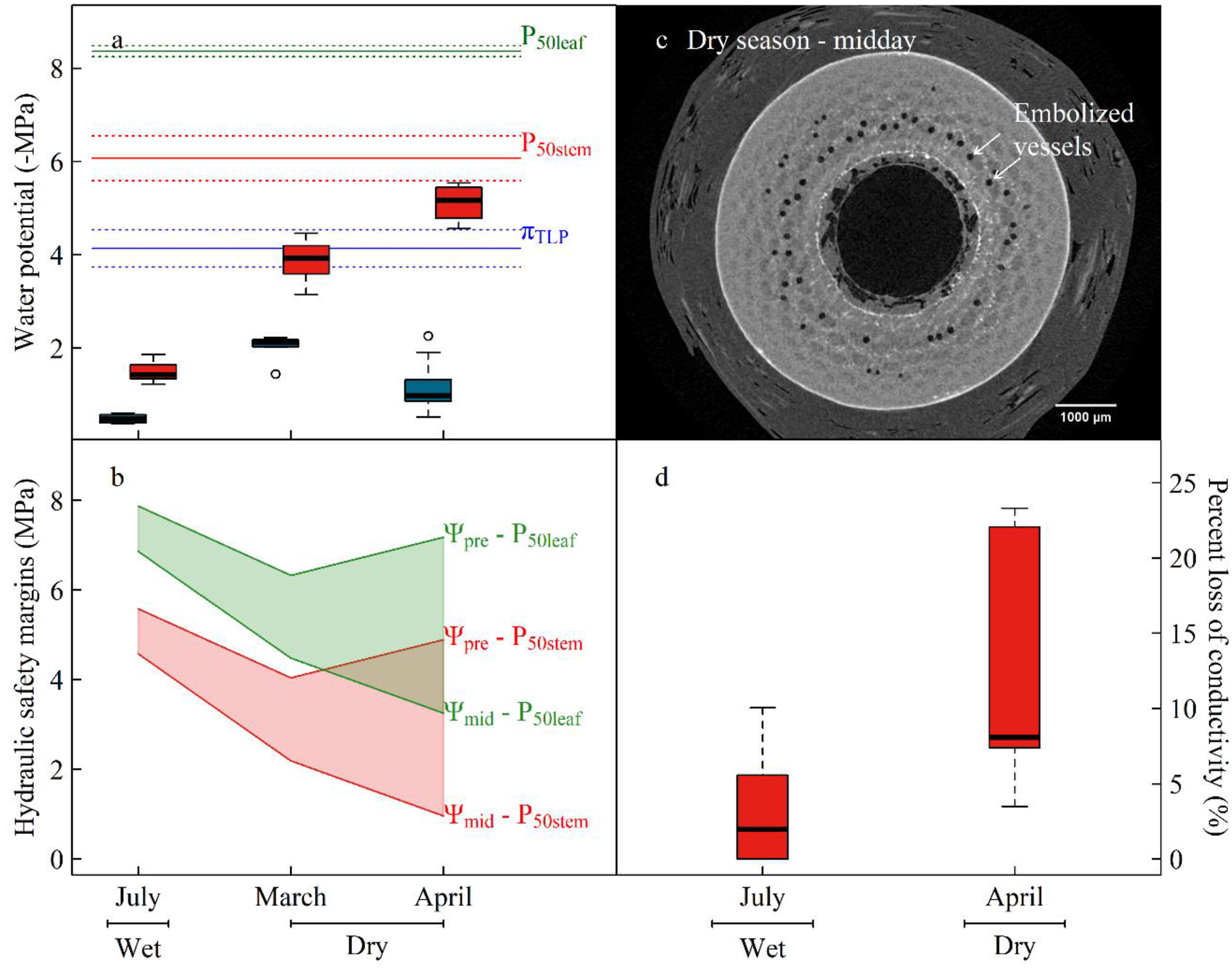
|
| Fig. 4 Predawn (Ψpre, blue boxplot) and midday leaf water potentials (Ψmid, red boxplot, a), hydraulic safety margins (b) and native embolism (c & d). The horizontal lines in (a) represent different hydraulic safety metrics and the 95% confidence intervals of P50stem, P50leaf, and πTLP. The green and red polygons in (b) illustrate the daytime range of leaf and stem hydraulic safety margins across seasons, respectively. (c) shows a MicroCT image with embolized vessels (white arrows) in the xylem in the dry season. |
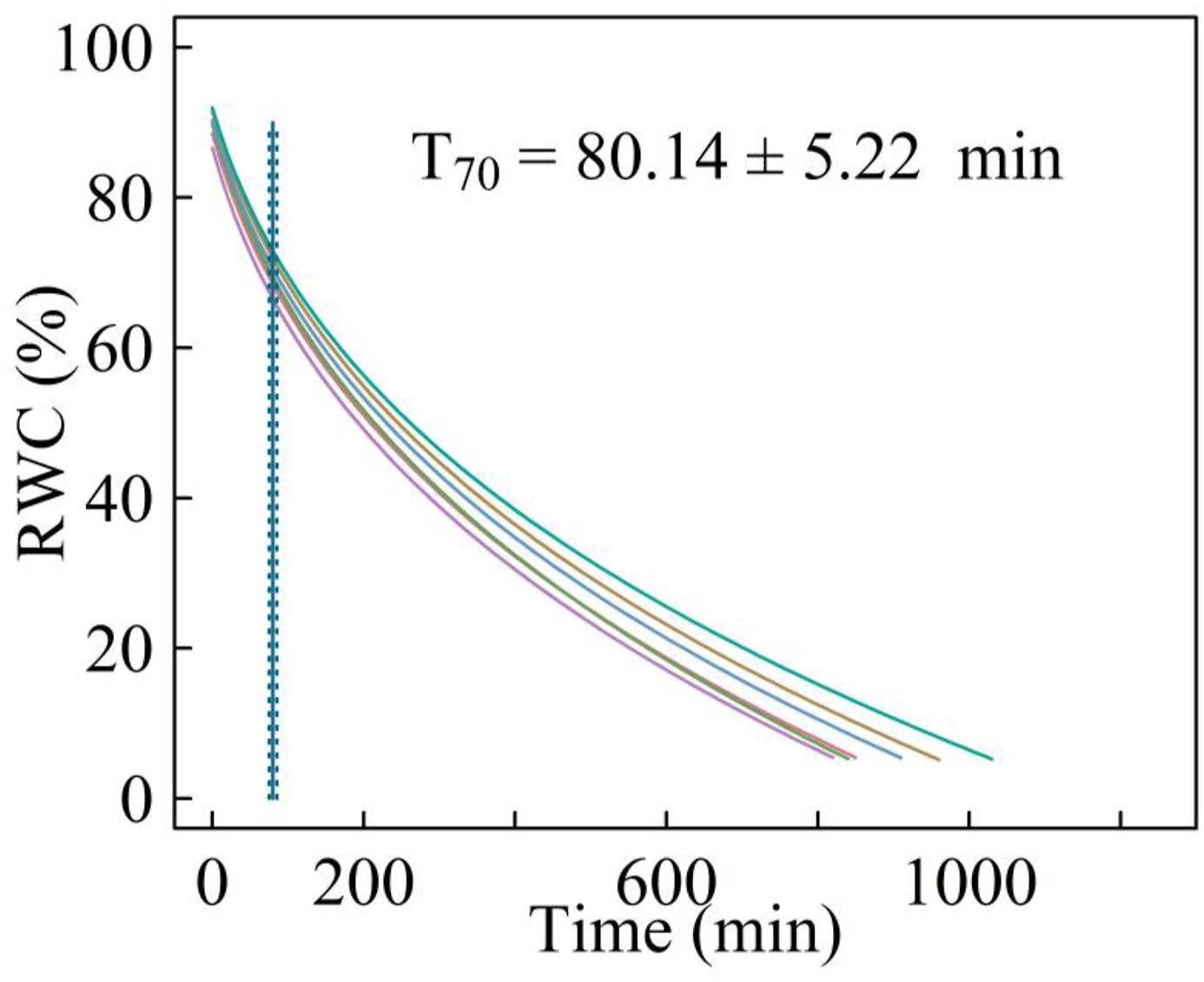
|
| Fig. 5 Leaf water releasing curves of the leaves of Cephalostachyum pergracile. RWC, relative water content. Each curve represents one replicate. The solid and dotted vertical lines are the mean and standard errors of the dehydration time to 70% RWC. |
The xylem water potential exhibited great diurnal and seasonal variations (Fig. 4a). The midday leaf water potential decreased from -1.42 MPa in July (wet season) to -3.93 MPa and -5.17 MPa in March (mid-dry season) and April (late dry season), respectively (Fig. 4a). Such water potential caused a 5.87 ± 2.33% loss of the conductivity of the stem xylem in the wet season and 12.87 ± 4.09% in the dry season at midday (Fig. 4d). This conductivity loss resulted from the embolism of numerous vessels in the dry season (Fig. 4c). However, the hydraulic safety margins of stems and leaves were 0.96 MPa and 3.25 MPa, represented by the difference between the minimum Ψmid and P50stem (Ψmid − P50stem) and between the minimum Ψmid and P50leaf (Ψmid − P50leaf), respectively (Fig. 4b). Additionally, water source analyses using stable isotopes indicated that 49.4% of the absorbed xylem water of C. pergracile came from upper 20 cm-deep topsoil layers (Fig. 6).
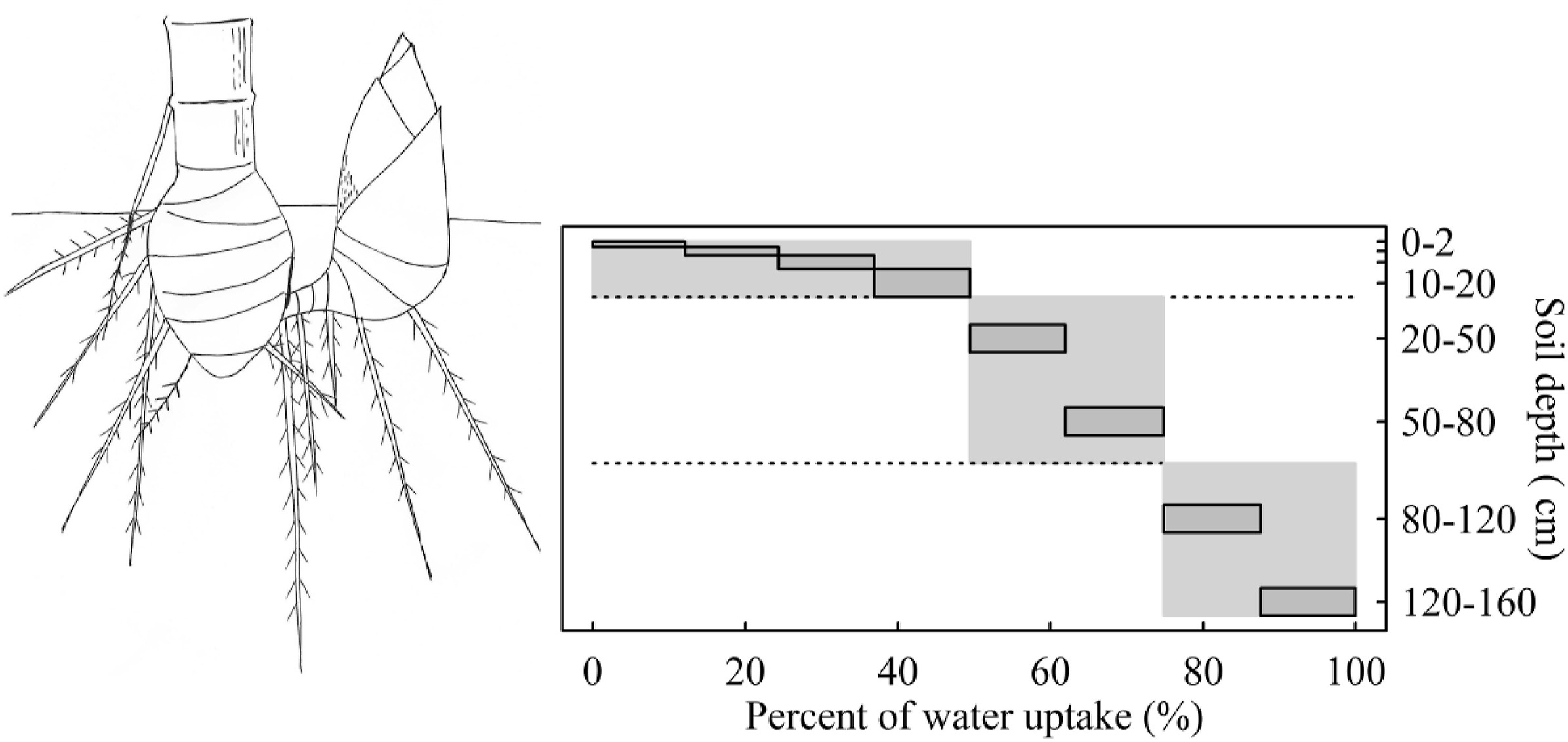
|
| Fig. 6 Water uptake depth distribution by Cephalostachyum pergracile at fine (dark grey boxes) and coarse (light grey boxes) depth resolution. |
This study shows high resistance of the stem and the leaf vein of Cephalostachyum pergracile to xylem embolism, as well as strong desiccation tolerance of its leaves, but the low capacity of its leaf epidermis to prevent water loss. Additionally, the lack of hydraulic vulnerability segmentation, the high permeability of the leaf epidermis, and the high reliance on shallow soil layer water make C. pergracile susceptible to water stress, particularly under extreme drought conditions when soil water availability is extremely low.
4.1. High hydraulic safety but highly permeable leaf in Cephalostachyum pergracileDespite the preference of C. pergracile for tropical humid valleys, it exhibited a high hydraulic safety in both leaves and stems (Fig. 3a and b). Its P50stem was more negative than that of 87.4% of the species listed in the xylem functional trait database (Fig. 7) (Choat et al., 2012). The P50stem value indicates that the xylem of C. pergracile can be more resistant to hydraulic failures than many tree species growing in dry habitats such as chaparral and Mediterranean regions (Jacobsen et al., 2014; Lobo et al., 2018). This suggests that the stem xylem of C. pergracile could keep most of its vessels functioning even under mild or moderate low water potentials (Hacke and Sperry, 2001). Similarly, C. pergracile exhibited extraordinary resistance to embolism in its leaves (Fig. 3b), indicating greater embolism resistance than the leaves of other woody species from various habitats worldwide (Fig. 7) (Yan et al., 2020). This also implies that the leaf veins of C. pergracile remained functional even under very low water potentials, theoretically aiding the plant in withstanding drought (Brodribb et al., 2016, 2021). The high hydraulic safety of C. pergracile is also confirmed by its notably negative leaf πTLP value (Fig. 4a), which was more negative than that of most tree species across various biomes in China (Fig. 7) (Zhu et al., 2018) and from tropical dry forests (Vargas et al., 2021). The πTLP of C. pergracile measured in this study was also far more negative than that of the annual monocot maize (Li et al., 2009). However, the high mortality rate despite high drought resistance of C. pergracile during the extreme drought period aligns with previous studies, which found that species with high embolism resistance exhibited higher top-kill and mortality rates when experiencing extreme drought events compared to species that employ a drought avoidance strategy (Anderegg et al., 2018; Kukowski et al., 2013).
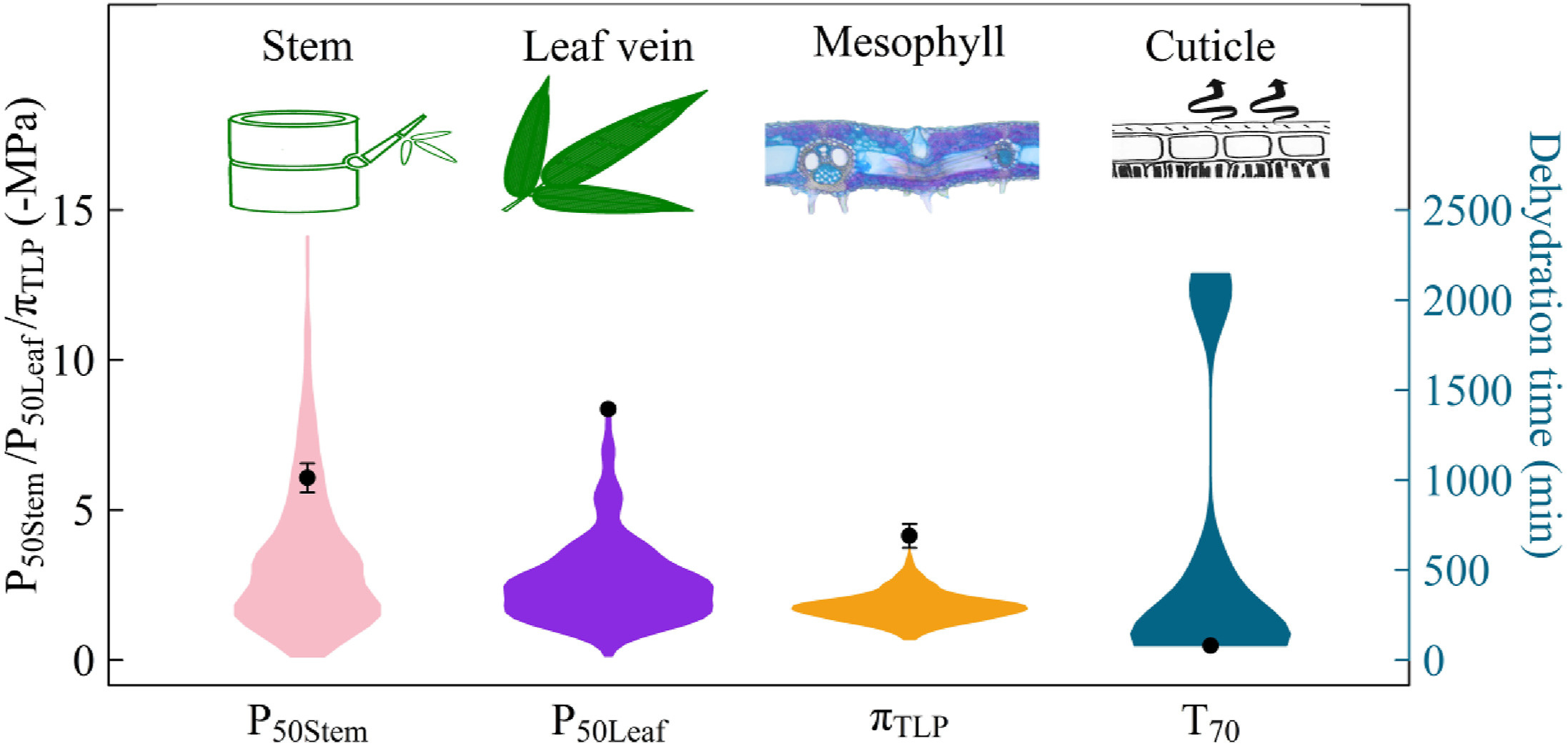
|
| Fig. 7 Comparison of different hydraulic safety-related traits of Cephalostachyum pergracile with data from the literature (Choat et al., 2012; Hao et al., 2010; Yan et al., 2020; Zhu et al., 2018). The points represent the mean value for C. pergracile, and the error bars are the standard errors. |
The leaf vein exhibited greater resistance to embolism than the stem xylem, suggesting a lack of effective hydraulic vulnerability segmentation in C. pergracile (Figs. 3a and b, 4a). In contrast, leaves are generally more vulnerable than the stem in most woody species (Pivovaroff et al., 2014; Johnson et al., 2016; Aritsara et al., 2022). This pattern may be attributed to its large vessels in the stem of C. pergracile, as revealed by the MicroCT images, which may limit its embolism resistance (Hacke et al., 2006). Alternatively, while πTLP was less negative than P50stem, the rapid daytime drop in water potential depicted its inefficiency in preventing water loss during drought (Fig. 4a), in contrast to trees (Scoffoni et al., 2017; Aritsara et al., 2022). Consequently, xylem tension at the leaves can easily spread to the stem (Pivovaroff et al., 2014). In such cases, hydraulic failure at leaves cannot protect the more expensive stem from embolism (Johnson et al., 2016; Zhu et al., 2016), which may further accelerate the propagation of embolism throughout the plant (Skelton et al., 2017). For instance, a 12.8% loss of conductivity was recorded in the stem xylem during a typical dry season in this study. Therefore, the lack of, even inverted, hydraulic vulnerability segmentation in C. pergracile may raise hydraulic risks during extreme drought.
Our results also showed that the safety margins remained positive but were smaller than 1 MPa (Fig. 4b), and were narrower than those of most tree species growing in tropical regions (Smith-Martin et al., 2023). The narrow safety margins measured in this study during the normal dry season suggest a high risk of hydraulic failure under severe drought conditions. Indeed, the extremely low water availability and high evapotranspiration demand during the drought events in 2019 and 2020 (Fig. 1) certainly exposed C. pergracile to much lower water potentials than the values measured in this study. Additionally, leaves of C. pergracile showed a rapid leaf dehydration rate (Fig. 5), which was much faster than that of co-occurring tree species (Fig. 7) (Hao et al., 2010). Thus, the high permeability of the leaf epidermis indicates the inability of the plant to control water loss under drought. Without such control, the plant continues to lose water at a high rate during drought, thus rapidly dropping the stem and leaf water potentials to values below P50stem and P50leaf, particularly when water supply from the soil is limited (Meinzer et al., 2009; Carminati et al., 2020). For instance, the midday leaf water potential of C. pergracile reached an exceptional value of -5.16 MPa in a normal dry season, leaving less than 1 MPa of safety margins for the stem to face extreme drought conditions (Fig. 4a and b). Therefore, although C. pergracile exhibits high resistance to drought-induced embolism and low πTLP, hydraulic failure may still occur when exposed to severe drought conditions.
Bamboo plants cannot replace embolized vessels like some ring-porous trees in temperate regions due to the lack of secondary growth (Clark et al., 2015; Liese and Köhl, 2015). Although bamboo plants can generate positive pressures from the root, thus repairing drought-induced embolism in aboveground organs (Cao et al., 2012), high root pressure to refill embolized vessels is physically unrealistic under severe drought conditions when soil water availability is extremely low. Additionally, frequent embolism-refilling cycles may also damage the xylem structure (Hacke et al., 2001). Further investigation of the xylem structure of C. pergracile, particularly the intervessel pit structure, is needed to fully understand the mechanism underlying its high xylem embolism resistance.
4.2. Shallow rooting: Limited water supply during the dry seasonThe water source partitioning using stable isotopes reveals that nearly half of the water absorbed by C. pergracile originated from the upper 20-cm depth soil layer, indicating its high reliance on surface water (Fig. 6). Considering that C. pergracile generally grows in well-drained loamy soils (Enarth Maviton and Sankar, 2023) and that the coarse texture of such soils imposes weak limitations to the vertical extension of the roots (Schenk and Jackson, 2005), the limited access to deep water sources is primarily inherent to the species and is less reliant on the environment. This pattern aligns with the steep depletion of soil water content in the upper 50-cm depth soil layer in a moso bamboo forest under drought (Zhang et al., 2019). In contrast, some tree species coexisting with C. pergracile in tropical humid regions can access water sources several meters belowground (Canadell et al., 1996). The restricted access to water by C. pergracile during the dry season is further supported by the substantial decline in leaf water potentials compared to the wet season, indicating that the amount of water exploitable by C. pergracile was insufficient to replenish water lost through transpiration (Watson, 1968). In addition to the high rate of water loss during drought, limited water supply from the soil further exacerbated the xylem tension in the plant, hastening the decline in water potential and increasing the risk of embolism in the xylem. While some vascular species can close the leaf stomata under water deficits (Carminati et al., 2020), the permeability of C. pergracile's leaf epidermis restricts the effectiveness of this protective mechanism. Therefore, the shallow rooting habit imposes a significant constraint on the survival of C. pergracile during drought.
Bamboo plants are characterized by their belowground rhizome system, and different aboveground individuals within the same clump are connected via the rhizome system to exchange water and nutrients (Banik, 2015). The rhizome system also ensures resource storage and distribution to support rapid growth and facilitates the regeneration of the entire clump (Liese and Köhl, 2015). In fact, drought and fire experiments on grasses showed that rhizomatous species resprouted faster than other species (Pausas and Paula, 2020). While high embolism resistance of the aerial organ can assist the culm and the leaves to remain operational during drought, fast water loss and limited water supply from the soil may lead to a severe depletion of the water pool in the rhizome system, which further limits the ability of bamboos to recover or regenerate new culms upon drought release, ultimately resulting in the death of the entire plant.
5. ConclusionsIn this study, Cephalostachyum pergracile exhibited high hydraulic safety, characterized by low P50leaf, P50stem, and turgor loss point, while no effective vulnerability segmentation mechanism was found between its leaves and stems. Nevertheless, C. pergracile may easily reach critical thresholds during severe drought due to its low water-retention capacity and reliance on surface water, suggesting that root pressure may be inefficient in refilling the embolized vessels during severe droughts. Our findings provide physiological evidence of the high mortality rate of a bamboo species during droughts, thereby helping to clarify the drought response mechanism of bamboo species.
AcknowledgmentsThe authors thank Peng-Yun Yang for his help during the measurements, Bin Yang and Xia Yuan in stable isotope analysis. We are also grateful for the support of the horticulture department of XTBG in sample collection and the National Forest Ecosystem Research Station at Xishuangbanna for providing the climatic data at the research site. This work was supported by the National Natural Science Foundation of China (Nos: 32071735, 32371576, 32350410420, 41861144016, and 31570406); CAS 'Light of West China' Program; The 14th Five-Year Plan of the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (E3ZKFF1K, E3ZKFF2B); Yunnan Provincial Science and Technology Department (2018HB068), and Yunnan Revitalization Talents Support Plan (YNWR-QNBJ-2019177).
Data accessibility statement
The data that support the findings of this study are openly available in the Science Data Bank at https://www.doi.org/10.57760/sciencedb.13284.
CRediT authorship contribution statement
Wanwalee Kongjarat: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis. Lu Han: Writing – review & editing, Writing – original draft, Resources, Methodology, Formal analysis, Data curation. Amy Ny Aina Aritsara: Writing – review & editing, Writing – original draft, Visualization, Software, Funding acquisition, Formal analysis, Data curation. Shu-Bin Zhang: Writing – review & editing, Validation, Formal analysis, Data curation. Gao-Juan Zhao: Writing – review & editing, Resources, Methodology, Data curation. Yong-Jiang Zhang: Writing – review & editing, Visualization, Validation, Methodology. Phisamai Maenpuen: Writing – review & editing, Resources, Methodology, Data curation. Ying-Mei Li: Writing – review & editing, Writing – original draft, Visualization, Methodology. Yi-Ke Zou: Writing – review & editing, Resources, Methodology, Data curation. Ming-Yi Li: Writing – review & editing, Visualization, Resources, Methodology, Data curation. Xue-Nan Li: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Lian-Bin Tao: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Ya-Jun Chen: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Adams, H.D., Zeppel, M.J.B., Anderegg, W.R.L., et al., 2017. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol., 1: 1285-1291. DOI:10.1038/s41559-017-0248-x |
Akinlabi, E.T., Anane-Fenin, K., Akwada, D.R., 2017. Bamboo - the Multipurpose Plant. Springer International Publishing, Cham, p. 262.
|
Allen, C., Macalady, A., Chenchouni, H., et al., 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag., 259: 660-684. DOI:10.1016/j.foreco.2009.09.001 |
Anderegg, W.R.L., Klein, T., Bartlett, M., et al., 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. U.S.A., 113: 5024-5029. DOI:10.1073/pnas.1525678113 |
Anderegg, W.R.L., Wolf, A., Arango-Velez, A., et al., 2018. Woody plants optimise stomatal behaviour relative to hydraulic risk. Ecol. Lett., 21: 968-977. DOI:10.1111/ele.12962 |
Aritsara, A.N.A., Wang, S., Li, B.N., et al., 2022. Divergent leaf and fine root "pressure–volume relationships" across habitats with varying water availability. Plant Physiol., 190: 2246-2259. DOI:10.1093/plphys/kiac403 |
Banik, R.L., 2015. Morphology and Growth, Bamboo: the Plant and its Uses. Springer, pp. 43-89.
|
Bartlett, M.K., Zhang, Y., Kreidler, N., et al., 2014. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett., 17: 1580-1590. DOI:10.1111/ele.12374 |
Brodersen, C.R., McElrone, A.J., Choat, B., et al., 2013. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiol., 161: 1820-1829. DOI:10.1104/pp.112.212712 |
Brodribb, T.J., Brodersen, C.R., Carriqui, M., et al., 2021. Linking xylem network failure with leaf tissue death. New Phytol., 232: 68-79. DOI:10.1111/nph.17577 |
Brodribb, T.J., Skelton, R.P., McAdam, S.A., et al., 2016. Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytol., 209: 1403-1409. DOI:10.1111/nph.13846 |
Canadell, J., Jackson, R.B., Ehleringer, J.B., et al., 1996. Maximum rooting depth of vegetation types at the global scale. Oecologia, 108: 583-595. DOI:10.1007/BF00329030 |
Cao, K.F., Yang, S.J., Zhang, Y.J., et al., 2012. The maximum height of grasses is determined by roots. Ecol. Lett., 15: 666-672. DOI:10.1111/j.1461-0248.2012.01783.x |
Carminati, A., Ahmed, M.A., Zarebanadkouki, M., et al., 2020. Stomatal closure prevents the drop in soil water potential around roots. New Phytol., 226: 1541-1543. DOI:10.1111/nph.16451 |
Chen, Y.J., Choat, B., Sterck, F., et al., 2021a. Hydraulic prediction of drought-induced plant dieback and top-kill depends on leaf habit and growth form. Ecol. Lett., 24: 2350-2363. DOI:10.1111/ele.13856 |
Chen, Y.J., Maenpuen, P., Zhang, Y.J., et al., 2021b. Quantifying vulnerability to embolism in tropical trees and lianas using five methods: can discrepancies be explained by xylem structural traits?. New Phytol., 229: 805-819. DOI:10.1111/nph.16927 |
Choat, B., Brodribb, T.J., Brodersen, C.R., et al., 2018. Triggers of tree mortality under drought. Nature, 558: 531-539. DOI:10.1038/s41586-018-0240-x |
Choat, B., Jansen, S., Brodribb, T.J., et al., 2012. Global convergence in the vulnerability of forests to drought. Nature, 491: 752-755. DOI:10.1038/nature11688 |
Clark, L.G., Londoño, X., Ruiz-Sanchez, E., 2015. Bamboo Taxonomy and Habitat. In: Liese, W., Köhl, M. (Eds.), Bamboo: the Plant and its Uses. Springer International Publishing, Cham, pp. 1-30.
|
Dai, A.G., 2011. Drought under global warming: a review. In: WIREs Climate Change, 2, pp. 45-65.
|
Ding, T., Gao, H., 2020. The record-breaking extreme drought in Yunnan province, Southwest China during spring-early summer of 2019 and possible causes. J. Meteorol. Res., 34: 997-1012. DOI:10.1007/s13351-020-0032-8 |
Dixon, H.H., Joly, J., 1895. On the ascent of sap. Philos. Trans. R. Soc. Lond. B-Biol. Sci., 186: 563-576. DOI:10.1098/rstb.1895.0012 |
Doughty, C.E., Keany, J.M., Wiebe, B.C., et al., 2023. Tropical forests are approaching critical temperature thresholds. Nature, 621: 105-111. DOI:10.1038/s41586-023-06391-z |
Duursma, R., Choat, B., 2017. Fitplc - an R package to fit hydraulic vulnerability curves. J. Plant Hydraul., 4: e002. DOI:10.20870/jph.2017.e002 |
Enarth Maviton, M., Sankar, V.R., 2023. Global Priority Species of Economically Important Bamboo. INBAR Technical Report No. 44. International Bamboo and Rattan Organization, Beijing, p. 210.
|
Ennajeh, M., Tounekti, T., Vadel, A.M., et al., 2008. Water relations and drought-induced embolism in olive (Olea europaea) varieties 'Meski' and 'Chemlali' during severe drought. Tree Physiol., 28: 971-976. DOI:10.1093/treephys/28.6.971 |
Fadrique, B., Gann, D., Nelson, B.W., et al., 2021. Bamboo phenology and life cycle drive seasonal and long-term functioning of Amazonian bamboo-dominated forests. J. Ecol., 109: 860-876. DOI:10.1111/1365-2745.13512 |
Feng, W.J., Leung, M.Y.T., Wang, D.X., et al., 2022. In: An extreme drought over South China in 2020/21 concurrent with an unprecedented warm Northwest Pacific and La Niña, 39, pp. 1637-1649.
|
Hacke, U.G., Sperry, J.S., 2001. Functional and ecological xylem anatomy. Perspect. Plant Ecol. Evol. Syst., 4: 97-115. DOI:10.1078/1433-8319-00017 |
Hacke, U.G., Sperry, J.S., Wheeler, J.K., et al., 2006. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol., 26: 689-701. DOI:10.1093/treephys/26.6.689 |
Hacke, U.G., Stiller, V., Sperry, J.S., et al., 2001. Cavitation fatigue, embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol., 125: 779-786. DOI:10.1104/pp.125.2.779 |
Hammond, W.M., Williams, A.P., Abatzoglou, J.T., et al., 2022. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth's forests. Nat. Commun., 13: 1761. DOI:10.1038/s41467-022-29289-2 |
Hao, G.Y., Sack, L., Wang, A.Y., et al., 2010. Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Funct. Ecol., 24: 731-740. DOI:10.1111/j.1365-2435.2010.01724.x |
Jacobsen, A., Pratt, R.B., Davis, S., et al., 2014. Geographic and seasonal variation in chaparral vulnerability to cavitation. Madrono, 61: 317-327. DOI:10.3120/0024-9637-61.4.317 |
Johnson, D.M., Wortemann, R., McCulloh, K.A., et al., 2016. A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiol., 36: 983-993. DOI:10.1093/treephys/tpw031 |
Kukowski, K.R., Schwinning, S., Schwartz, B.F., 2013. Hydraulic responses to extreme drought conditions in three co-dominant tree species in shallow soil over bedrock. Oecologia, 171: 819-830. DOI:10.1007/s00442-012-2466-x |
Levionnois, S., Ziegler, C., Jansen, S., et al., 2020. Vulnerability and hydraulic segmentations at the stem–leaf transition: coordination across Neotropical trees. New Phytol., 228: 512-524. DOI:10.1111/nph.16723 |
Li, Y.Y., Sperry, J.S., Shao, M.G., 2009. Hydraulic conductance and vulnerability to cavitation in corn (Zea mays L.) hybrids of differing drought resistance. Environ. Exp. Bot., 66: 341-346. DOI:10.1016/j.envexpbot.2009.02.001 |
Liese, W., Köhl, M., 2015. Bamboo - the Plant and its Uses. Springer, Cham, p. 356. Springer International Publishing Switzerland.
|
Lobo, A., Torres-Ruiz, J.M., Burlett, R., et al., 2018. Assessing inter- and intraspecific variability of xylem vulnerability to embolism in oaks. For. Ecol. Manag., 424: 53-61. DOI:10.1016/j.foreco.2018.04.031 |
McDowell, N.G., Sapes, G., Pivovaroff, A., et al., 2022. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ., 3: 294-308. DOI:10.1038/s43017-022-00272-1 |
Meinzer, F.C., Johnson, D.M., Lachenbruch, B., et al., 2009. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol., 23: 922-930. DOI:10.1111/j.1365-2435.2009.01577.x |
Pammenter, N.W., Vander Willigen, C., 1998. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol., 18: 589-593. DOI:10.1093/treephys/18.8-9.589 |
Pausas, J.G., Paula, S., 2020. Grasses and fire: the importance of hiding buds. New Phytol., 226: 957-959. DOI:10.1111/nph.15964 |
Perez-Harguindeguy, N., Diaz, S., Garnier, E., et al., 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot., 61: 167-234. DOI:10.1071/BT12225 |
Pivovaroff, A.L., Sack, L., Santiago, L.S., 2014. Coordination of stem and leaf hydraulic conductance in southern California shrubs: a test of the hydraulic segmentation hypothesis. New Phytol., 203: 842-850. DOI:10.1111/nph.12850 |
R Core Team, 2023. R: A Language and Environment for Statistical Computing (Vienna, Austria).
|
Sack, L., Pasquet-Kok, J., Contributors, P., 2011. Leaf Pressure-Volume Curve Parameters. Prometheus. |
Schenk, H.J., Jackson, R.B., 2002. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol., 90: 480-494. DOI:10.1046/j.1365-2745.2002.00682.x |
Schenk, H.J., Jackson, R.B., 2005. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma, 126: 129-140. DOI:10.1016/j.geoderma.2004.11.018 |
Schindelin, J., Arganda-Carreras, I., Frise, E., et al., 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods, 9: 676-682. DOI:10.1038/nmeth.2019 |
Scoffoni, C., Albuquerque, C., Brodersen, C.R., et al., 2017. Outside-xylem vulnerability, not xylem embolism, controls leaf hydraulic decline during dehydration. Plant Physiol., 173: 1197-1210. DOI:10.1104/pp.16.01643 |
Shen, J.X., Zhang, Y.J., Maenpuen, P., et al., 2022. Response of four evergreen savanna shrubs to an incidence of extreme drought: high embolism resistance, branch shedding and maintenance of nonstructural carbohydrates. Tree Physiol., 42: 740-753. DOI:10.1093/treephys/tpab150 |
Skelton, R.P., Brodribb, T.J., Choat, B., 2017. Casting light on xylem vulnerability in an herbaceous species reveals a lack of segmentation. New Phytol., 214: 561-569. DOI:10.1111/nph.14450 |
Smith-Martin, C.M., Muscarella, R., Hammond, W.M., et al., 2023. Hydraulic variability of tropical forests is largely independent of water availability. Ecol. Lett., 26: 1829-1839. DOI:10.1111/ele.14314 |
Stock, B.C., Semmens, B.X., 2016. MixSIAR GUI User Manual, 3.1 ed, p. 72.
|
Tao, S., Chave, J., Frison, P.L., et al., 2022. Increasing and widespread vulnerability of intact tropical rainforests to repeated droughts. Proc. Natl. Acad. Sci. U.S.A., 119: e2116626119. DOI:10.1073/pnas.2116626119 |
Terra, M.d.C.N.S., Prado-Júnior, J.A.d., Souza, C.R.d., et al., 2021. Tree species dominance in neotropical savanna aboveground biomass and productivity. For. Ecol. Manag., 496: 119430. DOI:10.1016/j.foreco.2021.119430 |
Tyree, M.T., 1997. The Cohesion-Tension theory of sap ascent: current controversies. J. Exp. Bot., 48: 1753-1765. |
Tyree, M.T., Zimmermann, M.H., 2002. Xylem Structure and the Ascent of Sap. Springer Berlin Heidelberg, Berlin, Heidelberg, p. 283.
|
Vargas, G.G., Brodribb, T.J., Dupuy, J.M., et al., 2021. Beyond leaf habit: generalities in plant function across 97 tropical dry forest tree species. New Phytol., 232: 148-161. DOI:10.3917/lp.407.0147 |
Vorontsova, M., Clark, L., Dransfield, J., et al., 2016. World Checklist of Bamboos and Rattans. INBAR, Beijing, p. 454.
|
Warton, D.I., Duursma, R.A., Falster, D.S., et al., 2012. Smatr 3- an R package for estimation and inference about allometric lines: the smatr 3 - an R package. Methods Ecol. Evol., 3: 257-259. DOI:10.1111/j.2041-210X.2011.00153.x |
Watson, D.J., 1968. A prospect of crop physiology. Ann. Appl. Biol., 62: 1-9. DOI:10.1111/j.1744-7348.1968.tb03845.x |
West, A.G., Dawson, T.E., February, E.C., et al., 2012. Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytol., 195: 396-407. DOI:10.1111/j.1469-8137.2012.04170.x |
Wheeler, J., Huggett, B., Tofte, A., et al., 2013. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Enrivon., 36: 1938-1949. DOI:10.1111/pce.12139 |
Yan, C.L., Ni, M.Y., Cao, K.F., et al., 2020. Leaf hydraulic safety margin and safety–efficiency trade-off across angiosperm woody species. Biol. Lett., 16: 20200456. DOI:10.1098/rsbl.2020.0456 |
Yang, Y.M., Wang, K.L., Pei, S.J., et al., 2004. Bamboo diversity and traditional uses in Yunnan, China. Mt. Res. Dev., 24: 157-165. DOI:10.1659/0276-4741(2004)024[0157:BDATUI]2.0.CO;2 |
Yang, D.M., Zhou, W., Wang, X.L., et al., 2023. An analytical complete model of root pressure generation: theoretical bases for studying hydraulics of bamboo. Plant Cell Environ, 47: 59-71. |
Yuan, X., Wang, L.Y., Wu, P.L., et al., 2019. Anthropogenic shift towards higher risk of flash drought over China. Nat. Commun., 10: 4661. DOI:10.1038/s41467-019-12692-7 |
Yuen, J.Q., Fung, T., Ziegler, A.D., 2017. Carbon stocks in bamboo ecosystems worldwide: estimates and uncertainties. For. Ecol. Manag., 393: 113-138. DOI:10.1016/j.foreco.2017.01.017 |
Zhang, M.X., Chen, S.L., Jiang, H., et al., 2019. Water-use characteristics and physiological response of Moso bamboo to flash droughts. Int. J. Environ. Res. Publ. Health, 16: 2174. DOI:10.3390/ijerph16122174 |
Zhou, M.Y., Zhang, Y.X., Haevermans, T., et al., 2017. Towards a complete generic-level plastid phylogeny of the paleotropical woody bamboos (Poaceae: Bambusoideae). Taxon, 66: 539-553. DOI:10.12705/663.2 |
Zhu, S.D., Chen, Y.J., Ye, Q., et al., 2018. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol., 38: 658-663. DOI:10.1093/treephys/tpy013 |
Zhu, S.D., Liu, H., Xu, Q.Y., et al., 2016. Are leaves more vulnerable to cavitation than branches?. Funct. Ecol., 30: 1740-1744. DOI:10.1111/1365-2435.12656 |



