b. University of Chinese Academy of Sciences, Beijing 100049, China
Co-flowering plants often share pollinators, which may result in interspecific pollen transfer (Feinsinger et al., 1988; Bell et al., 2005; Lazaro et al., 2009). Heterospecific pollen may interfere conspecific pollen in different reproductive stages, including adhesion and germination of pollen, pollen tube growth, ovule fertilization and seed development (Caruso and Alfaro, 1989; Wilcock and Neiland, 2002; Morales and Traveset, 2008). The impacts of interspecific pollen transfer are greater between plants that are more closely phylogenetically related, such as co-occurring and co-flowering species in the same genus (Streher et al., 2020).
Reproductive interference may be weakened when there are greater floral traits differences between species, resulted from character replacement or ecological sorting. For example, co-flowering Stylidium species in Western Australia is probably a result character displacement, where co-exist congeners evolved different "triggering" mechanisms that precisely bring styles and stigmas into contact with pollinators (Armbruster et al., 1994). Two species of Clarkia that are often found in multi-species communities in the southern Sierra foothills, they co-occur more frequently than a null model predicts (Eisen and Geber, 2018), providing a pattern consistent with the ecological sorting.
Alternatively, plants may also benefit from decreased floral trait variation within species, maintaining higher degree of floral constancy. The term floral constancy describes the phenomenon that pollinators (often social bees) tend to visit flowers of a single species successively, even ignoring alternative rewarding flowers during movements (Grant, 1950; Levin and Anderson, 1970). As Darwin mentioned, "no one will suppose that insects act in this manner for the good of the plant", but "it favours cross-fertilization of distinct individuals of the same species" (Darwin, 1876). Two non-exclusive hypotheses may explain the floral constancy phenomenon. Darwin's hypothesis suggests that pollinators are constant to minimize the costs of relearning flower-handling skills after every switch (see Woodward and Laverty, 1992). Empirical evidence indicated that bumblebees spend longer flower-handling time following a switch between flower types that need different handling skills (Gegear and Laverty, 1995, 1998). The searching image hypothesis suggests that pollinators are constant because they formed a searching image during foraging (Gegear and Laverty, 2001). Wilson and Stine (1996) found that bumble bees visited flowers from different species of similar colours, regardless of handling skills (Wilson and Stine, 1996). The extent of flower constancy is influenced by floral traits such as size, shape, scent and colour (Gegear and Laverty, 2001). To increase flower constancy, natural selection may favour distinct and constant floral traits.
Colour is an important floral trait that influences pollinators' decisions. On the one hand, pollinators were reported to successively visit one morph in species with polymorphic floral colour, which induced associative mating (Lebel et al., 2018). On the other hand, different species sharing similar floral colour (or other traits) may not be distinguished by pollinators. An example is the deceptive pollination, e.g., the non-reward orchid Eulophia zeyheriana imitates the colour of the rewarding Wahlenbergia cuspidata to attract pollinators (Garcia et al., 2020). These scenarios remind us to consider that the response of pollinators to flowers should depend on the community context, i.e., the similarity of co-flowering species (see Kagawa and Takimoto, 2016; Benadi and Gegear, 2018). Studies on floral colour in communities mainly focused on the inter-specific difference (McEwen and Vamosi, 2010) and its correlation with environment gradients ((Arnold et al., 2009). Intraspecific colour variation received far less attention and almost exclusively on discrete variation, i.e., colour polymorphism or colour change. Continuous colour variation within species, although much more common (Sapir et al., 2021; Trunschke et al., 2021), received far less attention (but see Paine et al., 2019; Whiteny et al., 2020).
Pedicularis (Orobanchaceae) is a large genus with over 600 species that are distributed in the northern hemisphere, with more than half species found in Southwest China (Hong et al., 1998). Pedicularis species in the Hengduan Mountains has great diversity in floral character (including size, shape and colour), but is almost exclusively pollinated by bumblebees and is mainly obligate outcrossers (Wang and Li, 1998; Macior et al., 2001; Yang et al., 2007; Huang and Fenster, 2007). Different communities have diverse assemblage of Pedicularis species, with various extents of floral trait similarity, providing different contexts that may influence phenotype variation. Therefore, this genus is an ideal model to understand the phenotypic variation in the context of pollinator-mediated species interaction. The assemblage of Pedicularis species in Southwestern China are suggested to be a result of pollination-related ecological sorting through selection against reproductive interference, where species with more divergent floral characters are more likely co-exist (Eaton et al., 2012). The work by Eaton et al. (2012) mentioned above investigated the floral diversity in Pedicularis communities in SW China, but treated floral colour as a discrete character and did not quantify the intraspecific variation. Furthermore, it categories colour by human perception, which is very different from the colour vision properties of the real pollinators.
This study focused on floral colour variation within and across communities, with consideration of the community composition. Specifically, we ask (1) whether the extent of floral colour variation differ between populations, and whether it correlates with the community composition; (2) what is the overall pattern of intraspecific floral colour variation as perceived by pollinators. To answer these questions, we investigated natural communities that include single or multiple Pedicularis species, and quantified floral colour based on colour perception of the pollinators. We predict that in communities that include two or more species sharing similar floral colours, the intraspecific variation should be lower, decreasing potential reproductive interference.
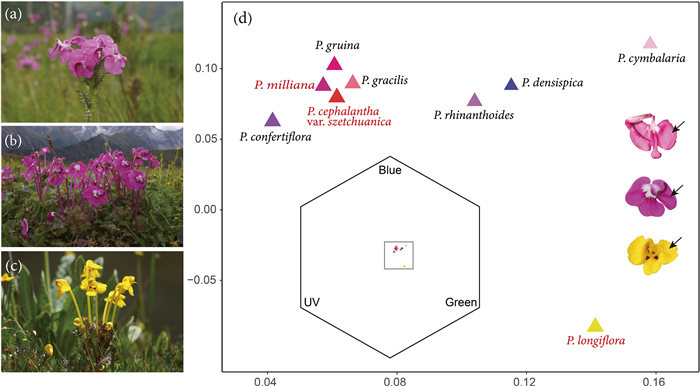
2. Materials and methods 2.1. Study species and communitiesThis study was conducted in the Hengduan Mountains (Northwestern Yunnan and Eastern Xizang) from July to September in 2021 and 2022. The three most common Pedicularis species, P. cephalantha var. szetchuanica Bonati, P. milliana W.B. Yu, D.Z. Li & H. Wang and P. longiflora Rudolph (Fig. 1a–c) are our focus species. Please note that three varieties of P. longiflora were recognized in Flora of China (Wu and Raven, 1998) (Yu, pers. comm.), these taxa were treated as a single species in this study. These species are all found in the alpine meadows and are pollinated by bumblebees.
|
| Fig. 1 Three focus Pedicularis species in this study from SW China (a–c), the distribution of the floral colours (the patch with the greatest surface area was selected) of nine Pedicularis species in the colour hexagon model for bumblebee vision and colour patches that are selected among the flowers of three focus Pedicularis species (d). (a) P. cephalantha var. szetchuanica; (b) P. milliana; (c) P. longiflora, (d) Colour loci of nine Pedicularis species involved in this study in colour hexagon, showing the colour similarity between them. The colour of the triangles is only used to distinguish between species; black arrows indicate colour patches we selected among the flowers of three focus Pedicularis species, we chose the same area of colour for each species. |
For each species, we tried to find two types of communities, one with merely the focus species, and the other coexisting with other Pedicularis species, with either similar or dissimilar floral colours. Finally, we collected 19 communities in this area (Appendix 1). In this context, a community refers to an area within the foraging range of bumblebees. The distance between the two communities is more than 20 km, and there are mountains that create a geographical barrier in between them.
Except for the three focus species, six others Pediculars species were found in these communities. Voucher specimens were collected for each species in each community and preserved at KUN (see Appendix 2).
2.2. UV-VIS images of flowersTraditionally, colour was quantified in terms of reflectance spectra, but we used an image-based colour analysis method instead in the present study for the following reasons. The intensity of reflectance spectra is very sensitive to the distance between the probe and the object. This distance should be kept constant between measurements to produce comparable repeats. To meet this requirement, the surface of objects should be flat. However, Pedicularis flowers have complex 3D structures and are not suitable for repeat reflectance measurements that estimate intraspecific variation. Digital photography can overcome these defects. Furthermore, data from all the samples in a community can be obtained in single image, reducing the experimental error caused by multiple operations. It has been tested that results based on reflectance spectra and digital images fit well (Troscianko and Stevens, 2015). We also did a similar test using Pediculars flowers, and got similar results between two methods (see Appendix 3).
To cover the spectrum that bumble bees perceives, we obtained flower images that includes both human visible (VIS) and UV light information. Specifically, a Samsung NX1000 camera was modified by removing the low-pass filter to increase sensitivity to UV light, and mounted with a Nikkor EL-80 mm lens (the exact same set-up as suggested by Troscianko and Stevens 2015). A standard set that included 10% and 80% reflectance polytetrafluoroethylene standards was placed alongside the samples for calibration. The aperture was set to f/8 and ISO at 800, the exposure time was adjusted according to the lighting conditions. We used a Baader UV/IR blocking filter that transmits light between 400 and 700 nm to capture the visible light image. For UV images, a Baader U-Venus-Filter filter that transmits light between 300 and 400 nm with a transmitting peak at 350 nm was used. Images were saved as RAW files (SRW files for Samsung cameras).
For each focus species from a community, we collected 50–60 fresh flowers, each from a different individual, for photographing. These flowers were fixed on a black background board immediately. Visible-light (400–700 nm) and UV-light (300–400 nm) photographs of flowers were then taken by the camera mounted on a tripod so that two photos were obtained from the same position. All photographs were taken in natural daylight conditions. To minimize the presence of shadows on the flowers, we typically take pictures in shaded areas at the edge of the forest under field conditions. Cloudy days, which provide diffuse sunlight but with plenty of UV rays, are the optimal conditions for taking these photographs.
2.3. Image-based colour analysesThe images were analysed by mica Toolbox, an ImageJ plugin for colour analysis. Generally, it utilizes ij-dcraw (Sacha, 2013) to extract pixel data from RAW image files linearly and generates multispectral files. Images can be normalized using white or grey standards with known reflectance values (10% and 80%). The calibrated images were then used for mapping from camera colour to cone-catch quanta in a specific animal model (Troscianko and Stevens, 2015).
Specifically, this toolbox divides visible and UV photos in RAW files into five channels of red, green, blue, UV blue and UV red, and then combine them into a multispectral image. To convert the multispectral images into cone catches of bumblebees, we established a visual model based on the photoreceptor sensitivity curve of Bombus terrestris. This is done by specifying Samsung NX1000 as the camera body and Nikkor EL-80 mm as the lens, B. terrestris as the receptors, D65 300–700 as the photography illuminant and model illuminant, and Natural Spectra 300–700 as the training spectra in the plugin. All processing is performed on 32-bit floating point images rather than commonly used 8-bit images, this increases precision and reduces the likelihood of data loss through rounding error or saturation of pixels (Troscianko and Stevens, 2015). We then manually selected regions of interest from each multispectral image. When there are multiple colour patches on the petals, the patch with the greatest surface area was selected (see Fig. 1d). Only the domain and uniform petal parts were used for colour analysis. We did not analyse the colour patches of the nectar guide because they often work after the pollinators have landed on the flowers, whereas the dominant colour patch plays a more important role in attraction. For different samples of the same species, we chose the same region for analysis. Photon capture numbers of each receptor channel were obtained for further calculation in the bee's colour vision model.
The colour hexagon model (CH model) is established for trichromatic vision and is suitable for Hymenoptera (Chittka, 1992). The model expresses the signal values of the three receptors with the coordinates of 120° to each other, producing a two-dimensional equilateral hexagon space that can analyse and visualize the colour perception of bumblebees. Any colour can be represented as a point in this space based on the signal it excites in the photoreceptor, and colour difference in the CH model is determined by the Euclidean distance (in CH units) between colour loci (Chittka, 1992). Floral colours of the nine species of Pedicularis involved in the study were plotted in the colour hexagon (Fig. 1d, this is done by the mean of all data of one species). We calculated the intraspecific floral colour distances of the focal species, with greater distances indicating higher variations.
2.4. Statistical analysisWe first examined the difference in intraspecific floral colour distances between communities, using Kruskal–Wallis tests and the Wilcoxon rank-sum test. We then tested the association between colour variation and community context, by dividing the 19 communities into two groups, those with single Pedicularis species and those with multiple Pedicularis species. The mean intraspecific colour distance between the two groups was examined using an independent samples t-test.
To test whether these intraspecific floral colour variations exceeded the bee visual threshold of 0.11 hexagon units (Dyer and Chittka, 2004), we calculated the coordinates of the centroid (geometric center) from the coordinates of the flowers of each population in the CH model, and then calculated the distance between flowers and the distance from the centroid to each flower. The evolutionary constraints were considered strong if more than 95% of the flower-flower pairwise distances were less than the threshold, and intermediate if more than 99% and 95% of the flower-centroid pairwise distances were less than the threshold. The constraint was considered weak or absent if more than 5% of the flower-centroid pairwise distances were greater than the threshold (Paine et al., 2019).
3. ResultsWe collected floral colour data from 19 communities, including 9 communities containing Pedicularis cephalantha var. szetchuanica (3 + 6, numbers of communities with single and multiple species), 7 communities containing P. milliana (3 + 4), and 9 communities containing P. longiflora (3 + 6), with some overlap between them (Appendix 2). Besides these focal species, six congeners found in these communities are P. densispica, P. rhinanthoides, P. cymbalaria, P. gracilis, P. gruina and P. confertiflora. In human eyes, these flowers range from pink, magenta to yellow, while in the eyes of bumblebees, they occupied the blue, blue-green and green region in the colour hexagon map (shown in Fig. 1d). Except for communities K and L, the floral colour similarity of Pedicularis species co-flowering in other communities was low (see Fig. 1d and Appendix).
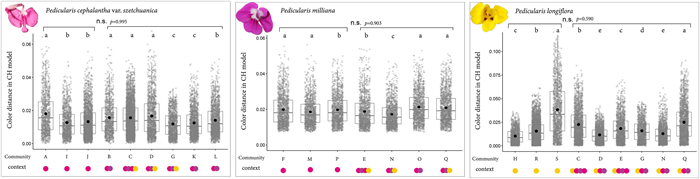
The intrapopulation colour variations (in terms of colour distances) as perceived by bumblebees range from less than 0.001 (Pedicularis cephalantha var. szetchuanica) to 0.09 (P. longiflora). For P. cephalantha var. szetchuanica, there are significant differences in intrapopulation colour variation between most communities (single: χ2 = 120.56, df = 2, p < 0.001, mixed: χ2 = 213.18, df = 5, p < 0.001, Fig. 2a). However, the difference between two types of communities, single or mixed, was not significant (Fig. 2a). Two other focal species, P. milliana and P. longiflora, showed similar patterns (single: χ2 = 16.092, df = 2, p < 0.001, mixed: χ2 = 260.1, df = 3, p < 0.001, Fig. 2b; single: χ2 = 188.17, df = 1, p < 0.001, mixed: χ2 = 824.39, df = 5, p < 0.001 Fig. 2c).
|
| Fig. 2 Intraspecific floral colour distances in communities with single or multiple Pedicularis species. Letters A to S are the community codes. Coloured circles below the horizontal axis indicates the number and floral colour of co-flowering Pedicularis species. (a) P. cephalantha var. szetchuanica; (b) P. milliana; (c) P. longiflora. |
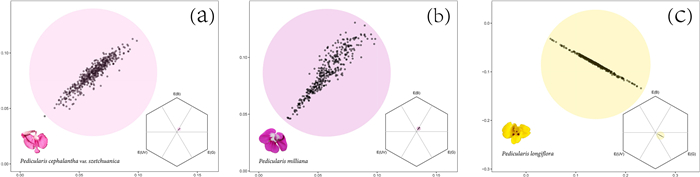
At the population level, more than 95% of flower–flower distances within populations were not discriminable for bumblebees (< 0.11 hexagon units) in all populations (25 of 25) examined, implying strong pollinator-related evolutionary constraints.
At the species level (pooled all the populations), we drew a circle that covers the region that is not discriminable by bumblebees, by setting the centroid as the center and 0.11 hexagon units as the diameter (Fig. 3). We found 99.79% and 100% floral loci of P. cephalantha var. szetchuanica and P. milliana are under the discrimination threshold of bumble bees (0.11 colour hexagon unite), implying "strong constraints" (Paine et al., 2019, Fig. 3a and b). For P. longiflora, 7.87% of flowers fall outside the circle in. So we drew a circle with the centroid as the center and 0.11 hexagon units as the radius, we found 100% floral loci of P. longiflora are within the circle, implying "intermediate constraint" (Fig. 3c).
|
| Fig. 3 The pattern of intraspecific floral colour variation. Each black dot represents the colour of a single flower. Each chart covers the flowers of all populations of each species. Regions that covered by the circle (a and b have a diameter of 0.11 hexagonal units, centered on the centroid of all points) are not discriminable by bumblebees. The circle of c has a radius of 0.11 hexagonal units, centered on the centroid of all points. (a) Pedicularis cephalantha var. szetchuanica; (b) P. milliana; (c) P. longiflora. |
Focused on three species of Pedicularis, we tested the association between intraspecific floral colour variation and the community context for the first time. In contrast to our expectations, the degree of intraspecific floral colour variation was independent of the community context. Specifically, Pedicularis species did not show lower colour variation in communities containing multiple congeners, compared with those containing a single Pedicularis species. It is possible that the history to form these herbaceous communities is not long enough to produce a detectable colour variation, or, more likely, the selection strength is not strong enough. Several reasons may lessen the selection strength on the intraspecific floral colour variation.
First, many of the co-flowering species in the mixed communities in this study have relatively large interspecific floral colour differences (Fig. 1d), which can be discriminated by bumble bees. Therefore, the selection towards a lower intraspecific colour variation may not be strong enough. Among all the communities we studied, species with very similar floral colour rarely coexist (i.e., P. milliana, P. cephalantha var. szetchuanica, P. gracilis and P. gruina, see Fig. 1d and Appendix 1). This may be a result of ecological sorting, where co-occur species assemblages are more dissimilar in floral traits than random samples from the species pool. Eaton et al. (2012) demonstrated this pattern in some flora traits of Pedicularis, but not in floral colour. Therefore, our study found a clue to support this process and deserves further attention.
Second, colour is an important but not all factor in maintaining flower constancy. Handling skills, for example, also influence the visiting behaviour of pollinators. When offered the blue or white flower with equally rewarding, pollinators visit the flowers with a lower handling time (shallow-well flowers) and are not constrained by flower colour (Sanderson et al., 2006). Besides colour, the genus Pedicularis has a very diversified floral structure, such as labellum and beak, that determines the position and posture of foraging pollinators. Although similar in colour, co-flowering Pedicularis species often have different floral structures (see Eaton et al., 2012). This is the case in the present study, where P. milliana and P. cymbalaria both have magenta flowers, but differ in the direction of their "beaks", which may be important to maintain flower constancy. As a reference in the same study region, bumblebees were found rarely switch between co-flowering Pedicularis species during the two years of visit observation (Tong and Huang, 2016). Therefore, the selection to promote flower constancy may not be reflected in the variation of floral colour.
Third, the selection on floral colour variation may also be influenced by the sensory property of pollinators. Bumblebees have trichromatic colour vision and showed flower constancy based on target colour (Gegear and Laverty, 2005), which implied strong pollinator-related evolutionary constraints on floral colour. As evidence, more than 95% of the intraspecific flower colour variation in the present study was below the discrimination threshold of pollinators. The limited studies that investigated the continuous colour variation within species had similar findings. In Sphaeralcea polychroma, Paine et al. (2019) found that the majority (70.6%) of the populations had > 95% floral colour variation that was visually indistinguishable from bees. Furthermore, the ability of colour discrimination by bumblebees is much worse than humans (Paine et al., 2019), which means they have a higher tolerance to colour variation. As a comparison, as much as 45.7% of the floral colour variation can be discriminable in the visual space of birds (Whitney et al., 2020). Given that the colour variation is already indiscriminate, it can hardly respond to selection by pollinators. This may explain the absence of colour variation differences between community contexts.
We noticed that, compared with the two Pedicularis species with pink flowers, the floral colour variations are relatively higher in P. longiflora, a species with yellow flower, with 7.87% variation exceeding the colour discrimination threshold of bumblebees. This is possibly a random result but may also have an explanation from the aspect of sensory ecology. Hymenopteran photoreceptors have maximum sensitivity values in the ultraviolet (ca. 340 nm), blue (ca. 430 nm) and green (about 535 nm), and have the best colour discrimination at wavelengths near 400 nm and 500 nm (von Helversen, 1972; Peitsch et al., 1992). The reflectance spectra of pink or magenta flowers have two peaks, in long (red) and short (blue) wavelength regions, respectively. As shown in the multi-spectra images, these flowers have relatively higher brightness in both red and blue channels and fall in the blue region in the colour hexagon map (Fig. 3a and b). Although bees are not very sensitive to the reflection in the red region, the reflections in the blue region fall into their best colour discrimination range. Therefore, it is possible that the selection pressure from pollinators on blue-pink flowers is stronger than on yellow ones (P. longiflora). The explanation can be tested in future studies that compare colour variation between taxa with blue-pink and yellow-white flowers on a larger scale.
In the present study, we investigated the intraspecific floral colour variation of three bumblebee-pollinated Pedicularis species in different communities, using an image-based colour analysis for bumblebee vision. We found that most of the intrapopulation floral colour variation was below the colour discrimination threshold of bumblebees, which implies strong selection by pollinators. Furthermore, there is no significant difference in intraspecific floral colour variation between different community contexts. This may be due to the fact that there are already large interspecific character differences caused by ecological sorting.
AcknowledgementThis research was funded by the West Light Foundation of The Chinese Academy of Sciences, national youth talent support program and Yunnan youth talents plan (YNWR-QNBJ-2018-183 to Y.N.). We thank Li-Shen Qian, Zi-Jue Ren, Wan-Yuan Dang and Tao Huang for help in field experiments, Li-Shen Qian for suggestion on graph preparation; Wen-Bin Yu for discussion on the taxonomy of P. longiflora.
Author contributions
YN, ZC and HS designed the research. QYZ performed the experiments and analyses. QYZ and YN wrote the paper. All authors approved the final paper.
Data availability statement
The data that support the findings of this study are openly available in the Science Data Bank at https://www.doi.org/.
Declaration of competing interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.03.011.
Armbruster, W.S., Edwards, M.E., Debevec, E.M., 1994. Floral character displacement generates assemblage structure of western Australian Triggerplants (Stylidium). Ecology, 75: 315-329. DOI:10.2307/1939537 |
Arnold, S.E.J., Savolainen, V., Chittka, L., 2009. Flower colours along an alpine altitude gradient, seen through the eyes of fly and bee pollinators. Arthropod-Plant Interacts., 3: 27-43. DOI:10.1007/s11829-009-9056-9 |
Bell, J.M., Karron, J.D., Mitchell, R.J., 2005. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology, 86: 762-771. DOI:10.1890/04-0694 |
Benadi, G., Gegear, R.J., 2018. Adaptive foraging of pollinators can promote pollination of a rare plant species. Am. Nat., 192: E81-E92. |
Caruso, C.M., Alfaro, M., 1989. Interspecific pollen transfer as a mechanism of competition: effect of Castilleja linariaefolia pollen on seed set of Ipomopsis aggregata. Can. J. Bot., 78: 600-606. |
Chittka, L., 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J. Comp. Physiol., 170: 533-543. |
Darwin, C., 1876. The Effects of Cross and Self-Fertilisation in the Vegetable Kingdom. Cambridge: Cambridge University Press.
|
Dyer, A.G., Chittka, L., 2004. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften, 91: 224-227. |
Eaton, D.A.R., Fenster, C.B., Hereford, J., et al., 2012. Floral diversity and community structure in Pedicularis (Orobanchaceae). Ecology, 93: S182-S194. |
Eisen, K.E., Geber, M.A., 2018. Ecological sorting and character displacement contribute to the structure of communities of Clarkia species. J. Evol. Biol., 31: 1440-1458. DOI:10.1111/jeb.13365 |
Feinsinger, P., Busby, W.H., Tiebout, H.M., 1988. Effects of indiscriminate foraging by tropical hummingbirds on pollination and plant reproductive success: experiments with two tropical treelets (Rubiaceae). Oecologia, 76: 471-474. |
Garcia, J.E., Phillips, R.D., Peter, C.I., et al., 2020. Changing how biologists view flowers-color as a perception not a trait. Front. Plant Sci., 11: 601700. |
Gegear, R.J., Laverty, T.M., 1995. Effect of flower complexity on relearning flower-handling skills in bumblebees. Can. J. Zool., 73: 2052-2058. DOI:10.1139/z95-241 |
Gegear, R.J., Laverty, T.M., 1998. How many flower types can bumble bees work at the same time. Can. J. Zool., 76: 1358-1365. |
Gegear, R.J., Laverty, T.M., 2001. The effect of variation among floral traits on the flower constancy of pollinators. In: Chittka, L., Thomson, J.D. (Eds. ), Cognitive Ecology of Pollination: Animal Behaviour and Floral Evolution, Chapter 1. Cambridge University Press, Cambridge, pp. 1-20.
|
Gegear, R.J., Laverty, T.M., 2005. Flower constancy in bumblebees: a test of the trait variability hypothesis. Anim. Behav., 69: 939-949. |
Grant, V., 1950. The flower constancy of bees. Bot. Rev., 16: 379-398. |
Hong, D.Y., Yang, H.B., Jin, C.L., et al., 1998. Pedicularis L. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds. ), Flora of China, 18. Missouri Botanical Garden Press, St. Louis, USA and Science Press, Beijing, China, pp. 105-224.
|
Huang, S.Q., Fenster, C.B., 2007. Absence of long-proboscid pollinators for long-corolla-tubed Himalayan Pedicularis species: implications for the evolution of corolla length. Int. J. Plant Sci., 168: 325-331. DOI:10.1086/510209 |
Kagawa, K., Takimoto, G., 2016. Inaccurate color discrimination by pollinators promotes evolution of discrete color polymorphism in food-deceptive flowers. Am. Nat., 187: 194-204. DOI:10.1086/684433 |
Lazaro, A., Lundgren, R., Totland, O., 2009. Co-flowering neighbors influence the diversity and identity of pollinator groups visiting plant species. Oikos, 118: 691-702. DOI:10.1111/j.1600-0706.2008.17168.x |
Lebel, M., Obolski, U., Hadany, L., et al., 2018. Pollinator-mediated selection on floral size and tube color in Linum pubescens: can differential behavior and preference in different times of the day maintain dimorphism?. Ecol. Evol., 8: 1096-10106. DOI:10.1002/ece3.3683 |
Levin, D.A., Anderson, W.W., 1970. Competition for pollinators between simultaneously flowering species. Am. Nat., 104: 455-467. |
Macior, L.W., Tang, Y., Zhang, J.C., 2001. Reproductive biology of Pedicularis (Scrophulariaceae) in the Sichuan Himalaya. Plant Species Biol., 16: 83-89. |
McEwen, J.R., Vamosi, J.C., 2010. Floral colour versus phylogeny in structuring subalpine flowering communities. Proc. R. Soc. B-Biol. Sci., 277: 2957-2965. DOI:10.1098/rspb.2010.0501 |
Morales, C.L., Traveset, A., 2008. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Crit. Rev. Plant Sci., 27: 221-238. DOI:10.1080/07352680802205631 |
Paine, K.C., White, T.E., Whitney, K.D., 2019. Intraspecific floral color variation as perceived by pollinators and non-pollinators: evidence for pollinator-imposed constraints?. Evol. Ecol., 33: 461-479. DOI:10.1007/s10682-019-09991-2 |
Peitsch, D., Fietz, A., Hertel, H., et al., 1992. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol., 170: 23-40. |
Sacha, J., 2013. Ij-Dcraw Plugin. V. 1.4.0. http://sourceforge.net/projects/ij-plugins/&cspan class.
|
Sanderson, C.E., Orozco, B.S., Hill, P.S.M., et al., 2006. Honeybee (Apis mellifera ligustica) response to differences in handling time. rewards and flower colours. Ethology, 112: 937-946. DOI:10.1111/j.1439-0310.2006.01245.x |
Sapir, Y., Gallagher, M.K., Senden, E., 2021. What maintains flower colour variation within populations?. Trends Ecol. Evol., 36: 507-519. |
Streher, N.S., Bergamo, P.J., Ashman, T.L., et al., 2020. Effect of heterospecific pollen deposition on pollen tube growth depends on the phylogenetic relatedness between donor and recipient. AoB Plants, 12: plaa016. |
Tong, Z.Y., Huang, S.Q., 2016. Pre- and post-pollination interaction between six co-flowering Pedicularis species via heterospecific pollen transfer. New Phytol., 211: 1452-1461. DOI:10.1111/nph.14005 |
Troscianko, J., Stevens, M., 2015. Image calibration and analysis toolbox - a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol. Evol., 6: 1320-1331. |
Trunschke, J., Lunau, K., Pyke, G.H., et al., 2021. Flower color evolution and the evidence of pollinator-mediated selection. Front. Plant Sci., 12: 617851. |
von Helversen, O., 1972. Zur spektralen unterschiedsempfindlichkeit der Honigbiene. J. Comp. Physiol., 80: 439-472. |
Wang, H., Li, D.Z., 1998. A preliminary study of pollination biology of Pedicularis (Scrophulariaceae) in Northwest Yunnan, China. Acta Bot. Sin., 40: 204-210. |
Whitney, K.D., Smith, A.K., White, T.E., 2020. Birds perceive more intraspecific color variation in bird-pollinated than bee-pollinated flowers. Front. Plant Sci., 11: 590347. |
Wilcock, C., Neiland, R., 2002. Pollination failure in plants: why it happens and when it matters. Trends Plant Sci., 7: 270-277. |
Wilson, P., Stine, M., 1996. Floral constancy in bumble bees: handling efficiency or perceptual conditioning?. Oecologia, 106: 493-499. |
Woodward, G.L., Laverty, T.M., 1992. Recall of flower handling skills by bumble bees: a test of Darwin's interference hypothesis. Anim. Behav., 44: 1045-1051. |
Wu, Z.Y., Raven, P.H. (Eds. ), 1998. Flora of China. Vol. 18 (Scrophulariaceae through Gesneriaceae). Science Press, Beijing and Missouri Botanical Garden Press, St. Louis.
|
Yang, C.F., Gituru, R.W., Guo, Y.H., 2007. Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): how does the same pollinator type avoid interspecific pollen transfer?. Biol. J. Linn. Soc., 90: 37-48. DOI:10.1111/j.1095-8312.2007.00709.x |



