b. University of Chinese Academy of Sciences, Yuquan Road, Beijing 100049, China
Human activities, such as fossil fuel burning, chemical fertilizer utilization, and animal husbandry development, have caused a sharp increase in the global atmospheric reactive nitrogen (N) deposition since the Industrial Revolution (Canfield et al., 2010). Global atmospheric N deposition has been projected to continually increase in the coming decades (Galloway and Cowling, 2021). Nitrogen enrichment, through atmospheric deposition (Stevens et al., 2004) or artificial applications (Clark et al., 2007; Bharath et al., 2020; Wilfahrt et al., 2021), has increased species biomass (Yue et al., 2020) but caused a decline in the plant species diversity in various grasslands. There are several non-exclusive mechanisms for these N-induced species losses in grasslands (Tian et al., 2020): soil eutrophication (Bobbink et al., 2010), acidification (Stevens et al., 2004; Tian and Niu, 2015; Wei et al., 2022), ammonium toxicity (Kleijn et al., 2008; Zhang et al., 2014b), cation imbalance (Clark et al., 2007; Midolo et al., 2019), metal ion toxicity (Tian et al., 2016, 2020; Bowman et al., 2018), and aboveground light restriction (Hautier et al., 2009; DeMalach et al., 2017; Xiao et al., 2021; Eskelinen et al., 2022).
It has been agreed that light restriction is considered the most acceptable aboveground factor affecting the decline in species diversity under nutrient addition in global grasslands (Hautier et al., 2009; Borer et al., 2014; Eskelinen et al., 2022). It is also widely accepted that species with a short stature are more likely to be lost under N-enrichment and N deposition owing to their weaker light competitiveness (Suding et al., 2005; Hautier et al., 2009; DeMalach et al., 2017; Wang et al., 2021; Eskelinen et al., 2022), compared with taller clonal grasses (Dickson and Gross, 2013; Dickson et al., 2014; Gross and Mittelbach, 2017). For perennial/clonal species, the plant stature can be measured by either the vegetative or reproductive module. The contribution of these vegetative and reproductive modules to plant growth is different. Vegetative modules play an important role in resource acquisition and allocation strategies, while reproductive modules are closely related to reproduction and viability (Segrestin et al., 2020). There is a close relationship between plant stature (average vegetative and reproductive height) and N-induced reduction in species richness (Gough et al., 2012; Virtanen et al., 2017). However, we know that a lower vegetative stature does not necessarily associate with a shorter reproductive height in a clonal species. Plant height regulates the species diversity by affecting the probability of species extinction and colonization during N enrichment (Yang et al., 2018; Zhu et al., 2020). Therefore, clarifying whether reproductive or vegetative height affects viability in N-rich environments is necessary.
Nitrogen-induced species diversity loss can be mediated by the frequency of N enrichment (Zhang et al., 2016; Cao et al., 2020), which is much higher than that in artificial fertilization that adds N once or several times in atmospheric deposition (Smith et al., 2009). Our previous studies have shown that the species colonization was greater under a low frequency (twice a year) of N addition (Zhang et al., 2016), resulting in a higher loss of species diversity at the community level (Zhang et al., 2014b). Additionally, the low N addition frequency caused a greater loss in clonal grasses (Zhang et al., 2014b, 2016). However, it is unknown whether the frequency of N addition affects the probability of extinction and colonization in clonal grasses, similarly to that in the community. Moreover, perennial grasses have clonal characteristics that are different from the other plant species in the community in response to N enrichment. Nitrogen addition also enhances interspecific competition in clonal species (Gough et al., 2012), resulting in the loss of those species with weak competitiveness (Gross and Mittelbach, 2017). It has been reported that in comparison with sexual reproduction, clonal propagation has its advantage undergoing unfavorite environmental conditions (Barrett, 2015; Stephens et al., 2020; Gao et al., 2020). For example, Li et al. (2021) found that N addition decreased sexual reproduction, but increased clonal propagation in the perennial grass dominated temperate grasslands, thus suggesting that more resources are allocated to vegetative modules in clonal grasses under N enrichment. Taken together, vegetative modules may play an important role in determining the survival ability of clonal grasses in an N-enriched environment. However, further studies are required.
Inner Mongolian grassland with a high plant diversity is located on the eastern edge of the Eurasian steppe. Clonal grasses, which are the main constructive and dominant species in grasslands, are sensitive to N enrichment (Song et al., 2012; Zhang et al., 2016, 2019). The grassland experienced a relatively low (below 1.0 g N m−2) annual atmospheric N deposition during the past 30 years (Yu et al., 2019). Therefore, this is the ideal model ecosystem to evaluate the impact of increasing N deposition on biodiversity and relative ecological processes. This study aimed to determine whether the frequency of N addition regulates the probability of extinction and colonization, and tease apart the effects of vegetative and reproductive plant height on this probability in determining species diversity of clonal grasses in Northern China. We hypothesized that: (ⅰ) the colonization probability is lower under a low frequency of N addition, while the extinction probability is similar between the two frequencies of N addition and is similar to the whole community (Zhang et al., 2016), because we have previously found that similar effects of the rate and frequency of N addition on the richness of grass and the whole community in the same experimental setup (Zhang et al., 2014b); and (ⅱ) plant height of the vegetative module, rather than the reproductive module, determines clonal grass loss as N addition decreased sexual reproduction but increased clonal propagation in temperate grasslands (Li et al., 2021).
2. Materials and methods 2.1. Study siteThe experimental grassland (43°13′ N, 116°14′ E), located on a temperate steppe in Inner Mongolia, China, near the Inner Mongolia Grassland Ecosystem Research Station, the Chinese Academy of Sciences, was fenced in 1999 to exclude large animal grazing. This site was relatively flat with an altitude of approximately 1255–1260 m. The long-term mean annual temperature was 1.1 ℃ (1982–2019), with the mean monthly temperatures ranging from −21.1 ℃ (January) to 20.0 ℃ (July). The long-term mean annual precipitation was 341.3 mm with approximately three-fourths falling during growing seasons (from May to August). Clonal grasses, such as Leymus chinensis, Stipa grandis, Achnatherum sibiricum, Agropyron cristatum, Cleistogenes squarrosa, Koeleria cristata, and Festuca dahurica, accounted for over 90% of the aboveground biomass in the whole community (Zhang et al., 2015). No fertilizer was added to the experimental grassland prior to our experiment. The total atmospheric N deposition was below 1.0 g N m−2 yr−1 during 1980–2015 (Yu et al., 2019).
2.2. Experimental designThe field N addition experimental design used in this study was previously reported in detail by Zhang et al. (2019). Briefly, the randomized complete block design field experiment started in 2008 and included two frequencies (twice yr−1 versus monthly) crossing with nine rates (0, 1, 2, 3, 5, 10, 15, 20, and 50 g N m−2 yr−1) of N addition. Nitrogen (NH4NO3; > 99%) addition started on September 1st, 2008, and thereafter, continued on the first day of each month for the high frequency treatment (12N additions yr−1). The low frequency N treatment started on November 1st, 2008, and thereafter, continued on the first day of June and November each year (2N additions yr−1). NH4NO3 was mixed with clean sand (0.5 kg yr−1) from November to next April (during daily average air temperature < 0 ℃) and in purified water (less than 1 mm yr−1) from May to October (during daily average air temperature ≥5 ℃). Therefore, the amount of N added following the monthly addition on August 1st (plant community peak biomass period; Zhang et al., 2018) was the same between the two frequencies (Zhang et al., 2014b). Hence, the experiments with N addition all added both purified water and clean sand. Moreover, not know, we set a control treatment (neither N nor water addition) to detect whether these added purified water and clean sand may affect the grassland ecosystem. Therefore, this experiment had 19 treatments including the control, with each containing 10 blocks (replicates), and an area of 8 m × 8 m for each plot. There was an interval of 1 m between the plots and 2 m between the blocks.
2.3. Field plant aboveground biomass and species richnessSpecies richness (the number of plant species) and plant aboveground net primary productivity (ANPP) were measured following Zhang et al. (2015). Briefly, the number of plant species and plant aboveground biomass was sampled each year from 2008 to 2013 between August 10–15th using one 0.5 m × 2 m quadrat per plot without spatial overlaps. After investigating species richness in per quadrat, plant aboveground biomass was harvested by cutting the green vegetation on the soil surface and separated by species. The green vascular parts of each species were oven-dried at 65 ℃ for 48 h to a constant weight, and these dry mass can be represented as plant ANPP, because all aboveground plants have died during the winter season (Zhang et al., 2018). Here, only species richness and ANPP of clonal grasses were used.
2.4. Soil sampling and measurementFollowing Zhang et al. (2016), concurrent with the period of above-ground plant sampling in 2012 and 2013, three soil cores (0–10 cm depth and 3 cm in diameter) were collected from each plot. In order to investigate the potential influence of soil acidification and ammonium (NH4+–N; mg kg−1) accumulation on the species diversity, soil pH and soil NH4+–N concentration were measured. The soil samples from each plot were thoroughly mixed and sieved through a 2-mm mesh to obtain one composite sample for laboratory analysis of soil ammonium and soil water content (%). Subsamples were air-dried for analysis of soil pH. For soil NH4+–N measurements, 10 g fresh soil subsamples were extracted with 50 mL 2.0 M KCl solution and then analyzed using a flow injection autoanalyzer (FLAstar 5000 Analyzer; Foss Tecator, Hillerod, Denmark). The soil pH was measured in water suspension (soil: water = 1:2.5) by a pH meter (FE20–FiveEasy).
2.5. Plant trait measurementsAll clonal grasses (Table 1) in this field experiment (Zhang et al., 2019) were selected to measure the plant traits. Five clumps were dug out for each species from the natural grassland near the experimental area in mid-August, 2020, during the peak aboveground biomass period in this area. Eight traits potentially affecting the species response to N enrichment were quantified (Yang et al., 2018; Zhu et al., 2020). These were plant heights, stem diameter and biomass, leaf biomass and number, and root depth, diameter, and biomass (Table S3). For example, plant height includes vegetative height (the length from soil surface to the vegetative stem top), reproductive height (the length from soil surface to the flower spike top) and average height (the average value of reproductive height and vegetative height).
| Scientific name | Family | Genus | Relative aboveground biomass in community (%) | Clonal/Non |
| Stipa grandis P. Smirn. | Gramineae | Stipa | 35.60 | Clonal |
| Leymus chinensis (Trin.) Tzvel. | Gramineae | Leymus | 25.22 | Clonal |
| Achnatherum sibiricum (L.) Keng | Gramineae | Achnatherum | 16.49 | Clonal |
| Agropyon cristatum Roshev. | Gramineae | Agropyon | 11.64 | Clonal |
| Cleistogenes squarrosa (Trin.) Keng. | Gramineae | Cleistogenes | 3.01 | Clonal |
| Koeleria cristata (L.) Pers. | Gramineae | Koeleria | 1.29 | Clonal |
| Festuca dahurica (St.-Yves) V. Krecz. et Bobr. | Gramineae | Festuca | 0.12 | Clonal |
Principal component analysis (PCA) for the species was carried out under three modules based on the plant traits: reproductive module, vegetative module, and their average value. The results for the PCA employing a FactoMineR package (Lê et al., 2008) were different (Fig. S1) when using the reproductive module traits (80.1%), vegetative module traits (75.3%), or average value of the plant traits (78.2%). This information provided basic confidence in distinguishing the contribution from the reproductive and vegetative plant height to changes in clonal grass richness under N enrichment.
Following the methods from Yang et al. (2018) and Zhu et al. (2020), the probability of species extinction and colonization, as well as species diversity (frequency) determination was calculated during 2008–2013. If a species presented in the ith survey of the same plot but disappeared in the ith + 1 survey, it was recognized as extinct, and the proportion of 10 plots under a certain treatment was the extinction probability of the species (Yang et al., 2018). Similarly, if a species was absent in the ith survey of the same plot but appeared in the ith + 1 survey, it was recognized as colonized, and the proportion of 10 plots under a certain treatment was the colonization probability of the species. The probability of species colonization and extinction was calculated annually with a 5-year N addition. Following Zhang et al. (2016), species frequency was calculated as the species appearance proportion of 10 plots under a certain treatment.
Repeated-measure covariance analysis was then employed for testing the effects of the rate and frequency of N addition, species, year, and their interactions, using the block as a random factor, on the following: (ⅰ) three-way covariance analysis used for species frequency following 5 years of N addition (2013), with the pre-treatment (2008) species frequency as the covariate; (ⅱ) repeated-measure variance analysis (ANOVA) used for the annual probability of species extinction and colonization; and (ⅲ) three-way ANOVA used for the probability of extinction and colonization during 2008–2013.
The generalized linear model was used to test the effects of the reproductive plant height, vegetative plant height, average plant height, and ANPP of the species in the control on the probability of species extinction and colonization, with the effectiveness of the model evaluated using the Akaike information criterion (AIC).
Multiple stepwise regression analysis was also used to test the effects of plant height (vegetative, reproductive and average height) and ANPP of the species in the control on the probability of species extinction and colonization. Before conducting the multiple stepwise regression analysis, collinearity predictor (average height) was removed (Variance Inflation Factors; VIF > 10) (O'brien, 2007).
Soil NH4+–N was log10-transformed to meet the assumptions of normality and homogeneity. Pearson correlation was employed to assess whether soil acidification, ammonium accumulation affected the species diversity. Analysis of covariance, with soil pH, NH4+–N as covariates, was used to distinguish the slopes between the two N addition frequencies on the species frequency, extinction probability and colonization probability. We found no significant differences of two slopes were detected in our measured variables between the two frequencies of N addition. Thus, the correlation was given with combined data. Data analysis and mapping were carried out in the R 4.2.2 software (R Core Team, 2020).
3. Results 3.1. Effects of N addition rates on species frequency and probability of species extinction and colonizationNitrogen addition affected both the ANPP and relative ANPP in these clonal grasses community during 2009–2013 (Fig. S2). For yearly species frequency, it decreased with the increasing N addition rates (Table S1; Figs. S3a and d; F1, 1242 = 50.6, P < 0.0001), and the negative effect of N addition was more evident with this increase in its duration (Table S1; Fig. S3a). Nitrogen addition significantly increased the species annual extinction probability (Table S1; Fig. S3e; F1, 1242 = 12.9, P = 0.0004), but did not alter their annual colonization probability (Table S1; Fig. S3f; F1, 1242 = 3.6, P = 0.0565).
Similarly, N addition significantly decreased species frequency over years of 2008–2013 (Table 2; Fig. 1a; F1, 1222 = 84.7, P < 0.0001) and increased species extinction probability (Table 2; Fig. 1b; F1, 1223 = 64.6, P < 0.0001), but did not change the species colonization probability (Table 2; Fig. 1c; F1, 1223 = 2.5, P = 0.1131).
| Species frequency | Extinction probability | Colonization probability | ||||||
| F | P | F | P | F | P | |||
| V0 | 446.1 | < 0.0001 | – | – | – | – | ||
| Block | 1.0 | 0.4446 | 0.8 | 0.6443 | 1.1 | 0.3938 | ||
| Rate of N addition (N) | 84.7 | < 0.0001 | 64.6 | < 0.0001 | 2.5 | 0.1131 | ||
| Frequency of N addition (F) | 13.4 | 0.0003 | 9.4 | 0.0022 | 4.6 | 0.0326 | ||
| Species (S) | 96.9 | < 0.0001 | 51.8 | < 0.0001 | 14.7 | < 0.0001 | ||
| N × F | 0.2 | 0.6901 | 1.2 | 0.2774 | 1.5 | 0.2209 | ||
| N × S | 12.6 | < 0.0001 | 0.6 | < 0.0001 | 1.3 | 0.2498 | ||
| F × S | 0.8 | 0.6090 | 0.7 | 0.6491 | 1.1 | 0.3509 | ||
| N × F × S | 1.2 | 0.3013 | 0.6 | 0.7389 | 0.7 | 0.6179 | ||
| Note: – indicates there is no covariate in the analysis. | ||||||||

|
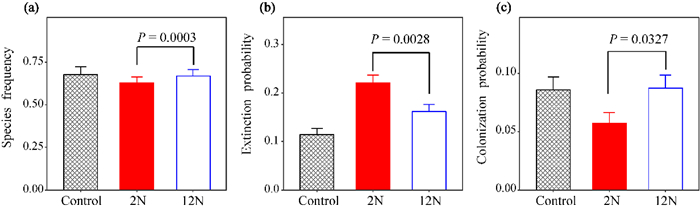
| Fig. 1 The effects of the rate of N addition on species frequency and the probability of species extinction and colonization across 2008–2013. (a) species frequency, (b) extinction probability and (c) colonization probability. Nine rates (0, 1, 2, 3, 5, 10, 15, 20, and 50 g N m−2 yr−1) crossed with two frequencies (twice yr−1 vs. monthly) of N addition, which gray polygon, red circles and bule circles represent the control (ambient N), the low (twice yr−1; 2N additions yr−1) and the high (monthly; 12N additions yr−1) frequency of N addition, respectively. P-values between the two frequencies of N addition were given. |
The frequency in N addition did not change the annual species frequency (Table S1; F1, 1242 = 0.4, P = 0.5202) or annual probability of species extinction (Table S1; F1, 1242 = 1.1, P = 0.3034) and colonization (Table S1; F1, 1242 = 3.1, P = 0.0785). The frequency of N addition and the year interacted with the annual species frequency (Table S1; F5, 6255 = 4.0, P = 0.0012).
Compared to the control, species frequency was reduced by 11.67% and 3.13% at the low and high frequency of N addition, respectively (Table 2; Fig. 2a; F1, 1222 = 13.4, P = 0.0003). Regardless of the frequency of N addition, N addition increased extinction probability but decreased colonization probability. Extinction probability (93.06% vs 41.67%) was significantly higher (Table 2; Fig. 2b; F1, 1223 = 9.4, P = 0.0022) and colonization probability (33.33% vs 1.85%) was lower at the lower frequency of N addition (Fig. 2c; F1, 1223 = 4.6, P = 0.0326).
|
| Fig. 2 The effects of the frequency of N addition on species frequency and the probability of species extinction and colonization across 2008–2013. (a) species frequency, (b) extinction probability and (c) colonization probability. Gray, red and bule represent the control (ambient N conditions), the low (twice yr−1; 2N additions yr−1) and the high (monthly; 12N additions yr−1) frequency of N addition, respectively. P-value between the two frequencies of N addition were given. |
Irrespective of the frequency of N addition, species frequency was positively correlated with soil pH (Fig. S6a; R2 = 0.84, P < 0.001) and negatively correlated with soil ammonium concentration (Fig. S6d; R2 = 0.84, P < 0.001). Also, the species extinction probability was negatively correlated with soil pH (Fig. S6b; R2 = 0.71, P < 0.001) and positively correlated with soil ammonium concentration (Fig. S6e; R2 = 0.74, P < 0.001) at two frequencies of N addition, while the relationship between the species colonization probability and soil pH and soil ammonium nitrogen was not significant (Figs. S6c and f; Ps ≥ 0.1446).
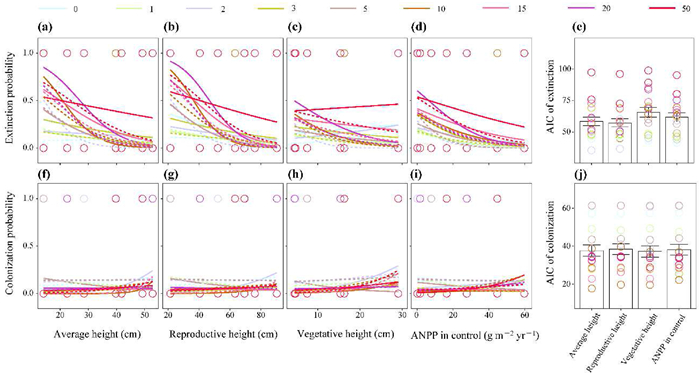
3.4. Relationship between plant height and the probability of extinction and colonizationPlant reproductive height (Fig. 3b, e; AIC = 57.4) was the best fit to species extinction probability compared to the average height (Fig. 3a; AIC = 58.5), vegetative height (Fig. 3c; AIC = 65.9), and ANPP (Fig. 3d; AIC = 61.9). Meanwhile, neither the reproductive height, vegetative height, average height, nor species ANPP correlated with the species colonization probability (Table S2; R2 < 0.10), with the lowest mean AIC observed in the vegetative height (Fig. 3f–j). There was no significant correlation between the species extinction/colonization probability and other measured traits, such as plant root depth (Figs. S5a–d).
|
| Fig. 3 Species extinction and colonization as functions of average height (a, f), reproductive height (b, g), vegetative height (c, h) and species ANPP in control (d, i) under N enrichment. AIC values show the fit goodness of different models (e, j). Different colors represent 9 rates of nitrogen additions (0, 1, 2, 3, 5, 10, 15, 20, and 50 g N m−2 yr−1); Solid lines and Dotted lines represent the low (twice yr−1; 2N additions yr−1) and the high (monthly; 12N additions yr−1) frequency of N addition, respectively. |
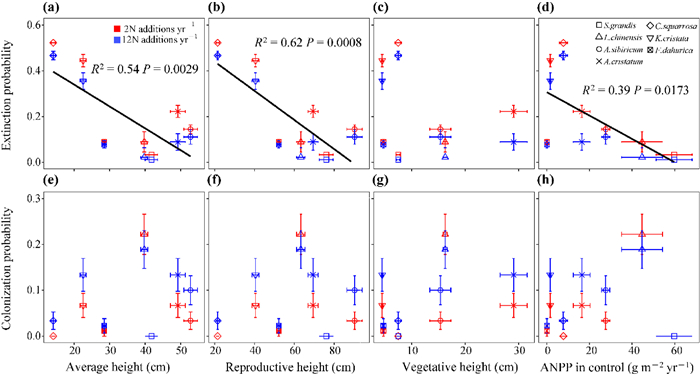
Moreover, the plant reproductive height (Fig. 4b; R2 = 0.62, P = 0.0008) was more closely correlated with species extinction probability as it had the highest R2 value compared with the average height (Fig. 4a; R2 = 0.54, P = 0.0029), vegetative height (Fig. 4c; R2 = 0.08, P = 0.3287), and species ANPP (Fig. 4d; R2 = 0.39, P = 0.0173), regardless of the N addition frequency (Figs. S4a–d). There was no significant correlation between the species colonization probability and plant reproductive, average, and vegetative heights, and species ANPP (Fig. 4e–h; Figs. S4e–h), suggesting that plant height and species ANPP are poor indices for species colonization probability with N addition.
|
| Fig. 4 The correlation relationship of species extinction probability and colonization probability and average height (a, e), reproductive height (b, f), vegetative height (c, g) and species ANPP in control (d, h) under N additions. Red and bule represent the low (twice yr−1; 2N additions yr−1) and the high (monthly; 12N additions yr−1) frequency of N addition, respectively. Different shapes represent species identity. Significant lines were shown. |
Finally, results of the multiple stepwise regression analysis showed that the reproductive height was the best individual predictor for species extinction probability with 62% of the variance explained (Table 3; F1, 12 = 19.7, P = 0.0008). However, similar with above-mentioned analysis, neither plant height nor ANPP was a significant factor for colonization probability (Table 3; P > 0.05).
| Response variable | Multiple stepwise regression model | AIC | F | R2 | P |
| Extinction probability (EP) | Model 1: EP = 0.002VH – 0.006RH – 0.002ANPP | −56.31 | 6.5 | 0.66 | 0.0104 |
| Model 2: EP = – 0.005RH – 0.002ANPP | −58.05 | 10.4 | 0.65 | 0.0029 | |
| Model 3: EP = – 0.006RH | −58.82 | 19.7 | 0.62 | 0.0008 | |
| Colonization probability (CP) | Model 1: CP = 0.004VH – 0.007RH + 0.001ANPP | −69.95 | 1.0 | 0.23 | 0.4372 |
| Model 2: CP = 0.004VH – 0.0003RH | −71.54 | 1.4 | 0.21 | 0.2819 | |
| Model 3: CP = 0.004VH | −73.40 | 3.0 | 0.20 | 0.1110 |
To the best of our knowledge, this is the first in situ study designed to tease apart the effects of reproductive height, vegetative height, and their average, and species biomass on the probability of species extinction and colonization, as the plant height and species biomass were the most commonly used to analyze the richness of clonal grasses in previous studies (Gough et al., 2012; Dickson and Gross, 2013; Gross and Mittelbach, 2017). Nitrogen addition significantly decreased the species frequency and increased species extinction probability, but did not significantly alter the species colonization probability. A low frequency of N addition reduced the species frequency and colonization probability, but showed a greater species extinction probability, partially supporting our first hypothesis. This finding suggests that this may overestimate the negative effect of atmospheric N deposition on clonal grass richness by overestimating extinction probability and underestimating colonization probability. Contrary to our second hypothesis, reproductive height was the best index for assessing the species extinction probability relative to vegetative height, average height, and species biomass. Therefore, clonal species with a low reproductive height face a higher extinction risk with an increasing N deposition.
4.1. Frequency of N addition on species extinction and colonization of clonal grassesThe species frequency and colonization probability were lower under the low frequency N addition (Table 2; Fig. 2a, c), which correlates with our previous results at the community level (Zhang et al., 2014b, 2016). These results may be related to the stronger effects of soil ammonium toxicity (Zhang et al., 2014b, 2016). Surprisingly, the low frequency N addition increased the clonal species extinction probability, which is inconsistent with our previous results, thus showing that the resident species loss number was greater between the two frequencies of N addition at the community level (Zhang et al., 2016). The higher clonal species extinction probability under the low frequency of N addition may also be related to an increased soil ammonium toxicity. Because we found that clonal species extinction probability was negatively correlated with soil pH and positively correlated with soil ammonium concentration at two frequencies of N addition, and during the experimental period, only the soil ammonium concentration was significantly higher at the low frequency of N addition (Zhang et al., 2014a, 2014b) due to the lower ammonia emissions from the soils (Zhang et al., 2014a). The higher soil ammonium nitrogen concentration may be toxic to seed germination, seedlings, and tillers (van Den Berg et al., 2005), thus reducing seedling recruitment and tiller survival whilst accelerating the replacement of alkaline cations such as Ca2+ and Mg2+ from the soil (Matschonat and Matzner, 1996; Bowman et al., 2008). This resulted in the loss of these cations through leaching (Tian and Niu, 2015). Therefore, ammonium ions may break the balance of basic cations in plants, resulting in indirect toxicity. A lack of alkaline cations reduces the N utilization efficiency (Chen et al., 2017) and photosynthetic efficiency (Bethers et al., 2009), causing secondary stress and weakening the resistance to diseases and pests (Kleijn et al., 2008).
All the clonal grasses in the grassland were aboveground bud species, and the aboveground tissues were dead during winter (Bai et al., 2004). Therefore, the low frequency of N addition with the stronger soil ammonium toxicity both directly and indirectly decreased the colonization probability and increased the extinction probability, resulting in a reduced species richness and frequency. Low frequency N addition significantly overestimated the decline in species richness and frequency by decreasing the colonization probability combined with an increasing extinction probability.
4.2. Effects of plant height on the species probability of extinction and colonizationNitrogen addition decreased the species frequency and increased extinction probability, which is consistent with the previous N-induced negative effects on species richness at the community level (Zhang et al., 2014b, 2016) and in the global grassland ecosystems (Clark and Tilman, 2008; Humbert et al., 2016; Seabloom et al., 2021; Muehleisen et al., 2023).
Average plant height (commonly used in previous studies) and species ANPP were negatively associated with the species extinction probability, indicating that either a low stature or rare species had a higher extinction probability under N-enriched conditions, which is consistent with previous studies in grasslands (Suding et al., 2005; Yang et al., 2018; Zhu et al., 2020). Low-stature species are more likely to be lost following N enrichment, which may be mainly attributed to the asymmetry in light resources (Saar et al., 2012; Yang et al., 2015; DeMalach et al., 2017; Xiao et al., 2021). In this study, the taller Leymus chinensis and Agropyon cristatum species had a lower extinction probability, whereas the lowest stature clonal grass (Cleistogenes squarrosa) had the highest extinction probability. Our findings support the classic "size advantage" theory that taller species are more competitive and tend to grow better in nutrient-enriched environments (Yang et al., 2011; Lines et al., 2012; Craine and Dybzinski, 2013; Gross and Mittelbach, 2017).
More importantly, the reproductive height relative to the vegetative height, average height, and species biomass (ANPP) more accurately explains the variation in the species extinction probability (largest R2 and lowest mean AIC). This suggests that the plant reproductive height may be a more accurate index for evaluating the extinction probability of clonal grasses under N-enrichment, compared to other indices. The resource trade-off between clonal and sexual reproduction is one of the most important characteristics in the plant life history (Chu et al., 2006), and N addition may change the balance (Loeppky and Coulman, 2001; Wang et al., 2019; Liu et al., 2021). Rhizome expansion in N-enriched environments may lead to an increase in the ramet density and intraspecific competition, and induce sexual reproduction to some extent (Rautiainen et al., 2004). Taller clonal species tend to have more sexual reproduction decisions (Hämmerli and Reusch, 2003) as seed transmission may weaken the intraspecific competition for resources and increase the survival advantage (Yang and Kim, 2016). Species with a taller reproductive stature tend to have a higher seed mass (Moles et al., 2004), shorter propagation distances (Marteinsdóttir, 2014), and lower extinction probability (Larson and Funk, 2016). Species with a taller reproductive height might be more conducive to alleviating the ammonium toxicity in the N-enriched environment because of the stronger silicon transport and accumulation ability (Pavlovic et al., 2021; Barreto et al., 2022). Monocotyledons, such as grasses, accumulate significant amounts of silicon (Epstein, 1999; Ma and Yamaji, 2015; de Tombeur et al., 2023), particularly in the hulls of spikes (Raven, 2003; Farooq and Dietz, 2015). Grasses with taller reproductive modules that develop spikes may have a superior silicon transport and accumulation to alleviate the ammonium toxicity and decrease the extinction probability.
Neither the height nor species biomass had a significant relationship with the species colonization probability. Previous studies showed that the species colonization limitation depends on litter mass and light penetration, but has no direct relationship with the aboveground biomass (Tilman, 1993). Taller species had a low colonization probability (Tracey and Aarssen, 2019), while smaller species may have a high colonization ability owing to the high abundance of small seeds (Hodgson et al., 2020). Plant height is more sensitive to light under N-enriched conditions, whereas seed mass (Spotswood et al., 2017), seed quantity (DeMalach et al., 2016; Eskelinen et al., 2021), and root type (Dickson et al., 2014) often show stronger responses to the soil environments. The aboveground and belowground parts jointly determine the richness of the species (Flores-Moreno et al., 2019; Weigelt et al., 2021). Therefore, it is necessary to explore the effective predictor (trait) of the clonal grass colonization under an increasing N deposition.
In summary, a low frequency of N addition might overestimate the effects on the probability of colonization and extinction, hence resulted in a higher loss of clonal grasses, which was attributable to soil ammonium toxicity, consistent with previous studies (Zhang et al., 2014b). These findings support our previous suggestion that simulating the atmospheric deposition should utilize a higher frequency of N addition (Zhang et al., 2014b). More importantly, for the first time to the best of our knowledge, by evaluating the effects of three plant heights (vegetative, reproductive and average) and aboveground biomass on the diversity of clonal grasses under N addition, we found that clonal grass reproductive height was the best index to predict the extinction probability of clonal grasses in N-enriched conditions. This indicates that clonal grasses with higher reproductive height have a lower extinction probability in N-enriched conditions. Further studies with various clonal species are needed to test these conclusions and improve the biodiversity conservation under human-caused environmental changes.
AcknowledgementsWe thank Dr. Nianpeng He, Dr. Guangming Zhang, Mr. Jianjun Chen, and Mr. Xiaoliang Wang for their assistance and discussion. We appreciate the Inner Mongolia Grassland Ecosystem Research Station for the experimental facility support. This study was supported by a grant from the National Natural Science Foundation of China (grant no. 32071603 and 32122055) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA26020101).
Author contributions
XGH and YHZ design of the field N experiment, YHZ and XC conceived the study, CX, HNL, ZRR, YQZ, RXL and YHZ collected the data, XC and HNL analyzed the data, XC and YHZ wrote the draft manuscript. All author contributed to the critical analysis and completion of the final manuscript and approve its submission.
Data availability statement
The data that support the findings of this study are openly available in the Science Data Bank at https://doi.org/10.57760/sciencedb.07174.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.04.003.
Bai, Y.F., Han, X.G., Wu, J.G., et al., 2004. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature, 431: 181-184. DOI:10.1038/nature02850 |
Barreto, R.F., de Mello Prado, R., Lúcio, J.C.B., et al., 2022. Ammonium toxicity alleviation by silicon is dependent on cytokinins in tomato cv. Micro-Tom. J. Plant Growth Regul., 41: 417-428. DOI:10.1007/s00344-021-10314-5 |
Barrett, S.C.H., 2015. Influences of clonality on plant sexual reproduction. Proc. Natl. Acad. Sci. U.S.A., 112: 8859-8866. DOI:10.1073/pnas.1501712112 |
Bethers, S., Day, M.E., Wiersma, G.B., et al., 2009. Effects of chronically elevated nitrogen and sulfur deposition on sugar maple saplings: nutrition, growth and physiology. For. Ecol. Manage., 258: 895-902. DOI:10.1016/j.foreco.2009.03.024 |
Bharath, S., Borer, E.T., Biederman, L.A., et al., 2020. Nutrient addition increases grassland sensitivity to droughts. Ecology, 101: e02981. DOI:10.1002/ecy.2981 |
Bobbink, R., Hicks, K., Galloway, J., et al., 2010. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol. Appl., 20: 30-59. DOI:10.1890/08-1140.1 |
Borer, E.T., Seabloom, E.W., Gruner, D.S., et al., 2014. Herbivores and nutrients control grassland plant diversity via light limitation. Nature, 508: 517-520. DOI:10.1038/nature13144 |
Bowman, W.D., Cleveland, C.C., Halada, Ĺ., et al., 2008. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci., 1: 767-770. DOI:10.1038/ngeo339 |
Bowman, W.D., Ayyad, A., de Mesquita, C.P.B., et al., 2018. Limited ecosystem recovery from simulated chronic nitrogen deposition. Ecol. Appl., 28: 1762-1772. DOI:10.1002/eap.1783 |
Canfield, D.E., Glazer, A.N., Falkowski, P.G., 2010. The evolution and future of earth's nitrogen cycle. Science, 330: 192-196. DOI:10.1126/science.1186120 |
Cao, J.R., Pang, S., Wang, Q.B., et al., 2020. Plant-bacteria-soil response to frequency of simulated nitrogen deposition has implications for global ecosystem change. Funct. Ecol., 34: 723-734. DOI:10.1111/1365-2435.13484 |
Chen, W., Xu, R., Hu, T., et al., 2017. Soil-mediated effects of acidification as the major driver of species loss following N enrichment in a semi-arid grassland. Plant Soil, 419: 541-556. DOI:10.1007/s11104-017-3367-x |
Chu, Y., Yu, F.-H., Dong, M., 2006. Clonal plasticity in response to reciprocal patchiness of light and nutrients in the stoloniferous herb Glechoma longituba L. J. Integr. Plant Biol., 48: 400-408. DOI:10.1111/j.1744-7909.2006.00237.x |
Clark, C.M., Tilman, D., 2008. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature, 451: 712-715. DOI:10.1038/nature06503 |
Clark, C.M., Cleland, E.E., Collins, S.L., et al., 2007. Environmental and plant community determinants of species loss following nitrogen enrichment. Ecol. Lett., 10: 596-607. DOI:10.1111/j.1461-0248.2007.01053.x |
Craine, J.M., Dybzinski, R., 2013. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol., 27: 833-840. DOI:10.1111/1365-2435.12081 |
DeMalach, N., Zaady, E., Weiner, J., et al., 2016. Size asymmetry of resource competition and the structure of plant communities. J. Ecol., 104: 899-910. DOI:10.1111/1365-2745.12557 |
de Tombeur, F., Raven, J.A., Toussaint, A., et al., 2023. Why do plants silicify?. Trends Ecol. Evol., 38: 275-288. DOI:10.1016/j.tree.2022.11.002 |
DeMalach, N., Zaady, E., Kadmon, R., 2017. Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett., 20: 60-69. DOI:10.1111/ele.12706 |
Dickson, T.L., Gross, K.L., 2013. Plant community responses to long-term fertilization: changes in functional group abundance drive changes in species richness. Oecologia, 173: 1513-1520. DOI:10.1007/s00442-013-2722-8 |
Dickson, T.L., Mittelbach, G.G., Reynolds, H.L., et al., 2014. Height and clonality traits determine plant community responses to fertilization. Ecology, 95: 2443-2452. DOI:10.1890/13-1875.1 |
Epstein, E., 1999. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol., 50: 641-664. DOI:10.1146/annurev.arplant.50.1.641 |
Eskelinen, A., Elwood, E., Harrison, S., et al., 2021. Vulnerability of grassland seed banks to resource-enhancing global changes. Ecology, 102: e03512. DOI:10.1002/ecy.3512 |
Eskelinen, A., Harpole, W.S., Jessen, M.-T., et al., 2022. Light competition drives herbivore and nutrient effects on plant diversity. Nature, 611: 301-305. DOI:10.1038/s41586-022-05383-9 |
Farooq, M.A., Dietz, K.-J., 2015. Silicon as versatile player in plant and human biology: overlooked and poorly understood. Front. Plant Sci., 6: 994. DOI:10.3389/fpls.2015.00994 |
Flores-Moreno, H., Fazayeli, F., Banerjee, A., et al., 2019. Robustness of trait connections across environmental gradients and growth forms. Global Ecol. Biogeogr., 28: 1806-1826. DOI:10.1111/geb.12996 |
Galloway, J.N., Cowling, E.B., 2021. Reflections on 200 years of nitrogen, 20 years later. Ambio, 50: 745-749. DOI:10.1007/s13280-020-01464-z |
Gao, Y., Zheng, J., Lin, X., Du, F., 2020. Distribution patterns of clonal plants in the subnival belt of the Hengduan Mountains, SW China. Plant Divers., 42: 386-392. DOI:10.1016/j.pld.2020.06.006 |
Gough, L., Gross, K.L., Cleland, E.E., et al., 2012. Incorporating clonal growth form clarifies the role of plant height in response to nitrogen addition. Oecologia, 169: 1053-1062. DOI:10.1007/s00442-012-2264-5 |
Gross, K.L., Mittelbach, G.G., 2017. Negative effects of fertilization on grassland species richness are stronger when tall clonal species are present. Folia Geobot., 52: 401-409. DOI:10.1007/s12224-017-9300-5 |
Hämmerli, A., Reusch, T.B.H., 2003. Inbreeding depression influences genet size distribution in a marine angiosperm. Mol. Ecol., 12: 619-629. DOI:10.1046/j.1365-294X.2003.01766.x |
Hautier, Y., Niklaus, P.A., Hector, A., 2009. Competition for light causes plant biodiversity loss after eutrophication. Science, 324: 636-638. DOI:10.1126/science.1169640 |
Hodgson, J.G., Marti, G.M., Šerá, B., et al., 2020. Seed size, number and strategies in annual plants: a comparative functional analysis and synthesis. Ann. Bot., 126: 1109-1128. DOI:10.1093/aob/mcaa151 |
Humbert, J.-Y., Dwyer, J.M., Andrey, A., et al., 2016. Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: a systematic review. Global Change Biol., 22: 110-120. DOI:10.1111/gcb.12986 |
Kleijn, D., Bekker, R.M., Bobbink, R., et al., 2008. In search for key biogeochemical factors affecting plant species persistence in heathland and acidic grasslands: a comparison of common and rare species. J. Appl. Ecol., 45: 680-687. DOI:10.1111/j.1365-2664.2007.01444.x |
Larson, J.E., Funk, J.L., 2016. Seedling root responses to soil moisture and the identification of a belowground trait spectrum across three growth forms. New Phytol., 210: 827-838. DOI:10.1111/nph.13829 |
Lê, S., Josse, J., Husson, F., 2008. Factominer: an R package for multivariate analysis. J. Stat. Software, 25: 1-18. DOI:10.18637/jss.v025.i01 |
Li, Z., Wu, J., Han, Q., et al., 2021. Nitrogen and litter addition decreased sexual reproduction and increased clonal propagation in grasslands. Oecologia, 195: 131-144. DOI:10.1007/s00442-020-04812-8 |
Lines, E.R., Zavala, M.A., Purves, D.W., et al., 2012. Predictable changes in aboveground allometry of trees along gradients of temperature, aridity and competition. Global Ecol. Biogeogr., 21: 1017-1028. DOI:10.1111/j.1466-8238.2011.00746.x |
Liu, L., Zuo, S., Ma, M., et al., 2021. Appropriate nitrogen addition regulates reproductive strategies of Leymus chinensis. Glob. Ecol. Conserv., 27: e01599. DOI:10.1016/j.gecco.2021.e01599 |
Loeppky, H.A., Coulman, B.E., 2001. Residue removal and nitrogen fertilization affects tiller development and flowering in meadow bromegrass. Agron. J., 93: 891-895. DOI:10.2134/agronj2001.934891x |
Ma, J.F., Yamaji, N., 2015. A cooperative system of silicon transport in plants. Trends Plant Sci., 20: 435-442. DOI:10.1016/j.tplants.2015.04.007 |
Marteinsdóttir, B., 2014. Seed rain and seed bank reveal that seed limitation strongly influences plant community assembly in grasslands. PLoS One, 9: e103352. DOI:10.1371/journal.pone.0103352 |
Matschonat, G., Matzner, E., 1996. Soil chemical properties affecting NH4+ sorption in forest soils. Z. Pflanz Bodenkunde, 159: 505-511. DOI:10.1002/jpln.1996.3581590514 |
Midolo, G., Alkemade, R., Schipper, A.M., et al., 2019. Impacts of nitrogen addition on plant species richness and abundance: a global meta-analysis. Global Ecol. Biogeogr., 28: 398-413. DOI:10.1111/geb.12856 |
Moles, A.T., Falster, D.S., Leishman, M.R., et al., 2004. Small-seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. J. Ecol., 92: 384-396. DOI:10.1111/j.0022-0477.2004.00880.x |
Muehleisen, A.J., Watkins, C.R.E., Altmire, G.R., et al., 2023. Nutrient addition drives declines in grassland species richness primarily via enhanced species loss. J. Ecol., 111: 552-563. DOI:10.1111/1365-2745.14038 |
O'brien, R.M., 2007. A caution regarding rules of thumb for variance inflation factors. Qual. Quantity, 41: 673-690. DOI:10.1007/s11135-006-9018-6 |
Pavlovic, J., Kostic, L., Bosnic, P., et al., 2021. Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci., 12: 697592. DOI:10.3389/fpls.2021.697592 |
R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. (Accessed 3 October 2022).
|
Rautiainen, P., Koivula, K., HyvÄrinen, M., 2004. The effect of within-genet and between-genet competition on sexual reproduction and vegetative spread in Potentilla anserina ssp. egedii. J. Ecol., 92: 505-511. DOI:10.1111/j.0022-0477.2004.00878.x |
Raven, J.A., 2003. Cycling silicon - the role of accumulation in plants - Commentary. New Phytol., 158: 419-421. DOI:10.1046/j.1469-8137.2003.00778.x |
Saar, L., Takkis, K., Pärtel, M., et al., 2012. Which plant traits predict species loss in calcareous grasslands with extinction debt?. Divers. Distrib., 18: 808-817. DOI:10.1111/j.1472-4642.2012.00885.x |
Seabloom, E.W., Adler, P.B., Alberti, J., et al., 2021. Increasing effects of chronic nutrient enrichment on plant diversity loss and ecosystem productivity over time. Ecology, 102: e03218. DOI:10.1002/ecy.3218 |
Segrestin, J., Navas, M.-L., Garnier, E., 2020. Reproductive phenology as a dimension of the phenotypic space in 139 plant species from the Mediterranean. New Phytol., 225: 740-753. DOI:10.1111/nph.16165 |
Smith, M.D., Knapp, A.K., Collins, S.L., 2009. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology, 90: 3279-3289. DOI:10.1890/08-1815.1 |
Song, M.-H., Yu, F.-H., Ouyang, H., et al., 2012. Different inter-annual responses to availability and form of nitrogen explain species coexistence in an alpine meadow community after release from grazing. Global Change Biol., 18: 3100-3111. DOI:10.1111/j.1365-2486.2012.02738.x |
Spotswood, E.N., Mariotte, P., Farrer, E.C., et al., 2017. Separating sources of density-dependent and density-independent establishment limitation in invading species. J. Ecol., 105: 436-444. DOI:10.1111/1365-2745.12686 |
Stephens, S., van Kleunen, M., Dorken, M.E., 2020. Patterns of pollen dispersal and mating in a population of the clonal plant Sagittaria latifolia. J. Ecol., 108: 1941-1955. DOI:10.1111/1365-2745.13399 |
Stevens, C.J., Dise, N.B., Mountford, J.O., et al., 2004. Impact of nitrogen deposition on the species richness of grasslands. Science, 303: 1876-1879. DOI:10.1126/science.1094678 |
Suding, K.N., Collins, S.L., Gough, L., et al., 2005. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. U.S.A., 102: 4387-4392. DOI:10.1073/pnas.0408648102 |
Tian, D., Niu, S., 2015. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett., 10: 024019. DOI:10.1088/1748–9326/10/2/024019 |
Tian, Q., Liu, N., Bai, W., et al., 2016. A novel soil manganese mechanism drives plant species loss with increased nitrogen deposition in a temperate steppe. Ecology, 97: 65-74. DOI:10.1890/15-0917.1 |
Tian, Q., Yang, L., Ma, P., et al., 2020. Below-ground-mediated and phase-dependent processes drive nitrogen-evoked community changes in grasslands. J. Ecol., 108: 1874-1887. DOI:10.1111/1365-2745.13415 |
Tilman, D., 1993. Species richness of experimental productivity gradients: how important is colonization limitation?. Ecology, 74: 2179-2191. DOI:10.2307/1939572 |
Tracey, A., Aarssen, L., 2019. Resident species with larger size metrics do not recruit more offspring from the soil seed bank in old-field meadow vegetation. J. Ecol., 107: 1067-1078. DOI:10.1111/1365-2745.13089 |
van Den Berg, L.J., Dorland, E., Vergeer, P., et al., 2005. Decline of acid-sensitive plant species in heathland can be attributed to ammonium toxicity in combination with low pH. New Phytol., 166: 551-564. DOI:10.1111/j.1469-8137.2005.01338.x |
Virtanen, R., Eskelinen, A., Harrison, S., 2017. Comparing the responses of bryophytes and short-statured vascular plants to climate shifts and eutrophication. Funct. Ecol., 31: 946-954. DOI:10.1111/1365-2435.12788 |
Wang, S., van Dijk, J., Wassen, M.J., 2019. Sexual reproduction traits of Holcus lanatus L. and Parnassia palustris L. in response to absolute and relative supply of nitrogen and phosphorus. Environ. Exp. Bot., 168: 103813. DOI:10.1016/j.envexpbot.2019.103813 |
Wang, P., Alpert, P., Yu, F.-H., 2021. Physiological integration can increase competitive ability in clonal plants if competition is patchy. Oecologia, 195: 199-212. DOI:10.1007/s00442-020-04823-5 |
Wei, Y., Jing, X., Su, F., et al., 2022. Does pH matter for ecosystem multifunctionality? An empirical test in a semi-arid grassland on the Loess Plateau. Funct. Ecol., 36: 1739-1753. DOI:10.1111/1365-2435.14057 |
Weigelt, A., Mommer, L., Andraczek, K., et al., 2021. An integrated framework of plant form and function: the belowground perspective. New Phytol., 232: 42-59. DOI:10.1111/nph.17590 |
Wilfahrt, P.A., Asmus, A.L., Seabloom, E.W., et al., 2021. Temporal rarity is a better predictor of local extinction risk than spatial rarity. Ecology, 102: e03504. DOI:10.1002/ecy.3504 |
Xiao, Y., Liu, X., Zhang, L., et al., 2021. The allometry of plant height explains species loss under nitrogen addition. Ecol. Lett., 24: 553-562. DOI:10.1111/ele.13673 |
Yang, Y.Y., Kim, J.G., 2016. The optimal balance between sexual and asexual reproduction in variable environments: a systematic review. J. Ecol. Environ., 40: 12. DOI:10.1186/s41610-016-0013-0 |
Yang, H.J., Li, Y., Wu, M., et al., 2011. Plant community responses to nitrogen addition and increased precipitation: the importance of water availability and species traits. Global Change Biol., 17: 2936-2944. DOI:10.1111/j.1365-2486.2011.02423.x |
Yang, Z., Hautier, Y., Borer, E.T., et al., 2015. Abundance- and functional-based mechanisms of plant diversity loss with fertilization in the presence and absence of herbivores. Oecologia, 179: 261-270. DOI:10.1007/s00442-015-3313-7 |
Yang, X., Yang, Z., Tan, J., et al., 2018. Nitrogen fertilization, not water addition, alters plant phylogenetic community structure in a semi-arid steppe. J. Ecol., 106: 991-1000. DOI:10.1111/1365-2745.12893 |
Yu, G., Jia, Y., He, N., et al., 2019. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci., 12: 424-429. DOI:10.1038/s41561-019-0352-4 |
Yue, K., Fornara, D.A., Li, W., et al., 2020. Nitrogen addition affects plant biomass allocation but not allometric relationships among different organs across the globe. J. Plant Ecol., 14: 361-371. DOI:10.1093/jpe/rtaa100 |
Zhang, Y.H., Han, X., He, N.P., et al., 2014a. Increase in ammonia volatilization from soil in response to N deposition in Inner Mongolia grasslands. Atmos. Environ., 84: 156-162. DOI:10.1016/j.atmosenv.2013.11.052 |
Zhang, Y.H., Lü, X.T., Isbell, F., et al., 2014b. Rapid plant species loss at high rates and at low frequency of N addition in temperate steppe. Global Change Biol., 20(11): 3520-3529. DOI:10.1111/gcb.12611 |
Zhang, Y.H., Feng, J.C., Isbell, F., et al., 2015. Productivity depends more on the rate than the frequency of N addition in a temperate grassland. Sci. Rep., 5: 12558. DOI:10.1038/srep12558 |
Zhang, Y.H., Stevens, C.J., Lü, X.T., et al., 2016. Fewer new species colonize at low frequency N addition in a temperate grassland. Funct. Ecol., 30: 1247-1256. DOI:10.1111/1365-2435.12585 |
Zhang, Y.H., Loreau, M., He, N.P., et al., 2018. Climate variability decreases species richness and community stability in a temperate grassland. Oecologia, 188: 183-192. DOI:10.1007/s00442-018-4208-1 |
Zhang, Y.H., Feng, J.C., Loreau, M., et al., 2019. Nitrogen addition does not reduce the role of spatial asynchrony in stabilising grassland communities. Ecol. Lett., 22: 563-571. DOI:10.1111/ele.13212 |
Zhu, J., Zhang, Y., Yang, X., et al., 2020. Synergistic effects of nitrogen and CO2 enrichment on alpine grassland biomass and community structure. New Phytol., 228: 1283-1294. DOI:10.1111/nph.16767 |



