b. Institute of Botany, Ulm University, Albert-Einstein-Allee 11, Ulm, 89081, Germany
Water movement in plants from the roots to the leaves is mainly driven by leaf transpiration, and this process is generally explained by the cohesion-tension theory (Dixon and Joly, 1985). Still, how plants achieve a stable sap transport without the constant formation of gas embolism under negative pressure remains largely unclear (Jansen and Schenk, 2015; Wheeler and Stroock, 2008). This question was given renewed attention by the discovery of xylem sap lipids, which provide a dynamic surface tension to sap, depending on the local concentration of surfactants at gas–liquid interfaces in xylem conduits (Schenk et al., 2017; Yang et al., 2020). Recent observations of lipids in xylem sap were also in line with earlier observations (Esau, 1965; Esau et al., 1966; Scott et al., 1960), showing that a thin layer of lipids occurs on the inner walls of xylem conduits after cell death (Schenk et al., 2017; Levionnois et al., 2022).
Lipids in xylem sap are either amphiphilic or hydrophobic. Although there are relatively few studies on lipids in xylem sap or apoplast fluids, they all point to the presence of lipids in xylem sap of the species tested (Gonorazky et al., 2012; Guan et al., 2022; Schenk et al., 2017, 2021). However, studies on the chemical composition of xylem sap may suffer from contamination, and it is therefore important to consider control samples. Polar lipids with an amphiphilic property, such as monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), phosphatidicacid (PA) and phosphatidylcholine (PC) were found to be the main lipid components in xylem sap according to Schenk et al. (2021) and Guan et al. (2022). In addition, a certain amount of the neutral, hydrophobic lipid triacylglycerol (TAG) has been found in xylem sap of temperate tree species (Guan et al., 2022). These amphiphilic lipids are mostly attached to inner conduit walls, pit borders as well as inter-conduit pit membranes, and are difficult to detach by sap extraction (Guan et al., 2022; Schenk et al., 2017, 2018). Polar lipids may exist in xylem sap as coatings on nanobubbles, and/or nanoparticles and may coat nanobubbles during gas–liquid–surfactant interactions in pit membranes, which is essential to stabilise nanobubbles and to prevent embolism under a certain range of xylem water potentials (Ingram et al., 2021; Schenk et al., 2015). Although more research is needed to test the functional implications of xylem sap lipids for embolism formation, various basic questions remain poorly addressed. For instance, it is largely unclear if species from different phylogenetic lineages and biomes differ in their lipid concentrations and composition. Additional information on lipid characteristics in xylem sap across various biomes and climate conditions would thus be useful, and may contribute to our understanding of the biological significance of xylem sap lipids.
Because of typically high evaporation rates in hot and humid climates, tropical rainforest species tend to have wider and longer vessels than temperate diffuse-porous species (Hacke et al., 2017). Species with large vessels are also likely to have a high proportion of axial parenchyma surrounding these vessels (Morris et al., 2018). Woody plants in tropical savannas have rather narrow vessels and a relatively high resistance to xylem embolism (Chen et al., 2021; Wargowsky et al., 2021). Interestingly, the width and length of cut-open vessels in stem segments has been shown to affect the lipid concentration in extracted xylem sap (Guan et al., 2022; Schenk et al., 2021). Although plant hydraulic strategies that integrate xylem anatomy with stomatal behaviour and embolism resistance has been studied in many woody species from tropical environments (Bucci et al., 2004; Chen et al., 2021; Wang et al., 2023; Zhang et al., 2016), we are not aware of earlier work on lipidomic analyses of xylem sap from tropical species.
The savanna is an important biome with a wide distribution (~20% of land surface) across all continents (Ratnam et al., 2016; Scholes and Archer, 1997). Due to the influence of southwestern monsoons and the rain-shadow effect of the Himalayan Mountains, valley savannas are formed in the river-valley regions in Southwest China (Yao et al., 2012). Tropical savanna species experience their lowest water potentials during dry seasons, and these seasonal periods of severe drought may trigger the formation of gas embolisms (Shen et al., 2022). Seasonal variation in lipid composition of xylem sap may be affected by the formation of new vessels, enzyme activity of the apoplast, or even lipid transport from vessel-associated parenchyma cells to conduits (Schenk et al., 2021). It could therefore be suggested that seasonal changes in the composition and concentration of polar lipids in xylem sap may reflect seasonal adaptation of savanna species to periods of long and intense drought. However, evidence of seasonal variation in the lipid composition between summer and winter was lacking for xylem sap of temperate tree species (Guan et al., 2022), with only Geijera parviflora showing a significantly higher proportion of sap galactolipids (DGDG and MGDG) in March than in July (Schenk et al., 2021).
In the present study, we analysed the lipidomic content of xylem sap extracted from 12 angiosperm species growing in a tropical valley–savanna and a tropical seasonal rainforest. We aimed to address the following questions:
1) Does the lipid concentration and composition of xylem sap differ in tropical tree species growing in different environments? Significant differences in lipid composition in the xylem sap between savanna and rainforest species would suggest that lipid composition plays a functional role in plant adaption to seasonal drought.
2) Do the concentration and composition of polar lipids in xylem sap differ between the dry season and rainy season, and between deciduous and evergreen species for tropical savanna trees? We expect no significant difference between these two seasons, because polar lipids likely originate as cytoplasmic remnants from living cells, and cannot be formed in dead, mature conduits (Esau et al., 1966; Scott et al., 1960). However, it is unclear whether lipid concentration and composition differ between deciduous and evergreen species.
3) Are there differences in the lipid composition across angiosperm phylogeny? If differences could be found among plant orders, lipid composition would be a good reference for studying the evolutionary processes of plant drought adaption. Available evidence suggests that lipid composition is largely conserved across the major angiosperm clades, although only 11 species have been studied thus far (Schenk et al., 2021; Guan et al., 2022)
4) Is there a correlation between polar lipid concentration and vessel anatomy? Two previous studies have showed a relationship between lipid concentration and the total vessel wall perimeter at the cut xylem surface, and between the lipid concentration and the open vessel volume (Guan et al., 2022; Schenk et al., 2021). We therefore expect to find a similar trend in tropical angiosperm species tested in this study.
2. Materials and methods 2.1. Study sites and plant materialsThis study was conducted at the Yuanjiang Savanna Ecosystem Research Station (Savanna, 23°27’N, 102°10’E) and the Tropical Rainforest Ecosystem Research Station (Rainforest, 21°41’N, 101°25’E) of the Xishuangbanna Tropical Botanical Garden (Chinese Academy of Sciences), which are both located in Yunnan Province, Southwest China. Both sites have distinct dry and rainy seasons, with the dry season lasting from November to April, and the rainy season lasting from May to October. 80–85% of the annual rainfall occurs during the rainy season. According to the climate records of the last five years (2018–2022), the mean annual precipitation (MAP) was about 1550 mm in the rainforest, and about 750 mm at the savanna field site. In the rainforest, mean annual temperature (MAT) was around 21.9 ℃, with the monthly mean temperature ranging from 14.8 ℃ (January) to 25.5 ℃ (July); at the savanna site, MAT was around 25 ℃, ranging from 16 ℃ (January) to 32 ℃ (July or August). Detailed information on climatic characteristics is shown in Fig. 1.
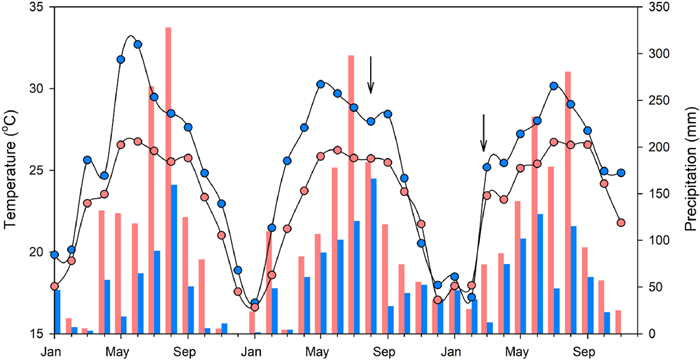
|
| Fig. 1 Monthly patterns of air temperature (℃; indicated by dots with lines) and precipitation (mm; bars) at the two field sites studied: a tropical seasonal rainforest (red colours) and a savanna (blue colours). Climate data (from January 2020 to November 2022) were obtained from climate stations near the two sites. Arrows indicate the two sampling periods. |
A total of 12 angiosperm tree species (five species from the rainforest, and seven species from the savanna), representing the most common species in the two habitats, were selected for this study (Table 1). For each species, three healthy and mature individuals were labelled. We sampled fresh branches and extracted xylem sap from the tagged individuals in August 2021 (only for the seven savanna species) and March 2022 (for all the 12 species), corresponding to rainy and dry season, respectively.
| Species | Abbreviation | Site | Growth form | Leaf habit | Height (m) | DBH (cm) | Open vessel volume (mL) | Ψdry (MPa) |
| Diospyros yunnanensis | DIYU | Savanna | Tree | Evergreen | 3.8 | 26 | 1.28 | −5.11 |
| Haldina cordifolia | HACO | Savanna | Tree | Deciduous | 8.3 | 13 | 0.63 | −5.63 |
| Lannea coromandelica | LACO | Savanna | Tree | Deciduous | 5.5 | 22 | 0.58 | −3.43 |
| Olea ferruginea | OLFE | Savanna | Tree | Evergreen | 5.2 | 15 | 1.16 | −3.98 |
| Polyalthia cerasoides | POCE | Savanna | Tree | Semi-deciduous | 5.1 | 26 | 0.71 | −3.83 |
| Tarenna depauperata | TADE | Savanna | Shrub | Evergreen | 2.5 | 6 | 0.46 | −1.62 |
| Terminalia franchetii | TEFR | Savanna | Tree | Deciduous | 6.4 | 27 | 0.61 | −6.48 |
| Barringtonia fusicarpa | BAFU | Rainforest | Tree | Evergreen | 36.5 | 52 | na | −1.32 |
| Gironniera subaequalis | GISU | Rainforest | Tree | Deciduous | 35.0 | 35 | na | −1.37 |
| Parashorea chinensis | PACH | Rainforest | Tree | Evergreen | 25.0 | 38 | na | −1.98 |
| Phoebe lanceolata | PHLA | Rainforest | Tree | Evergreen | 7.2 | 14 | 0.99 | −1.14 |
| Pseuduvaria indochinensis | PSIN | Rainforest | Tree | Deciduous | 27.2 | 49 | 1.17 | −1.85 |
Branch collection and xylem sap extraction followed procedures described in Schenk et al. (2021) and Guan et al. (2022). To ensure that plants were relatively well hydrated, branches between 1.5 and 2 m in length were collected before 6 a.m. The cut ends of branches were wrapped with moist tissue and covered with parafilm. Branch terminals were also covered with black plastic bags. This reduced water loss from leaf transpiration and the cut ends during sample transportation. After arriving at the experimental location, we re-cut the basipetal end of the branch under water to obtain freshly cut surfaces. We then removed the bark tissue about 5 cm in length using a ring stripper, which was cleaned in advance with deionised water. We also used a clean razor blade to trim the cut surface to ensure the conduits were clearly cut open and not compressed. To remove cellular debris and cytoplasmic residues from the bark tissue, we flushed the cut surfaces of branches and the entire xylem cylinder for approximately 90 s with a high-pressure dental flosser filled with deionised water and set at the fastest flow rate.
Prior to extracting xylem sap, the cut end of the xylem was immersed in a glass vial containing 1 mL of deionised water for 60 s to collect contamination control samples. Comparing the concentration and composition between contamination control samples and actual xylem sap samples was important to test if the lipids extracted originated from the xylem sap, instead of living cells such as cut parenchyma cells. The apparatus for xylem sap extraction was assembled according to Schenk et al. (2021). Briefly, the cut end of the branch was inserted through a perforated rubber stopper into a small brown glass bottle filled with ice in a Bucher flasher. The proximal end of the branch was placed under vacuum by connecting the flasher to a vacuum pump. After removing all the leaves from the branch, small segments of ca. 1–2 cm were cut off from the distal end, and the samples was successively shortened. The cutting was stopped and the pump was switched off until a desired volume of sap (ca. 2 mL) was collected. The sap extraction process usually took five to 8 min for each branch. Xylem sap was transferred to glass vials using clean glass pipettes and stored at −20 ℃.
2.3. Mass spectrometry analysisOne mL of each xylem sap sample and contamination control sample was freeze-dried using a laboratory freeze drying system (Alpha 1–4 LSCplus, Christ, Germany). Subsequently, 1 mL of chloroform/methanol/water (5:5:1) solution was added to isolate lipids in the samples. The mixture was vortexed for 60 s and centrifuged at 3500 rpm for 8 min. This process was repeated twice, and the three supernatants were transferred to a new glass vial and combined. The supernatant solutions were lyophilised to further remove solution and the isolated lipids were stored at −80 ℃. The samples were sent with dry ice to Shanghai Applied Protein Technology Co., Ltd. for mass spectrometry (MS) analysis.
After slow thawing at 4 ℃, 200 μL of methanol, 20 μL of internal standard mixture and 800 μL of methyl tert-butyl ether (MTBE) were added sequentially to the sample, vortexed and mixed. The mixture was sonicated in a low-temperature water bath for 20 min, then allowed to stand at room temperature for 30 min. Then 200 μL of ultrapure water for mass spectrometry was added, vortexed and mixed, and centrifuged at 14,000 rpm for 15 min at 4 ℃. The upper organic phase was collected and dried with nitrogen. For mass spectrometry, 200 μL of 90% isopropanol/acetonitrile was added to dissolve the lipid residues, vortexed thoroughly and centrifuged at 14,000 rpm for 15 min at 4 ℃. The supernatant was injected into the ultra-performance liquid chromatograph (UHPLC Nexera LC-30A, SHIMADZU, Japan) and the AB Sciex 6500+ QTRAP Hybrid Tandem Quadrupole-Linear Ion Trap Mass Spectrometer (AB SCIEX, MA, USA) for further analysis. We quantified the following lipids: galactolipids (including digalactosyldiacylglycerol DGDG, monogalactosyldiacylglycerol MGDG), phospholipids (including lysophosphatidylcholine LPC, lysophosphatidylglycerol LPG, phosphatidicacid PA, phosphatidylcholine PC, phosphatidylethanolamine PE, phosphatidylglycerol PG, phosphatidylinositol PI, phosphatidylserine PS), and the neutral lipid triacylglycerol (TAG). Response factors were applied to correct for differences in the mass spectral responses of biological lipid molecular species compared to the mass spectral responses of the internal standards for the lipid classes, except for 18:2-containing TAG, which is reported as units of normalised mass spectral intensity, where the intensity of 1 unit corresponded to the intensity of 1 nmol of internal standard.
2.4. Predawn water potential and stem xylem anatomyWater potentials at predawn (Ψdry, MPa) were measured in the late dry season of 2022 (March) for all species at the two study sites. Three sun-exposed, healthy leaves or leafy twigs were cut from plant individuals to determine Ψdry. Twigs were sealed in plastic bags and taken to the laboratory. The water potentials were measured using a pressure chamber (PMS, Corvallis, OR, USA), and all the measurements were completed within 1 h.
The open vessel volume (mL) was calculated for nine out of twelve species studied following Guan et al. (2022).
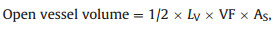
|
where the stem xylem surface area as observed in transverse sections (AS, cm2) was measured based on the stem segments collected in March 2022. The average vessel length (LV, cm) and vessel fraction (VF, %) were retrieved from Aritsara et al. (unpublished data), which were based on individuals of the same species from the same field sites.
2.5. Data analysisAfter testing the data for normal distribution and homogeneity of variance, independent t-tests were used to examine differences in concentrations and proportions of the target lipids between the two sites, and between the dry and rainy season within species. The phylogenetic tree of 23 species, 12 species from this study, and 11 species from Guan et al. (2022) and Schenk et al. (2021), was constructed based on the mega-tree ‘GBOTB.extended.tre’ using the V.PhyloMaker package (Jin and Qian, 2019), except for Tridica sebifera, whose phylogenetic position was not included in this mega-tree. Comparison of the lipid composition between xylem sap samples collected from different habitats and seasons was performed based on a principal component analysis (PCA) using the factoextra package. Standard major axis analyses between lipid concentrations and open vessel volume were conducted using the lmodel2 package. Statistical analyses were performed using SPSS 29 (IBM, Armonk, New York, USA) and R (v.4.2.2, R Core Team, 2022).
3. ResultsFor the 12 species studied, the total lipid concentration varied from 0.094 ± 0.030 nmol/mL (Gironniera subaequalis) to 0.262 ± 0.119 nmol/mL (Lannea coramandelica). Polar lipids in xylem sap and contamination controls included a small proportion of galactolipids (i.e., DGDG, and MGDG), and a higher proportion of phospholipids, such as PC, PA, PG and PI (Table S1). In contrast, a low concentration of phospholipids, including PE, LPC, and LPG, was found in xylem sap (Fig. S1). Normalized mass spectral intensity of 18:2-containing TAG was also detected in all twelve species, ranging from 10 to 30% of the total intensity of the lipids tested.
Lipid concentration in xylem sap was generally twice that of the contamination control samples (Table S1); furthermore, compared to the controls, the xylem sap showed a large difference in lipid composition (Fig. S1). For instance, in the contamination controls, PS accounted for an average of 20% of total lipids, whereas in the xylem sap PS accounted for a low percentage of total lipids. In contrast, large amounts of PC and PA were found in xylem sap but not in contamination controls (Fig. S1).
Total lipid concentration of xylem sap collected from savanna species and from rainforest species in March 2022 did not differ significantly, although two savanna species, Lannea coromandelica and Tarenna depauperata, had considerably higher lipid concentrations than the other species. The lipid composition of xylem sap collected at the savanna site largely overlapped with that of the sap collected at the rainforest site, based on the loadings of the individual scores of the PCA, suggesting a similar lipid composition between the two sites (Fig. 3a). A similar pattern was shown for deciduous and evergreen species, indicating that leaf phenology had no effect on the lipid composition of xylem sap (Fig. S2).
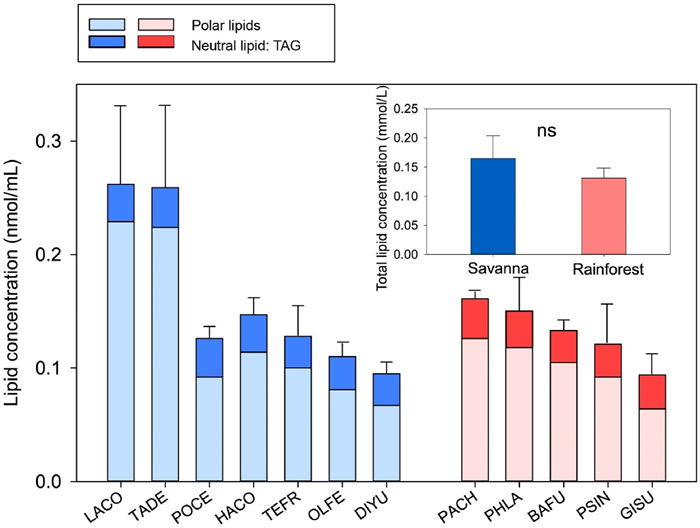
|
| Fig. 2 Lipid concentrations in xylem sap from 12 tropical angiosperm species collected during the dry season of 2022 (March). Shown are polar lipids and neutral lipids (TAG = triacylglycerol). Inset: mean values of the total lipid concentration for tropical savanna and rainforest species, respectively. NS indicates no significant difference between the two biomes (p = 0.05). Error bars are standard errors. Abbreviations for each species are listed in Table 1. For each species, three specimens were studied. |

|
| Fig. 3 Principal components analysis based on the lipid composition (%) in xylem sap of each individual studied. (a) Specimens from the rainforest and savanna site were labelled in red and blue, respectively. Xylem sap from a total of 12 species was collected during the dry season (March 2022), and 3 specimens per species were studied. (b) Score plot graph of xylem sap of seven species collected during the rainy season (August 2021) and the dry season (March 2022) at the savanna site. Each symbol represents a plant specimen, and three individuals were collected for each species. The elliptical areas represent 95% confidence regions. |
Similarly, lipid composition of xylem sap of the seven savanna species was largely similar between samples collected during the rainy season and the dry season, although samples collected at the dry season were more variable along the first principal axis (Fig. 3b). The results of an independent sample t-test suggested no intraspecific difference in lipid composition between sap collected in the rainy and dry season for Haldina cordifolia and T. depauperata (Table S2). The xylem sap collected in the dry season showed a significantly lower concentration of DGDG compared to that collected during the rainy season for L. coromandelica, Olea ferruginea and Terminalia franchetii. A lower concentration of PC was found in D. yunnannesis and Polyalthia cerasoides, and a lower concentration of PE was found in T. franchetii in the dry season sample than in the rainy season (Table S2).
When pooling all the present lipidomic data of 25 angiosperm trees (including the present and two previously published studies), we found that six out of seven savanna species, together with rainforest species Phoebe lanceolata and Parashorea chinensis were clustered. The savanna species had higher levels of PC, PA, DGDG, and MGDG (Fig. 4). However, the lipid composition appeared to be similar across all phylogenetic lineages included in our analysis. Thus, no phylogenetic signals were found in lipid proportions of the 23 species for which the phylogenetic position was available (Fig. S3).
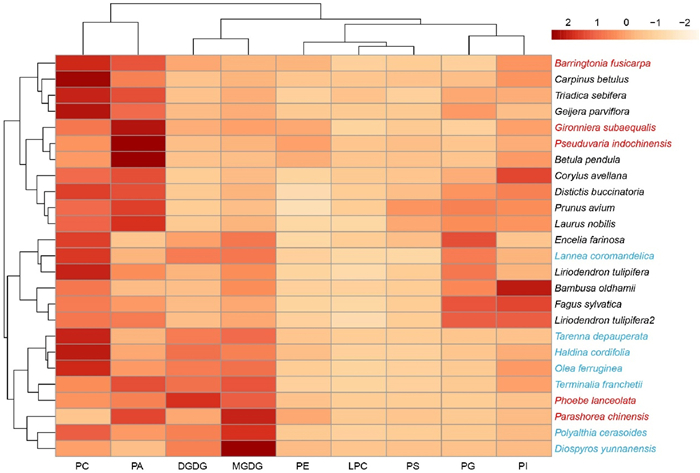
|
| Fig. 4 A heatmap of polar lipid composition for 25 species, including 12 species from this study, 7 species from Schenk et al. (2021), and 6 species from Guan et al. (2022) based on normalised data, Euclidean distance measures, and a Ward clustering algorithm. Note that Liriodendron tulipifera was studied in both published papers and therefore reported twice in this heatmap. Tropical savanna species are in blue, tropical seasonal rainforest species are in red, and temperate species are in black. Colours of the corresponding rectangle indicates normalised lipid proportion. Each column represents a lipid type, i.e., DGDG, digalactosyldiacylglycerol; MGDG, monogalactosyldiacylglycerol. LPC, lysophosphatidylcholine; PA, phosphatidicacid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine. |
The cut open vessel volume ranged from 0.46 to 1.28 mL for the nine tropical species measured, and a significant, negative relationship was found between the polar lipid concentration and the open vessel volume (Fig. 5a). This trend was consistently found for the total lipid concentration and the open vessel volume (Fig. 5b).

|
| Fig. 5 Relationship (a) between the cut-vessel volume (mL) and the polar lipid concentration (nmol/ml) in extracted xylem sap, and (b) between the cut-vessel volume and the total lipid concentration per xylem sap amount extracted. Each symbol represents the mean value of one species. Data from this study and Guan et al. (2022) are shown in green and red, respectively. Solid lines show a standard major axis regression. |
All the species tested in this study showed higher lipid concentration in xylem sap compared to the contamination control samples (Table S1), suggesting that the lipid contents extracted did not represent cell debris originating from cut parenchyma cells or living fibres. This finding is consistent with other lipidomic results from previous studies (Guan et al., 2022; Schenk et al., 2017, 2021). Moreover, a second argument why the lipids studied in xylem sap represent no artefact is that the lipid composition in the control samples was largely different from that in xylem sap. A high proportion of PS, for instance, was characteristic of most control samples. In addition, a much narrower variation of the lipid composition in the xylem sap than in the control samples also provided solid evidence for the presence of natural lipids in xylem sap (Fig. S1).
Polar lipids, such as the phospholipids PC, PA and the galactolipids MGDG and DGDG, form large proportions of the xylem sap lipids (Fig. S1). PC, PA, PE, PS and PI are structural phospholipids with distinct and specific distributions in cellular membranes. PC, for instance, constitutes over half of all membranes in eukaryotic cells (van Meer et al., 2008). MGDG and DGDG, however, have been found to be associated exclusively with plastids (Hölzl and Dörmann, 2019). Hence, the relatively high amount of MGDG and DGDG in xylem sap is rather surprising (Guan et al., 2022; Schenk et al., 2021). Membrane breakdown and disintegration associated with programmed cell death during vessel development may account for the presence of phospholipids and galactolipids in xylem sap.
A certain proportion of triacylglycerol (TAG) was consistently found for all tropical species tested (Table S1 and Fig. S1), which agrees with a previous study on temperate species (Guan et al., 2022). Distribution of neutral lipid droplets in xylem parenchyma cells has been found in some tropical species with low starch storage in parenchyma (Herrera-Ramírez et al., 2021). The presence of lipid droplets in vessels has also been reported for Myrothamnus flabellifolia and for species in the Betula genus (Schneider et al., 2003; Westhoff et al., 2008). However, neutral lipids such as TAGs differ considerably in their origin and behaviour from polar, amphiphilic lipids (Guan et al., 2022; Jansen and Schenk, 2021).
4.2. Habitat and seasonal variation in lipid composition of xylem sapLong distance sap transport in plant xylem is not gas free, but contains numerous bubbles at nanoscale (Schenk et al., 2015, 2017). Nanobubbles are coated with polar lipids, which reduce the surface tension of gas–liquid interfaces and keep the bubbles relatively small (Schenk et al., 2015). How nanobubbles expand and lead to gas embolism is unknown (Schenk et al., 2015). Although species growing in arid habitats experience much greater negative water potentials during the dry season (Table 1), we found that lipid concentration and composition in tropical savanna and rainforest species were not significantly different in these two habitats (Figs. 2 and 3a). An overall similarity in the lipid composition was also shown between deciduous and evergreen species (Fig. S2), suggesting that the lipid composition may not be related to the hydraulic strategies of plants to cope with seasonal drought stress. Moreover, when integrating all published lipidomic results of xylem sap and this study, we found no phylogenetic signals for lipid composition. Nevertheless, most savanna species were clustered due to the relatively high proportion of PC, PA and galactolipids in these species (Figs. 4 and S3). Whether and how lipid xylem sap composition affects gas–liquid–surfactant interactions in plant conduits remains unclear. Therefore, it is unclear why the sap composition of these savanna species differs from the xylem sap in species from other biomes. Yet, a difference in polar lipid composition may affect the dynamics of surface tension, gas–liquid interactions, and gas solubility. Dissolution of gas in liquid phases changes with temperature and pressure (Mercury et al., 2003; Pereira et al., 2022; Schenk et al., 2016), and surface tension of xylem sap lipids also changes with bubble size (Yang et al., 2020). Plants live under much more complex conditions than model conditions, and modelling the behaviour of lipid-coated nanoscale gas bubbles under negative pressure remains a challenge (Ingram et al., 2021).
An overall similarity in polar lipid composition of tropical savanna species was found between xylem sap collected during dry and rainy season (Fig. 3b). Significant changes in lipid concentrations were mainly detected in PC and DGDG for a few species (Table S2). These findings suggest a lack of seasonal variation in xylem sap lipid composition for tropical species. A previous study based on six temperate species has also reported little variation in lipid composition between summer and winter sap samples (Guan et al., 2022). High PA concentrations may be attributed to phospholipase D activity in cut living cells in response to wounding (Bargmann et al., 2009). However, enzymatic changes in lipid composition are unlikely to happen across seasons; in addition, wounding response was not relevant to our study. Moreover, the tiny pore sizes in pit membranes (Zhang et al., 2020; Kaack et al., 2021) likely prevent xylem sap lipids from passing with the sap flow between conduits, which may explain why the amount and composition of lipids remains fairly constant over consecutive seasons.
4.3. Xylem sap lipid concentration and xylem anatomyThe concentration of polar lipids and total lipids was negatively related to open vessel volume for nine tropical species (Fig. 5). A lower concentration of lipids was collected from vessels with a larger cut vessel volume, which is in agreement with results based on temperate tree species (Guan et al., 2022). This trend continued when published data and new measurements were pooled, and the slopes of the linear regressions were similar (Fig. 5). Yet, a functional, mechanistic explanation is unclear. Since lipids appear to be mainly distributed on inter-vessel walls and pit borders, it is likely that they are difficult to remove from the vessel lumen surface, and that the lipids collected via sap extraction under vacuum represent only a small fraction of the total amount of all lipids within a conduit (Guan et al., 2022; Schenk et al., 2021). Therefore, the variation in lipid concentration may not necessarily indicate the intrinsic differences in lipid concentration among species. In addition, vessel volume has been shown to affect lipid concentration due to a dilution effect, which is especially relevant when the amount of extracted xylem sap is much larger than the cut vessel volume (Guan et al., 2022). The volume of xylem sap extracted was 1.5–2.5 mL for each branch, which is larger than the cut vessel volume (Fig. 5a). A dilution effect, however, was not tested in our samples. Approaches such as mass spectrometry imaging (i.e., MALDI-MS) on vessel walls could improve our understanding of the natural distribution of lipids in vessels (Sturtevant et al., 2016), as well as the potential biological significance of lipids in stabilizing nano-scale gas bubbles.
5. ConclusionsIn summary, our results have extended our understanding of sap lipids to tropical tree species and provide support for the presence of polar lipids in the xylem sap of species from different biomes. Lipid composition appears to be largely conservative across angiosperm phylogeny. The lack of major differences in lipid concentration and composition between tropical habitats and across seasons suggests that lipids are derived from the living vessel elements, remain trapped in individual conduits, and do not change after cell death when conduits become functional for water transport. The conservative lipid composition of various angiosperm species suggests that polar lipids may play a similar role in reducing surface tension of the coated nanobubbles in xylem sap. It appears that xylem sap lipids do not have adaptational significance.
AcknowledgementWe thank Jun-Lian Zhuang for her help with sampling and sap extraction, and the staff from the Xishuangbanna Tropical Botanical Garden, and the Yuanjiang Savanna Ecosystem Research Station, Chinese Academy of Sciences, for their assistance in sampling plant material. The facility centre of the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources is acknowledged for providing mass spectrometry equipment. This study was supported by the Natural Science Foundation of China (project number 31861133008). SJ acknowledges financial support from the Deutsche Forschungsgemeinschaft (German Research Foundation, DFG, project number 410768178).
Author contribution
L.-B.H. conducted experiments and preliminary data analyses; X.G. analysed the data and wrote the draft of the manuscript. S.J. and K.-F.C. conceived and supervised the project. All authors were involved in editing the manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.07.001.
Bargmann, B.O., Laxalt, A.M., ter Riet, B., et al., 2009. Reassessing the role of phospholipase D in the Arabidopsis wounding response. Plant Cell Environ., 32: 837-850. DOI:10.1111/j.1365-3040.2009.01962.x |
Bucci, S.J., Goldstein, G., Meinzer, F.C., et al., 2004. Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiol., 24: 891-899. DOI:10.1093/treephys/24.8.891 |
Chen, Y.J., Choat, B., Sterck, F., et al., 2021. Hydraulic prediction of drought-induced plant dieback and top-kill depends on leaf habit and growth form. Ecol. Lett., 24: 2350-2363. DOI:10.1111/ele.13856 |
Dixon, H.H., Joly, J., 1985. On the ascent of sap. Phil. Trans. Roy. Soc. Lond. B, 186: 563-576. |
Esau, K., 1965. Plant Anatomy. second ed. New York: John Wiley & Sons.
|
Esau, K., Cheadle, V., Gill, R., 1966. Cytology of differentiating tracheary elements Ⅱ. Structures associated with cell surfaces. Am. J. Bot., 53: 765-771. DOI:10.1002/j.1537-2197.1966.tb06832.x |
Gonorazky, G., Laxalt, A.M., Dekker, H., et al., 2012. Phosphatidylinositol 4-phosphate is associated to extracellular lipoproteic fractions and is detected in tomato apoplastic fluids. Plant Biol., 14: 41-49. DOI:10.1111/j.1438-8677.2011.00488.x |
Guan, X., Schenk, H.J., Roth, M.R., et al., 2022. Nanoparticles are linked to polar lipids in xylem sap of temperate angiosperm species. Tree Physiol., 42: 2003-2019. |
Hacke, U.G., Spicer, R., Schreiber, S.G., et al., 2017. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ., 40: 831-845. DOI:10.1111/pce.12777 |
Herrera-Ramírez, D., Sierra, C.A., Römermann, C., et al., 2021. Starch and lipid storage strategies in tropical trees relate to growth and mortality. New Phytol., 230: 139-154. DOI:10.1111/nph.17239 |
Hölzl, G., Dörmann, P., 2019. Chloroplast lipids and their biosynthesis. Annu. Rev. Plant Biol., 70: 51-81. DOI:10.1146/annurev-arplant-050718-100202 |
Ingram, S., Salmon, Y., Lintunen, A., et al., 2021. Dynamic surface tension enhances the stability of nanobubbles in xylem sap. Front. Plant Sci., 12: 732701. |
Jansen, S., Schenk, H.J., 2015. On the ascent of sap in the presence of bubbles. Am. J. Bot., 102: 1561-1563. DOI:10.3732/ajb.1500305 |
Jansen, S., Schenk, H.J., 2021. Not all lipids in xylem conduits are artefacts. A reply to Yamagishi et al. IAWA J., 42: 384-385. DOI:10.1163/22941932-00002186 |
Jin, Y., Qian, H., 2019. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography, 42: 1353-1359. DOI:10.1111/ecog.04434 |
Kaack, L., Weber, M., Isasa, E., et al., 2021. Pore constrictions in intervessel pit membranes provide a mechanistic explanation for xylem embolism resistance in angiosperms. New Phytol., 230: 1829-1843. DOI:10.1111/nph.17282 |
Levionnois, S., Kaack, L., Heuret, P., et al., 2022. Pit characters determine drought-induced embolism resistance of leaf xylem across 18 Neotropical tree species. Plant Physiol., 190: 371-386. DOI:10.1093/plphys/kiac223 |
Mercury, L., Azaroual, M., Zeyen, H., et al., 2003. Thermodynamic properties of solutions in metastable systems under negative or positive pressures. Geochem. Cosmochim. Acta, 67: 1769-1785. DOI:10.1016/S0016-7037(02)01406-0 |
Morris, H., Gillingham, M.A.F., Plavcová, L., et al., 2018. Vessel diameter is related to amount and spatial arrangement of axial parenchyma in woody angiosperms. Plant Cell Environ., 41: 245-260. DOI:10.1111/pce.13091 |
Pereira, L., Jansen, S., Miranda, M.T., et al., 2022. Dynamic changes in gas solubility of xylem sap reiterate the enigma of plant water transport under negative pressure. bioRxiv: 475193. |
Ratnam, J., Tomlinson, K.W., Rasquinha, D.N., et al., 2016. Savannahs of Asia: antiquity, biogeography, and an uncertain future. Philos. Trans. R. Soc. Lond. B Biol. Sci., 371: 20150305. DOI:10.1098/rstb.2015.0305 |
R Core Team. 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.Rproject.org/.12c
|
Schenk, H.J., Espino, S., Rich-Cavazos, S.M., et al., 2018. From the sap’s perspective: the nature of vessel surfaces in angiosperm xylem. Am. J. Bot., 105: 172-185. DOI:10.1002/ajb2.1034 |
Schenk, H.J., Espino, S., Romo, D.M., et al., 2017. Xylem surfactants introduce a new element to the Cohesion-Tension theory. Plant Physiol., 173: 1177-1196. DOI:10.1104/pp.16.01039 |
Schenk, H.J., Espino, S., Visser, A., et al., 2016. Dissolved atmospheric gas in xylem sap measured with membrane inlet mass spectrometry. Plant Cell Environ., 39: 944-950. DOI:10.1111/pce.12678 |
Schenk, H.J., Michaud, J.M., Mocko, K., et al., 2021. Lipids in xylem sap of woody plants across the angiosperm phylogeny. Plant J., 105: 1477-1494. DOI:10.1111/tpj.15125 |
Schenk, H.J., Steppe, K., Jansen, S., 2015. Nanobubbles: a new paradigm for air-seeding in xylem. Trends Plant Sci., 20: 199-205. |
Schneider, H., Manz, B., Westhoff, M., et al., 2003. The impact of lipid distribution, composition and mobility on xylem water refilling of the resurrection plant Myrothamnus flabellifolia. New Phytol., 159: 487-505. |
Scholes, R.J., Archer, S.R., 1997. Tree-grass interactions in savannas. Annu. Rev. Ecol. Systemat., 28: 517-544. DOI:10.1146/annurev.ecolsys.28.1.517 |
Scott, F.M., Sjaholm, V., Bowler, E., 1960. Light and electron microscope studies of the primary xylem of Ricinus communis. Am. J. Bot., 47: 162-173. |
Shen, J.X., Zhang, Y.J., Maenpuen, P., et al., 2022. Response of four evergreen savanna shrubs to an incidence of extreme drought: high embolism resistance, branch shedding and maintenance of nonstructural carbohydrates. Tree Physiol., 42: 740-753. DOI:10.1093/treephys/tpab150 |
Sturtevant, D., Lee, Y.-J., Chapman, K.D., 2016. Matrix assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI) for direct visualization of plant metabolites in situ. Curr. Opin. Biotechnol., 37: 53-60. |
van Meer, G., Voelker, D.R., Feigenson, G.W., 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol., 9: 112-124. DOI:10.1038/nrm2330 |
Wang, Y.-Q., Song, H.-Q., Chen, Y.-J., et al., 2023. Hydraulic determinants of drought-induced tree mortality and changes in tree abundance between two tropical forests with different water availability. Agric. For. Meteorol., 331: 109329. |
Wargowsky, I.K., Nesmith, J.E., Holdo, R.M., 2021. Root vascular traits differ systematically between African savanna tree and grass species, with implications for water use. Am. J. Bot., 108: 83-90. DOI:10.1002/ajb2.1597 |
Westhoff, M., Schneider, H., Zimmermann, D., et al., 2008. The mechanisms of refilling of xylem conduits and bleeding of tall birch during spring. Plant Biol., 10: 604-623. DOI:10.1111/j.1438-8677.2008.00062.x |
Wheeler, T.D., Stroock, A.D., 2008. The transpiration of water at negative pressures in a synthetic tree. Nature, 455: 208-212. DOI:10.1038/nature07226 |
Yang, J.L., Michaud, J.M., Jansen, S., et al., 2020. Dynamic surface tension of xylem sap lipids. Tree Physiol., 40: 433-444. DOI:10.1093/treephys/tpaa006 |
Yao, Y.F., Bruch, A.A., Cheng, Y.M., et al., 2012. Monsoon versus uplift in southwestern China–Late Pliocene climate in Yuanmou basin, Yunnan. PLoS One, 7: e37760. DOI:10.1371/journal.pone.0037760 |
Zhang, S.B., Zhang, J.L., Cao, K.F., 2016. Divergent hydraulic safety strategies in three co-occurring Anacardiaceae tree species in a Chinese savanna. Front. Plant Sci., 7: e2075. |
Zhang, Y., Carmesin, C., Kaack, L., et al., 2020. High porosity with tiny pore constrictions and unbending pathways characterize the 3D structure of intervessel pit membranes in angiosperm xylem. Plant Cell Environ., 43: 116-130. DOI:10.1111/pce.13654 |



