b. Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China;
c. State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China;
d. Center for Research and Education on Biological Evolution and Environments, School of Earth Sciences and Engineering, Nanjing University, Nanjing 210023, China
The Tibetan Plateau, known as 'the roof of the world', is an ideal natural laboratory for studying biological evolution in the Cenozoic (Chang and Miao, 2016). The biogeographic history of the Tibetan Plateau, especially regarding its connections with other regions in the Northern Hemisphere during the Cenozoic, is quite complex. Researchers have made explored the molecular phylogenetic and biogeographic history of the Tibetan Plateau based on modern species from this region (Renner, 2016; Ding et al., 2020). In recent years, several hypotheses have been proposed on the biogeographic significance of the Tibetan Plateau: Into Tibet, Out of Tibet, Out of India, and Into and Out of Africa (Deng et al., 2011; Wen et al., 2014; Zhou et al., 2022). Most of these were proposed to explain the biogeographical connections between the Tibetan Plateau and other regions during the Neogene or Quaternary. A series of excellent works in recent years have revealed high terrestrial biodiversity on the Tibetan Plateau during the Paleogene (Sun et al., 2015; Wu et al., 2017; Ai et al., 2018; Xu et al., 2018; Su et al., 2018, 2019, 2020; Deng et al., 2019a, 2019b, 2020; Liu et al., 2019; Low et al., 2019; Tang et al., 2019; Huang et al., 2020). Recently, fossil assemblages from the middle Eocene of central Tibet indicated a relatively free floristic exchange across the Northern Hemisphere (Su et al., 2020). Fossil plants collected from different strata in Tibet indicate extensive biogeographical connections with other floras of the Northern Hemisphere and make Tibet a crossroad for global floristic exchange in the Paleogene (Zhou et al., 2022). It is crucial to reconstruct the pathways for the floristic linkage between the Tibetan Plateau and other Northern Hemisphere regions during the Paleogene (Tang et al., 2019).
The Qaidam Basin is the largest Cenozoic terrestrial intermountain basin on the Tibetan Plateau (Fang et al., 2007; Miao et al., 2016). It has been regarded as a key region to study the paleoclimatic and paleobiological evolution of the northern Tibetan Plateau (Wang et al., 2007). Angiosperms play an important role in global ecosystems today; meanwhile, fossil angiosperms can provide important information on Cenozoic climate change (Sun et al., 2002). However, compared with abundant Cenozoic vertebrate fossils found in the basin (Wang et al., 2007, 2015; Chang et al., 2008; Yang et al., 2018, 2022), the fossil record of angiosperms from the Paleogene Qaidam Basin is relatively small. The plant records published thus far include Simaroubaceae Candolle, Leguminosae Jussieu, Salicaceae Mirb., and Juglandaceae DC. ex Perleb, and are mainly from the Huatugou area and Dahonggou area, which are located in the northwestern and northern margins of the basin, respectively (Yan et al., 2018; Han et al., 2020; Song et al., 2020; Yang et al., 2021a, Yang et al., 2021b). Information on the Paleogene paleovegetation in the basin is still incomplete. Nevertheless, preliminary work has indicated that in the late Paleogene the vegetation of this region was very different from that of modern times.
Betulaceae Gray, which consists of six genera (Carpinus L., Corylus Ascherson, Ostrya Scop., Ostryopsis Decne, Alnus Mill., and Betula L.) and 150–200 species, is an important component of the deciduous broad-leaf forest in the north temperate zone today (Li and Skvortsov, 1999). Betulaceous fossils are common in Cenozoic floras of the Northern Hemisphere (Crane, 1981; Chen, 1994a, 1994b; Liu, 1996; Lin et al., 2010; Liu et al., 2014; Xue et al., 2020). They are often found in different floras such as northern temperate deciduous broad-leaf forest, subtropical deciduous broad-leaf forest, and subtropical evergreen and deciduous broad-leaf mixed forest (Tao and Du, 1987). Betulaceous fossils not only provide information about paleovegetation, but also contribute to reconstructing Cenozoic paleobiogeograhic and paleoclimatic history (Chen, 1994a, 1994b). Herein, fossil leaves and fruits of Betulaceae from the Oligocene Shangganchaigou Formation of northwestern Qaidam Basin, including Betula and Carpinus, are described from the Oligocene Shangganchaigou Formation of northwestern Qaidam Basin. The fossil fruits are assigned to Betula subgenus Betula, and the fossil leaves are attributed to Carpinus grandis Unger emend. Heer. based on morphological comparisons. These fossils, together with other reported fossil plants from the same locality, not only enrich our knowledge of the paleovegetation in the Qaidam Basin, but also provide insights into the intercontinental floristic linkage between the Tibetan Plateau and other regions of the Northern Hemisphere during the Oligocene.
2. Materials and methodsThe new specimens were collected from the Huatugou region (N38°24′41″, E90°53′14″, and N38°24′38″, E90°53′9″), the middle portion of the Shangganchaigou Formation, Qinghai Province, NW China at an elevation of about 3500 m a.s.l. All of the fossil leaves were unearthed from the same locality in the same layer, whereas the fossil fruits were collected from a different layer that is stratigraphically ~100 m lower (Fig. 1). The formation, which was deposited in a lacustrine environment, is lithologically composed of conglomerates, sandstones, siltstones, mudstones, micritic carbonates, and gypsum beds. Our fossils were collected from light-green and light-yellow silty mudstone. Recent magnetostratigraphic and paleobotanic studies have dated the Shangganchaigou Formation to the Oligocene (Sun et al., 2005; Lu and Xiong, 2009; Chang et al., 2015; Ji et al., 2017; Song et al., 2020). According to the 'interpolation method' (He et al., 2021), if the lithofacies and deposition rates are assumed to be uniform, then the bottom and top ages of the formation can be used as primary markers, and the thickness relative to the top or base of the unit can be used to interpolate the ages. The lithofacies and deposition rate of Shangganchaigou Formation in the Huatugou Section, according to detailed study (Chang et al., 2015) and our observation in the geological survey, are uniform; so, we estimate that the age of our betulaceous fossils is about 28–26 Ma (early Chattian) based on their position in the section.
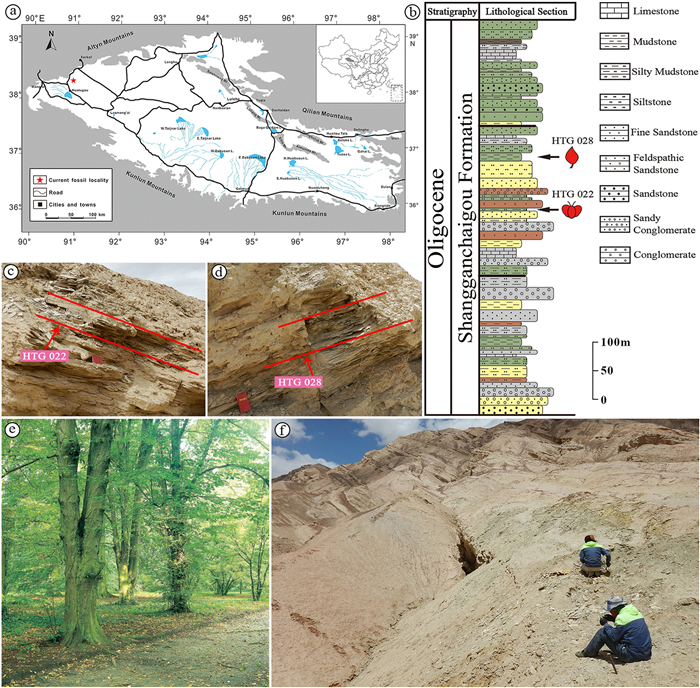
|
| Fig. 1 (a) Map showing detailed location of the fossil site in the Qaidam Basin, Qinghai Province, China. (b) Stratigraphic column of the Oligocene Shangganchaigou Formation. (c–d) The fossil-bearing layer in sites HTG022 and HTG028. (e) Living Carpinus betulus about 120 years old in the arboretum Kornik, Poland (from Boratynski, 2014). (f) Local topographic features surrounding the present fossil site, Huatugou (approximately 3500 m a.s.l.). |
All the specimens described in this study were observed under a Leica MZ 12.5 stereo microscope and figures were prepared using Adobe Photoshop CC 2015. Specimens of Betula and Carpinus used for comparison were retrieved from the Trinity Herbarium, Trinity College (Dublin, Ireland), Xishuangbanna Tropical Botanical Garden (Mengla, China), and Herbarium of Lanzhou University (Lanzhou, China). Cleared leaves of extant species were prepared following the method of Foster (1952). The terminology used to describe leaf morphology follows Liu (1996) and the Leaf Architecture Working Group (Ellis et al., 2009).
We compared our fossil materials with modern Carpinus species and we performed a hierarchical clustering analysis to determine the similarity in leaf morphology between current fossils and modern species. Pictures of extant Carpinus were referenced from the Chinese Virtual Herbarium (http://www.cvh.ac.cn/), the virtual herbaria of the French National Museum of Natural History (https://science.mnhn.fr/institution/mnhn/item/search/form), the virtual herbaria of the Royal Botanic Garden Edinburgh (https://data.rbge.org.uk/), and an image data set of cleared leaves (Wilf et al., 2021). The hierarchical clustering analysis was performed in PAST. The abbreviation 'HTG' refers to the fossil site, Huatugou town, Qinghai Province, China.
3. Results 3.1. Systematic paleontologyOrder Fagales Engler.
Family Betulaceae Gray.
Genus Carpinus L.
Section Carpinus L.
Species C. grandis Unger emend. Heer.
Specimens checked: HTG28001 (Fig. 2a and b), HTG28301 (Fig. 2c), HTG28003 (Fig. 2d), HTG28004 (Fig. 2e), HTGHTG28308 (Fig. 2f), and HTG28002 (Fig. 2g).

|
| Fig. 2 Fossil leaves of Carpinus grandis from site HTG028 in the Huatugou area. Partial sketches show the higher-order venation of leaf and areoles of the current fossils and three extant genera in Betulaceae. (a)–(b) HTG28001. (c) HTG28304. (d) HTG28003. (e) HTG28004. (f) HTG28308. Preserved areoles can be observed in the dotted region in Fig. 3e. (g) HTG28002. In (d) and (e), black arrows denote well-developed external veins. (h) Enlargement of leaf apex in (f). Black arrow denotes the acuminate leaf apex. (i) Enlargement of the laminar base in (a). Black arrows denote the basal vein. (j) Enlargement of (a). White arrows denote the opposite tertiary veins. Black arrows denote well-developed pectinal veins. (k) Enlargement of laminar margin in (c). Black arrows denote the teeth and acute sinuses. (l) Enlargements of (d). Black arrows denote the secondary veins slightly curved and bending sharply upwards when close to the leaf margin. White arrow denotes the acute sinuses. Scale bars = 10 mm in (a)–(g), 1 mm in (h)–(l). |
Repository: The Paleontology Laboratory of the School of Earth Sciences, Lanzhou University, China.
Locality: Fossil site HTG028, the Oligocene Shangganchaigou Formation, Huatugou Town, Qinghai Province, China.
Description: Leaves are elliptic, 3.5–7.0 cm long by 2.0–3.5 cm wide, the widest part being in the middle; leaf base acute-cuneate (Fig. 2a, d, g); leaf apex acuminate without drip tip (Fig. 2f, h). Leaf margin is doubly serrate and somewhat irregular, with small teeth, apical side of each tooth convex or concave, and basal side is straight or slightly convex; tooth apices are obtuse, rarely slightly sharp, with acute sinuses between teeth (Fig. 2k); about 3–5 teeth present in interval between two consecutive secondary veins. Pinnate primary vein is straight and moderately thick, craspedodromous secondary veins are straight or slightly upwardly curved; secondary veins form an angle of 30–50° with primary vein. In HTG28002 and 28003, 11 pairs of secondary veins are preserved, and 8 pairs are preserved in HTG28001, spaced at 0.2–0.8 cm; based on the leaf morphology, leaf apex shape and the space between contiguous two secondary veins (Fig. 2), we deduce that the numbers of secondary veins are 12–13. Pectinal veins and external veins are well-developed (Figs. 2j and 3a). Close to the leaf margin the secondary veins slightly curved and branched, entering teeth basally and bending sharply upwards, terminating in apices or bases of teeth; branches of secondary veins also entering the nearest teeth (Fig. 2l). Basal vein is proximally contiguous with the margin (Fig. 2i). Tertiary venation opposite percurrent, tertiaries are almost parallel to each other and nearly perpendicular to the secondary veins (Fig. 2j). Higher-order veins are mostly orthogonally reticulate, areoles commonly regular, veinlets are almost absent or simple without branch if they are present (Fig. 3e and f).
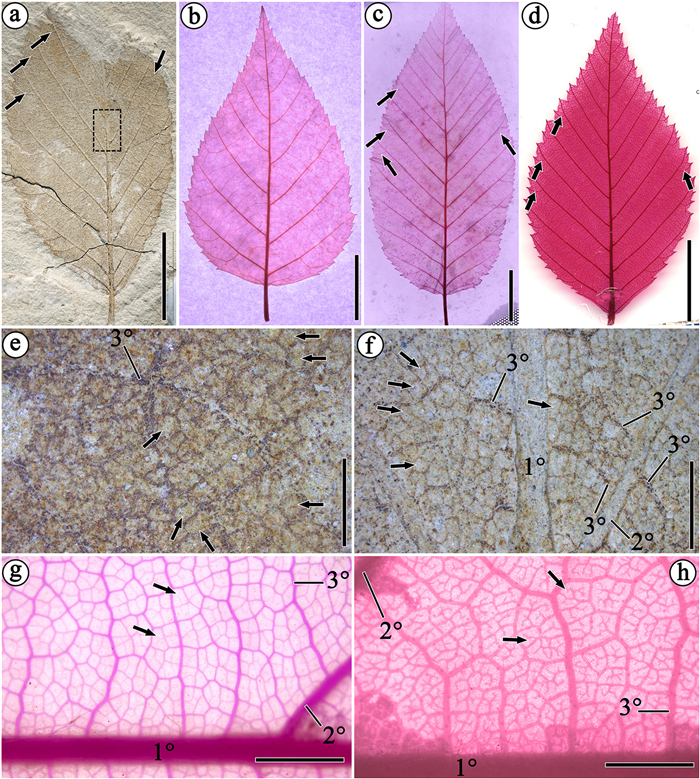
|
| Fig. 3 Comparison of fossil leaves of Carpinus grandis from Huatugou area and extant species of Carpinus and Betula. (a) HTG28001 showing preserved areoles in the dotted region. (b) Betula utilis. (c) C. betulus. (d) C. turczaninowii. In (a), (c) and (d), black arrows denote well-developed external veins. (e) Enlargement of the dotted region in Fig. 2f. (f) Enlargement of the dotted region in (a). In (e)–(f), black arrows denote orthogonally reticulate areoles and simple veinlets. (g) Enlargement of the areoles in C. betulus. Black arrows denote orthogonally reticulate areoles and simple veinlets (h) Enlargement of the areoles in B. utilis. Black arrows denote multiple branched veinlets. 1° = midvein; 2° = secondary vein; 3° = tertiary vein. Scale bars = 10 mm in (a)–(d), 1 mm in (e)–(h). |
Comparison: Within Betulaceae, the sharp apical bend of the secondary veins on entering the teeth is indicative of Betula, Carpinus, and Ostrya (Wolfe and Wehr, 1987), in which this character is present in current specimens (Fig. 2l). Compared with these extant genera, Ostrya can be firstly excluded. Manchester and Crane (1987) pointed out that leaves of Ostrya are typically distinguished by secondary veins that are gently curved and/or undulating in course and that have successive prominent abmedial branches to the subsidiary teeth. We examined some cleared Ostrya leaves from a recent data set (Wilf et al., 2021) and found that their features support this suggestion, and we found these undulating secondary veins were often present at the base of the leaf. Thereby, Ostrya is different from our fossil leaves, which have straight secondary veins. These fossil leaves (Figs. 2 and 3) are characterized by an elliptical leaf shape, doubly serrulate leaf margin, craspedodromous secondary veins, percurrent tertiary veins with well-developed areoles, mostly orthogonal reticulate higher-order venation, and the development of free ending veinlets (FEVs); all these features are characteristics of leaves of extant species of Carpinus (Liu, 1996; Worobiec and Szynkiewicz, 2007). Some detailed studies have shown that there are two major morphological differences between the leaves of Betula and Carpinus: 1) Within Carpinus, FEVs in areoles are almost absent, or simple if they are present. In contrast, within Betula, FEVs are well-developed and multiple branched (Wolfe and Wehr, 1987; Liu, 1996; Worobiec and Szynkiewicz, 2007). 2) Within Carpinus, the external veins are well-developed, but they are mostly absent in Betula (Liu, 1996). In the current fossil materials, some FEVs can be observed but they are all simple, differing from the multi-branched veinlets in Betula (Fig. 3e–h). Additionally, the external veins are well-developed in the current fossils, as those in extant Carpinus (Figs. 2d, e, h, and 3a, c, d). In our fieldwork during recent years, no Betula fruits have been found at the site of this fossil leaf (HTG028), and these fruits seem to be only concentrated at the fossil site HTG022. Therefore, we think it is better to assign these fossil leaves to Carpinus.
The Huatugou leaves possess about 12 or 13 pairs of secondary veins, like the species of section Carpinus (7–15 pairs), which is different than section Distegocarpus (20–34 pairs) (Krüssmann, 1976). We compared the leaf morphology of the fossils with modern hornbeams in section Carpinus (Table 1). Our comparative analysis indicates that the current fossils are distinguished from most extant species by the acute-cuneate base. In extant Carpinus, species with an acute or cuneate base are rare. A summary of leaf architecture in Betulaceous genera indicated that the most obvious characteristic is that Carpinus, Corylus, Ostrya and Ostryopsis typically possess a cordate leaf base (Hickey, 1977); an acute or cuneate base, as observed in the current fossil, is more typical of Alnus and Betula. However, Crane (1981) pointed out that certain leaves with an acute base do occur in species of Carpinus. We further compared the current fossils with species possessing broadly-cuneate or rounded-cuneate bases in this section. Carpinus monbeigiana Hand.-Mazz., C. tschonoskii Maxim., C. polyneura Franch., C. mollicoma Hu, and C. rupestris A. Carmus can be excluded because they have more than 14 pairs of secondary veins and setiform teeth. Carpinus omeiensis Hu has a broadly-cuneate base and 12–16 pairs of secondary veins, but the serration is simple; C. turczaninowii Hance always has smaller and ovate leaves, different from the current fossils; C. londoniana H. Winkl. and C. kweichowensis Hu have cuneate bases but the leaf shape of the former is always lanceolate and the latter have a larger leaf size than our fossils. In the reported fossil species of Carpinus, those with cuneate bases are rare as well; C. cf. lanceolata Hand.-Mazz. from the Miocene Shanwang formation (Shandong, China) has an acute-cuneate base, but the broadly lanceolate leaf shape is different from the current fossils (WGCPC, 1978); C. cf. fargesiana H. Winkl. from the late Eocene Lawula formation (Xizang, China) has elliptic leaves and a broadly-cuneate base, but the angle between secondary veins and the primary vein is distinctly unequal on both sides of the primary vein (Tao and Du, 1987). Among extant species, the leaf morphology of the Huatugou fossils most closely resemble C. betulus L. (Fig. 3), but their tertiary veins are somewhat different. The tertiary veins of our fossil leaves are almost opposite but those of C. betulus are usually alternate or mixed; in the Huatugou fossils, the number of tertiary veins along the secondaries in the middle of leaves is denser (> 15/cm) than in C. betulus (≈10/cm). Among previously reported fossils, our new materials can be assigned to the European fossil species C. grandis, which is well known in the Cenozoic floras of Europe and Asia from the middle Oligocene to early Pliocene (Li and Guo, 1976; Mai, 1981; Tao and Du, 1987; Mai and Walther, 1988, 1991; Hummel, 1991; Zastawniak and Walther, 1998; Worobiec and Szynkiewicz, 2007; Dai et al., 2013). C. grandis most probably is a collective morphotype species and includes several species of fossil leaves; the differences in structure of epidermis in different morphotypes also point to an evolutionary series comprising several species (Worobiec and Szynkiewicz, 2007). Leaves of C. grandis are characterized by the elliptic or oblong-ovate leaf blades and 10–12 pairs of secondary veins, with an acute, obtuse or rounded, rarely cordate base, well-developed and regular areoles, simple veinlets, secondaries slightly curve, tertiary venation percurrent and opposite, tertiaries are nearly perpendicular to the secondary veins, external veins and pectinal veins well-developed, tertiary veins along the secondaries in the middle of leaves dense. All of these characters are similar to the Huatugou fossils. We think it is better to assign the current Oligocene forms into the fossil species rather than to a living species.
| Species | Leaf shape | Size (cm) | L/W | Apex | Base | Margin | 2nd veins | Fossil/extant |
| C. grandis (in this paper) | Elliptic | 3.5–7 × 2–3.5 | 1.6–2.5 | ? | Acute-cuneate | Doubly serrate | 12–13 | Fossil; Oligocene |
| C. wulongensis | Elliptic to ovate-lanceolate | 4–7 × 1.2–2.5 | 2.2–3.1 | Acuminate | Rounded, subcordate | Doubly serrate | 12 | Fossil; Miocene |
| C. grandis | Ovate-long ovate | 6 × 3–4 | 1.5–2 | ? | Rounded or subcordate | Doubly serrate | ? | Fossil; Miocene |
| C. subcordata | Long ovate | 3.1–11 × 1.9–4.9 | 1.7–2.9 | Acuminate | Cordate or subcordate | Doubly serrate | 14–20 | Fossil; Miocene |
| C. miofangiana | Ovate-lanceolate, lanceolate | 6.7–11.3 × 3–4.7 | 2.1–3.5 | Acuminate | Rounded or subcordate | Doubly serrate | 15–20 | Fossil; Miocene |
| C. chaneyi | Long ovate | 11 × 5.1 | 2.2 | Caudate-acuminate | Rounded | Doubly serrate | 11 | Fossil; Miocene |
| C. latifolia | Long ovate | 8 × 4.2 | 1.9 | Acuminate | Obliquely rounded | Simply serrate | 9 | Fossil; Miocene |
| C. cf. lanceolata | Broadly lanceolate | 4.7–9.7 × 1.7–2.7 | 2.7–3.6 | Caudate-acuminate | Cuneate | Doubly serrate | 9–12 | Fossil; Miocene |
| C. cf. fargesiana | Ovate-elliptic | 5 × 2.8 | 1.5 | Acuminate | Broadly cuneate | Doubly minutely serrate | 13 | Fossil; late Eocene |
| C. grandis | Ovate or long ovate | 6 × 3–4 | 1.5–2 | ? | ? | Doubly serrate | 10 | Fossil; late Eocene |
| C. grandis | Ovate or elliptic | 6–7 × 3–4.7 | 1.5–2.4 | Attenuate | Rounded, obtuse, cuneate, rarely cordate | Doubly serrate | 10–12 | Fossil; middle Miocene |
| C. caroliniana | Oblong or ovate | 2.8–5.2 × 1.3–3 | 1.7–2.6 | Acuminate | Rounded or convex | Doubly serrate | 12–15 | Fossil; Pliocene, Pleistocene |
| C. tengchongensis | Ovate-oblong or elliptic | 7–13 × 4.9–8.3 | 1.5–1.9 | Acuminate | Obliquely cordate | Irregularly and doubly minutely serrate | 13–15 | Fossil; late Pliocene |
| C. mioviminea | Ovate | 4.3 × 1.7 | 2.5 | Acuminate | Asymmetrically cordate | Doubly serrate | 13 | Fossil; early Oligocene |
| C. oblonga | Oblong, elliptic-oblong | 6.4 × 3.4 | 1.9 | Acute or obtuse | Auricular | Irregularly and doubly serrate | 9–13 | Fossil; middle Miocene |
| C. eliptica | Elliptic, elliptic-oblong | 4.6 × 2.6 | 1.8 | Acute or obtuse | Auricular | Doubly serrate | 10–15 | Fossil; middle Miocene |
| C. cordata | Ovate or ovate-oblong | 8–15 × 4–5 | 1.9–3.2 | Acuminate or caudate acuminate | Asymmetrically cordate | Doubly setiform serrate | 15–20 | Extant |
| C. tientaiensis | Ovate or elliptic | 5–10 × 3–5.5 | 1.6–2.8 | Acute | Subcordate or subrounded | Doubly and obtusely shortly serrate | 12–15 | Extant |
| C. londoniana | Elliptic-lanceolate, oblong or lanceolate | 6–12 × 1.7–3.5 | 2.5–4.8 | Acuminate or caudate-acuminate | Rounded-cuneate, cuneate, subrounded | Irregularly and doubly mucronate serrate | 11–13 | Extant |
| C. viminea | Elliptic, oblong or ovate-lanceolate | 6–11 × 3–5 | 1.9–2.7 | Acuminate, acute or caudate | Subcordate or subrounded | Doubly mucronate serrate | 12–15 | Extant |
| C. putoensis | Elliptic or broadly elliptic | 5–10 × 3.5–5 | 1.7–2.5 | Acuminate or acute | Rounded or broadly cuneate | Irregularly and doubly setiform serrate | 11–14 | Extant |
| C. kweichowensis | Elliptic, narrowly elliptic, oblong or narrowly oblong | 8–12 × 3.5–5.5 | 2.3–3.1 | Acuminate or acute | Subrounded or rounded-cuneate | Irregularly and doubly minutely serrate | 10–16 | Extant |
| C. turczaninowii | Ovate, ovate-elliptic, broadly ovate, or ovate-rhombic | 2.5–5 × 1.5–3.5 | 1.7–2.5 | Acute or acuminate | Subrounded, broadly cuneate or subcordate | Irregularly doubly serrate or simply serrate | 8–12 | Extant |
| C. kawakamii | Ovate-lanceolate or oblong-lanceolate | 4–5 × 1.8–2.5 | 1.6–2.5 | Acute or caudate acuminate | Subcordate or subrounded | Regularly and doubly serrate | 10–15 | Extant |
| C. tsaiana | Elliptic, oblong-lanceolate, oblong, or ovate-lanceolate | 7–14 × 4.5–6 | 1.6–2.5 | Acuminate | Obliquely cordate or cordate | Irregularly and doubly minutely serrate | 14–16 | Extant |
| C. chuniana | Elliptic, obovate-oblong or oblong | 7–11 × 5–5.5 | 1.5–2.1 | Acute or acuminate | Cordate | Irregularly or regularly doubly minutely serrate | 14–18 | Extant |
| C. shensiensis | Oblong or obovate-oblong | 6–9 × 3–4.5 | 1.6–2.7 | Acute or acuminate | Cordate or subrounded | Regularly and doubly minutely serrate | 14–16 | Extant |
| C. pubescens | Oblong, oblong-lanceolate, ovate-lanceolate or elliptic | 5–10 × 2–3.5 | 2.3–3.5 | Acuminate | Subrounded-cuneate or subrounded | Regularly and doubly minutely serrate | 12–14 | Extant |
| C. monbeigiana | Oblong-lanceolate, ovate-lanceolate or elliptic | 5–10 × 2.5–4 | 2.2–3.3 | Acute or caudate-acuminate | Rounded, subcordate or rounded-cuneate | Irregularly and doubly setiform serrate | 14–18 | Extant |
| C. fargesiana | Ovate-lanceolate, elliptic, ovate-elliptic or oblong | 2.5–7.5 × 2–2.5 | 1.9–3.2 | Acute or acuminate | Rounded or subcordate | Irregularly and doubly mucronate serrate | 12–16 | Extant |
| C. purpurinervis | Oblong-lanceolate, narrowly lanceolate or lanceolate | 2–6 × 1–1.7 | 1.8–3.6 | Acuminate | Subrounded or subcordate | Irregularly mucronate serrate | 11–13 | Extant |
| C. hebestroma | Lanceolate or ovate-lanceolate | 5–5.5 × 1.4–1.8 | 3.2–3.9 | Acuminate | Subrounded | Irregularly and simply serrate | 11–12 | Extant |
| C. tschonoskii | Elliptic, oblong or ovate-lanceolate | 5–12 × 2.5–5 | 2.7–3.6 | Acuminate or caudate-acuminate | Subrounded or subrounded-cuneate | Doubly setiform serrate | 14–16 | Extant |
| C. polyneura | Elliptic-lanceolate, oblong-lanceolate or lanceolate | 4–8 × 1.5–2.5 | 2.8–4.1 | Acuminate | Broadly cuneate or subrounded | Doubly and regularly setiform serrate | 16–20 | Extant |
| C. mollicoma | Oblong-lanceolate or elliptic-lanceolate | 5–6.5 × 2–2.5 | 2.5–3.2 | Acuminate or caudate-acuminate | Rounded or rounded-cuneate | Irregularly recurved setiform serrate | 14–17 | Extant |
| C. omeiensis | Elliptic or ovate-elliptic | 6–8 × 2.5–3.5 | 2.6–3.5 | Acuminate or caudate-acuminate | Rounded or broadly cuneate | Simply setiform serrate | 12–16 | Extant |
| C. rupestris | Oblong-lanceolate or lanceolate | 4–5 × 1.5–2 | 2.3–3 | Acuminate | Subrounded or broadly cuneate | Minutely simply setiform serrate | 14–17 | Extant |
| C. austro-sinensis | Elliptic or Ovate | 3.8–3.9 × 1.6–2 | 1.7–2.5 | Acute or acuminate | Rounded and cordate | Doubly serrate | 8–12 | Extant |
| C. betulus | Elliptic | 4.9–10.8 × 2.9–4.8 | 1.7–2.2 | Acuminate | Rounded and cuneate | Doubly serrate | 15–18 | Extant |
| C. carpinoides | Elliptic | 6.1 × 3.0 | 2.0 | Acute or acuminate | Rounded or cordate | Doubly serrate | 16–18 | Extant |
| C. chowii | Elliptic or ovate | 5.4 × 2.1 | 2–2.54 | Acuminate | Rounded or cuneate | Simply serrate | 11–12 | Extant |
| C. eximia | Elliptic | 9.5 × 4.8 | 1.7–2.1 | Acuminate | Rounded | Doubly serrate | 16–17 | Extant |
| C. faginea | Elliptic or ovate | 7.9 × 3.5 | 2.28 | Acuminate | Rounded or cordate | Simply serrate | 16–19 | Extant |
| C. fargesii | Elliptic or oblong | 8.1 × 3.7 | 2.2 | Acuminate | Cordate | Doubly serrate | 11–14 | Extant |
| C. handelii | Ovate | 6.2 × 2.4 | 2.6 | Acute or acuminate | Cordate | Simply serrate | 16–18 | Extant |
| C. henryana | Elliptic or ovate | 8.4 × 4.2 | 2.1 | Acute or acuminate | Rounded or cordate | Simply serrate | 13–15 | Extant |
| C. japonica | Elliptic | 3.9–4.2 × 2.1–2.2 | 1.8–2.0 | Acuminate | Cordate | Simply serrate | 15–17 | Extant |
| C. lanceolata | Elliptic or lanceolate | 6 × 1.7 | 3.5 | Acuminate | Cuneate | Doubly serrate | 10–12 | Extant |
| C. laxiflora | Elliptic or ovate | 6.6–9.3 × 3.2–4.6 | 1.9–2.2 | Acuminate | Rounded or cordate | Doubly serrate | 12–17 | Extant |
| C. minutiserrata | Elliptic or oblong | 6.1 × 1.7 | 3.6 | Acute or acuminate | Rounded or cordate | Doubly serrate | 12–14 | Extant |
| C. orientalis | Elliptic or ovate | 3–3.6 × 1.7 | 1.9–2.1 | Acuminate | Cuneate or cordate | Doubly serrate | 13–14 | Extant |
| C. poilanei | Elliptic or ovate | 8.3 × 2.8 | 3.0 | Acute or acuminate | Cuneate | Doubly serrate | 11–14 | Extant |
| C. seemeniana | Elliptic or ovate | 4.8 × 3 | 1.6 | Acute or acuminate | Rounded or cordate | Simply serrate | 10–13 | Extant |
| C. firmifolia | Elliptic or ovate | 2.9–5.1 × 1.8–2.4 | 1.6–2.2 | Acuminate | Rounded, cuneate or cordate | Simply serrate | 8–13 | Extant |
| C. mollis | Elliptic | 2.9–7.5 × 1.6–3.7 | 1.6–2.1 | Acuminate | Cordate | Doubly serrate | 16–19 | Extant |
| C. tropicalis | Elliptic or ovate | 5.8–11.1 × 2.6–3.7 | 1.2–2.9 | Acute or acuminate | Cordate | Doubly serrate | 11–13 | Extant |
| C. tungtzeensis | Elliptic or ovate | 2.4–8.6 × 2.8–3.6 | 1.3–2.5 | Acuminate | Rounded, cuneate or cordate | Simply serrate | 9–12 | Extant |
| C. yedoensis | Elliptic | 2.7–8.5 × 1.5–4.3 | 1.3–2.3 | Acuminate | Rounded or cuneate | Doubly serrate | 10–12 | Extant |
Genus: Betula L.
Subgenus: Betula L.
Species: Betula sp.
Specimens checked: HTG22031 (Fig. 4a), HTG22032 (Fig. 4b), HTG22033 (Fig. 4c and d), and HTG28410 (Fig. 4g).
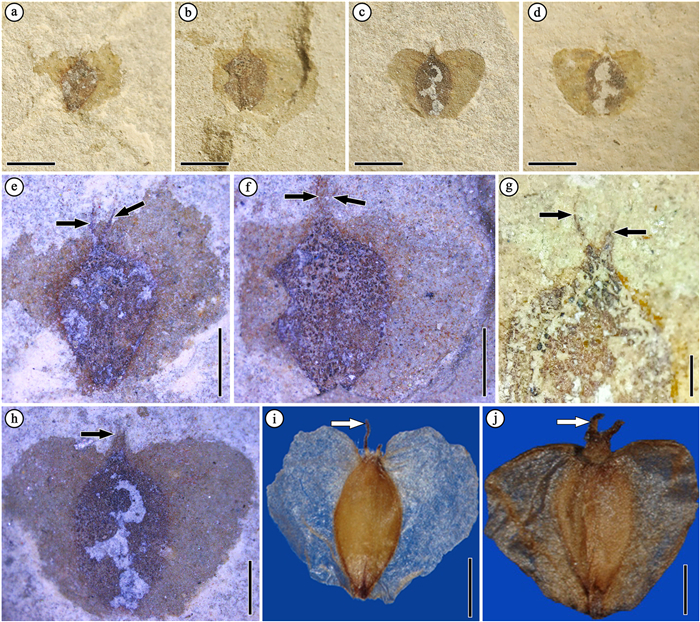
|
| Fig. 4 Fossil fruits of Betula from site HTG022 in the Huatugou area, and similar extant species of Betula and Alnus. Pictures of extant species were downloaded from the Chinese Virtual Herbarium (http://www.cvh.ac.cn/). (a) HTG22031. (b) HTG22032. (c)–(d) HTG22033a and its counterpart 22033b. (e)–(g) Enlargement of (a)–(c) and HTG28410. Black arrows denote the paired and thin persistent styles at apex. (i) Extant Betula pubescens. (j) Extant Alnus crispa. In (i) and (j), white arrows denote different persistent styles at apex. Scale bars = 2 mm in (a)–(d), 1 mm in (g)–(j). |
Repository: The Paleontology Laboratory of the School of Earth Sciences, Lanzhou University, China.
Locality: Fossil site HTG022, the Oligocene Shangganchaigou Formation, Huatugou Town, Qinghai Province, China.
Description: Fruits are 2.5–3.0 mm in width and 3.5–5.0 mm in length. Pericarp is ovoid with two thin persistent styles at apex (Fig. 4e–g) and two membranous wings on its lateral side. Nutlets are oval or obovate in shape. Membranous wings are 1.6–1.9 mm in width and 2.8–3.3 mm in length; and the width and length of nutlets are 1.4–1.8 mm and 2.3–3 mm respectively. The wings and nutlets are almost equal in width, and the latter are shorter. The widest part of wings is situated at the upper region.
Comparison: The fossil fruits are characterized by two membranous wings, which are as wide as nutlets, or slightly wide. The widest parts of wings are at the upper region. The fruit morphology of these fossils is very similar to those of living Betula species (Fig. 4), although there are some species in Alnus Mill. that also have membranous and wide wings. The two genera can be distinguished by the morphology of persistent styles. They are usually slender in Betula but stout in Alnus (Fig. 4i and j). Recent molecular research on the phylogenetic relationship among birch trees divided the genus Betula into two subgenera, Aspera and Betula, noting that the former has no or very narrow seed wings whereas the latter has obvious seed wings (Wang et al., 2021). Furthermore, the subgenus Betula has been divided into four sections: Acuminatae, Betula, Costatae, Dahuricae (Wang et al., 2021). Among these, species who have wider wings can be excluded, including Betula alnoides Buch.-Ham. ex D. Don and B. luminifera H. Winkl. It is hard to further identify the current fossil fruits because of poor preservation and a lack of related fossil leaves at this locality. Consequently, we tentatively describe them as an undefined species in subgenus Betula.
4. Discussion 4.1. Nearest living relatives of the Huatugou fossil leavesWe performed hierarchical clustering analysis to determine the similarity in leaf morphology between the fossil leaves and modern Carpinus species, based on a data matrix including 33 modern species and 16 leaf characters. Detailed information on the hierarchical clustering is provided in Supplementary data. Before the clustering, we used the principal component analysis (PCA) to assess these characters, gathering the top 12 components with a sum of variance greater than 95% as new variables and obtaining a tree diagram for clustering (for detailed information, see Supplementary data). These analyses indicate that the morphology of the Huatugou fossil leaves most resembles C. betulus (Fig. 5). Thus, we considered C. betulus as the nearest living analogue of the current fossil leaves. This is consistent with a previous viewpoint (Utescher and Mosbrugger, 2015). Unfortunately, associated fruits of Carpinus, which would help confirm this relationship, have not been found at the Huatugou sites thus far.
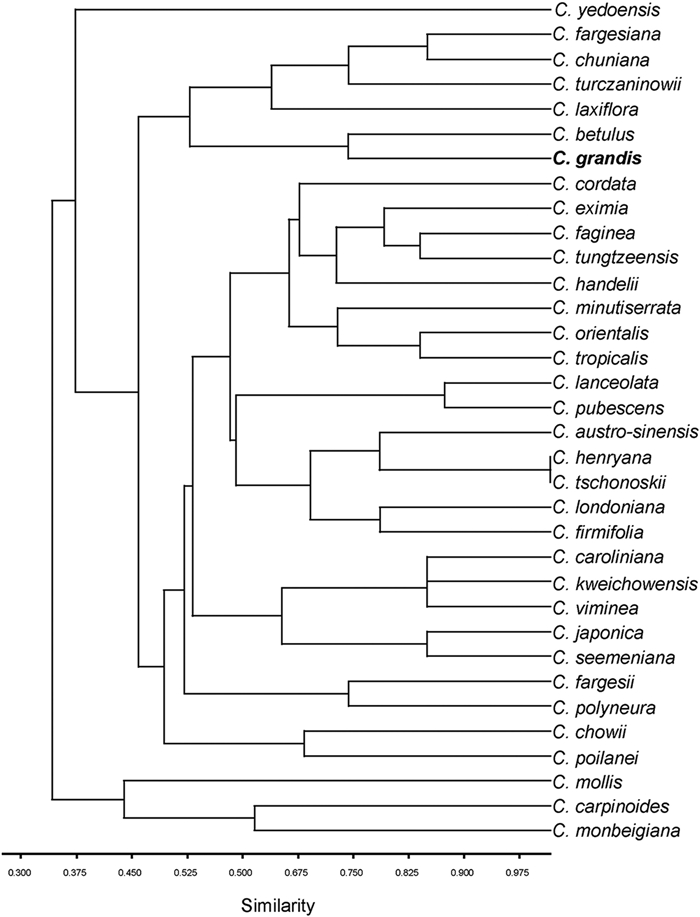
|
| Fig. 5 Cluster results showing the morphological similarity among current fossil leaves and 33 extant species of Carpinus. For detailed characters and matrix, see Table S1. |
Recent discoveries revealed a considerably high biodiversity and a humid subtropical ecosystem in central Tibet during the middle Eocene (Liu et al., 2019; Su et al., 2019, 2020; Tang et al., 2019). Several Paleogene taxa from this region also revealed a relatively free exchange of lineages across the Northern Hemisphere (Su et al., 2020). For example, Ailanthus maximus (Liu et al., 2019), Cedrelospermum (Jia et al., 2019) and Lagokarpos tibetensis (Tang et al., 2019) indicated an obvious floristic linkage between the Tibetan Plateau and European and North American floras in the late Paleogene, a discovery that laid the foundation for exploring the biogeographic significance of the Tibetan Plateau during the early Paleogene. However, more comprehensive information of floristic exchanges between the Tibetan Plateau and other regions in the late Paleogene are still required.
To study the exchange between the current fossil plants and other contemporaneous floras in the Northern Hemisphere, we first gathered the fossil records of Betula and Carpinus in the Paleogene (Table 2). Eocene megafossils of Carpinus are found in northwestern North America, China (Liaoning and Mangkang), and Japan (Fig. 6). The earliest unequivocal fruit record of Carpinus comes from the middle Eocene (50–49 Ma) of North America (Pigg et al., 2003). Based on fossil records, it is reasonable to assume that the genus dispersed via the Bering Land Bridge, an important corridor for many temperately disjunct lineages until 5.5–5.4 Ma (Milne and Abbott, 2002). The earliest reliable fossil of Betula was collected from the Paleocene in northeastern China and Kazakhstan (Tao and Xiong, 1986). The fossil record of Betula shows a similar distribution pattern to that of Carpinus, which likewise suggests an exchange between Northern America and East Asia via the Bering Land Bridge (Fig. 6). In addition, the floristic linkage between East Asia and Europe might be weak in the Eocene, given that Turgai Strait still separated Europe and Asia at that time (Mennecart et al., 2018).
| Species | Locality | Age | Reference |
| Betula prisca | Heilongjiang, China; Kazakhstan | Paleocene | Tao and Xiong (1986) |
| B. leopoldae | Southern British Columbia, Canada | Middle Eocene | Crane and Stockey (1987) |
| B. leopoldae | Northeastern Washington, America | middle Eocene | Wolfe and Wehr (1987) |
| B. dissecta | Liaoning, China | Middle to late Eocene | WGCPC (1978) |
| B. fushunensis | Liaoning, China | Middle to late Eocene | WGCPC (1978) |
| B. populoides | Liaoning, China | Middle to late Eocene | WGCPC (1978) |
| B. speciosa | Liaoning, China | Middle to late Eocene | WGCPC (1978) |
| B. subpubescens | Liaoning, China | Middle to late Eocene | WGCPC (1978) |
| Betula sp. | Liaoning, China | Middle to late Eocene | WGCPC (1978) |
| B. mankongensis | Tibet, China | Late Eocenea | Tao and Du (1987) |
| B. cf. utilis | Tibet, China | Late Eocene | Tao and Du (1987) |
| B. cf. vera | Tibet, China | Late Eocene | Tao and Du (1987) |
| B. buzekii | Czech | Oligocene | Kvacek and Walther (2006) |
| Betula sp. | Qaidam, China | Oligocene | This paper |
| B. prisca | Kazakhstan | early Oligocene | Zhilin (1989) |
| Betula sp. | Southwestern Siberia, Russia | Late Oligocene | Denk et al. (2020) |
| B. dissecta | Liaoning, China | Oligocene | Jin and Shang (1998) |
| B. fushunensis | Liaoning, China | Oligocene | Jin and Shang (1998) |
| Betula sp. | Tibet, China | Late Oligocene | Ai et al. (2018) |
| B. kleinsaubernitzensis | Kleinsaubernitz, Germany | Oligocene | Walther (2003) |
| B. dryadum | Enspel, Germany | Oligocene | Koehler (2003) |
| B. angustifolia | Oregon, America | Oligocene | Meyer and Manchester (1997) |
| Betula sp. | Yunnan, China | Early Oligocene | Linnemann et al. (2018) |
| Carpinus perryae | Washington, America | Middle Eocene (49–50 Ma) | Pigg et al. (2003) |
| Carpinus-like bracts | Eckfeld, Germany | Middle Eocene | Wilde and Frankenhäuser (1998) |
| Carpinus sp. | Yellowstone National Park, America | Middle Eocene | Wheeler et al. (1977) |
| C. latifolia | Liaoning, China | Middle to late Eocene | WGCPC (1978) |
| Carpinus sp. | Japan | Late Eocene | Tanai (1972) |
| C. cf. fargesiana | Tibet, China | Late Eocene | Tao and Du (1987) |
| C. grandis | Tibet, China | Late Eocene | Tao and Du (1987) |
| Carpinus sp. | Czech | Oligocene | Kvacek and Walther (2006) |
| C. grandis | Qaidam, China | Oligocene | This paper |
| C. cordataeformis | Czech | Oligocene | Kvacek and Walther (2006) |
| C. grandis | Czech | Oligocene | Kvacek and Walther (2006) |
| C. mediomontana | Czech | Oligocene | Kvacek and Walther (2006) |
| Carpinus sp. | Kazakhstan | Early Oligocene | Zhilin (1989) |
| C. subcordata | Kazakhstan | Early Oligocene | Zhilin (1989) |
| C. subcordata | Kazakhstan | Late Oligocene | Zhilin (1989) |
| C. cf. grandis | Southwestern Siberia, Russia | Late Oligocene | Denk et al. (2020) |
| Carpinus sp. | Japan | Oligocene | Tanai (1991) |
| C. kushiroensis | Japan | Oligocene | Tanai (1970) |
| Carpinus sp. | Liaoning, China | Oligocene | Jin and Shang (1998) |
| Carpinus sp. | Lanzhou, China | Oligocene | Geng et al. (2001) |
| C. mioviminea | Yunnan, China | Early Oligoceneb | Huang (2017) |
| Carpinus sp. | Tibet, China | Late Oligocene | Ai et al. (2018) |
| C. grandis | Kleinsaubernitz, Germany | Oligocene | Walther (2003) |
| C. cordataeformis | Enspel, Germany | Oligocene | Koehler (2003) |
| C. mediomontana | Enspel, Germany | Oligocene | Koehler (2003) |
| C. grandis | Enspel, Germany | Oligocene | Koehler (2003) |
| a The age of Mangkang flora (Tibet, China) was previously considered to be late Miocene (Tao and Du, 1987) and then revised to be late Eocene (Su et al., 2018). b The age of Wenshan flora (Yunnan, China) was previously considered to be middle Miocene and then revised to be early Oligocene (Tian et al., 2021). | |||
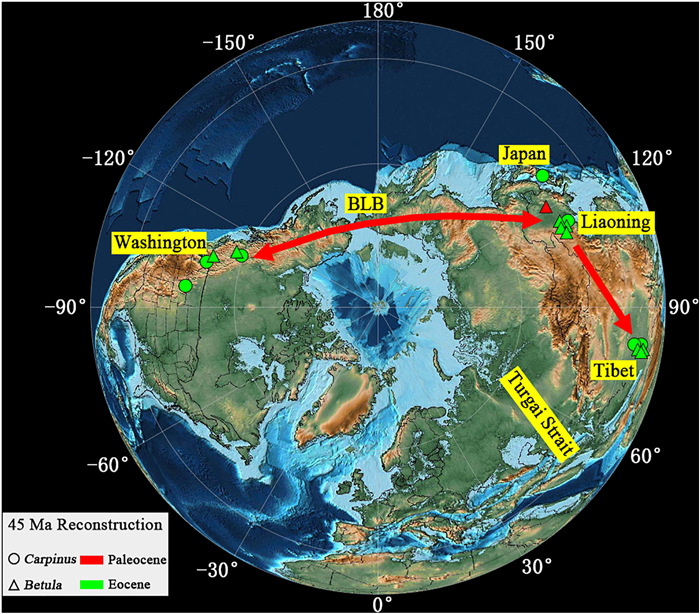
|
| Fig. 6 Paleographic map of the world during the early Paleogene (45 Ma). The map was modified from the PALEOMAP PaleoAtlas for Gplates (Scotese, 2016). The circles denote fossil records of Carpinus. Triangles denote records of Betula in northwestern North America, Europe, and East Asia. The red line indicates the possible dispersal path. BLB, Bering Land Bridge. |
The Betulaceous fossils and other associated fossil angiosperms from the Oligocene of the Qaidam Basin provide further evidence for Paleogene floral interchanges between the Tibetan Plateau and other regions in the Northern Hemisphere. Abundant fossil records indicate that Betula and Carpinus were widely distributed in the Oligocene Eurasia (Fig. 7a). As mentioned, the fossil leaves in this study have been assigned to C. grandis, which is a common fossil morphospecies in the Cenozoic deposits of the Northern Hemisphere (Li and Guo, 1976; Mai, 1981; Tao and Du, 1987; Mai and Walther, 1988, 1991; Hummel, 1991; Zastawniak and Walther, 1998; Worobiec and Szynkiewicz, 2007). In Asia, C. grandis has been found in late Eocene Mangkang and middle Miocene Namling of Tibet (Tao and Du, 1987; Guo et al., 2019); and in Europe, this fossil species appears to have had a more extensive distribution since the Oligocene, including the modern-day Czech Republic, southern Russia, and Germany (see Table 2). As the nearest living equivalent of the current fossil leaves, C. betulus is also naturally distributed in central Europe today (Boratynski, 2014). We speculate that the current fossil Carpinus from the northern Tibetan Plateau might be closely related to European taxa. This speculation is corroborated by the presence of other Oligocene fossil angiosperms found in the Huatugou region. For example, Podocarpium, an extinct genus well-documented in the Cenozoic deposits around Eurasia (Wang et al., 2007; Xu et al., 2015), is abundant in the Oligocene Qaidam Basin (Yan et al., 2018; Han et al., 2020; Song et al., 2020). It is also known from the Oligocene of south China, central Europe, and western Europe (Fig. 7a). Recent research has revealed that Podocarpium may have originated in East Asia, migrated into the central valley of Tibet in the late Eocene, and then spread westward to Europe (Li et al., 2022). Many fossil samaras and leaves of Ailanthus confucii have been found at the Huatugou fossil site (Yang et al., 2021a, Yang et al., 2021b). This is a species that been discovered in Oligocene deposits from Europe to East Asia. Another fossil species, A. tardensis Hably, which is found in Oligocene sediments in Europe and South China, indicates a subsequent diversification in Europe or South Asia (Song et al., 2014). The enhanced floristic affinity between Europe and Asia during the Oligocene can be attributed to the continuous retreat of the Turgai Strait at that time. In addition, enhanced moisture transport via westerlies provided suitable climatic conditions for the exchange of these plants during the Oligocene (Wu et al., 2021). The flora in the Oligocene Huatugou area also shares some components with the contemporaneous floras of Yunnan Province at the southeastern edge of the Tibetan Plateau (Huang et al., 2016; Huang, 2017; Tian et al., 2021). With consideration of the higher global temperature (Zachos et al., 2001) and the moderate elevation of the southeastern plateau during the late Eocene to early Oligocene (Li et al., 2015; Spicer et al., 2020; Xiong et al., 2020), the floras in the northeastern and southeastern plateau might have been interconnected at that time.
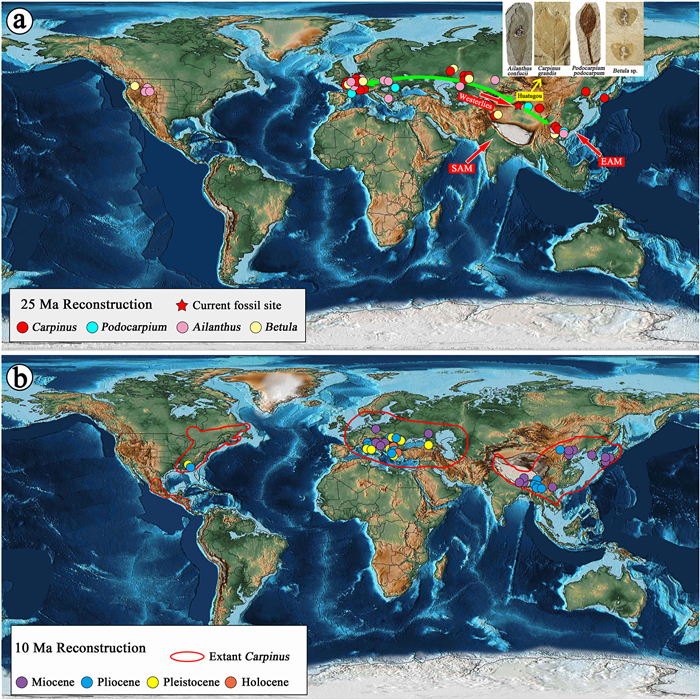
|
| Fig. 7 Paleographic map of the world during the late Paleogene (25 Ma; a) and middle Neogene (10 Ma; b). The map was modified from the PALEOMAP PaleoAtlas for Gplates (Scotese, 2016). In panel a, the red, green, pink, and light-yellow circles denote the Oligocene fossil records of Carpinus, Podocarpium, Ailanthus, and Betula in the Northern Hemisphere respectively. The red star denotes the site of our current fossil findings in Huatugou. Red lines indicate the possible dispersal route between Huatugou and other regions. The red arrows denote the Westerlies and Asian Monsoon. SAM, South Asian Monsoon; EAM, East Asian Monsoon. In b, the purple, blue, yellow, and orange circles denote fossil records of Carpinus in the Miocene, Pliocene, and Pleistocene, and Holocene in the Northern Hemisphere respectively. The red line denotes the distribution range of extant Carpinus (modified from Chen et al., 1994b). |
Overall, the records of plant fossils indicate a close floristic linkage between the Qaidam Basin and Europe during the Oligocene, which can be attributed to the retreat of the Turgai Strait and the favorable climatic conditions provided by the enhanced moisture transport via westerlies. Thus, the Qaidam Basin might represent a pathway on the northern plateau for the free floristic exchange between the Tibetan Plateau and Europe during the late Paleogene. Our findings enrich our knowledge of the biogeographic significance of plant fossils from the Qaidam Basin. Moreover, these fossils reveal the potential influence of westerlies on the formation of paleovegetation in the late Paleogene northern Tibetan Plateau.
Although Carpinus is a common element in the modern North American vegetation, it exhibits an unusual and somewhat perplexing fossil distribution. Reliable records of Carpinus are extremely rare throughout post-Eocene North American sediments (Manchester, 1999). It still remains uncertain if Carpinus was present but left no clear fossil record or if the genus went extinct in North America and migrated back to the continent recently (Stults et al., 2002; Stults and Axsmith, 2009). After the Paleogene, Carpinus was still widespread in Europe and East Asia; its modern distribution was mostly shaped since the Neogene. Under the influence of the increasing elevation of the southern Tibetan Plateau and consequent climatic changes (Spicer et al., 2003), this genus was only distributed at low latitudes on and around the plateau (Fig. 7b).
4.3. Paleoenvironmental implicationsMany Betulaceae species are important components of the deciduous broad-leaf forests in the north temperate zone (Li and Skvortsov, 1999). Carpinus betulus, the nearest living equivalent of current fossil leaves, is naturally distributed at low-elevation (not exceeding 2300 m) warm and moist regions in central Europe today (Boratynski, 2014). In China, the species of section Carpinus live in similar environments, with mean annual temperature (MAT) values ranging from −1.8 to 24.1 ℃, mean annual precipitation (MAP) values ranging from 320 mm to 2992 mm, and elevations not exceeding 2800 m (Li and Skvortsov, 1999; Fang et al., 2011). Betula also commonly inhabits the mid-latitude region in the Northern Hemisphere (Chen, 1994b), and some act as keystone species of forests across Eurasia and North America (Ashburner and McAllister, 2013).
During our fieldwork in the Huatugou area over the past few years, many other fossil plants were also found from the continuous layer in the lower and middle parts of Shangganchaigou Formation (Yang, 2022). Desmanthus Willd. (Leguminosae), collected from lower Shangganchaigou Formation, is a pantropical legume genus native to the New World, revealing that the northwestern Qaidam was much warmer in the early Oligocene (Yang et al., 2021a, Yang et al., 2021b). A. confucii Unger (Simarubaceae), which was found from a nearby layer and is the most common member among fossil Ailanthus, is thought to have shared a similar ecological niche with extant A. altissima in the past, also indicating that climate conditions at the fossil site were much warmer and more humid during the early Oligocene than currently (Yang et al., 2021b). In addition, some fossil fruits and leaves from this region, including Podocarpium (A. Braun) Herendeen, Acer L., Ulmus L., Cupressus L., Chamaecyparis Spach, Thuja L., Adiantum L., and Equisetum L., have been previously reported (Yan et al., 2018; Chen et al., 2021; Yang, 2022). Preliminarily research based on the coexistence approach suggests that the mean annual temperature (MAT) and mean annual precipitation (MAP) was about 14 ℃ and 600–1200 mm, respectively (Yang, 2022), which is partly consistent with a recent study (Song et al., 2020) focused on coetaneous northeastern Qaidam Basin (MAT = 11.6 ± 2.4 ℃, MAP > 1000 mm).
4.4. Warm and humid forests in Oligocene Qaidam for floristic exchangeIn addition to the fossil plants mentioned above, a paleobotanical study in the western Qaidam Basin noted that Quercus L. is common in the Shangganchaigou Formation (Zhong, 2007). Palynological data from the Dahonggou Section in the northeastern Qaidam Basin also showed an abundance of Quercus in this formation (Lu et al., 2010). In addition, recent research in the Dahonggou Section indicated that the leaf fossil assemblages from the early Oligocene (30.8 Ma) were dominated by Populus L. and Podocarpium, with the occasional Cyclocarya Iljinsk (Song et al., 2020). These fossil plants collectively suggest that there used to be a warm and humid deciduous broad-leaf forest during the Oligocene in Qaidam. Few trees today can survive such an elevation at this latitude on the plateau (Fig. 1f). The regional eradication of these plants might have resulted from the rising elevation and decreasing temperatures and precipitation since the Miocene (An et al., 2001; Tiffney and Manchester, 2001; Garzione et al., 2005).
The global cooling at the Eocene–Oligocene transition (Zachos et al., 2001; Zanazzi et al., 2007) not only profoundly changed the climatic conditions and floristic composition in southeastern Tibet, i.e., from subtropical/warm temperate in the late Eocene to cool temperate in the early Oligocene (Su et al., 2018), but this climatic event and consequent cooling and aridification (Dupont-Nivet et al., 2007) have also made an impact on the succession of fish fauna in Huatugou (Yang et al., 2018, 2022). After the Eocene–Oligocene transition, global temperatures gradually rose (Zachos et al., 2001). In western Qaidam, aridity decreased, which has been attributed to increased moisture from westerlies (Wu et al., 2021). Together, these conditions created a suitable environment in the Qaidam Basin for plants that inhabit warm, humid forest to flourish and eventually spread.
5. ConclusionsIn this study, we describe megafossils of Betulaceae collected from the Oligocene western Qaidam Basin (Huatugou area), including Carpinus leaves and Betula fruits. Our main conclusions are as follows: (1) Based on detailed morphological comparison, the fossil leaves were assigned to C. grandis and the fossil fruits were identified as Betula sp. They represent the first fossil record of Betulaceae in the Qaidam Basin. (2) These Betulaceous fossils, together with previously reported fossil plants from the same locality, reveal a close floristic linkage between the Qaidam Basin and Europe during the Oligocene. The Qaidam Basin might represent a pathway on the northern plateau for the free floristic exchange between the Tibetan Plateau and Europe during the late Paleogene. (3) Fossil plants collected from Huatugou area suggest a warm and humid deciduous broad-leaf forest during the Oligocene. The westerlies provided suitable climatic conditions for such a warm and humid forest, allowing the Qaidam Basin to act as a region for these plants to flourish and spread.
AcknowledgementsWe thank to Dr. Junling Dong (Chengdu University of Technology, Chengdu), and Dr. Pengju He (Lanzhou University, Lanzhou) for substantial suggestions; Dr. Haylin Chan and Pallas Cate (Sun Yatsen University, Guangzhou) for helpful remarks and assisting with English; Prof. Steven R. Manchester (University of Florida, U.S.A.) for providing helpful suggestions and literature; Dr. Jianwu Li, Mr. Bing Wang (Xishuangbanna Tropical Botanical Garden, Mengla), and Prof. Trevor Hodkinson (Trinity College, Ireland) for providing some extant specimens of Carpinus and Betula. We sincerely thank Dr. Bian Wang (IVPP, Beijing) for improving the language. This research was conducted under the China Postdoctoral Science Foundation (No. 2022M723151); the Second Tibetan Plateau Scientific Expedition Research Program (No. 2019QZKK0704); the National Natural Science Foundation of China (No. 42172005, 41272026, 41972008, 31870200); and the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB26000000).
Author contributions
D.Y. and T.Y. designed research and led the expeditions; J.C., H.C., L.H., L.Z., W.L., J.W., and X.L. collected the fossil and extant specimens; Y.D., W.L., and S.X. performed research; T.Y. and J.C. analyzed data; and D.Y. and T.Y. wrote the paper.
Declaration of competing interest
The authors declare they have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.03.007.
Ashburner, K., McAllister, H., 2013. The Genus Betula. A taxonomic revision of birches. Univ. Chicago Press, pp. 1-432.
|
An, Z.S., Kutzbach, J.E., Prell, W.L., et al., 2001. Evolution of Asian monsoons and phased uplift of the Himalaya-Tibetan plateau since late Miocene times. Nature, 411: 62-66. DOI:10.4319/lo.2001.46.1.0062 |
Ai, K.K., Shi, G.L., Zhang, K.X., et al., 2018. The uppermost Oligocene Kailas flora from southern Tibetan Plateau and its implications for the uplift history of the southern Lhasa terrane. Paleogeogr. Paleoclimatol. Paleoecol., 515: 143-151. |
Boratynski, A., 2014. Carpinus betulus. In: Stimm, B., Roloff, A., Lang, U.M., Weisgerber, H. (Eds.), Enzyklopädie der Holzgewächse: Handbuch und Atlas der Dendrologie.
|
Chang, M.M., Wang, X.M., Liu, H.Z., et al., 2008. Extraordinarily thick-boned fish linked to the aridification of the Qaidam Basin (northern Tibetan Plateau). Proc. Natl. Acad. Sci. U.S.A., 105: 13246-13251. DOI:10.1073/pnas.0805982105 |
Chang, H., Li, L.Y., Qiang, X.K., et al., 2015. Magnetostratigraphy of Cenozoic deposits in the western Qaidam Basin and its implication for the surface uplift of the northeastern margin of the Tibetan Plateau. Earth Planet. Sci. Lett., 430: 271-283. DOI:10.1016/j.epsl.2015.08.029 |
Chang, M.M., Miao, D.S., 2016. Review of the Cenozoic fossil fishes from the Tibetan Plateau and their bearings on paleoenvironment. Chin. Sci. Bull., 61: 981-995. DOI:10.1360/n972015-01372 |
Chen, H.Y., Yang, T., Han, L., et al., 2021. The Oligocene Equisetum from Qaidam Basin, northeastern Tibetan plateau in China and its implications. Hist. Biol., 33: 2845-2853. DOI:10.1080/08912963.2020.1830280 |
Chen, Z.D., 1994a. Phylogeny and phytogeography of the Betulaceae. Acta Phytotaxon. Sin., 32: 1-31. |
Chen, Z.D., 1994b. Phylogeny and phytogeography of the Betulaceae (CONT). Acta Phytotaxon. Sin., 32: 101-153. |
Crane, P.R., 1981. Betulaceous leaves and fruits from the British upper Palaeocene. Bot. J. Linn. Soc., 83: 103-136. DOI:10.1111/j.1095-8339.1981.tb01224.x |
Crane, P.E., Stockey, R.A., 1987. Betula leaves and reproductive structures from the middle Eocene of British columbia, Canada. Can. J. Bot., 65: 2490-2500. DOI:10.1139/b87-338 |
Dai, J., Sun, B.N., Xie, S.P., et al., 2013. A new species of Carpinus (Betulaceae) from the Pliocene of Yunnan Province, China. Plant Syst. Evol., 299: 643-658. DOI:10.1007/s00606-012-0750-1 |
Denk, T., Bouchal, J.M., Smirnov, P., et al., 2020. Late Oligocene leaf and pollen flora of southwestern Siberia: taxonomy, biogeography and palaeoenvironments. Hist. Biol., 33: 2951-2976. |
Deng, T., Wang, X.M., Fortelius, M., et al., 2011. Out of Tibet: Pliocene woolly rhino suggests high-plateau origin of Ice Age megaherbivores. Science, 333: 1285-1288. DOI:10.1126/science.1206594 |
Deng, T., Wang, X.M., Wu, F.X., et al., 2019a. Review: implications of vertebrate fossils for paleo-elevations of the Tibetan Plateau. Global Planet. Change, 174: 58-69. DOI:10.1016/j.gloplacha.2019.01.005 |
Deng, T., Wu, F.X., Wang, S.Q., et al., 2019b. Significant shift in the terrestrial ecosystem at the Paleogene/Neogene boundary in the Tibetan Plateau. Chin. Sci. Bull., 64: 2894-2906. DOI:10.1360/TB-2019-0053 |
Ding, W.N., Ree, R.H., Spicer, R.A., et al., 2020. Ancient orogenic and monsoon-driven assembly of the world's richest temperate alpine flora. Science, 369: 578-581. DOI:10.1126/science.abb4484 |
Dupont-Nivet, G., Krijgsman, W., Langereis, C.G., et al., 2007. Tibetan plateau aridification linked to global cooling at the Eocene-Oligocene transition. Nature, 445: 635-638. DOI:10.1038/nature05516 |
Ellis, B., Daly, D.C., Hickey, L.J., et al., 2009. Manual of Leaf Architecture. New York: Cornell University Press.
|
Fang, J., Wang, Z., Tang, Z., 2011. Atlas of Woody Plants in China: Distribution and Climate. Higher Education Press, Springer.
|
Fang, X.M., Zhang, W.L., Meng, Q.Q., et al., 2007. High-resolution magnetostratigraphy of the Neogene Huaitoutala section in the eastern Qaidam Basin on the NE Tibetan plateau, Qinghai Province, China and its implication on tectonic uplift of the NE Tibetan plateau. Earth Planet Sci. Lett., 258: 293-306. DOI:10.1016/j.epsl.2007.03.042 |
Foster, A.S., 1952. Foliar venation in angiosperms from an ontogenetic standpoint. Am. J. Bot., 39: 752-766. DOI:10.1002/j.1537-2197.1952.tb13099.x |
Garzione, C.N., Ikari, M.J., Basu, A.R., 2005. Source of Oligocene to Pliocene sedimentary rocks in the Linxia Basin in northeastern Tibet from Nd isotopes: implications for tectonic forcing of climate. Geol. Soc. Am. Bull., 117: 1156-1166. DOI:10.1130/B25743.1 |
Geng, B.Y., Tao, J.R., Xie, G.P., 2001. Early tertiary fossil plants and paleoclimate of Lanzhou basin. J. Syst. Evol., 39: 105-115. |
Guo, S.X., Spicer, R.A., Widdowson, M., et al., 2019. The composition of the middle Miocene (15 Ma) Namling paleoflora, south central Tibet, in the context of other Tibetan and Himalayan floras. Rev. Palaeobot. Palynol., 271: 104088. DOI:10.1016/j.revpalbo.2019.06.011 |
Han, F., Yang, T.L., Zhang, K.X., et al., 2020. Early Oligocene Podocarpium (Leguminosae) from Qaidam Basin and its paleoecological and biogeographical. Rev. Palaeobot. Palynol., 282: 104309. DOI:10.1016/j.revpalbo.2020.104309 |
He, P.J., Song, C.H., Wang, Y.D., et al., 2021. Early Cenozoic activated deformation in the Qilian Shan, northeastern Tibetan Plateau: insights from detrital apatite fission-track analysis. Basin Res., 33: 1731-1748. DOI:10.1111/bre.12533 |
Hickey, L.J., 1977. Stratigraphy and paleobotany of the golden valley formation (early tertiary) of western north Dakota. Geol. Soc. Am. Memoirs, 150. |
Huang, J., 2017. The Middle Miocene Wenshan Flora, Yunnan, Southwestern China and its Palaeoenvironment Reconstruction. Ph. D. Dissertation. University of Chinese Academy of Sciences.
|
Huang, J., Su, T., Li, S.F., et al., 2020. Pliocene flora and paleoenvironment of Zanda basin, Tibet, China. Sci. China Earth Sci., 63: 212-223. DOI:10.1007/s11430-019-9475-2 |
Huang, Y.J., Jia, L.B., Wang, Q., et al., 2016. Cenozoic plant diversity of Yunnan: a review. Plant Divers., 38: 271-282. DOI:10.1016/j.pld.2016.11.004 |
Hummel, A., 1991. The Pliocene leaf flora from Ruszów near zary in lower Silesia, south-west Poland. Part Ⅱ. (Betulaceae). Acta Palaeobot., 31: 73-151. |
Ji, J.L., Zhang, K.X., Clift, P.D., et al., 2017. High-resolution magnetostratigraphic study of the Paleogene-Neogene strata in the northern Qaidam Basin: implications for the growth of the northeastern Tibetan Plateau. Gondwana Res., 46: 141-155. DOI:10.1016/j.gr.2017.02.015 |
Jia, L.B., Su, T., Huang, Y.J., et al., 2019. First fossil record of Cedrelospermum (Ulmaceae) from the Qinghai–Tibetan plateau: implications for morphological evolution and biogeography. J. Syst. Evol., 57: 94-104. DOI:10.1111/jse.12435 |
Jin, J.H., Shang, P., 1998. Discovery of early tertiary flora in Shenbei coalfield, Liaoning. Acta Sci. Nat. Univ. Sunyatseni, 37: 129-130. |
Koehler, J., 2003. Oligocene macroflora of Enspel (Germany). Pangaea. DOI:10.1594/PANGAEA.106541 |
Krüssmann, G., 1976. Handbuch der Laubgeholze, vol. 1. Paul Parey, Belin-Hamburg.
|
Kvacek, Z., Walther, H., 2006. Oligocene macroflora of Bechlejovice (Czech Republic). Pangaea. DOI:10.1594/PANGAEA.510779 |
Li, H.M., Guo, S.X., 1976. The Miocene flora from Namling of Xizang. Acta Palaeontol. Sin., 15: 7-18. |
Li, P.Q., Skvortsov, A.K., 1999. Betulaceae. Beijing: Science Press. In: Wu, C.Y., Raven, P.H., Hong, D.Y. (Eds.), Flora of China Vol. 4. Missouri Botanical Garden Press, St. Louis, pp. 286-313.
|
Li, S.Y., Currie, B.S., Rowley, D.B., et al., 2015. Cenozoic paleoaltimetry of the SE margin of the Tibetan Plateau: constraints on the tectonic evolution of the region. Earth Planet Sci. Lett., 432: 415-424. DOI:10.1016/j.epsl.2015.09.044 |
Lin, Z.C., Sun, B.N., Lomax, B.H., et al., 2010. Leaf megafossils of Betula yunnanensis sp. nov. (Betulaceae) from the Mangbang Formation, SW China and its taphonomic implications. Rev. Palaeobot. Palynol., 163: 84-103. DOI:10.1016/j.revpalbo.2010.10.006 |
Linnemann, U., Su, T., Kunzmann, L., et al., 2018. New U-Pb dates show a Paleogene origin for the modern Asian biodiversity hot spots. Geology, 46: 3-6. |
Li, W.C., Huang, J., Chen, L.L., et al., 2022. Podocarpium (Fabaceae) from the late Eocene of central Tibetan Plateau and its biogeographic implication. Rev. Palaeobot. Palynol., 305: 104745. DOI:10.1016/j.revpalbo.2022.104745 |
Liu, J., Su, T., Spicer, R.A., et al., 2019. Biotic interchange through lowlands of Tibetan Plateau suture zones during Paleogene. Paleogeogr. Paleoclimatol. Paleoecol., 524: 33-40. DOI:10.1016/j.palaeo.2019.02.022 |
Liu, X.Y., Manchester, S.R., Jin, J.H., 2014. Alnus subgenus Alnus in the Eocene of western North America based on leaves, associated catkins, pollen, and fruits. Am. J. Bot., 101: 1925-1943. DOI:10.3732/ajb.1400228 |
Liu, Y.S., 1996. Foliar architecture of Betulaceae and a revision of Chinese betulaceous megafossils. Palaeontogr. Abteilung B-Palaeophytol. Palaeobot.-Palaeophytol., 239: 23-57. |
Low, S.L., Su, T., Spicer, T., et al., 2019. Oligocene Limnobiophyllum (Araceae) from the central Tibetan Plateau and its evolutionary and palaeoenvironmental implications. J. Syst. Palaeontol., 18: 415-431. DOI:10.1037/dev0000621 |
Lu, H.J., Xiong, S.F., 2009. Magnetostratigraphy of the Dahonggou section, northern Qaidam Basin and its bearing on cenozoic tectonic evolution of the Qilian Shan and Altyn tagh fault. Earth Planet Sci. Lett., 288: 539-550. DOI:10.1016/j.epsl.2009.10.016 |
Lu, J.F., Song, B.W., Chen, R.M., et al., 2010. Palynological assemblage of Eocene-Oligocene pollen and their biostratigraphic correlation in Dahonggou, Daqaidam area, Qaidam Basin. J. China Univ. Geosci., 35: 839-848. |
Mai, D.H., 1981. Entwicklung und klimatische Differenzierung der Laubwaldflora Mitteleuropas im Tertiär. Flora, 171: 525-582. DOI:10.1016/S0367-2530(17)31304-X |
Mai, D.H., Walther, H., 1988. Die pliozänen floren von Thüringen, Deutsche Demokratische Republik. Quartarpalaontologie, 7: 55-297. DOI:10.1515/9783112652541-003 |
Mai, D.H., Walther, H., 1991. Die oligozänen und untermiozänen Floren Nordwest-Sachsens und des Bitterfelder Raumes. Abh. Staatl. Mus. Mineral. Geol. Dresd., 38: 1-230. |
Manchester, S.R., 1999. Biogeographical relationships of North American tertiary floras. Ann. Mo. Bot. Gard., 86: 472-522. DOI:10.2307/2666183 |
Manchester, S.R., Crane, P.R., 1987. A new genus of Betulaceae from the Oligocene of western North America. Bot. Gaz., 148: 263-273. DOI:10.1086/337654 |
Mennecart, B., Geraads, D., Spassov, N., et al., 2018. Discovery of the oldest European ruminant in the late Eocene of Bulgaria: Did tectonics influence the diachronic development of the Grande Coupure?. Paleogeogr. Paleoclimatol. Paleoecol., 498: 1-8. DOI:10.1016/j.palaeo.2018.01.011 |
Meyer, H.W., Manchester, S.R., 1997. The Oligocene Bridge Creek Flora of the John Day Formation, Oregon, vol. 141. Univ. California Press Geol. Sci., pp. 1-195
|
Miao, Y.F., Song, C.H., Fang, X.M., et al., 2016. Late Cenozoic genus Fupingopollenites development and its implications for the Asian summer monsoon evolution. Gondwana Res., 29: 320-333. DOI:10.1016/j.gr.2014.12.007 |
Milne, R.I., Abbott, R.J., 2002. The origin and evolution of Tertiary relict floras. Adv. Bot. Res., 38: 281-314. |
Pigg, K.B., Manchester, S.R., Wehr, W.C., 2003. Corylus, Carpinus and Palaeocarpinus (Betulaceae) from the middle Eocene Klondike mountain and Allenby formations of northwestern North America. Int. J. Plant Sci., 164: 807-822. DOI:10.1086/376816 |
Renner, S.S., 2016. Available data point to a 4-km-high Tibetan Plateau by 40 Ma, but 100 molecular-clock papers have linked supposed recent uplift to young node ages. J. Biogeogr., 43: 1479-1487. DOI:10.1111/jbi.12755 |
Song, B.W., Spicer, R.A., Zhang, K.X., et al., 2020. Qaidam Basin leaf fossils show northeastern Tibet was high, wet and cool in the early Oligocene. Earth Planet Sci. Lett., 537: 116175. DOI:10.1016/j.epsl.2020.116175 |
Scotese, C.R. PALEOMAP PaleoAtlas for GPlates and the PaleoData Plotter Program, PALEOMAP Project. http://www.earthbyte.org/paleomap-paleoatlas-for-gplates/.
|
Song, Z.Q., Shi, G.L., Chen, Y.F., et al., 2014. Winged fruits of Ailanthus (Simaroubaceae) from the Oligocene Ningming formation of Guangxi, and there taxonomic and biogeographic implications. Acta Palaeontol. Sin., 53: 191-200. |
Spicer, R.A., Harris, N.B.W., Widdowson, M., et al., 2003. Constant elevation of Southern Tibet over the past 15 million years. Nature, 412: 622-624. |
Stults, D.Z., Axsmith, B.J., Haywick, D., 2002. Evidence of Carpinus (Betulaceae) in the late tertiary (Pliocene) of Alabama. Am. J. Bot., 89: 1547-1549. DOI:10.3732/ajb.89.9.1547 |
Spicer, R.A., Su, T., Valdes, P.J., et al., 2020. Why the 'uplift of the Tibetan plateau' is a myth. Natl. Sci. Rev., 8: nwaa091. |
Stults, D.Z., Axsmith, B.J., 2009. Betulaceae from the Pliocene and Pleistocene of southwest Alabama, southeastern United States. Rev. Palaeobot. Palynol., 155: 25-31. DOI:10.1016/j.revpalbo.2009.01.001 |
Su, T., Spicer, R.A., Li, S.H., et al., 2018. Uplift, climate and biotic changes at the Eocene-Oligocene transition in south-eastern Tibet. Natl. Sci. Rev., 5: 642-652. DOI:10.1093/nsr/nwy048 |
Su, T., Farnsworth, A., Spicer, R.A., et al., 2019. No high Tibetan plateau until the Neogene. Sci. Adv., 5: eaav2189. DOI:10.1126/sciadv.aav2189 |
Su, T., Spicer, R.A., Wu, F.X., et al., 2020. A middle Eocene lowland humid subtropical "Shangri-La" ecosystem in central Tibet. Proc. Natl. Acad. Sci. U.S.A., 7: 32989-32995. DOI:10.1073/pnas.2012647117 |
Sun, B., Wang, Y.F., Li, C.S., et al., 2015. Early Miocene elevation in northern Tibet estimated by palaeobotanical evidence. Sci. Rep., 5: 10379. DOI:10.1038/srep10379 |
Sun, G., Ji, Q., Dilcher, D.L., et al., 2002. Archaefructaceae, a new basal angiosperm family. Science, 296: 899-904. DOI:10.1126/science.1069439 |
Sun, Z.M., Yang, Z.Y., Pei, J.L., et al., 2005. Magnetostratigraphy of Paleogene sediments from northern Qaidam Basin, China: implications for tectonic uplift and block rotation in northern Tibetan Plateau. Earth Planet Sci. Lett., 237: 635-646. DOI:10.1016/j.epsl.2005.07.007 |
Tanai, T., 1970. The Oligocene floras from the Kushiro coal field, Hokkaido, Japan. J. Fac. Sci. Hokkaido Univ. Ser. IV Geol. Mineral., 14: 383-514. |
Tanai, T., 1972. Tertiary history of vegetation in Japan. In: Graham, A. (Ed.), Floristics and Palaeofloristics of Asia and Eastern North America. Elsevier, Amsterdam.
|
Tanai, T., Uemura, K., 1991. The Oligocene Noda flora from the Yuya-wan area of the western end of Honshu, Japan. Part Ⅰ. Bull. Natl. Sci. Mus., 17: 57-80. |
Tang, H., Liu, J., Wu, F.X., et al., 2019. Extinct genus Lagokarpos reveals a biogeographic connection between Tibet and other regions in the Northern Hemisphere during the Paleogene. J. Syst. Evol., 57: 670-677. DOI:10.1111/jse.12505 |
Tao, J.R., Du, N.Q., 1987. Miocene flora from Markam county and fossil record of Betulaceae. Acta Bot. Sin., 29: 649-655. |
Tao, J.R., Xiong, X.Z., 1986. The latest cretaceous flora of Heilongjiang Province and the floristic relationship between East Asia and North America. Acta Phytotaxon. Sin., 24: 121-135. |
Tian, Y.M., Spicer, R.A., Huang, J., et al., 2021. New early Oligocene zircon U-Pb dates for the 'Miocene' Wenshan basin, Yunnan, China: biodiversity and paleoenvironment. Earth Planet Sci. Lett., 565: 116929. DOI:10.1016/j.epsl.2021.116929 |
Tiffney, B.H., Manchester, S.R., 2001. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci., 162: S3-S17. DOI:10.1086/323880 |
Utescher, T., Mosbrugger, V., 2015. The Palaeoflora Database. At. http://www.geologie.unibonn.de/Palaeoflora.
|
Walther, H., 2003. Oligocene macroflora of Kleinsaubernitz (Germany). Pangaea. DOI:10.1594/PANGAEA.126036 |
Wang, N., Wu, F.X., 2015. New Oligocene cyprinid in the central Tibetan Plateau documents the pre-uplift tropical lowlands. Ichthyol. Res., 62: 274-285. DOI:10.1007/s10228-014-0438-3 |
Wang, N., Kelly, L.J., McAllister, H.A., et al., 2021. Resolving phylogeny and polyploid parentage using genus-wide genome-wide sequence data from birch trees. Mol. Phylogenet. Evol., 160: 107126. DOI:10.1016/j.ympev.2021.107126 |
Wang, Q., Dilcher, D.L., Lott, T.A., 2007. Podocarpium A. Braun ex Stizenberger 1851 from the middle Miocene of Eastern China, and its palaeoecology biogeography. Acta Palaeobot., 47: 237-251. |
Wen, J., Zhang, J.Q., Nie, Z.L., et al., 2014. Evolutionary diversifications of plants on the Qinghai-Tibetan plateau. Front. Genet., 5: 4. |
Wheeler, E., Scott, R.A., Barghoorn, E.S., 1977. Fossil dicotyledonous woods from Yellowstone National Park. J. Arn. Arb., 58: 280-306. DOI:10.5962/p.185801 |
Wilde, V., Frankenhäuser, H., 1998. The middle Eocene plant taphocoenosis from Eckfeld (Eifel, Germany). Rev. Palaeobot. Palynol., 101: 7-28. DOI:10.1016/S0034-6667(97)00067-5 |
Wilf, P., Wing, S.L., Meyer, H.W., et al., 2021. An image dataset of cleared, x-rayed, and fossil leaves vetted to plant family for human and machine learning. PhytoKeys, 187: 93-128. DOI:10.3897/phytokeys.187.72350 |
Wolfe, J.A., Wehr, W., 1987. Middle Eocene dicotyledonous plants from Republic, northeastern Washington. US Geol. Surv. Bull., 1597: 1-25. |
Worobiec, G., Szynkiewicz, A., 2007. Betulaceae leaves in Miocene deposits of the Bełchatów Lignite mine (Central Poland). Rev. Palaeobot. Palynol., 147: 28-59. DOI:10.1016/j.revpalbo.2007.06.001 |
Writing Group of Cenozoic Plants of China (WGCPC), 1978. Cenozoic Plants from China, Fossil Plants of China, vol. 3. Science Press, Beijing.
|
Wu, F.X., Miao, D.S., Chang, M.M., et al., 2017. Fossil climbing perch and associated plant megafossils indicate a warm and wet central Tibet during the late Oligocene. Sci. Rep., 7: 878. DOI:10.1038/s41598-017-00928-9 |
Wu, M.H., Zhuang, G.S., Hou, M.Q., et al., 2021. Expanded lacustrine sedimentation in the Qaidam Basin on the northern Tibetan Plateau: Manifestation of climatic wetting during the Oligocene icehouse. Earth Planet Sci. Lett., 565: 116935. DOI:10.1016/j.epsl.2021.116935 |
Xiong, Z.Y., Ding, L., Spicer, R.A., et al., 2020. The early Eocene rise of the Gonjo Basin, SE Tibet: from low desert to high forest. Earth Planet Sci. Lett., 543: 116312. DOI:10.1016/j.epsl.2020.116312 |
Xu, H., Su, T., Zhou, Z.K., 2018. Leaf and infructescence fossils of Alnus (Betulaceae) from the late Eocene of the southeastern Qinghai-Tibetan plateau. J. Syst. Evol., 57: 105-113. |
Xu, Q.Q., Qiu, J., Zhou, Z.K., et al., 2015. Eocene Podocarpium (Leguminosae) from south China and its biogeographic implications. Front. Plant Sci., 6: 938-951. |
Xue, L., Jia, L.B., Nam, G., et al., 2020. Involucre fossils of Carpinus, a northern temperate element, from the Miocene of China and the evolution of its species diversity in East Asia. Plant Divers., 42: 155-167. DOI:10.1016/j.pld.2020.01.001 |
Yan, D.F., Zhang, L., Han, L., et al., 2018. Podocarpium from the Oligocene of NW Qaidam Basin, China and its implications. Rev. Palaeobot. Palynol., 259: 1-9. DOI:10.1016/j.revpalbo.2018.09.009 |
Yang, T., Zhang, L., Li, W.J., et al., 2018. New schizothoracine from Oligocene of Qaidam Basin, northern Tibetan plateau, China, and its significance. J. Vertebr. Paleontol., 38: 1-12. |
Yang, T., Han, L., Chen, H.Y., et al., 2021a. Oligocene Desmanthus (Leguminosae) from the Qaidam Basin in northeastern Tibetan plateau, China, and its implications for paleoclimate and paleoelevation. Hist. Biol., 33: 2744-2754. DOI:10.1080/08912963.2020.1826471 |
Yang, T., Jia, J.W., Chen, H.Y., et al., 2021b. Oligocene Ailanthus from northwestern Qaidam Basin, northern Tibetan plateau, China and its implications. Geol. J., 56: 616-627. DOI:10.1002/gj.3904 |
Yang, T., Liang, W.Y., Cai, J.H., et al., 2022. A new cyprinid from the Oligocene of Qaidam Basin, north-eastern Tibetan plateau, and its implications. J. Syst. Palaeontol., 19: 1161-1182. |
Yang, T., 2022. Fossil Schizothoracines from the Oligocene of Northwestern Qaidam Basin and Their Implications. Ph. D. thesis. Lanzhou University, Lanzhou.
|
Zachos, J., Pagani, M., Sloan, L., et al., 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292: 686-693. DOI:10.1126/science.1059412 |
Zanazzi, A., Kohn, M.J., MacFadden, B.J., et al., 2007. Large temperature drop across the Eocene-Oligocene transition in central North America. Nature, 445: 639-642. DOI:10.1038/nature05551 |
Zastawniak, E., Walther, H., 1998. Betulaceae from Sośnica near Wrocław (Poland) – a revision of Goeppert's original materials and a study of more recent collections. Acta Palaeobot., 38: 87-145. |
Zhilin, S.G., 1989. History of the development of the temperate forest flora in Kazakhstan, U.S.S.R. from the Oligocene to the Early Miocene. Bot. Rev., 55: 205-330. DOI:10.1007/BF02858522 |
Zhong, X., 2007. Discovery of plant fossils of Shangganchaigou Formation in south slope of Arkin Mountain in Qaidam Basin and their geological significance. Gansu Geology, 16: 26-30. |
Zhou, Z.K., Liu, J., Chen, L.L., et al., 2022. Cenozoic plants from Tibet: an extraordinary decade of discovery, understanding and implications. Sci. China Earth Sci., 66: 205-226. DOI:10.1007/s11430-022-9980-9 |



