b. University of Chinese Academy of Sciences, Beijing 100049, China;
c. Sino-Africa Joint Research Center, Chinese Academy of Sciences, Wuhan 430074, China;
d. Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun 666303, China;
e. Botany Department, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya;
f. East African Herbarium, National Museums of Kenya, P.O. Box 451660-0100, Nairobi, Kenya
The potential effects of climate change on biodiversity have received considerable attention. This is because climate change is expected to alter the growth and natural distribution of species, as well as increase biodiversity loss in natural habitats, resulting in a loss of species diversity and germplasm resources (Wan et al., 2021; Ngarega et al., 2021, 2022a). By the year 2050, Africa is expected to experience a 2.6–3.0 ℃ increase in average temperatures, and large areas of northern and southern Africa are projected to be > 3.5 ℃ above pre-industrial levels (Serdeczny et al., 2017; Lee et al., 2020; Mkala et al., 2022). It is projected that with the continued increase in global average temperatures and precipitation, Africa remains extremely vulnerable to climate change (Sintayehu, 2018).
Climate variables influence the composition and distribution of vegetation, which can be a direct reflection of global climate change (Yan et al., 2021). Accordingly, researchers are focused on the linkages between biodiversity, ecosystem services, and climate change. These biological-climate predictions can serve as a foundation for hypothetical studies on species origin, vegetation partitioning, and floristic creation, as well as play a key role in biotechnology and species introduction and domestication (Zhang et al., 2020). However, most developing countries lack a comprehensive biodiversity inventory, as well as a sufficient grasp of particular species to identify those in danger (Burke, 2004; Çoban et al., 2020). The classification of large geographic areas into biomes allows for an evaluation of vegetation types based on the principal climatic restrictions and related prevalent growth patterns (Burke, 2004; Dar et al., 2020).
Climate change is estimated to be a major factor in the extinction of many animal and plant species (Nzei et al., 2021; Mkala et al., 2022). Species near a biome boundary and at the edge of their distribution ranges are likely to be the first to respond to climate changes (Thomas, 2010). Thus, we can effectively target conservation initiatives to offset the effects of climate change by modeling their distributions (Merow and Jr, 2014). Species distribution models (SDMs) are important for analyzing the spatial and temporal processes that make up a species' life cycle. Additionally, SDMs for historical timelines can also offer ecological and evolutionary data on the historical changes of species distribution over time (Park et al., 2022). Past models can be used to explain phylogeographic patterns and speciation processes, as well as to predict historical hotspots and potential migration routes (Park et al., 2022). Additionally, studies on how species have adapted to past climate change offer important insights into how species will respond to climate change in the future (Pearson, 2006). Future distribution models can forecast habitat suitability and provide information on the likelihood of range shifts or population changes (Sinclair et al., 2010; Ngarega et al., 2022b). However, for the great majority of species, occurrence data is generally limited, especially for uncommon or extremely rare species (Merow and Jr, 2014). MaxEnt is a popular tool for predicting the distribution of species based solely on occurrence point information (Merow et al., 2013). In addition, it can also predict habitat suitability for species in different habitats based on presence-only data (Elith et al., 2011; Gomes et al., 2018). Even though models do not precisely reflect the mechanisms that lead to observed distributions, they are useful in describing the relationships between species and environmental variables at wide spatial scales (Merow and Jr, 2014).
Xerophyta Juss., is a monocotyledonous genus of the Velloziaceae family that is found on the African mainland, Madagascar, and the Arabian Peninsula (Beentje, 1994; Elith et al., 2011; Farrant et al., 2015; Gomes et al., 2018). In mainland Africa, there are approximately 45 species, one species is found on the Arabian Peninsula, and 25 species are found in Madagascar (Behnke et al., 2013). The closest relative of Xerophyta is the genus Vellozia found in South America, primarily Brazil (Mcpherson et al., 1997; Mello-Silva et al., 2005; Alcantara et al., 2015; Wanga et al., 2021). The family Velloziaceae has been shown to have a Gondwanan origin, with the separation between African and South American species corresponding to the continents dividing roughly 100 million years ago (Mello-Silva et al., 2011). All African species are desiccation tolerant, i.e., resurrection plants (Alcantara et al., 2018). Most of the Xerophyta spp., except Xerophyta elegans, are poikilochlorophyllous, i.e., they lose their chlorophyll during desiccation, having protective mechanisms that are fully activated in the leaves (Mello-Silva et al., 2011; Behnke et al., 2013; Farrant et al., 2015). Most research on the genus has focused on the whole genome and the mechanisms that underlie desiccation tolerance in these species (Farrant et al., 2015; Costa et al., 2017; Radermacher et al., 2019; Wanga et al., 2020; 2021), which can be key in tackling food security amidst the global warming threats. Recent reports indicating the high frequency of fire occurrence and human exploitation (i.e., brooms or brushes), medicinal products and fuelwood have been recorded to impact the habitat of Xerophyta species, necessitating urgent conservation actions (Goodman, 2021; IUCN, 2023). To date, there have been no studies that have simulated or projected the distribution of Xerophyta species. This lack of attention may be attributed to the fact that these species grow in inaccessible areas, hence they are botanically unexploited (Behnke et al., 2013). As a result, examining the factors that impact the distribution and evolution of Xerophyta in Africa could offer valuable insights into how xerophytic species respond to changes in climate.
In this study, we explored the potential distribution of six Xerophyta species under past, current and future climates using the MaxEnt model. We utilized two Representative Concentration Pathway (RCPs) scenarios representing moderate (RCP 4.5) and extreme greenhouse gas emissions (RCP 8.5) to model the potential distribution of Xerophyta species in the 2070s. The past climate was represented by the Last Glacial Maximum (LGM, 22,000 ya) and the Mid-Holocene (MH, 6000 ya). The goals of the study were to (1) understand the geographical distribution patterns and future changes of Xerophyta spp. in Africa, (2) determine the dominant bioclimatic variables that limit the habitat distribution of Xerophyta spp., and (3) model suitable habitat of Xerophyta spp. under past, current and future climate change scenarios and provide a scientific basis for the protection of Xerophyta spp. resources.
2. Materials and methods 2.1. Species occurrence dataThere are ca. 70 Xerophyta species native to tropical Africa and Madagascar (Wanga et al., 2021). However, several species lack adequate distribution data to allow for successful modeling (Stockwell and Peterson, 2002). In this study, six species with adequate and validated occurrences were studied. We obtained species occurrence points from the Global Biodiversity Information Facility (GBIF, http://www.gbif.org/; accessed in March 2021). Because some of these occurrences lacked geographical information, Google Earth was utilized to supplement the latitude and longitude information. Spatial thinning of occurrence data is a simple and easy-to-implement strategy for mitigating the impacts of sampling bias (Aiello-Lammens et al., 2015). We used the spThin package (Aiello-Lammens et al., 2015) in the R environment to ensure that only one occurrence record per grid cell at a resolution of 2.5 arc-min. After spatial thinning of occurrences, points obtained were used for modeling; 69 for Xerophyta dasylirioides, 33 for X. elegans, 63 for X. humilis, 30 for X. pectinata, 92 for X. viscosa, and 21 for X. villosa (Table S1; Fig. 1). ArcGIS 10.5 (Esri, Redlands, CA, USA, http://www.esri.com) was then used to create maps of the remaining species occurrences (Fig. 1).
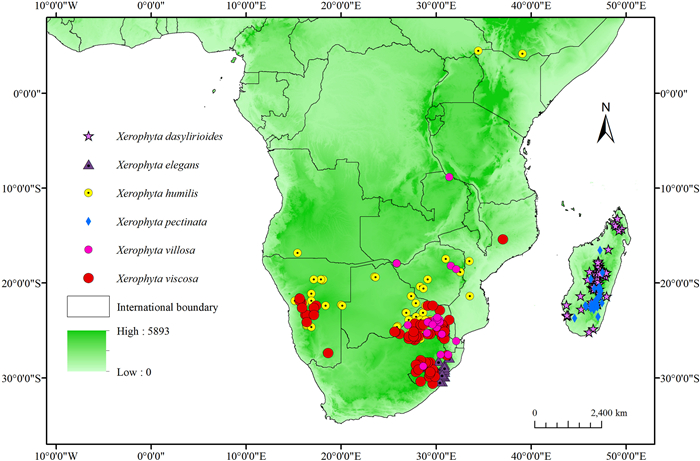
|
| Fig. 1 Locality and occurrence records of Xerophyta species employed in the modelling. |
We employed 'bioclimatic' characteristics from the WorldClim2.1 database (https://www.worldclim.org/data/worldclim21.-html) to characterize climatic conditions relevant to biological species (Table S2; Fick and Hijmans, 2017). This data set is based on monthly consensus climatologies spanning 1970 to 2000. The data set was obtained at a spatial resolution of 2.5 arc-min or 5 km2, which roughly corresponds to the uncertainty inherent in the occurrence data. We removed nine data layers (described in Table S3) because they contained artifacts that would otherwise cause sharp variances and multicollinearity between neighboring pixels. We excluded the variables with correlation coefficients of more than 0.7. This analysis was achieved through the Variance Importance Factor (VIF) procedure in the usdm package available in R platform v.3.6.2 (R Core Team, 2022). Data for the past and future scenarios were obtained from WorldClim1.4 and WorldClim2.1, respectively, with a spatial resolution of 2.5 arc-min (Hijmans et al., 2005; Fick and Hijmans, 2017). We utilized one global climate model (GCM), the Community Climate System Model version 4 (CCSM4; Gent et al., 2011), to download the climate data for the future (2070s) and for the past. Climate data for the past came from 22,000 years ago, reflecting the LGM, and 6000 years ago, reflecting the MH. This GCM (CCSM) has been recognized as one of the most appropriate GCMs for the African region (Nzei et al., 2021; Ngarega et al., 2022a). The RCP scenarios consist of four pathways, including RCP 2.6, RCP 4.5, RCP 6.0, and RCP 8.5 (Remya et al., 2015). RCP 4.5 and RCP 6.0 are both moderate greenhouse gas emission scenarios, with RCP 4.5 representing a scenario in which a higher priority is given to mitigation than in RCP 6.0 (Wei et al., 2018). RCP 8.5 implies that no climate policies would be implemented in the future or the present, and it reflects very high emissions scenarios (1370-ppm CO2 equivalent by 2100; Van Vuuren et al., 2011). Therefore, we chose RCP 4.5 (moderate scenario) and RCP 8.5 (extreme scenario) to simulate the suitable habitat distribution of Xerophyta spp. for the future time frame.
2.3. Model calibration and developmentWe utilized MaxEnt 3.4.4 (Elith et al., 2011; Phillips and Dudík, 2008) to predict the distribution of Xerophyta species. MaxEnt was firstly used to build a model, and then the predict function was used in conjunction with a specified set of independent variables to calculate the likelihood of occurrence. The potential distribution of our focal taxa was predicted using the climatic variables and occurrence localities described in the earlier steps. For model calibration and development, 25% of the occurrence data were chosen at random as test sets and 75% as training sets. Following the 'Subsample' strategy, the model was duplicated ten times. The background region represents accessible space that can be colonized or has previously been inhabited by the species being modeled (Barve et al., 2011). Like prior studies, we used 10,000 random background points (Nzei et al., 2021). The output format was set to 'Logistic', with a maximum iteration of 5000. The default settings for the rest of the parameters were kept. The area under the receiver operating characteristic (ROC) curve (AUC; Area under the ROC Curve) and True Skill Statistics (TSS) were used to assess the accuracy of the MaxEnt models (Allouche et al., 2006; Warren and Seifert, 2011; Ngarega et al., 2022b). AUC values vary from 0 to 1, and the closer value is to 1, the better the model's performance. The accuracy of the model is rated in five categories: Fail (0.5–0.6), Poor (0.6–0.7), Fair (0.7–0.8), Good (0.8–0.9), and Excellent (0.9–1.0), with a value less than 0.5 equaling randomness (Swets, 1988). The jackknife test was also used to assess the impact of variables on the Xerophyta species. Results of jackknife tests show the weight of different environmental factors affecting the habitat suitability of Xerophyta species. The final outputs were continuous suitability layers made up of the mean of the predictions from the ten model runs. To illustrate the differences and convergences between each model, the average continuous logistic output predictions were transformed into binary maps using the Maximum sum of training sensitivity and specificity (MTSS) threshold value that maximizes sensitivity and specificity (Table S4; Liu et al., 2016).
2.4. Changes in habitat suitability and niche overlap analysisWe used the Python-based GIS toolkit SDMtoolbox (Brown et al., 2017) in ArcGIS v.10.5, to investigate the differences between the current (baseline) and past or future climate distribution areas. We imported the binary maps of suitability described in the previous steps into ArcGIS v.10.5 and used the SDMtoolbox to analyze and map the spatial range changes of the suitable habitat area of Xerophyta spp.
The examination of niche overlap allows Xerophyta species to estimate the niche they share. The logistic output maps for the six Xerophyta species pairwise pairings were compared to analyze the niche overlap between the species using Schoener's D metrics (Warren et al., 2008). This analysis was implemented in ENM tools v.1.4.4 (Warren et al., 2010). With values ranging from 0 to 1, this metric assesses the niche overlap of two species, with 1 indicating similarities in the two niches and 0 indicating no overlap (Warren et al., 2008).
2.5. Multivariate environmental similarity surface (MESS) analysisWhen creating models to determine how climate change affects species' suitable areas, a common challenge is understanding to what extent future climatic conditions differ from contemporary climatic variables used in the model. One method to measure this is by using multivariate environmental similarity surface (MESS), as outlined by Elith et al. (2010). The MESS method involves identifying a reference layer of environmental variables and calculating the similarity (S) between the point set of environmental variables in the reference layer and those under different climatic conditions. A positive S value indicates a climate difference at the point, with a smaller S value indicating a more significant difference. Conversely a negative S value shows that at least one environmental variable at that point is outside the reference range, indicating a considerable environmental change. We applied the MESS procedure by utilizing the 'density.tools.novel' tool in the maxent.jar file and plotted the results for the past and future climate scenarios.
3. Results 3.1. Performance of the model and variable contributionAfter correlation analyses, the remaining variables included the mean diurnal range (Bio2), isothermality (Bio3), mean temperature of wettest quarter (Bio8), mean temperature of driest quarter (Bio9), precipitation of wettest month (Bio13), precipitation of driest month (Bio14), precipitation of warmest quarter (Bio18), and precipitation of driest quarter (Bio19) (Tables S2 and S3). The modeling of the current distribution of Xerophyta species yielded strong evaluation scores for AUC and TSS values greater than 0.902, indicating that the MaxEnt model was very effective in forecasting the potential distribution for the Xerophyta species (Table 1). Jackknife results indicated that when used in isolation, precipitation of warmest quarter (Bio18) influenced the distribution of X. dasyliroides and X. pectinata most, indicating that this variable contains considerable information absent in other variables (Fig. 2). The impact of bioclimatic conditions on species distribution was assessed using the percent contribution (Table 2). The minimum temperature of the coldest quarter (Bio9) and precipitation of the warmest quarter (Bio18) were the principal bioclimatic variables impacting the potential distribution of the majority of the species. For all the studied species, the precipitation of the coldest quarter (Bio19) and the precipitation of the warmest quarter (Bio8) had a low contribution. This implies that the minimum temperature and precipitation of the warmest quarter are the most critical factors influencing the Xerophyta species' potential distribution. On the other hand, jackknife analyses showed that when used in isolation, isothermality (Bio3), precipitation of warmest quarter (Bio18), and mean temperature of driest quarter (Bio9) had the most gains for different species (Fig. 2).
| Species | AUC (SD) | TSS |
| Xerophyta dasylirioides | 0.991 (0.002) | 0.902 |
| X. elegans | 0.992 (0.004) | 0.911 |
| X. humilis | 0.954 (0.010) | 0.910 |
| X. pectinata | 0.988 (0.006) | 0.916 |
| X. viscosa | 0.978 (0.006) | 0.920 |
| X. villosa | 0.970 (0.006) | 0.906 |
| Note: SD, Standard deviation. | ||
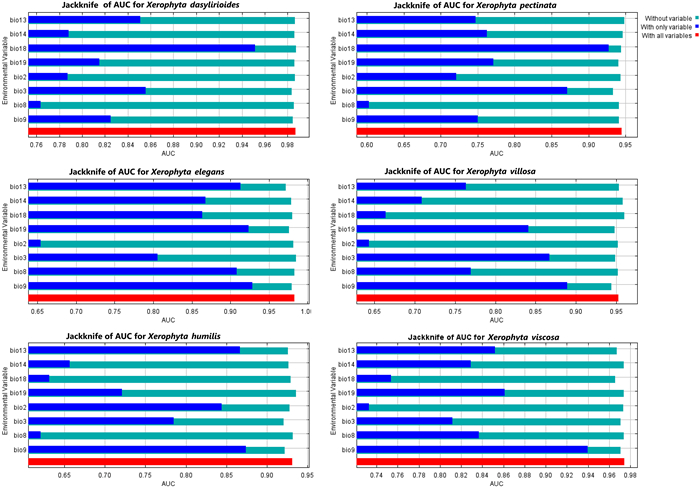
|
| Fig. 2 Jackknife results for Xerophyta spp. distribution using MaxEnt models. |
| Species | Bio2 | Bio3 | Bio8 | Bio9 | Bio13 | Bio14 | Bio18 | Bio19 |
| Xerophyta dasylirioides | 0.4 | 7.5 | 0.9 | 20.3 | 0.3 | 9.2 | 56.7 | 4.9 |
| X. elegans | 8.7 | 0.6 | 0.8 | 39.9 | 6.4 | 20 | 16.1 | 7.6 |
| X. humilis | 12.3 | 8.9 | 0.2 | 48.0 | 16.3 | 5.5 | 2.4 | 6.5 |
| X. pectinata | 1.6 | 12.1 | 0.1 | 21.9 | 1.8 | 11.3 | 50.5 | 0.7 |
| X. viscosa | 0.7 | 3.3 | 0.3 | 72.1 | 3.9 | 3.8 | 14.8 | 1.1 |
| X. villosa | 0.3 | 12.1 | 0.2 | 49.2 | 9.0 | 4.2 | 14.5 | 10.4 |
| Note: Bold values show the three most important variables for each species. Bio2 = mean diurnal range (℃), Bio3 = isothermality (℃), Bio8 = mean temperature of the wettest quarter (℃), Bio9 = mean temperature of the driest quarter (℃), Bio13 = precipitation of the wettest month (mm), Bio14 = driest month precipitation (mm), Bio18 = precipitation of warmest quarter (mm), Bio19 = precipitation of coldest quarter (mm). | ||||||||
The potential distribution of Xerophyta in the past (LGM and MH), present, and future (RCP 4.5 and RCP 8.5), are shown in Fig. 3. Suitability regions were classified into high, medium (moderate), low and no species suitability regions. Environmental conditions during the LGM were less suitable for Xerophyta species than were those of the MH. However, environmental conditions between these two past periods did not significantly differ for X. pectinata, X. humilis and X. villosa. This allowed the geographic expansion, reduction and even the stable regions for the different Xerophyta species under current climatic conditions.
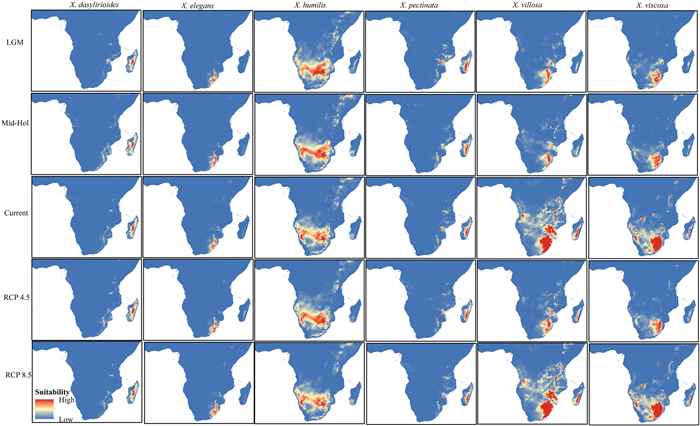
|
| Fig. 3 Xerophyta spp. prediction maps for the Last Glacial Maximum (LGM), Mid-Holocene (Mid-Hol), current climatic conditions, future, RCPs 4.5 and 8.5. Species distribution in their habitats are color coded differently to show high, medium and low suitability areas. Red indicates high suitability, brown indicates medium suitability, white indicates low suitability and blue indicates no species distributed in the area. Habitat suitability ranges from 0 (blue, low suitability) to 1 (red, high suitability). |
Baseline current models revealed that the moderate and high-suitability zones for Xerophyta are mostly found in tropical Africa and correspond to the recorded occurrence localities. X. humilis, X. villosa, and X. viscosa were projected to have a continuous high-suitability distribution, whereas that of X. pectinata was fragmented. The potential distribution for these taxa varied across the region. For instance, X. villosa was projected to have potential distribution areas in Zambia, Zimbabwe and South Africa, whereas X. humilis was projected to have potential distributions in Angola, Botswana, Ethiopia, Mozambique, Namibia, Northern Provinces, Sudan, Swaziland, Zambia, and Zimbabwe (Fig. 3). The species' potential current suitable habitats for Xerophyta ranged from 166.05 × 103 km2 (X. elegans) to 1915.75 × 103 km2 (X. humilis) (Table S5).
3.3. Patterns of change in habitat suitabilityTo clarify how geographic distribution patterns of Xerophyta species may change and identify predicted migration paths, we calculated the change in suitability compared to current suitability for each grid cell and scenario. These useful changes are shown in Fig. 3, which show areas where contraction (decrease in fitness over time) and expansion (increase in fitness over time) occur. The range possibilities for suitability changes cover four different situations: large expansions from low suitability, large contraction from high suitability, constant high suitability, and constant low suitability. Range change differed between past and the two future scenarios depending on each species' response. For most species, range loss was predicted to be larger than range gain, irrespective of the scenario (Fig. 4), however, the greatest losses were predicted under the RCP 8.5 scenario (Table S5). Specifically, under RCP 8.5, X. pectinata (35.40%), was predicted to lose the largest portions of its suitable ranges, whereas X. dasylirioides (129.1%) was predicted to have the highest gains in suitability.
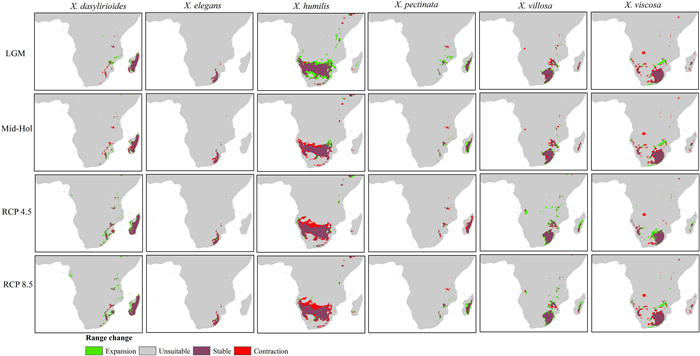
|
| Fig. 4 Representation of the past and future distribution maps of Xerophyta species range change for Last Glacial Maximum (LGM), 20,000 ya, Mid-Holocene (Mid-Hol), 6000 ya, RCP 4.5 and RCP 8.5. Stable areas are shown in purple, areas of loss are shown in red, areas of gain are shown in green, and unsuitable areas are shown in grey. |
According to Schoener's D metrics, the niches of Xerophyta species overlap considerably (Table 3). The highest niche overlap was predicted in X. pectinata and X. dasylirioides (0.635). Other species, such as X. humilis and X. pectinata, however, are projected to occupy different ecological niches.
| Species | X. dasylirioides | X. elegans | X. humilis | X. pectinata | X. villosa | X. viscosa |
| X. dasylirioides | – | 0.211 | 0.163 | 0.635 | 0.306 | 0.210 |
| X. elegans | X | – | 0.212 | 0.246 | 0.456 | 0.452 |
| X. humilis | X | x | – | 0.112 | 0.456 | 0.534 |
| X. pectinata | X | x | x | – | 0.318 | 0.187 |
| X. villosa | X | x | x | x | – | 0.538 |
| X. viscosa | X | x | x | x | x | – |
The region where the climate anomaly (S < 0) could potentially occur was small in reference to the potential distribution area of all Xerophyta species, as shown in Fig. 5, under the past and future climate scenarios. Notably, compared to all other climate scenarios for all the Xerophyta species, the RCP 8.5 was observed to have the largest degree of extrapolation (Fig. 4), indicating that this scenario had the highest degree of anomaly.
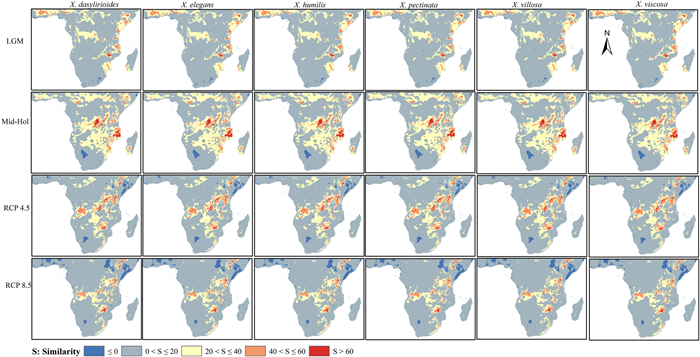
|
| Fig. 5 The multivariate environmental similarity surface (MESS) map by data source (Xerophyta spp. localities) and considering the environmental variables as reference environmental area for the African region for the past (LGM and Mid-Hol) and future distribution (RCPs 4.5 and 8.5). |
Niche modeling is a useful tool for predicting the potential impacts of climate change on the distribution of species as well as their responses (Ngarega et al., 2022b). This is the first study to use a niche modeling approach to model the distribution of Xerophyta in Africa. The resulting outputs of our study may provide valuable information for conservation, management and research of the genus. Here, we specifically utilized the MaxEnt modeling approach to project areas that have suitable climates for six Xerophyta species under the current baseline period and evaluated the impacts of climate change on their past and future potential habitat distribution. The MaxEnt models had very high predictive capacities (AUC and TSS values above 0.902; Table 1), which is comparable to various studies done in the region using MaxEnt modeling approach (Nzei et al., 2021; Wan et al., 2021), as well as other studies beyond the African region (Yan et al., 2021).
4.1. Significant variables affecting Xerophyta distributionThe potential distribution of plant species is generally affected by climate variables, which contribute significantly to their growth and abundance (Beamount et al., 2005). We evaluated the key bioclimatic variables determining the variance in Xerophyta species distribution based on the results of the jackknife test and the contribution rates obtained by the MaxEnt model (Wiens et al., 2009; Zhu et al., 2013). Our results indicate that the distributions of Xerophyta species are mainly affected by the following variables: (1) minimum temperature of the coldest quarter (Bio9), and (2) precipitation of the warmest quarter (Bio18) (Table 2). These results indicate that both temperature and precipitation variables limit Xerophyta distribution. These findings are consistent with a previous study that showed freely drained sandy or rocky soils, high temperatures and seasonal rain regimes favor the establishment of resurrection and drought-resistant plants (Alcantara, 2018).
Species of the genus Xerophyta are well-adapted to xeric environments and all tested species display vegetative desiccation tolerance with most of the species being poikilochlorophyllous and others homoichlorophyllous (Lyall et al., 2020). This may explain the variations that occurs in the range change responses of the species, with most species having a restriction to the inselbergs during extreme conditions hence forming terrestrial islands for survival (Chen et al., 2019). Recent studies have shown that most species of Xerophyta exhibit a series of changes in their physiologies when exposed to high temperatures, including chlorophyll degradation, cessation of growth, and senescence, an expression of desiccation tolerance (Costa et al., 2017; Radermacher et al., 2019). Moreover, some species have adapted to these conditions, e.g., X. viscosa was shown to express the gene XVSAP1 at both high and low temperatures, which conferred higher adaptability to these hostile environments (Garwe et al., 2003). Finally, the harsh chasmophytic habitat of most of its species leads to frequent periods of severe water deficit, even during the wet seasons (Farrant et al., 2015). Therefore, in high temperature and high precipitation areas of Africa, the distribution patterns of the Xerophyta species are predicted to frequently shift in the future.
4.2. Ecological niche suitability projections for the Last Glacial Maximum and Mid-HoloceneThe habitat suitability projected for the LGM and MH scenarios differed from those obtained for current climate conditions. The habitat suitability projected for the LGM and MH scenarios varied among the Xerophyta species with most species preferring LGM environmental conditions for persistence. According to the LGM projections, the species occurred in the tropical Africa, Southern and Central Africa, and the Madagascar regions, however, they did not differ much with the MH period, except for a reduction in range size. These regions were considered suitable putative cryptic refugia that persisted during the LGM. Refugia are areas in which a population can survive through an extended period of unfavorable conditions, and have hence facilitated the persistence of components of biodiversity over long periods of time and through changing climatic conditions (Keppel et al., 2011). Notably, when environmental conditions turn out to be more suitable for the species, they could potentially expand from within these refugia. During the LGM, the climate was cooler and drier, hence no rainfall; thus, vegetation only occurred in high-elevation refugia regions due to the formation of mist (Leal, 2004). However, during the MH the temperature became warmer (Park et al., 2019), hence those species that were widely adapted could spread. Additionally, the difference between the two climate scenarios of the past could be a result of desiccation tolerance speciation, evolution and adaptation for the Xerophyta species, which would explain the differences in the species responses among the regions (Lyall et al., 2020).
4.3. Implications of future climate scenarios to species distribution and conservationClimate change affects species distribution, and future projections suggest that it will become more intense, leading to global warming (Putten et al., 2010). In addition, the impacts of climate change depend on various combinations of traits in plant species, which determine their abundance, resistance to disturbance and ability to respond to changes in environmental conditions. Our results showed that the current suitable habitat for Xerophyta spp. ranges from 166.05 × 103 km2 (X. elegans) to 1915.75 × 103 km2 (X. humilis) (Table S5). In addition, models showed that highly suitable habitats are in the tropical regions, which are concurrent with recorded species localities. The prediction models also indicate that highly suitable habitat is currently available in Eastern, Central and Southern Africa for most of the Xerophyta species.
Our models predicted that habitat loss, gain, and stability would vary in the future for different Xerophyta species. For instance, X. pectinata, and X. humilis were projected to lose the largest portions of their suitable ranges, whereas X. dasylirioides had the greatest range gains (Table S5; Fig. 4). The varying responses under the high concentration emission scenario, RCP 8.5, could be due to the poikilochlorophyllous and homoiochlorophyllous nature of species in this genus. The results clearly show that predicted future climatic changes will have a significant impact on the geographic distribution of Xerophyta; the ability of plants in this genus to adapt or move to new locations with more favorable environmental conditions will be critical for the survival of the genus in the future. As a result, desiccation tolerance mechanisms may play important roles in the future distribution of the species, thereby warranting further investigations. It is also possible that Xerophyta will not adapt to new developments, and the loss of their habitat range, as predicted in this study, may be a representation of the disappearance of geographic loss. It is important to note that climate change effects on the genus might differ among individual species owing to differences in genetic and physiological tolerance of the different species. Some Xerophyta species share similar habitats and niches since their ranges overlap. Considering the significance of the mean temperature of the driest quarter (Bio9) and precipitation of warmest quarter (Bio18) in determining the habitat suitability of X. pictinata and X. dasylirioides, it is reasonable to observe a high degree of overlap in their niches as demonstrated in Table 3.
The current study evaluated the potential impacts of past and future climate change on the distribution of six Xerophyta species in Africa, thereby, providing basic information on this little-known genus. The high level of agreement between the predicted distributions and the botanical collections revealed that the climatic variables studied are certainly crucial in determining Xerophyta distribution patterns. However, the accuracy of our MaxEnt model predictions may be limited by various factors, including the methodology and data applied herein. For example, our data were mostly gathered from GBIF and online databases, corresponding to low occurrence points for several species, which may have resulted in biases in the modeling approach. Secondly, MaxEnt modelling may not account for other factors impacting local adaptation, dispersal, and lack of precise knowledge about changes in land use throughout the predicted range of Xerophyta spp. Due to the paucity of research in these inselbergs where the Xerophyta species occurs, we were unable to identify any of the additional variables impacting distribution, adaptability, and evolution, which should be considered in future research. As a result, while the results offer insights into the distribution of Xerophyta, the model outputs of our study should be treated with caution.
5. ConclusionIn the present study, we modeled the past and current distribution of suitable habitats for six Xerophyta spp. and evaluated how climate change would affect their distribution in the future using the Maxent algorithm. Our findings reveal that the habitats suitability among the Xerophyta species are predicted to vary owing to climate change. Nonetheless, with the changing climate, the suitable habitat of Xerophyta species may be compromised. In addition, with the low records and poor sampling of Xerophya species in Africa, aggressive species documentation and taxon sampling may be required to enhance our knowledge of the occurrence extent and area of occupancy for the genus. Finally, additional studies combining ecology, phylogeography and behavior are required to improve the understanding of the ecology and evolution of Xerophyta in Africa.
AcknowledgementsWe are sincerely thankful to the anonymous reviewers for comments and recommendations that helped improve this manuscript. This work was supported by grants from the International Partnership Program of Chinese Academy of Sciences (151853KYSB20190027), Sino-Africa Joint Research Center, CAS (SAJC202101) and The ANSO Scholarship for Young Talents, PhD Fellowship Program University of Chinese Academy of Sciences, China.
Data availability statement
The data sets presented in this study can be found in the online repositories (GBIF) and supplementary materials.
Author contributions
VW, BN, VN, GWH, RG: conceptualization. GWH and RG: funding acquisition. GWH, MG and RG: supervision. VW and BN: methodology. VW, BN, MO, EM, CN, CO, GO and WO. VW, BN, GWH, MG, MO, CO and EM revised the draft manuscript. All authors have read and agreed to the final version of the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.05.001.
Aiello-Lammens, M.E., Boria, R.A., Radosavljevic, A., et al., 2015. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography, 38: 541-545. DOI:10.1111/ecog.01132 |
Alcantara, S., de Mello-Silva, R., Teodoro, G.S., et al., 2015. Carbon assimilation and habitat segregation in resurrection plants: a comparison between desiccation- and non-desiccation-tolerant species of Neotropical Velloziaceae (Pandanales). Funct. Ecol., 29: 1499-1512. DOI:10.1111/1365-2435.12462 |
Alcantara, S., Ree, R.H., Mello-silva, R., 2018. Accelerated diversification and functional trait evolution in Velloziaceae reveal new insights into the origins of the campos rupestres' exceptional floristic richness. Ann. Bot., 122: 165-180. DOI:10.1093/aob/mcy063 |
Allouche, O., Tsoar, A., Kadmon, R., 2006. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol., 43: 1223-1232. DOI:10.1111/j.1365-2664.2006.01214.x |
Barve, N., Barve, V., Jiménez-Valverde, et al., 2011. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. model., 222: 1810-1819. DOI:10.1016/j.ecolmodel.2011.02.011 |
Beaumont, L.J., Hughes, L., Poulsen, M., 2005. Predicting species distributions: use of climatic parameters in BIOCLIM and its impact on predictions of species' current and future distributions. Ecol. Model., 186: 251-270. DOI:10.1016/j.ecolmodel.2005.01.030 |
Behnke, H.D., Hummel, E., Hillmer, S., et al., 2013. A revision of African Velloziaceae based on leaf anatomy characters and rbcL nucleotide sequences. Bot. J. Linn. Soc., 172: 22-94. DOI:10.1111/boj.12018 |
Brown, J.L., Bennett, J.R., French, C.M., 2017. SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ, 5: e4095. DOI:10.7717/peerj.4095 |
Burke, A., 2004. From plains to inselbergs: species in special habitats as indicators for climate change?. J. Biogeogr., 31: 831-841. DOI:10.1046/j.1365-2699.2003.00984.x |
Beentje, H., Adamson, J., Bhanderi, D., 1994. Kenya Trees, Shrubs, and Lianas. National Museums of Kenya. P.O. Box 40658, Nairobi, Kenya.
|
Chen, Y., Shi, X., Zhang, L., et al., 2019. Effects of increased precipitation on the life history of spring-and autumn-germinated plants of the cold desert annual Erodium oxyrhynchum (Geraniaceae). AoB Plants, 11: plz004. DOI:10.1093/aobpla/plz004 |
Çoban, H.O., Örücü, Ö. K., Arslan, E.S., 2020. MaxEnt modeling for predicting the current and future potential geographical distribution of Quercus libani Olivier. Sustainability, 12: 2671. DOI:10.3390/su12072671 |
Costa, M.C.D., Artur, M.A., Maia, J., et al., 2017. A footprint of desiccation tolerance in the genome of Xerophyta viscosa. Nat. Plants, 3: 1-10. DOI:10.1038/nplants.2017.38 |
Dar, J.A., Subashree, K., Bhat, N.A., et al., 2020. Role of major forest biomes in climate change mitigation: an eco-biological perspective. In: Roychoudhury, R.N., Nautiyal, S., Agarwal, S., Baksi, S. (Eds.), Socio-economic and Eco-Biological Dimensions in Resource Use and Conservation. Springer, Cham, pp. 483-526. https://doi.org/10.1007/978-3-030-32463-6_24.
|
De Mello-Silva, R., 2005. Morphological analysis, phylogenies and classification in Velloziaceae. Bot. J. Linn. Soci., 148: 157-173. DOI:10.1111/j.1095-8339.2005.00399.x |
Elith, J., Phillips, S.J., Hastie, T., et al., 2011. A statistical explanation of MaxEnt for ecologists. Divers. Distrib., 17: 43-57. DOI:10.1111/j.1472-4642.2010.00725.x |
Elith, J., Kearney, M., Phillips, S., 2010. The art of modelling range-shifting species. Methods Ecol. Evol., 1: 330-342. DOI:10.1111/j.2041-210X.2010.00036.x |
Farrant, J.M., Cooper, K., Hilgart, A., et al., 2015. A molecular physiological review of vegetative desiccation tolerance in the resurrection plant Xerophyta viscosa (Baker). Planta, 242: 407-426. DOI:10.1007/s00425-015-2320-6 |
Fick, S.E., Hijmans, R.J., 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol., 37: 4302-4315. DOI:10.1002/joc.5086 |
Garwe, D., Thomson, J.A., Mundree, S.G., 2003. Molecular characterization of XVSAP1, a stress-responsive gene from the resurrection plant Xerophyta viscosa Baker. J. Exp. Bot., 54: 191-201. DOI:10.1093/jxb/erg013 |
Goodman, E.A., 2021. Sleeping Beauty of the Plant World? Xerophyta elegans: a rare flowering perennial resurrection plant. https://conservancy.umn.edu/bitstream/handle/11299/225165/Xerophyta%20elegans%20by%20Elizabeth%20Goodman.pdf?sequence=1.
|
Gent, P.R., Danabasoglu, G., Donner, L.J., et al., 2011. The community climate system model version 4. J. Clim., 24: 4973-4991. DOI:10.1175/2011JCLI4083.1 |
Gomes, V.H., IJff, S.D., Raes, N., et al., 2018. Species Distribution Modelling: contrasting presence-only models with plot abundance data. Sci. Rep., 8: 1-12. DOI:10.1038/s41598-017-18927-1 |
Hijmans, R.J., Cameron, S.E., Parra, J.L., et al., 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol., 25: 1965-1978. DOI:10.1002/joc.1276 |
IUCN, 2023. The IUCN Red List of Threatened Species. Version 2022-2. https://www.iucnredlist.org.
|
Keppel, G., Van Niel, K.P., Wardell-Johnson, G.W., et al., 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr., 21: 393-404. DOI:10.1111/j.1466-8238.2011.00686.x |
Leal, M., 2004. The African Rain Forest during the Last Glacial Maximum, an Archipelago of Forests in a Sea of Grass. Ph. D. thesis. University, Wageningen.
|
Lee, H.K., Lee, S.J., Kim, M.K., et al., 2020. Prediction of plant phenological shift under climate change in South Korea. Sustainability, 12: 1-14. DOI:10.3390/su12219276 |
Liu, C, Ikeda, K, Rasmussen, R, et al., 2016. Continental-scale convection-permitting modeling of the current and future climate of North America. Clim. Dyn., 23: 91-105. DOI:10.1504/IJSPACESE.2016.081562 |
Lyall, R., Schlebusch, S.A., Proctor, J., et al., 2020. Vegetative desiccation tolerance in the resurrection plant Xerophyta humilis has not evolved through reactivation of the seed canonical LAFL regulatory network. Plant J., 101: 1349-1367. DOI:10.1111/tpj.14596 |
McPherson, G., van der Werff, H., Keating, R.C., 1997. A new species of Xerophyta (Velloziaceae) from Madagascar. Novon, 7: 387-394. DOI:10.2307/3391770 |
Mello-Silva, R., Santos, D.Y.A.C., Salatino, M.L.F., et al., 2011. Five vicarious genera from Gondwana: the Velloziaceae as shown by molecules and morphology. Ann. Bot., 108: 87-102. DOI:10.1093/aob/mcr107 |
Merow, C.,, 2014. A comparison of Maxlike and Maxent for modelling species distributions. Methods Ecol. Evol., 5: 215-225. DOI:10.1111/2041-210X.12152 |
Merow, C., Smith, M.J., Silander, J.A., 2013. A practical guide to MaxEnt for modeling species' distributions: what it does, and why inputs and settings matter. Ecography, 36: 1058-1069. DOI:10.1111/j.1600-0587.2013.07872.x |
Mkala, E.M., Mutinda, E.S., Wanga, V.O., et al., 2022a. Modeling impacts of climate change on the potential distribution of three endemic Aloe species critically endangered in East Africa. Ecol. Inf., 71: 101765. DOI:10.1016/j.ecoinf.2022.101765 |
Mkala, E.M., Jost, M., Wanke, S., et al., 2022b. How vulnerable are holoparasitic plants with obligate hosts to negative climate change impacts?. Ecol. Inf., 69: 101636. DOI:10.1016/j.ecoinf.2022.101636 |
Ngarega, B.K., Masocha, V.F., Schneider, H., 2021. Forecasting the effects of bioclimatic characteristics and climate change on the potential distribution of Colophospermum mopane in southern Africa using Maximum Entropy (Maxent). Ecol. Inf., 65: 101419. DOI:10.1016/j.ecoinf.2021.101419 |
Ngarega, B.K., Nzei, J.M., Saina, J.K., et al., 2022a. Mapping the habitat suitability of Ottelia species in Africa. Plant Divers., 44: 468-480. DOI:10.1016/j.pld.2021.12.006 |
Ngarega, B.K., Gikonyo, F.N., Wanga, V.O., et al., 2022b. Threatened Fabaceae taxa in coastal East Africa: current and future modelled distributions and conservation priorities. South Afr. J. Bot., 150: 779-788. DOI:10.1016/j.sajb.2022.08.033 |
Nzei, J.M., Ngarega, B.K., Mwanzia, V.M., et al., 2021. The past, current, and future distribution modeling of four water lilies (Nymphaea) in Africa indicates varying suitable habitats and distribution in climate change. Aquat. Bot., 173: 103416. DOI:10.1016/j.aquabot.2021.103416 |
Park, H.S., Kim, S.J., Stewart, A.L., et al., 2019. Mid-holocene northern hemisphere warming driven by arctic amplification. Sci. Adv., 5: eaax8203. DOI:10.1126/sciadv.aax8203 |
Park, I.K., Borzée, A., Park, J., et al., 2022. Past, present, and future predictions on the suitable habitat of the Slender racer (Orientocoluber spinalis) using species distribution models. Ecol. Evol., 12: 1-13. DOI:10.1002/ece3.9169 |
Pearson, R.G., 2006. Climate change and the migration capacity of species. Trends Ecol. Evol., 21: 111-113. DOI:10.1016/j.tree.2005.11.022 |
Phillips, S.J., Dudík, M., 2008. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography, 31: 161-175. DOI:10.1111/j.0906-7590.2008.5203.x |
R Core Team, 2022. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved from. https://www.R-project.org/.
|
Radermacher, A.L., du Toit, S.F., Farrant, J.M., 2019. Desiccation-driven senescence in the resurrection plant Xerophyta schlechteri (Baker) N.L. Menezes: comparison of anatomical, ultrastructural, and metabolic responses between senescent and non-senescent tissues. Front. Plant Sci., 10: 1-16. DOI:10.3389/fpls.2019.01396 |
Remya, K., Ramachandran, A., Jayakumar, S., 2015. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng., 82: 184-188. DOI:10.1016/j.ecoleng.2015.04.053 |
Serdeczny, O., Adams, S., Baarsch, F., et al., 2017. Climate change impacts in Sub-Saharan Africa: from physical changes to their social repercussions. Reg. Environ. Change, 17: 1585-1600. DOI:10.1007/s10113-015-0910-2 |
Sinclair, S.J., White, M.D., Newell, G.R., 2010. How useful are species distribution models for managing biodiversity under future climates?. Ecol. Soc., 15: 13. DOI:10.5751/ES-03089-150108 |
Sintayehu, D.W., 2018. Impact of climate change on biodiversity and associated key ecosystem services in Africa: a systematic review. Ecosys. Health Sustain., 4: 225-239. DOI:10.1080/20964129.2018.1530054 |
Stockwell, D.R., Peterson, A.T., 2002. Effects of sample size on accuracy of species distribution models. Ecol. Modell., 148: 1-13. DOI:10.1016/S0304-3800(01)00388-X |
Swets, J.A., 1988. Measuring the accuracy of diagnostic systems. Science, 240: 1285-1293. DOI:10.1126/science.3287615 |
Thomas, C.D., 2010. Climate, climate change and range boundaries. Divers. Distrib., 16: 488-495. DOI:10.1111/j.1472-4642.2010.00642.x |
Van der Putten, W.H., Macel, M., Visser, M.E., 2010. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Phil. Trans. Royal Soci. B: Biol. Sci., 365: 2025-2034. DOI:10.1098/rstb.2010.0037 |
Van Vuuren, D.P., Edmonds, J., Kainuma, M., et al., 2011. The representative concentration pathways: an overview. Clim. Change, 109: 5-31. DOI:10.1007/s10584-011-0148-z |
Wan, J.N., Mbari, N.J., Wang, S.W., et al., 2021. Modeling impacts of climate change on the potential distribution of six endemic baobab species in Madagascar. Plant Divers., 43: 117-124. DOI:10.1016/j.pld.2020.07.001 |
Wanga, V.O., Dong, X., Oulo, M.A., et al., 2020. The complete chloroplast genome sequence of Xerophyta spekei (Velloziaceae). Mitochondrial DNA Part. B, 5: 100-101. DOI:10.1080/23802359.2019.1698365 |
Wanga, V.O., Dong, X., Oulo, M.A., et al., 2021. Complete chloroplast genomes of Acanthochlamys bracteata (China) and Xerophyta (Africa) (Velloziaceae): comparative genomics and phylogenomic placement. Front. Plant Sci., 12: 1-16. DOI:10.3389/fpls.2021.691833 |
Warren, D.L., Glor, R.E., Turelli, M., 2010. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography, 33: 607-611. DOI:10.1111/j.1600-0587.2009.06142.x |
Warren, D.L., Glor, R.E., Turelli, M., 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution, 62: 2868-2883. DOI:10.1111/j.1558-5646.2008.00482.x |
Warren, D.L., Seifert, S.N., 2011. Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecol. Appl., 21: 335-342. DOI:10.1890/10-1171.1 |
Wei, B., Wang, R., Hou, K., et al., 2018. Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob. Ecol. Conserv., 16: e00477. DOI:10.1016/j.gecco.2018.e00477 |
Wiens, J.A., Stralberg, D., Jongsomjit, D., et al., 2009. Niches, models, and climate change: assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. U.S.A., 106: 19729-19736. DOI:10.1073/pnas.0901639106 |
Yan, X., Wang, S., Duan, Y., et al., 2021. Current and future distribution of the deciduous shrub Hydrangea macrophylla in China estimated by MaxEnt. Ecol. Evol., 11: 16099-16112. DOI:10.1002/ece3.8288 |
Zhang, K., Liu, H., Pan, H., et al., 2020. Shifts in potential geographical distribution of Pterocarya stenoptera under climate change scenarios in China. Ecol. Evol., 10: 4828-4837. DOI:10.1002/ece3.6236 |
Zhu, G.P., Li, G.Q., Bu, W.J., et al., 2013. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci., 21: 90-98. DOI:10.3724/sp.j.1003.2013.09106 |



