b. Institute of Evolution and Ecology, School of Life Sciences, Central China Normal University, Wuhan 430079, China;
c. School of Biological Sciences, University of Portsmouth, Portsmouth PO12DY, UK;
d. Institute of Arctic Biology, University of Alaska Fairbanks, Fairbanks, AK 99775, USA
It has been proposed that the evolution of floral traits is moulded by selection mediated by the most abundant and effective local pollinators (Stebbins, 1970; Fenster et al., 2004). Evolutionary transitions from short to long corolla tubes or nectar spurs have been observed in diverse plant lineages and are usually associated with the changes in the lengths of the tongues of major pollinators. Because nectar is generally available at the base of corolla tubes, only foragers with long enough tongues would be able to access the nectar. Floral manipulations of the depth of nectar within the corolla tube or spur have shown that the morphological matching between corolla tube length and pollinator tongue length is beneficial to both plant sexual reproduction and pollinator foraging efficiency (Nilsson, 1988; Anderson and Johnson, 2008; Muchhala and Thomson, 2009). A recent community survey showed that the plant-pollinator 'arms-race' leading to deeper flowers and longer proboscides could be maintained only if deeper flowers also offer larger nectar rewards (Klumpers et al., 2019). Thereafter, the evolution of long corolla tubes could be driven by the morphological adaptations of long-tongued pollinators to accessing reward (Nilsson, 1988, 1998; Wasserthal, 1997; Amorim et al., 2014; Johnson et al., 2017).
Specialized plant-pollinator mutualisms, when obligate, engender high risk of extinction of one or both partners when one partner becomes scarce or goes extinct locally. From the plant perspective, sexual reproduction would fail if its reproductive success depends completely on one specialized pollinator species. In response to such events, plants with specialized pollination may increase reproduction by cloning (Ferrero et al., 2020), evolve to be autonomously self-pollinating, or evolve to be less specialized in the pollinators utilized (Armbruster and Baldwin, 1998; Fenster and Martén-Rodríguez, 2007; Zhang and Li 2008; Roels and Kelly, 2011; Petanidou et al., 2012; Ward and Blum, 2012).
Unlike most members of the largely tropical Zingiberaceae, Roscoea is an exclusively temperate genus extending into high-elevation regions of the southern Himalayas. Roscoea species are characterized by purple flowers with corolla tubes ranging from 2 to 13 cm in length. Although previous studies have revealed a geographically mosaic pattern of coevolution in Roscoea purpurea and a long-tongued fly pollinator (Philoliche longirostris) in Nepal (Paudel et al., 2015, 2016), long-tongued insect pollinators have not been seen on long-tubed Roscoea species in the eastern Himalayas of China during the past 15+ years of observations (Zhang and Li, 2008; Zhang et al., 2011, and subsequent unpublished observation). Indeed, the eastern Himalayas appear to be outside the distribution area of these long-tongued flies. With a length of over 6 cm, the corolla tubes of most Roscoea species are much longer than the proboscis length of any insect recorded in the eastern Himalayas. In the eastern groups, autonomous self-pollination in R. schneideriana (Zhang and Li, 2008) and R. debilis (Fan and Li, 2012) has been observed, differing from the various pollination systems observed in western groups in Nepal, which includes pollination by short-tongued bumblebees (R. tujimensis) and beetles (R. alpina), as well as long-tongued flies (R. purpurea) (Paudel et al., 2017; 2019).
The reverse transition, from long to short corolla tubes, and the concomitant shift to pollination by short-tongued pollinators, seems to be very rare or absent in Roscoea. In the present study, we wish to understand the maintenance of long corolla tubes in regions apparently lacking long-tongued pollinators. We calculate coefficients of variation (CV) in floral traits to see if the corolla-tube length might be more variable than other floral traits in the absence of selection by long-tongued pollinators (see Pérez-Barrales et al., 2007).
Our initial field surveys of Roscoea species in the eastern Himalayas showed four unusual features about the flowers. (1) The lower portion of the corolla tube is largely fused with the style, precluding accumulation of nectar (were there any) in this region, making the length of corolla tube from outside appear much longer than the real corolla-tube depth on the inside; (2) The styles of some Roscoea species bend or coil within the corolla tube (see Fig. 1); (3) No effective pollinators were seen over hundreds of hours of observations in the field populations; (4) The main study species treated here, R. schneideriana, has nectarless flowers, despite having corollas with long tubes suggestive of pollination by long-tongued flies or lepidopterans, providing a rare opportunity to examine whether the degree of the style curvature is related to the corolla-tube length.

|
| Fig. 1 Plants and measurements of morphological traits in Roscoea schneideriana. Measurement of plant height of flowering individuals in natural habitat in Shangri-La population (a); A flowering individual showing its coiling style (b); and measurement of stigma height and the constricted corolla tube, noting the pink top corolla-tubes are hollow (measured as tube depth, TD) and that white bottom tubes (CCT) are filled by the style, with no room for nectar (c). |
Roscoea (Zingiberaceae) comprises 18 species and is the only genus in the Zingiberaceae occurring at high elevation. They are found along the Himalayas from Kashmir in the west, to Southwest China in the east (Cowley, 1982; Ngamriabsakul et al., 2000). After the early uplift of the Himalayas and rapid extrusion of Indochina in Middle to late Quaternary, the Himalayan Grand Canyon has become a natural barrier dividing Roscoea species into two distinct Himalayan and Chinese clades, with six species in the western region and 12 species in eastern region (Ngamriabsakul et al., 2000; Zhao et al., 2016). Roscoea species are perennial herbs with orchid-like hermaphroditic flowers. Each flower has three petals (usually pink) outside, two lateral staminodes, one labellum, a long style and one anther included in a tubular calyx and a long corolla tube as in other members of the Zingiberaceae. The lower part of corolla tubes of Roscoea species is enclosed in white calyx and covered by a sheathing bract (Fig. 1). We investigated R. schneideriana in three sites: Sicun (3320 m a.s.l., 27°80′N, 99°80′E), Shangri-La/Zhongdian (3268 m a.s.l., 27°90′N, 99°64′E), and Lijiang (2605 m a.s.l., 27°01′N, 100°19′E), northwestern Yunnan Province, Southwest China.
2.2. Measurements of traits and CVsSix traits in Roscoea schneideriana were measured with digital calipers (0.01 mm precision) or a rule (1 mm precision). These traits were plant height, corolla-tube length, corolla-tube depth, constricted corolla-tube length, style length and stigma height (Fig. 1). To estimate relative distance to accessible nectar (if any), the ratio of corolla-tube depth to corolla tube-length was calculated for each flower. To gain insight into possible stabilizing selection (or lack thereof) mediated by pollinators, we calculated for each floral trait coefficients of variation (CV) as the standard deviation divided by the mean.
2.3. Evaluation of style coiling in Roscoea schneiderianaDifferent from straight or bowed styles in most Roscoea species, the style in R. schneideriana is compressed into coils, the curvature being most obvious in the upper portion (Fig. 1C). To investigate the covariation of style coiling with corolla tube length, we measured one fresh flower from each of 66, 50 and 45 individuals from Lijiang, Shangri-La and Sicun populations, respectively. We defined a style coiling index, which was calculated as Coiling ratio = Style length/Stigma height. In a total of 161 (66 + 50 + 45) flowers, we measured the vertical length of the styles both in the curled state (i.e., stigma height) and restored straight state (i.e., style length).
To assess whether style coiling was related to corolla-tube length, we subjected the data to generalized linear modelling, where the coiling ratio was the dependent variable (assumed to have a reasonably normal distribution), source population was a fixed factor, and corolla-tube length was a covariate. All statistics were performed in SPSS 21.0 or SPSS 26 (IBM Inc., New York, USA).
3. Results 3.1. Variation of traitsOf the six traits measured, plant height varied the most (CV = 0.338) and style length varied the least (CV = 0.078) (Table 1). Corolla-tube length (0.134) was much more variable than style length (0.078), suggesting different selective factors (or genetic-developmental constraints) acting on an 'outside' pollinator-attracting trait and inside post-pollination trait. Corolla tube depth (CV = 0.156) was less variable than constricted corolla tube length (0.165), and the CV of the ratios of corolla tube depth/length was as low as 0.114, suggesting the distance to access nectar (corolla tube depth) was under stable selection from pollinators, rather than the constricted part of the corolla tube (CCT on Fig. 1A), which may be under the selection of unpredictable sources.
| Traits | Mean ± SE (mm) | CV |
| Plant height | 126.61 ± 4.37 | 0.338 |
| Corolla tube length | 40.00 ± 0.55 | 0.134 |
| Corolla tube depth | 14.21 ± 0.23 | 0.156 |
| Constricted corolla tube length | 25.78 ± 0.43 | 0.165 |
| Corolla tube depth/corolla tube length | 0.35 ± 0.00 | 0.114 |
| Stigma height | 57.62 ± 0.61 | 0.104 |
| Style length | 64.76 ± 0.52 | 0.078 |
The corolla-tube length varied from 21 to 47 mm in Shangri-La, from 31 to 42 mm in Sicun, and from 32 to 58 mm in Lijiang populations. In the three populations, the style coiling index was not significantly correlated with corolla-tube length (partial correlation analysis controlling for plant height, Shangri-La: r = -0.051, p = 0.727, Sicun: r = 0.013, p = 0.932, Lijiang: r = -0.214, p = 0.087). However, generalized linear modelling of the pooled data (taking population and corolla-tube length into account simultaneously) suggested a weak, but real, negative relationship between coiling index (0.1532 ± 0.0059, n = 161, Mean ± s.e.) and corolla-tube length (Fig. 2; AICc of model including corolla-tube length = -492.17, vs. -490.88 without corolla-tube-length term, and vs. -488.31 for model including both terms and their interaction). Thus, in Roscoea schneideriana the degree of style coiling tended to increase as the corolla tubes got shorter (Fig. 2).
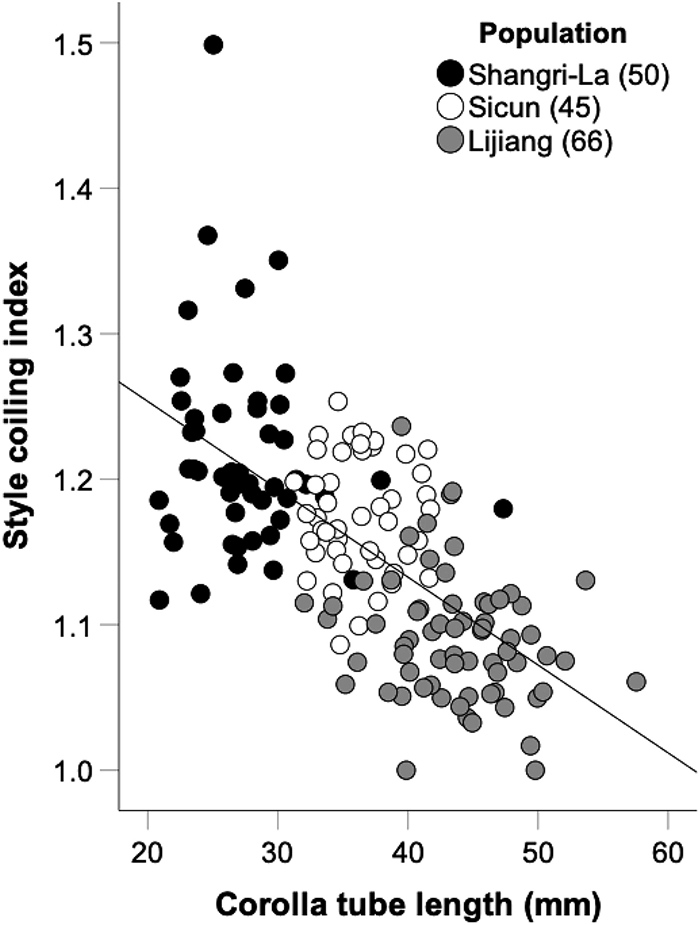
|
| Fig. 2 Relationship between corolla tube length and style coiling index in Roscoea schneideriana. Filled, and grey dots indicate three populations in Shangri-La, Sicun, and Lijiang, respectively. Numbers in brackets represent sampled individuals in that population. |
The CVs in style length were 0.106 in Shangri-La, 0.062 in Sicun, and 0.080 in Lijiang, which are all lower than the corresponding CVs for corolla-tube length (0.170, 0.082, and 0.111, respectively). These results suggest stronger stabilizing selection on style length than on corolla-tube length in this species.
4. DiscussionThe lower CVs in the style length than in corolla-tube length observed in three field populations of Roscoea schneideriana suggest stronger stabilizing selection on style length. Considering the fact that the flowers lack nectar (Zhang and Li, 2008) and that its pollinators are yet to be observed, it seems likely that maintenance of longer styles reflects selection for effective pollen-tube filtering and thus increased offspring fitness (Moore and Pannell, 2011).
The style length is generally isometric with corolla tube length with increasing flower size (Wang et al., 2016; Newman and Johnson, 2021). Our field survey in Roscoea schneideriana, however, revealed greater style coiling in flowers with shorter corolla tubes. The coiled styles of Roscoea species might be regarded as a response to selection for a reduced corolla-tube depth in the face of stabilizing selection for long styles. This suggests that style length has lagged other traits in the evolutionary history of the group; that is, reduction of style length has lagged behind reduction of corolla-tube length. The reason for corolla-tube length not co-evolving with pollinator proboscis length might be some constraining irreversibility (e.g., Dollo's law; Gould, 1970). The curvature of styles seems uncommon in flowering plants but is widespread in Pedicularis (Ju Tang and Shuang-Quan Huang, unpublished data), a superdiverse genus with variable corolla tube length, apparently also associated with loss of long-tongued pollinators (Huang and Fenster 2007).
Phytogeographic studies in Roscoea suggested that long-tongue pollinators failed to colonize or went extinct in the regions (Zhao et al., 2016). The absence of long-tongued pollinators in the eastern Himalayas (Zhang and Li, 2008; Zhang et al., 2011; Fan and Li, 2012) may have resulted from the historical uplift of the Himalayas preventing the dispersal and persistence of the specialized long-tongued pollinators seen on the south side of the Himalayan region in Nepal, where long-tongued flies serve as dependable pollinators of Roscoea species (Paudel et al., 2016). Thus, the shortening of the effective corolla-tube depth by constriction of the tube base may be a novel mechanism allowing Roscoea species to adapt to locally available, short-tongued pollinators, such as bees and beetles. Combining the fact that the CV in the constricted corolla tube is much higher than the tube depth, the two parts of the corolla tube might have been under differential selection. The low variation in the corolla tube depth (TD), a functional trait for nectar accessibility by pollinators, may reflect a history of more stable selection than the constricted corolla tube (CCT; Fig. 1C), given that its most closely related species (nectar-producing R. alpina) is insect-pollinated (Paudel et al., 2017).
Considering the fact that many Roscoea species are self-compatible and/or able to autonomously self-pollinate, and that effective pollinators have never been observed (Zhang and Li, 2008; Fan and Li, 2012), it seems likely that the natural pollinators have been lost over much of the group's current distribution in the eastern Himalayas. Most commonly, we observed western honey bees collecting pollen from Roscoea flowers, although these bees are not native to the habitat and their collection and storage of pollen in corbiculae may actually reduce plant reproductive success (Hargreaves et al., 2009). Clearly, more detailed studies of plant reproductive biology in Roscoea species in the eastern Himalayas are needed.
AcknowledgementsWe thank members of the Institute of Evolution and Ecology at CCNU for help in the field. This work was supported by the National Natural Science Foundation of China (grant nos. 31270281, 32030071) to SQH, and NNSFC (32071671) to ZYT.
Author contributions
SQH, WSA and XFW planned and designed the research. BW, ZYT, WSA, and YZX performed experiments, conducted fieldwork, or analyzed data. SQH, ZYT, and WSA wrote the manuscript, and all authors commented. BW and ZYT contributed equally to this work.
Data availability
All raw data are available in Excel files that will be updated during revision and post-acceptance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Amorim, F.W., Wyatt, G.E., Sazima, M., 2014. Low abundance of long-tongued pollinators leads to pollen limitation in four specialized hawkmoth-pollinated plants in the Atlantic rain forest, Brazil. Naturwissenschaften, 101: 893-905. DOI:10.1007/s00114-014-1230-y |
Anderson, B., Johnson, S.D., 2008. The geographical mosaic of coevolution in a plant–pollinator mutualism. Evolution, 62: 220-225. |
Armbruster, W.S., Baldwin, B.G., 1998. Switch from specialized to generalized pollination. Nature, 394: 632. DOI:10.1038/29210 |
Cowley, E.J., 1982. A revision of Roscoea (Zingiberaceae). Kew Bull., 36: 747-777. DOI:10.2307/4117918 |
Fan, Y.L., Li, Q.J., 2012. Stigmatic fluid aids self-pollination in Roscoea debilis (Zingiberaceae): a new delayed selfing mechanism. Ann. Bot., 110: 969-975. DOI:10.1093/aob/mcs169 |
Fenster, C.B., Martén-Rodríguez, S., 2007. Reproductive assurance and the evolution of pollination specialization. Int. J. Plant Sci., 168: 215-228. DOI:10.1086/509647 |
Fenster, C.B., Armbruster, W.S., Wilson, P., et al., 2004. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst., 35: 375-403. DOI:10.1146/annurev.ecolsys.34.011802.132347 |
Ferrero, V., Navarro, L., Castro, S., et al., 2020. Global patterns of reproductive and cytotype diversity in an invasive clonal plant. Biol. Invasions, 22: 1691-1703. DOI:10.1007/s10530-020-02213-9 |
Gould, S.J., 1970. Dollo on Dollo's law: irreversibility and the status of evolutionary laws. J. Hist. Biol., 3: 189-212. DOI:10.1007/BF00137351 |
Hargreaves, A.L., Harder, L.D., Johnson, S.D., 2009. Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biol. Rev., 84: 259-276. DOI:10.1111/j.1469-185X.2008.00074.x |
Huang, S.Q., Fenster, C.B., 2007. Absence of long-proboscid pollinators for long-corolla-tubed Himalayan Pedicularis species: implications for the evolution of corolla length. Int. J. Plant Sci., 168: 325-331. DOI:10.1086/510209 |
Johnson, S.D., Moré, M., Amorim, F.W., et al., 2017. The long and the short of it: a global analysis of hawkmoth pollination niches and interaction networks. Funct. Ecol., 31: 101-115. DOI:10.1111/1365-2435.12753 |
Klumpers, S.G., Stang, M., Klinkhamer, P.G., 2019. Foraging efficiency and size matching in a plant–pollinator community: the importance of sugar content and tongue length. Ecol. Lett., 22: 469-479. DOI:10.1111/ele.13204 |
Moore, J.C., Pannell, J.R., 2011. Sexual selection in plants. Curr. Biol., 21: R176-R182. DOI:10.1016/j.cub.2010.12.035 |
Muchhala, N., Thomson, J.D., 2009. Going to great lengths: selection for long corolla tubes in an extremely specialized bat–flower mutualism. Proc. Roy. Soc. B., 276: 2147-2152. DOI:10.1098/rspb.2009.0102 |
Newman, E., Johnson, S.D., 2021. A shift in long-proboscid fly pollinators and floral tube length among populations of Erica junonia (Ericaceae). South Afr. J. Bot., 142: 451-458. DOI:10.1016/j.sajb.2021.07.014 |
Ngamriabsakul, C., Newman, M.F., Cronk, Q.C.B., 2000. Phylogeny and disjunction in Roscoea (Zingiberaceae). Edinb. J. Bot., 57: 39-61. DOI:10.1017/S0960428600000032 |
Nilsson, L.A., 1988. The evolution of flowers with deep corolla tubes. Nature, 334: 147-149. DOI:10.1038/334147a0 |
Nilsson, L.A., 1998. Deep flowers for long tongues: reply from LA Nilsson. Trends Ecol. Evol., 13: 509. |
Paudel, B.R., Shrestha, M., Burd, M., 2016. Coevolutionary elaboration of pollination-related traits in an alpine ginger (Roscoea purpurea) and a tabanid fly in the Nepalese Himalayas. New Phytol., 211: 1402-1411. DOI:10.1111/nph.13974 |
Paudel, B.R., Shrestha, M., Dyer, A.G., 2017. Ginger and the beetle: evidence of primitive pollination system in a Himalayan endemic alpine ginger (Roscoea alpina, Zingiberaceae). PLoS One, 12: e0180460. DOI:10.1371/journal.pone.0180460 |
Paudel, B.R., Shrestha, M., Dyer, A.G., et al., 2015. Out of Africa: evidence of the obligate mutualism between long corolla tubed plant and long-tongued fly in the Himalayas. Ecol. Evol., 5: 5240-5251. DOI:10.1002/ece3.1784 |
Paudel, B.R., Kessler, A., Shrestha, M., et al., 2019. Geographic isolation, pollination syndromes, and pollinator generalization in Himalayan Roscoea spp. (Zingiberaceae). Ecosphere, 10: e02943. DOI:10.1002/ecs2.2943 |
Pérez-Barrales, R., Arroyo, J., Armbruster, W.S., 2007. Differences in pollinator faunas generate geographic differences in floral morphology and integration in Narcissus papyraceus (Amaryllidaceae). Oikos, 116: 1904-1918. DOI:10.1111/j.0030-1299.2007.15994.x |
Petanidou, T., Godfree, R.C., Song, D.S., 2012. Self-compatibility and plant invasiveness: comparing species in native and invasive ranges. Perspect. Plant Ecol. Evol. Syst., 14: 3-12. DOI:10.1016/j.ppees.2011.08.003 |
Roels, S.A.B., Kelly, J.K., 2011. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution, 65: 2541-2552. DOI:10.1111/j.1558-5646.2011.01326.x |
Stebbins, G.L., 1970. Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annu. Rev. Ecol. Syst., 1: 307-326. DOI:10.1146/annurev.es.01.110170.001515 |
Wang, X.P., Yu, W.B., Sun, S.G., et al., 2016. Pollen size strongly correlates with stigma depth among Pedicularis species. J. Integr. Plant Biol., 58: 818-821. DOI:10.1111/jipb.12477 |
Ward, J.L., Blum, M.J., 2012. Exposure to an environmental estrogen breaks down sexual isolation between native and invasive species. Evol. Appl., 5: 901-912. DOI:10.1111/j.1752-4571.2012.00283.x |
Wasserthal, L.T., 1997. The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Bot. Acta, 110: 343-359. DOI:10.1111/j.1438-8677.1997.tb00650.x |
Zhang, Z.Q., Li, Q.J., 2008. Autonomous selfing provides reproductive assurance in an alpine ginger Roscoea schneideriana (Zingiberaceae). Ann. Bot., 102: 531-538. DOI:10.1093/aob/mcn136 |
Zhang, Z.Q., Kress, W.J., Xie, W.J., et al., 2011. Reproductive biology of two Himalayan alpine gingers (Roscoea spp., Zingiberaceae) in China: pollination syndrome and compensatory floral mechanisms. Plant Biol., 13: 582-589. DOI:10.1111/j.1438-8677.2010.00423.x |
Zhao, J.L., Gugger, P.F., Xia, Y.M., et al., 2016. Ecological divergence of two closely related Roscoea species associated with late Quaternary climate change. J. Biogeogr., 43: 1990-2001. DOI:10.1111/jbi.12809 |



