b. State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China;
c. Shaanxi Key Laboratory of Early Life and Environments, Northwest University, Xi'an 710069, China;
d. Exploration Company, Shaanxi Yanchang Petroleum (Group) Co., LTD., Yan'an 716099, China;
e. Beijing Youth Education SciTech Co., LTD., Beijing 100089, China
Pinus L., the largest genus within the family Pinaceae Lindley and even all extant conifers, is of famously great importance in ecology, forestry and economy across the globe (Price et al., 1998; Gernandt et al., 2005). This group currently includes some 113 modern species usually growing as evergreen trees (rarely shrubs) and widely distributed over large swaths of land in the Northern Hemisphere (Fig. 1A; Richardson and Rundel, 1998; Farjon and Filer, 2013; Jin et al., 2021). Some of these arboreal plants occur or co-dominate with other coniferous trees (e.g. evergreen spruces, deciduous larches) in northern temperate zones forming notably the taiga (Jin et al., 2021), whereas the great majority of them extend into subtropical and/or even tropical montane forests in low-middle latitudes (Price et al., 1998; Richardson and Rundel, 1998), creating two major diversity centers in mid-low latitudes of North and Central America (ca. 70 species, especially Mexico, California and the southeastern USA) and eastern Asia (ca. 25 species, especially central and southern China) (Fig. 1A; Price et al., 1998; Farjon and Filer, 2013; Farjon, 2018). Such a present-day diversity highly concentrated in low-middle latitudes rather than equatorial tropics markedly differs from the mode generally exhibited by woody plants in angiosperms, i.e., latitudinal diversity gradient (Jin et al., 2021). Understanding how this geographic pattern has been shaped largely depends on reliable megafossil pine records from different geological time intervals and associated geo-environmental processes.

|
| Fig. 1 Geography of extant Pinus and its phylogeny. (A), Present-day geographical distribution of Pinus. The global distribution of extant Pinus (shaded area) is modified from Critchfield and Little (1966) and Farjon and Filer (2013). (B), Recent cladogram of Pinus not only showing an ancient origin for the whole genus but a post-Oligocene origin for about 90% of extant pine species alike. The simplified transcriptome-based phylogenetic tree is reproduced after Jin et al. (2021) and the species-level taxa thereof are from the original publication. The main diagnostic characters and phytogeographical range of different sub-groups are combined from Price et al. (1998), Fu et al. (1999), Frankis (2002), Gernandt et al. (2005) and Jin et al. (2021). P., Pliocene; Q., Quaternary; NA and Mex represent North America north of Mexico, and Mexico, Central America, and the Caribbean, respectively; W/E NA, E Asia and NW Himalayas represent Western/Eastern North America north of Mexico, Eastern Asia and Northwestern Himalayas, respectively. |
Compared with pinaceous genera and other living conifers, Pinus has very distinct morphological features. Pine seed cones are biennial (rarely triennial) with woody, distally thickened seed scales helically arranged around the axes. The inflated exposure of the seed scale diagnostic for species identification comprises two varying parts, viz., umbo and apophysis (Miller, 1976). The umbo starts to grow during the first year and often terminates in a persistent spine (prickle or mucro; Ding et al., 2013: plate III, 4), which gradually forms a comparatively clear border after pollination in the second year (our field observations; Price et al., 1998; Fu et al., 1999; Farjon, 2021). Their thin leaves likewise feature multiple needles per fascicle ranging from 1 to 8 and bearing persistent or deciduous basal sheaths. These autapomorphic characters indicate that Pinus is a monophyletic taxon markedly differing from the remaining taxa of Pinaceae (e.g., Gernandt et al., 2005; Zidianakis et al., 2016).
The sub-classification of Pinus has been progressively well-resolved by morphological and phylogenetic studies. As a single genus placed under the subfamily Pinoideae Pilger (Florin, 1963; Cheng et al., 1978; Price et al., 1987; Frankis, 2002), this group is currently subdivided into two monophyletic subgenera predominantly on account of a mosaic combination of trait differences in foliage or wood (e.g., number of fibrovascular bundles per needle): subgenus Pinus L. (diploxylon or hard pines) and subgenus Strobus Lemmon (haploxylon or soft pines) (Price et al., 1998). This view is echoed in many molecular findings (Gernandt et al., 2005; Jin et al., 2021). Within this current binary system, each subgenus contains two sections, i.e., the predominantly Eurasian-Mediterranean section Pinus and the North American section Trifoliae Duhamel nested within the subgenus Pinus as well as the North American section Parrya Mayr and the Eurasian-North American section Quinquefoliae Duhamel within the subgenus Strobus (D. Don) Lemmon (Fig. 1B; Table 1). Although recent schemes at the intra-subgeneric level to a great degree focus on gene sequencing data (Zeb et al., 2020; Jin et al., 2021), these phylogenetically updated circumscriptions within the two subgenera also show a considerable number of trait variations (Cheng et al., 1978; Klaus, 1980; Price et al., 1998; Gernandt et al., 2005), which contribute to the recognition of fossils within the extant taxonomic framework (Fig. 1B; Grote and Srisuk, 2021; Jin et al., 2021). The systematic taxonomy of pine fossils can provide evidence that attests to the evolutionary events of this conifer hypothesized by molecular botany.
| Taxon | Mature cones | Seed scale | Umbo | Geographic distribution | |
| Position | Prickle (mucro) | ||||
| Subgenus Pinus (seed scale with a sealing band) | |||||
| Section Trifoliae (NA) | |||||
| Subsect. Australes | Open, closed | Thick | Dorsal | Variable, centromucronate | NA & Mex |
| Subsect. Contortae | Closed, open | Thick | Dorsal | Variable, centromucronate | W & E NA & Mex |
| Subsect. Attenuatae | Closed, irregularly shaped | Thick | Dorsal | Variable, centromucronate | Cal. & Baja Cal. |
| Subsect. Ponderosae | Open | Thick | Dorsal | Variable, centromucronate | W NA & Mex |
| Subsect. Sabinianae | Open, large | Thick | Dorsal | Variable (spiny), centromucronate | Cal. |
| Section Pinus (Laurasia & Tethyan) | |||||
| Subsect. Pinus | Open | Thick | Dorsal | Variable, excentromucronate | Eurasia & E NA |
| Subsect. Pinaster | Open (closed) | Thick | Dorsal | Absent | Mediterranean (Himalayas) |
| Subgenus Strobus (seed scale without a sealing band) | |||||
| Section Quinquefoliae (Laurasia) | |||||
| Subsect. Strobus | Open | Thin | Terminal | Absent | NA, Mex, Eurasia |
| Subsect. Krempfianae | Open | Thick | Dorsal | Absent | Central Vietnam |
| Subsect. Gerardianae | Open | Thick | Dorsal | Variable (mucro)/absent | E Asia & NW Himalayas |
| Section Parrya (NA) | |||||
| Subsect. Cembroides | Open | Thick | Dorsal | Absent, tectoid, parvimueronate | W NA & Mex |
| Subsect. Balfourianae | Open | Thick | Dorsal | Variable, erectoexcentro-mucronate | W NA |
| Subsect. Nelsoniae | Open | Thick | Dorsal | Absent | Mex |
| Morphological data of extant groups are sourced from Klaus (1980, 1989), Frankis (2002), Gernandt et al. (2005), Farjon (2021), Jin et al. (2021) and the present investigation. All abbreviations for the geographic ranges are the same as in Fig. 1. Cal., California. | |||||
Phylogenetic studies integrating fossil and extant taxa showcase the extensive evolutionary history of Pinus, which traces back to the Early Cretaceous or even Late Jurassic (Leslie et al., 2018). The origin of Pinus (stem group) is very ancient (ca. 155 Ma) and its subgeneric divergence time is near the Early-Late Cretaceous boundary (ca. 98.7 Ma), with a subsequent extensive expansion of early representatives during the Late Cretaceous across the Northern Hemisphere (Jin et al., 2021). However, as deduced by previous molecular data (Hernández-León et al., 2013; Jin et al., 2021) and evidenced by European, eastern Asian and North American fossil records (Table S1), it is remarkable and intriguing that the Miocene, which features environmental geodiversity transitioning towards modern conditions (e.g. mountain topography and climate) (Steinthorsdottir et al., 2021), is probably a key time interval for the origin and diversification of extant members of this ecologically significant tree genus (Fig. 1B). In other words, extant pines originated much later than previously thought (Jin et al., 2021). Tracking the evolutionary history of Pinus in greater resolution and over a more extensive spatiotemporal range still requires deep research on more geological records (e.g., plant fossils) with definite chronological constraints from key regions. In contrast to other fossil vegetative remains, seed cones of Pinus are characteristic and much easier to accurately recognize under modern systematic schemes based on the trait combination of apophyses and umbos (Klaus, 1980; Mai, 1986; Xu et al., 2015b; Zidianakis et al., 2016). Here, we describe two well-preserved female cones from the Late Miocene Shengxian Formation in Tiantai County of Zhejiang, a southeastern Chinese coastal province, where a subtropical monsoon climate currently dominates. These specimens uniformly show a rhomboidal apophysis with a dorsal denticulatomucronate umbo, a diagnostic character for members of subgenus Pinus section Pinus subsection Pinus. Extensive comparison of known extant and fossil members of this group displays adequate morphological disparities, and allows us to recognize the present reproductive organs as two species, Pinus shengxianica X.C. Li, Y. Hu & L. Xiao sp. nov. and Pinus speciosa Li. The new species P. shengxianica shares a striking cone similarity to Pinus latteri Mason and Pinus merkusii Jungh. & de Vriese from tropical southeast Asia in having annular bulges around the umbo on the apophysis, and is the first fossil cone with such a unique trait in southeastern mainland Asia and even all across the world up to now. The discovery of this new pine gives us an outstanding opportunity to learn about a historical biogeographic link between these two Southeast Asian low-latitude pines and their probable precursors from higher latitudes like never before. This unusual occurrence from the Miocene of Zhejiang in East Asian mid-low latitudes also provides insights into the generic evolutionary trajectory reflected by the pine fossil history and molecular data, marking the Miocene (rather than earlier times) as a key period for the origin and evolution of most extant pines globally (Jin et al., 2021). The existence of Pinus present in the paleoflora, together with numerous previously published coniferous and broadleaved taxa from the same horizon facilitates our better understanding of regional coniferophyte diversity and complex vegetation structure in the Miocene.
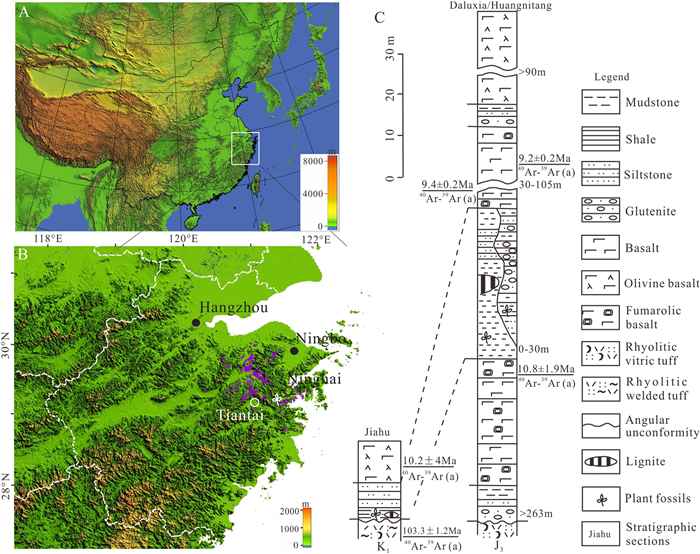
2. Materials and methodsThe mummified cones of Pinus in this study were unearthed from shale layers of a strip diatomite mine to the north of Jiahu Village in Tiantai County (29°09′N, 121°14′E, 280 m a.s.l., Fig. 2A and B), eastern Zhejiang, China. These fossil-containing horizons here are intercalated within basalts and they all resolve as the Shengxian Formation based on geologic correlation. Regionally, this unit outcrops on a land area of approximately 550 km2 and is made up primarily of diatomaceous mudstone, shale, argillaceous siltstone and basalts (Fig. 2B and C; Li et al., 2021). Currently, the fossiliferous layers amongst the Shengxian Formation are interpreted to be of Late Miocene age on the basis of 40Ar/39Ar dating of overlying and underlying basalts (10.8–10.2/9.2 Ma; Fig. 2C; Ho et al., 2003; Li et al., 2014, 2021; He, 2017). Previous paleobotanical findings of different fossil remains from the same strata include fern, coniferous or angiospermous leaves (Li and Guo, 1982; Li et al., 2010; Xiao et al., 2011; He et al., 2012; Wang et al., 2014), epiphyllous fungi (Ding et al., 2011; Du et al., 2012), cones or fruits (Ding et al., 2013, 2021a, 2021b; Jia et al., 2020; Li et al., 2015, 2021; Xu et al., 2015b), pollen grains (Liu et al., 2007, 2008; Yang et al., 2015, 2018), and rare angiospermous flowers (Li, 2010) as well as Coleoptera insects, cyprinids and cervids (Li et al., 2021), indicating a highly diverse biota.
|
| Fig. 2 Location and horizon of the present fossil cones of Pinus in eastern Zhejiang. (A, B) Rock exposures of the Shengxian Formation (purple area) and the fossil locality at Jiahu of Tiantai County (white leaf symbol). Physiographic base maps were sourced from Resource and Environment Data Cloud Platform (http://www.resdc.cn/) and constructed by ArcGIS 10.2. (C) Stratigraphic succession at the Jiahu and adjoining Daluxia/Huangnitang Villages. Regional stratigraphic correlations are modified from Li et al. (2014, 2021). The matrix from which the present cones were obtained is shale. The geological dating of (a) in C is from He (2017). |
In preparation for morphological study, fossil materials were isolated directly from country rock during fossil collection or disaggregated in water at the laboratory to remove sediments, and then immersed in 10% HCl solution for 2 days to remove fine carbonates. Subsequently, the specimens were neutralized in distilled water and in turn immersed in 50% HF solution for approximately 2 days to remove fine silicates. Finally, they were neutralized in distilled water again and any sediment still adhering to the fossil cones was manually removed with a soft-haired brush. The fossil cones were photographed and examined in detail with a digital camera, Leica M165FC stereomicroscope (Wetzlar, Germany) and/or KEYENCE VHX-1000 digital microscope (Osaka, Japan) illuminated by low, oblique incident light at Chang'an University (Xi'an, China). Some cone scales were mounted on a stub and coated with gold; then anatomical characters were observed and documented under a FEI Quanta 650 scanning electron microscope (Hillsboro, Oregon, United States) at Chang'an University.
Brightness and contrast of digital images were manipulated using Adobe Photoshop CS6. All specimens studied here were soaked in glycerin to avoid shrinking during the natural drying process and housed together with SEM stubs and photographs at the Geological Museum of Chang'an University, Xi'an, China. Morphological comparisons with extant Pinus species were made chiefly using online databases, including the Gymnosperm Database (https://www.conifers.org/pi/Pinus.php), the National Plant Specimen Resource Center (CVH, http://www.cvh.ac.cn/), Global Plants (https://plants.jstor.org/), National Museum of Natural History, Smithsonian Institution, Washington, D.C (http://collections.nmnh.si.edu/search/paleo/), the Virtual Herbarium at the New York Botanical Garden (http://sweetgum.nybg.org/science/vh/), Arboretum de Villardebelle (http://www.pinetum.org/) and eFloras (http://www.efl http://oras.org). Data from all online sources were used cautiously and only when the identification and character details were deemed reliable. The terminology for cone morphology and anatomy is adapted mainly from Klaus (1980, 1989) (e.g., umbo and apophysis), Li et al. (2022) and Yoshie and Sakai (1985). Infrageneric classification of the genus Pinus follows the recent scheme of Gernandt et al. (2005) and Jin et al. (2021).
3. Systematic paleobotanyOrder: Pinales Gorozh.
Family: Pinaceae Lindley.
Genus: Pinus L.
Subgenus: Pinus L.
Section: Pinus L.
Subsection: Pinus L.
Species: P. shengxianica X.C. Li, Y. Hu & L. Xiao sp. nov.
Specific diagnosis: Seed cone conical-ovoid, apex tapered, base obtuse. Seed scales woody and thick, essentially suboblong-obovoid, helically arranged around the center axis of the cone. Apophyses planarly pentagonal or rhombic, flat or slightly swollen, nearly pyramidal, evidently transversely keeled, and bearing 2 to 3 rings of pronounced ridges encircling the umbo (i.e., annular bulges). An obvious sealing band existing on the lower side of each apophysis. Umbos dorsal, avallate, with a recurved mucro (short prickle) on the apical apophysis defined as the denticulatomucronate umbo type.
Holotype: JH3-134 (Fig. 3).

|
| Fig. 3 Pinus shengxianica sp. nov. from the Miocene of eastern Zhejiang Province, Southeast China. (A) Female cone showing the gross morphology. Holotype, specimen no. JH3-134. (B) Same specimen as an opposite side of (A) after removing the sediments. (C) Close-up of an apophysis from the middle-apical portion of (B) showing a denticulatomucronate umbo. Note the short and recurved excentric mucro. (D, E) Close-up of an apophysis from the basal portion of (B) showing a transverse keel and 3 rings of ridges around the umbo (annular bulges indicated by green arrowheads). (F) Close-up of an apophysis showing 3 rings of ridges (green arrowheads) around the umbo and the sealing band (red arrowhead). (G) Apophysis surface showing distribution of elongated epidermal cells, a few waxes and stomatal openings with poorly-developed Florin rings. (H) Cross section of the seed scale showing internal structures. Note vascular bundles present in the center (red circle) and neighboring resin canals (green arrowheads) near the abaxial (right) and adaxial (left) sides of the scale. Scale bars: (A, B) = 1 cm; (C–F) = 2 mm; (G) = 100 μm; (H) = 200 μm. |
Etymology: The epithet shengxianica represents the strata, Shengxian Formation from which the fossil cone was recovered.
Type locality: Jiahu Village in Tiantai County of Zhejiang Province, China (Fig. 2A and B).
Type strata and age: Shengxian Formation, late Miocene (Fig. 2C).
Repository: Geological Museum of Chang'an University, Xi'an, China.
Description: The ovuliferous cone is symmetrical and conical-ovoid in general outline with a tapered apex (Fig. 3A and B), 6.8 cm long and 3.1 cm wide (at the widest part). Roughly 50 to 60 woody seed scales are helically arranged around the center axis of the cone. These scales are suboblong-obovate with a thickened apical exposure forming an apophysis (Fig. 3B). The apophyses are pentagonal or rhombic in planar view (Fig. 3A–E), 0.8–1.3 cm wide and 0.7–0.9 cm high with a width-to-height ratio of 1.1–1.4; the basal apophyses are smaller and more flattened than the apical apophyses, which are slightly swollen, short pyramidal (Fig. 3C–F). A conspicuous horizontal keel traverses the apophysis, and radiating ridges are absent except a pair of kind of inconspicuous longitudinal ones. An obvious sealing band is present near the border between the exposed apophysis and the seed scale base superimposed by the subtending seed scales (Fig. 3F; when the overlapping parts of the seed scales are open after cones mature, this imbricate character is easily accessible for observation; for details see Fig. 5). The umbo is dorsal and vaguely rhombic to elliptic (Fig. 3C–E) due to no prominent boundary delimiting the umbo and the remaining part of the apophysis; consequently, the umbo field is poorly developed and not easily distinguished from the apophysis (Fig. 3A–E). Notably, double or triple rings of pronounced ridges encompass the centered umbo (Fig. 3A–F). Among the apical apophyses, a short but stout recurved prickle is located above the horizontal keel near the upper edge of the umbo, typical of the denticulatomucronate umbo type (Fig. 3C); the basal apophyses often exhibit a tectoid umbo type (Fig. 3D). The apophysis surface is primarily glabrous with slightly elongate epidermal cells and sporadic stomata randomly arranged (Fig. 3G); the cells encircling the stomatal pore form poorly-developed Florin rings with little relief (Fig. 3G). A vascular bundle is present in the middle part of the apophysis and the external resin canals can be seen (Fig. 3H).

|
| Fig. 5 Seed scales of subg. Strobus and Pinus (A) and cones of P. merkusii and P. massoniana Lamb. from Southeast Asia (B–D). (A) The illustration was redrawn after Frankis (2002) and shows the main morphological differences of the extant subgenera. Note that the area between the dotted and orange lines of Inset c is indicative of the position of the sealing band. (B) A complete cone of P. merkusii showing the denticulatomucronate umbo (black arrowhead) and annular ridges (red arrowheads) around the umbo (Global Plants; P00731198, French National Museum of Natural History, MNHN). (C, D) A cone of P. massoniana showing the sealing band (black arrowheads) (NY04278105, New York Botanical Garden). Note that the sealing bands are easily accessible for observation as shown by (A, C, D) when removing the covers of seed scales to expose the overlapping abaxial surface. Scale bars: (B, C) = 1 cm, (D) = 5 mm. |
Species: Pinus speciosa Li.
Holotype: PB7639, Li and Guo (1982): Pl. 135, Fig. 17 (stored in NIGPCAS).
Type locality: Lüjia Village in Ninghai County of Zhejiang Province, China.
Type strata and age: Xiananshan Formation, Miocene (already reviewed as Shengxian Formation).
Specimen checked here: JH1-042 (Fig. 4) from the Late Miocene Shengxian Formation of Jiahu Village in Tiantai County of Zhejiang Province, China (Fig. 2).

|
| Fig. 4 Pinus speciosa from the Miocene of eastern Zhejiang Province, Southeast China. (A) Female cone showing the gross morphology. Specimen no. JH1-042. (B) Same specimen as and same side of (A) after removing the sediments. (C, D) Close-up of an apophysis from the middle-apical portion of (B) showing a denticulatomucronate umbo and a transverse keel (green arrowheads). Note the short and recurved excentric mucro. (E) Close-up of a slightly sunken umbo. (F) Close-up of an apophysis showing a vallum (green arrowhead) around the umbo and the weak sealing band (red arrowhead). (G) Apophysis surface showing distribution of papillary ornaments, a few waxes and stomatal openings without Florin rings. (H) Cross section of the seed scale showing internal structures. Note resin canals (green arrowheads) near the sides of the scale. Scale bars: (A, B) = 1 cm; (C, D, F) = 2 mm; (E) = 1 mm; (G) = 100 μm; (H) = 200 μm. |
Repository: Geological Museum of Chang'an University, Xi'an, China.
Supplemented description: The fossil cone is symmetrical and broadly ovoid with a tapered apex (Fig. 4A and B), 6.3 cm long and 3.2 cm wide. Roughly 60 to 70 woody cone scales are helically arranged around the cone axis. Each scale is characterized by an apophysis. Apophyses are slightly swollen. In planar view, the apophyses are broadly rhombic or irregularly pentagonal with a size of 1–1.3 cm wide × 0.7–0.9 cm long in the upper half of the cone and almost pyramidal with a size of 0.7–0.9 cm wide × 0.8 cm long near the base. Hence, the upper apophyses of the cone tend to be wider than the basal apophyses (Fig. 4A and B). Each apophysis has a prominent transverse keel (Fig. 4B). An inconspicuous longitudinal ridge can be seen in some apophyses (Fig. 4B). A weak sealing band delimits the apophysis from the rest of the cone scale (Fig. 4C, D and 4F; for details see Fig. 5). The umbos are dorsal, elliptic to rhombic in planar view, centrally sunken, 2.0 mm broad and 1.0 mm high and surrounded by an evident vallum (Fig. 4F). The slender prickle is recurved, and located above the transverse keel on the upper umbo field, characteristic of denticulatomucronate umbo type (Fig. 4C–F). The apophysis surface is primarily glabrous with papillary ornaments and sporadic stomata randomly arranged (Fig. 4F and G). A vascular bundle is not clearly shown in the middle part of the apophysis but the external resin canals can be seen (Fig. 4H).
4. Discussion 4.1. Systematic determination 4.1.1. Subsection identitiesThe female cones of Pinus are very distinctive from other pinaceous taxa (Miller, 1976). Their seed scales are always helically arranged surrounding the center axis in Fibonacci number ratios (Zeng and Wang, 2009) and the exposure of each seed scale unexceptionally consists of both umbo and apophysis parts (Miller, 1976; Fu et al., 1999; Gernandt et al., 2005; Farjon, 2021). These autapomorphic characters also observable in the present fossil cones enable us to place them within the monophyletic genus Pinus. The further comparisons are mainly considered in the context of the latest classification proposed by Jin et al. (2021).
As for the recently recognized thirteen subsections of four sections of two subgenera of this genus circumscribed by Jin et al. (2021), each sub-taxon has its own combination of distinctive traits, e.g., umbo position, presence of umbo prickle (mucro) (Fig. 1B; Table 1; Klaus, 1989; Farjon, 2021). First, subsections Strobus Loudon and Krempfianae Little et Crichfield of section Quinquefoliae, and subsections Cembroides Engelmann and Nelsoniae van der Burgh of section Parrya within the subgenus Strobus possess no umbo prickle (Table 1). This is also the case in subsection Pinaster Loudon of section Pinus of the subgenus Pinus (Table 1; Farjon, 2021); Li et al. (2022) stated that three species in this subsection (Pinus canariensis C.Sm. ex D.C., Pinus roxburghii Sarg. and Pinus heldreichii H. Christ) also have geniculate umbos (Klaus 1980; for details see Li et al., 2022), an umbo type with a clear knot-shaped thickening (not a mucro) at the root of the tectum (Klaus, 1980: Fig. 1). Moreover, the subsection Strobus bears a terminal umbo and a thin seed scale in texture (Table 1; Figs. 1B and 5A; Farjon, 2021). In contrast, both of our fossil cones display a recurved mucro (short prickle) above the transverse keel on the umbo (Fig. 3A–C and 4A–F), which was defined by Klaus (1980) as denticulatomucronate. Thus, these five subsections can be readily excluded from the candidate taxa for the taxonomic position of the present fossil cones due to a total lack of prickles.
Second, section Trifoliae within the subgenus Pinus and section Parrya within the subgenus Strobus are two strict North American monophyletic taxa (Table 1; Fig. 1B; Gernandt et al., 2005). The five subsections of section Trifoliae and subsection Balfourianae Engelmann of section Parrya are characterized by variable prickles (Fig. 1B; Table 1; Gernandt et al., 2005), but other cone characters (e.g., cone shape, size, symmetry and umbo mucro position) (Klaus, 1980; Li et al., 2022) will suffice to distinguish these groups from our fossil cones that have a denticulatomucronate umbo. For instance, Li et al. (2022) and Klaus (1980) pointed out that the sections Trifoliae generally bear a cone with centromucronate umbos (Fig. 3 in the Li et al., 2022). Furthermore, the two subsections Sabinianae and Attenuatae have an extremely large, heavy cone with highly pyramid-like apophyses and usually a long spine on the umbo, and an irregularly-shaped asymmetrical cone, respectively (Fig. 1B, plus our investigations) (Stockey, 1983; Jin et al., 2021).
Finally, the subsection Gerardianae Loudon only includes three extant species native to the northwestern Himalayas, northeast Yunnan, and northeastern and central China. Likewise, their cones can be easily discriminated from ours in having a centromucronate umbo (Figs. 2 and 10 in the Klaus, 1989). Additionally, Frankis (2002) concluded that cones of the subgenus Pinus develop a sealing band on the lower margin of the apophysis, contrasting with those of the subgenus Strobus (Fig. 5; Table 1); this is a key feature for pine diagnosis at the subgeneric level (Xing et al., 2010; Xu et al., 2015a). As mentioned above, Klaus (1980, 1989) pointed out that centromucronate umbos can be generally seen in the American pines of the subgenus Pinus and excentromucronate umbos are present in all the Eurasian pines of the subgenus Pinus (Table 1). According to the original description of Klaus (1980), the excentromucronate umbo denotes that the mucro does not originate from the transverse keel but above it in the upper area of the umbo. Notably, Li et al. (2022) and Klaus (1980) proposed that the section Pinus, including subsections Pinus and Pinaster, generally bears a cone with excentromucronate umbos (Table 1; Fig. 3 in the Li et al., 2022). Within the infrageneric classification recently re-circumscribed by Jin et al. (2021), the subsection Pinus of the section Pinus bears a cone with an excentromucronate umbo (Fig. 6b in the Li et al., 2022), differing from its sister subsection Pinaster, which always possesses geniculate umbos (Li et al., 2022) and commonly lacks the umbo mucro (Table 1) (Gernandt et al., 2005). Therefore, combined with other shared character combinations (e.g., dorsal umbo, thick seed scale, mucro; Table 1), the systematic position for the present fossil cones can be specifically narrowed down to subsection Pinus of section Pinus of subgenus Pinus, especially with an emphasis of their ties of kinship close to extant Eurasian species.
4.1.2. Comparison with extant species of subsect. PinusBased on the character details (e.g., size, location, orientation and number of mucros), the excentromucronate mucros of the subgenus Pinus are classified into four groups, namely denticulatomucronate, erectoexcentromucronate, perexcentromucronate and duplomucronate (Klaus, 1980, 1989). As for the denticulatomucronate umbo, the mucro-tip protrudes freely and obliquely forwards and downwards like a sharp tooth. In parallel, the transversal keel running underneath, mostly reduced and sunken in the middle, is not touched by the mucro-tip (e.g., Fig. 3C and Fig. 4C–4F). Here, the umbos of the seed cones of P. shengxianica and P. speciosa from the Miocene of Zhejiang are clearly denticulatomucronate (Fig. 3A–F and Fig. 4A–F), one of the aforementioned three types (excluding duplomucronate) of excentromucronate umbos seen in the subsection Pinus (Fig. 6 in the Li et al., 2022). Nevertheless, the former's umbos are characterized by double or triple circular ridges (Fig. 3C–F) and the latter's umbos are usually sunken (Fig. 4C–F). These diagnostic distinctions definitely suggest both likely represent two different species.
The extant subsection Pinus includes sixteen Eurasian species and two eastern North American species (Jin et al., 2021). There are only eight extant species among them whose excentromucronate umbo can be recognized as the denticulatomucronate type (Table 2; Li et al., 2022) on the basis of the mucro position relative to the umbo field by Klaus (1980, 1989). Thus, to determine the systematic relationship of the specimens studied here, we only compared our fossil cones to species of subsection Pinus with a denticulatomucronate umbo. The predominantly Mediterranean Pinus uncinata Ramond ex DC. possesses a strongly asymmetrical cone with both denticulatomucronate and erectoexcentromucronate umbos (Table 2; Li et al., 2022). The predominantly Mediterranean Pinus nigra J.F. Arnold and South Korean and Japanese Pinus thunbergii usually bear an asymmetrical cone (Table 2). Conversely, our two fossil cones are symmetrical (Fig. 3A, B, 4A and 4B). The Cuban Pinus tropicalis Morelet and Maritime Southeastern Asian P. merkusii possess a cone with cross and radial ridges on the apophyses (Fig. 5B), and this is the case in Pinus mugo Turra from southern Europe. P. tropicalis lacks an umbo prickle (Cheng et al., 1978; Fu et al., 1999). The Southwest Chinese Pinus densata Mast. displays a very prominent apophysis (Cheng et al., 1978; Fu et al., 1999), whereas Eurasian Pinus sylvestris L. has a cone with a small mucro (Li et al., 2022: Fig. 7d–f; Table 2); their cones also display cross and radial ridges on the apophyses (Cheng et al., 1978). In contrast, our two fossil cones lack radial ridges (Fig. 3A-F and 4A-4F). P. latteri is a species in tropical Mainland Southeast Asia and closely related to Sumatran pine P. merkusii and, thus, both were occasionally treated as conspecifics by some publications (Grote and Srisuk, 2021). These two low-latitude species differ from the present fossil cones in more prominent apophyses with well-developed umbo fields and radial ridges (Table 2; Fu et al., 1999; Plate III, 2 in the Grote and Srisuk, 2021). Overall, these aforementioned species, except P. merkusii and P. latteri, can also be discriminated from the present fossil specimen with annular bulges (P. shengxianica sp. nov.) in utterly lacking two or three circular ridges around the umbo (Table 2; Figs. 3 and 5), even irrespective of other cone morphological details. As stated by Klaus (1980), the southeastern Asian P. merkusii and P. latteri and eastern Asian P. thunbergii have a cone with annular bulges surrounding the umbo (Fig. 5B; Grote and Srisuk, 2021: Plate III, 2). Unexpectedly, our extensive investigations suggest that P. thunbergii lacks this key character. Thus, the present fossil species P. shengxianica is akin to the southeastern Asian P. merkusii and P. latteri in this respect, indicating that it probably represents an ancestral type bearing a close affinity to the two Southeast Asian extant species. Moreover, another co-occurring fossil species studied here, P. speciosa, appears to be extinct, because the currently available character combinations show no extant closely related equivalents within Pinus.
| Species | Cone | Apophyses | Keel type | Umbo | |||
| Symmetry | Shape | Size (cm) | Position | Mucro (prickle) | |||
| P. shengxianicaa | Symmetrical | Conical-ovoid | 6.8 × 3.1 | Rhombic or pentagonal | Conspicuously horizontally keeled with circular ridges | Dorsal | With a short but stout recurved prickle |
| P. speciosaa | Symmetrical | Ovoid or ellipsoidal with a round base | 6.3 × 3.2 | Broadly rhombic or irregularly pentagonal | Prominently transversely keeled with inconspicuous longitudinal ridges | Dorsal | Centrally sunken, with a slender, recurved prickle |
| P. densata | Symmetrical | Ovoid | 4–6 × 4–7 | Prominent, rhombic | Sharply transversely keeled | Dorsal | Prominent with a short prickle |
| P. merkusii | Symmetrical | Cylindrical or long-ovate | 4.8–11 | Rhomboid, thick, glossy, annularly furrowed/ridged, obviously radially ridged | Prominently transversely keeled and radially ridged | Dorsal | Slightly concave |
| P. latteri | Symmetrical | Conical or ovoid-cylindric | 5–10 | Subrhombic or pentagonal-rhombic, slightly swollen, annularly furrowed/ridged | Obviously transversely keeled and radially ridged | Dorsal | Slightly sunken |
| P. mugo | Symmetrical | Ovoid | 1.8–5.5 × 1.4–2.8 | Rhomboidal, thin, flat | Sharply transversely and longitudinally keeled | Central | Prominent with a short prickle |
| P. nigra | Asymmetrical | Ovoid | (3.5-)5–10 (-12) × 2–4 | Rhomboidal, thin, woody, rigid | Slightly raised and transversely keeled | Central | Small, unarmed or with a tiny deciduous prickle |
| P. sylvestris | Symmetrical or nearly so | Conic | 3–6 | Broadly rhombic, flat or shortly pyramidal | Slightly raised and transversely keeled | Dorsal | Small, blunt (minutely mucronate) |
| P. tropicalis | Symmetrical or nearly so | Ovoid with a flattened base | 5–8 × 4–5.5 | Flat or slightly raised | Cross ridged | Dorsal | Flat or slightly raised, rhombic, without a prickle |
| P. thunbergii | Asymmetrical | Conical-ovoid or ovoid | 4–7 × 3.5–6.5 | Rhomboidal, slightly swollen or flattened | Transversely keeled | Dorsal | Slightly concave, with a short-mucronate umbo |
| P. uncinata | Strongly asymmetrical | Ovoid | 2.5–6 × 2–4 | Thick, strongly pyramidal | Transversely keeled | Nearly apical | Often with a pronounced 1 mm prickle |
| a The fossil taxa are studied here. Characters of extant species sourced from Cheng et al. (1978), Fu et al. (1999), the Gymnosperm Database and our observations. | |||||||
Currently, there are only nine known fossil cone species with a denticulatomucronate umbo from the Cenozoic of Central Europe and East Asia based on the latest fossil record summarized by Li et al. (2022), and most of these representatives are reported from the Miocene (Table 3). In reality, several symmetrical seed cones from the upper Miocene of Gifu Prefecture of central Japan were initially named Pinus fujiii auct. non (Yasui) Miki and later revised as Pinus mikii T. Yamada, M. Yamada et Tsukagoshi (Yamada et al., 2015); it has a perexcentromucronate mucro on the umbo (a type with extremely eccentric and obtuse mucro and a transverse keel continuously across the umbo) (Yamada et al., 2014, 2015) rather than the denticulatomucronate one as misquoted by Li et al. (2022). The conserved P. fujiii (Yasui) Miki includes asymmetrical female cones from the early Miocene to early Pleistocene of Honshu and Kyushu, Japan; this species is characterized by a centromucronate umbo and a prominent prickle, suggesting that it was closely related to the North American section Trifoliae (Figs. 2 and 3 in the Yamada et al., 2015). Dozens of fossil cones and needles from the early Miocene of Weichang, Hebei in northern China were described as Pinus weichangensis T.-M. Yi & C.-S. Li and differ from the present cone with annular ridges (P. shengxianica) in having no annular ridges on the umbo (Table 3; Fig. 4 in the Li et al., 2022). This uncommon character is also absent from the fossil denticulatomucronate species from the Cenozoic of central Europe published by Mai (1986) (Table 3). Moreover, these European fossil pines show more morphological differences. For example, Pinus dixoni (Bowerbank) Gardner and Pinus ornata (Sternberg) Brongniart have a long seed cone with a larger size than our specimens (Plate LIV, Figs. 1–8 and plate LIX, Figs. 5–7 in the Mai, 1986; Li et al., 2022); Pinus thomasiana (Goeppert) Reichenbach and Pinus urani (Unger) Schimper possess an asymmetric seed cone (plate LVI, Figs. 1–8, plate LX, Figs. 1–4, plate LI, Figs. 11–13, plate LII, Figs. 1–5, plate, LIII, Figs. 1–4 and plate LIX, Figs. 8–9 in the Mai, 1986). The cone of Pinus nodosa Ludwig overlaps with the two cones presented here in the size, but it is very asymmetrical (plate LI, Figs. 1–10 in the Mai, 1986); Pinus hampeana (Unger) Heer has several cone characters comparable with the present fossil species but, critically, lacks annular bulges (plate LV, Figs. 1–13 and plate LIX, Figs. 10–12 in the Mai, 1986).
| Species | Cone | Apophyses | Keel type | Umbo | Age, locality | |||
| Symmetry | Shape | Size (cm) | Detail | Mucro (prickle) | ||||
| P. shengxianica sp. nov.a | Symmetrical | Conical-ovoid | 6.8 × 3.1 | Rhombic or pentagonal | Conspicuously horizontally keeled with inconspicuous longitudinal ridges | vaguely rhombic to elliptic | With a short but stout recurved prickle | Late Miocene; Jiahu, Tiantai, Zhejiang, Southeast China |
| P. speciosaa, b | Symmetrical | Ovoid or ellipsoidal with a round base | 6.3 × 3.2 or 6.4 × 3.4 | Broadly rhombic or irregularly pentagonal, slightly swollen | Prominently transversely keeled with longitudinal ridges | Depressed | Centrally sunken, with a slender, recurved prickle (beak-like) | Late Miocene; Jiahu, Tiantai, Zhejiang, Southeast China |
| P. weichangensisc | Symmetrical | Ovoid | 3.0–6.6 × 1.9–3.5 | Rhombic or polygonal, flat or shortly pyramidal | Sharply transversely keeled | Elliptic, protruding or slightly sunken | With a short prickle | Early Miocene; Weichang, North China |
| P. dixoni (Bowerbank) Gardnerd | Symmetrical | Ovoid to cylindrical, large, long | > 13.0 | Flat basally, more convex apically | Somewhat transversely keeled | Flatly convex to convex, sunken, vallate | Very slightly protruding | Middle to Late Eocene; Kayna-Süd and Böhlen, Germany |
| P. hampeana (Unger) Heerd | Symmetrical | Elongate-ovoid | 4.0–8.0 | Flattened | Slightly or obtusely keeled | Flattened | Mucronate | Middle Miocene; Wiesa, Sandförstgen and Klettwitz, Germany |
| P. nodosa Ludwigd | Very asymmetrical | Ovoid | 6.5 × 4.0 | Rhombic, basally hooked and thorny | Weakly transversely keeled | Nodularly thickened | Small, blunt or mucronate | Early Miocene; Rockenberg, Germany |
| P. thomasiana (Goeppert) Reichenbachd | Asymmetrical, curved | Ovoid | > 8.0 | Pyramidal (arched) | weakly transversely keeled | Arched | Mucronate | Late Eocene, Early to Mdiddle Oligocene; Samland, Russia; Poland; Germany |
| P. urani (Unger) Schimperd, e | Asymmetrical | Ovoid to cylindrical | > 9.0 | Broadly rhombic to pentagonal | obviously transversely keeled | Rhombic to button-shaped | Small or absent | Middle to Late Miocene; Eschweiler and Zülpich, Germany |
| P. ornata (Sternberg) Brongniarte | Symmetrical | Long ovoid to cylindric | 8.2–13.3 × 2.8–6.1 | Rhombic, flattened to slightly arched | Distinctly transversely keeled | Flattened to slightly arched | Small, indistinct | Early Miocene; Most Basin of Czech Republic |
| References: aPresent paper; bLi and Guo (1982); cLi et al. (2022); dMai (1986); eTeodoridis and Sakala (2008). | ||||||||
To date, four species of Pinus have been documented from varying sites of the Miocene Shengxian Formation of Zhejiang Province in southeastern China (Fig. 2). Several isolated shoots, needles and seed cones and one specimen in attachment from the same site as the present fossils were described by Ding et al. (2013) as Pinus premassoniana Su-Ting Ding et Bai-Nian Sun; this species can be distinguished by having a cone with a perexcentromucronate umbo (Ding et al., 2013: Plate I). The type specimen of the aforementioned P. speciosa was unearthed from Lüjia Village of Ninghai County (Plate 135, Fig. 17 in the Li and Guo, 1982), bordering Tiantai County, where our cones were discovered (Fig. 2B). The second cone presented here (Fig. 4A–F) produces a vast array of characters completely comparable with P. speciosa, e.g., size, symmetry, seed scale details, and thus can be assigned to this fossil record. Fossil cones from Huangnitang and Daluxia Villages of Ninghai County were designated as Pinus preyunnanensis X.H. Xu & B.N. Sun and Pinus prekesiya Xing, Liu & Zhou, respectively; the former can also be differentiated in bearing a perexcentromucronate umbo and a range of radial ridges around the umbo (Xu et al., 2015b: Plate I, 1–6), whereas the latter appears to display a denticulatomucronate umbo rather than the so-called perexcentromucronate umbo originally described by Xu et al. (2015b: Plate I, 9–12). Overall, these regional, concurrent fossil taxa consistently lack multiple rings of ridges around the umbo (i.e., annular bulges; Fig. 3A–F).
The extensive comparisons mentioned above demonstrate the morphological differences and similarities existing between known extant and fossil members of Pinus and our specimens, which can be used to designate the present fossil reproductive organs as the two species P. shengxianica sp. nov. and P. speciosa Li within the subgenus Pinus section Pinus subsection Pinus.
4.2. Phytogeography and fossil historyThere are 39 living species (16 introduced, 7 endemic) of Pinus in China (Fu et al., 1999), and most species assemble in central and southern China with topographically heterogeneous environments (Figs. 1A and 6; Hou, 1983; Fang et al., 2011), which is globally regarded a major center of diversity following North and Central America (Price et al., 1998; Farjon and Filer, 2013; Farjon, 2018). Today, just two wild pines, Pinus massoniana Lamb. and Pinus taiwanensis Hayata (subgenus Pinus subsection Pinus), grow in Zhejiang (Zhang and Zhang, 1986), while there are only three species native to Southeast Asia, i.e., Pinus kesiya Royle ex G. Gordon, and P. latteri and P. merkusii (Grote and Srisuk, 2021).
As discussed above, the sister species P. latteri and P. merkusii are native to mountainous areas of tropical southernmost China (Hainan, Guangxi and Guangdong) and the Indochinese Peninsula, and Sumatra, Luzon and Mindoro Islands, respectively (Fig. 6; Businský, 2014; Grote and Srisuk, 2021). Surprisingly, P. merkusii has colonized a few lands just south of the equator in central Sumatra, western Indonesia (Figs. 1A and 6; Critchfield and Little, 1966; Mirov, 1967; Farjon and Filer, 2013). The two southeastern Asian species share a key feature with the present fossil species P. shengxianica, i.e., annular bulges surrounding the umbo (Figs. 3 and 5B; Klaus, 1980), which has rarely been observed before in other extant or fossil pines. In contrast with well-developed umbo fields in these two extant members (Fig. 5B; Grote and Srisuk 2021: plate III, 2), the poorly-developed umbo field (Fig. 3) implies the fossil species is more primitive. Thus, this novel character combination suggests that P. shengxianica may represent one of ancestral conifers ever living during the early Neogene re-diversification, which are extinct and closely related to these eastern Asian survivors. This is remarkable, as Zhejiang today, located in middle subtropical China (Fig. 6), is devoid of pines with similar characters. Although fossilized cones from the Cenozoic are not new, this discovery stands out for the astonishing quality of preservation (Figs. 3 and 5B), linking the Miocene fossil pine with the two surviving pines distributed in tropical Southeast Asia (Figs. 1A and 6), and implies that some members of Pinus extended southwards from probable mid-low latitudes of origin in East Asia into tropical Southeast Asia during the Miocene.

|
| Fig. 6 Geographical ranges of Pinus latteri and P. merkusii in Southeast Asia and eastern Asian pine diversity center (after Price et al., 1998; Farjon and Filer, 2013; Farjon, 2018). Base maps were constructed with ArcGIS 10.2 based on data from the Resource and Environment Data Cloud Platform (http://www.resdc.cn/). The Roman numerals designate latitudinal vegetation zonation in Southeastern Asia in response to tropical/temperate transitions (after Ohsawa, 1993; EBVMC, 2007): I, Warm temperate deciduous broadleaved forest; II, Subtropical evergreen broadleaved forest; III, Tropical monsoon rainforest and rainforest; IV, Tibetan Plateau alpine vegetation. The distributions of P. latteri and P. merkusii refer to Fu et al. (1999), Businský (2014), Grote and Srisuk (2021) and the Global Biodiversity Information Facility. The star represents the fossil site where the present species P. shengxianica was uncovered. Arrows represent routes of range expansion for some pines in eastern Asia following its re-diversification during the Miocene. |
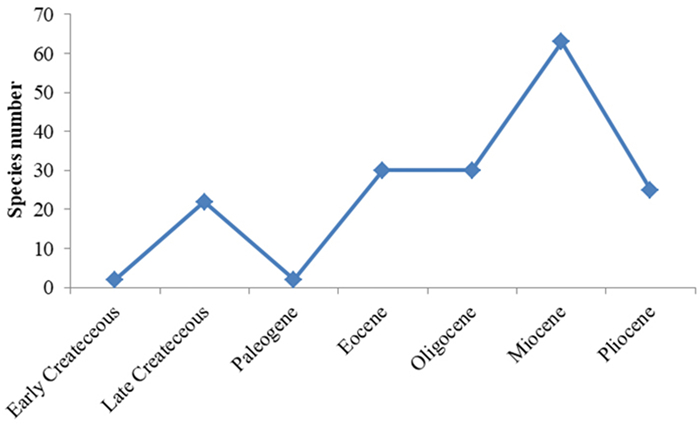
Similar to other common gymnospermous taxa (e.g., cycads; Nagalingum et al., 2011; Renner, 2011), the molecular data hypothesized that the overwhelming majority of extant species of Pinus were not continuously derived from a more ancient origin but from a rapid evolution and radiation occurring nearly synchronously across the globe in the Miocene (Jin et al., 2021). An evolutionary trend represented by the nearly complete fossil record from the Cretaceous onwards (Fig. 7; Table S1; Hu et al., 2022) is concordant with the molecular data, confirming this scenario.
|
| Fig. 7 Historical diversity of Pinus as indicated by megafossils (data sourced from Table S1). |
The rich Pinus fossils are well known from the Cretaceous and Cenozoic of North America, Europe and eastern Asia as wood, winged seeds, needles, twigs, cones or pollens (Fig. 7; Table S1). Falcon-Lang et al. (2016) described the so-called oldest known fossil named Pinus mundayi Falcon-Lang, Mages & Collinson based on the Early Cretaceous (Valanginian, ca. 140–133 Ma) charred long-shoots of Nova Scotia, southeastern Canada. However, regardless of its overestimated geological constraints, this fossil taxon has been largely questioned by Hilton et al. (2016) on the original anatomical misinterpretation therein resulting in a taxonomically inaccurate assignment to the genus Pinus. Another early record Pinus yorkshirensis Ryberg, Stockey, Hilton, Mapes, Riding et Rothwell was established on the basis of a fossil seed cone (Ryberg et al., 2012). This ancient species is arguably of Pinus, but the type specimen has no definite provenance; thus, the dating of the fossil (Hauterivian‒Barremian, ca. 131–129 Ma) was somewhat criticized because palynological sampling is not from the field horizon but directly from the attached rocks of the specimen (Falcon-Lang et al., 2016). A fossil cone species, Pinus belgica Alvin from the Wealden Formation of Belgium (Barremian–Aptian) (Alvin, 1960) is less debated than the other two Early Cretaceous representatives. These ancient fossil pines known from the Early Cretaceous of Western Europe seemingly suggest that the trans-North Atlantic mid-latitudes isolated by shallow seas at that time are the place of origin for Pinus.
Paleocene fossil occurrences are rare, suggesting that the preceding terminal-Cretaceous mass extinction likely destroyed the pine early biodiversity once widely-established in the Late Cretaceous Laurasia (Fig. 7; Table S1). With the onset of the Eocene, this coniferous taxon gradually recovered through time from the collapse of ancient lineages impacted by the famous catastrophe (Nichols and Johnson, 2008), ensuingly radiated rapidly in response to varying global climates towards ice-house conditions (Zachos et al., 2001), and peaked in past diversity during the Miocene, as indicated by the fossil evidence reviewed and illustrated in Fig. 7 (for details see Table S1), even though the potential paleobotanical sampling biases inevitably exist. The fossil history of Pinus in taxonomic terms is largely aligned with the evolutionary trajectory predicted by molecular studies (Jin et al., 2021).
More fossil records are spotted from the post-Eocene deposits of the current diversity centers in mid-low latitudes than from other latitudinal regions (Fig. 1A; Table S1; Price et al., 1998), e.g., southern China (Xing et al., 2010; Zhang et al., 2014; Xu et al., 2015a; An et al., 2019), southeastern and southwestern regions of USA (Axelrod, 1986; Stults et al., 2010), indicating that these fossil pines akin to derivatives living today were possibly assembling towards the subtropical middle-low latitudes of the Old and New Worlds rather than maintaining mid-latitudinal ancestral patterns. This biogeographical shift towards lower latitudes is similar to the conclusion of Jin et al. (2021) that Pinus species at mid-latitudes (acting as an evolutionary museum) are much older than those at other latitudes. Based on the shared traits (Figs. 3 and 5B; Grote and Srisuk, 2021: plate III, 2), extant P. merkusii and P. latteri from tropical Southeast Asia are closely related to the present fossil P. shengxianica, suggesting they possibly originated from East Asian mid-low latitude ancestors during this rapid generic re-diversification in the Miocene (Fig. 6), as was the case for the evolution of southwestern Chinese Pinus yunnanensis (Millar, 1998; Xu et al., 2015b). The global cooling (Zachos et al., 2001; Utescher et al., 2015) and increasing tectonic activities (Molnar and England, 1990; Raymo and Ruddiman, 1992) that shaped diverse regional topographies and habitats in the late Cenozoic (Jin et al., 2021) reportedly contributed to such a biogeographic dispersal event, that is, some lineages of Pinus extended from higher latitudes into lower latitudes in eastern Asia through geological time (Fig. 6).
4.3. Implications for the Miocene habitat in ZhejiangThe Miocene Shengxian Formation flora of eastern Zhejiang is renowned for diverse plant remains, amongst which pine cones and needles are very common. As mentioned above, four fossil species excluding our specimens were previously described from varying fossil sites of the equivalent strata based on morphological distinctions, that is, P. premassoniana from Jiahu in Tiantai (Ding et al., 2013), P. speciosa from Lüjia in Ninghai (Li and Guo, 1982) and P. preyunnanensis from Huangnitang in Ninghai and P. prekesiya from Daluxia in Ninghai (Xu et al., 2015b). Additionally, there are several coniferous taxa documented from the equivalent strata in eastern Zhejiang, e.g., deciduous Pseudolarix Gordon (Bai and Li, 2017), and evergreen Nothotsuga Hu ex C.N. Page (Ding et al., 2021a), Tsuga Carrière (Ding et al., 2021b), Calocedrus Kurz (Zhang et al., 2015) and Cunninghamia R. Brown (Du et al., 2012). These diverse, coeval fossil records indicate that eastern Zhejiang was one of the hot spots for coniferophyte diversity during the late Miocene.
Additionally, the Shengxian Formation flora yielded abundant broadleaved angiospermous plants and a few ferns exhibiting different growth habits, e.g., arborescent Quercus L. (Jia et al., 2015), Ormosia Jacks (Li et al., 2021), Choerospondias Burtt et Hill (Xiao et al., 2022) and Liquidambar L. (Xiao et al., 2011), liana Diploclisia Miers (Jia et al., 2020) and Smilax L. (Ding et al., 2011), shrubby Ilex L. (Li et al., 2010), aquatic Salvinia Seg. and Trapa L. (Li and Guo, 1982). The paleo-community consisting of coexisting broadleaved and needled plants with various niche preferences indicates that diverse vegetation types and an elevational zonation occurred in the Miocene of eastern Zhejiang due to varying environmental conditions (e.g., Zhe-Min Highland, Wang, 1985) that were spawned by late Cenozoic active tectonic processes and volcanism in the coastal mainland East Asia (Ho et al., 2003; Yin, 2010).
5. ConclusionsBased on extensive morphological comparisons with extant and fossil taxa, two well-preserved cones from the Miocene Shengxian Formation of Zhejiang, southeastern China are recognized with confidence as a new species P. shengxianica and a known fossil species P. speciosa, respectively. P. shengxianica shares a striking cone similarity to the two southeastern Asian species P. merkusii and P. latteri (Pinus subsection Pinus) in having annular bulges around the umbo on the apophysis, which indicates that these two low-latitude pines in Southeast Asia probably possess a close affinity with this new species and originated from East Asian mid-low latitude ancestors during the generic re-diversification that took place in the Miocene. The worldwide megafossil record from the Cretaceous onwards supports the hypothesis that the Miocene was a key period for the origin and evolution of most extant pines. The co-occurrences of coniferous taxa (e.g., evergreen Pinus, Nothotsuga, Tsuga, Calocedrus and Cunninghamia, and deciduous Pseudolarix) in this paleoflora indicate that eastern Zhejiang was one of the hot spots for coniferophyte diversity during the late Miocene. Simultaneously, the co-occurring broadleaved and needled taxa preferring diverse ecological niches confirmed that a needled-broadleaved mixed forest with complex vegetation structure and an altitudinal zonation occurred in the Miocene here.
AcknowledgmentsWe thank two anonymous reviewers and Dr. Jianwen Zhang and Raymond Porter (editors) for constructive comments and suggestions, Wenlong He and Zhiwei Li for assistance in fieldwork at the Jiahu localities, Ya Li for providing several early publications and Duobin Shan for processing map data. This work was funded in part by the National Natural Science Foundation of China (No. 41872017), the Foundation of State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (Nos. 193113 and 183125), the Fundamental Research Funds for the Central Universities, CHD (Nos. 300102272206, 300102271402 and 300102271403) and the Undergraduate Innovation and Entrepreneurship Project (No. S202210710194).
Author Contributions
X.L., Y.H., X.Z. and L.X. planned and designed the research. X.L., Y.H., L.X., L.L., R.Z. and L.Q. participated in the research. X.L., Y.H., X.Z., L.X. and L.L. wrote the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.12.002.
Alvin, K.L., 1960. Further conifers of the Pinaceae from the Wealdon Formation of Belgium. Mem. Inst. R. Sci. Natl. Belg., 146: 1-39. |
An, P.-C., Tang, D.-L., Chen, H., et al., 2019. Pliocene white pine (Pinus subgenus Strobus) needles from western Yunnan, southwestern China. Hist. Biol., 31: 1412-1422. |
Axelrod, D.I., 1986. Cenozoic history of some western American pines. Ann. Mo. Bot. Gard., 73: 565-641. DOI:10.2307/2399194 |
Bai, Y., Li, X., 2017. Late Miocene Pseudolarix amabilis bract-scale complex from Zhejiang, East China. PLoS One, 12: e0180979. DOI:10.1371/journal.pone.0180979 |
Businský, R., 2014. The Pinus merkusii agg. (Pinaceae): literature review, taxa delimitation and typifications. Phyton, 54: 1-26. |
Cheng, W. -C., Fu, L. -K., Law, Y. -W., et al., 1978. Pinaceae. In: Cheng, W. -C., Fu, L. -K. (Eds.), Fl. Reipubl. Popularis Sin. 7. Science Press, Beijing, pp. 32-281.
|
Critchfield, W.B., Little, E.L., 1966. Geographic Distribution of the Pines of the World. US Department of Agriculture and Forest Service, Washington, D.C.
|
Ding, S.-T., Chen, S.-Y., Ruan, S.-C., et al., 2021a. First fossil record of Nothotsuga (Pinaceae) in China: implications for palaeobiogeography and palaeoecology. Hist. Biol., 33: 3617-3624. DOI:10.1080/08912963.2021.1881781 |
Ding, S.-T., Sun, B.-N., Wu, J.-Y., et al., 2011. Miocene Smilax leaves and associated epiphyllous fungi from Zhejiang, East China and their paleoecological implications. Rev. Palaeobot. Palynol., 165: 209-223. DOI:10.1016/j.revpalbo.2011.03.004 |
Ding, S.-T., Wu, J.-Y., Chen, J.-L., et al., 2013. Needles and seed cones of Pinus premassoniana sp. nov., and associated pollen cone from the upper Miocene in East China. Rev. Palaeobot. Palynol., 197: 78-89. DOI:10.1016/j.revpalbo.2013.05.004 |
Ding, S.-T., Wu, J.-Y., Tang, D.-L., et al., 2021b. Seed cones of Tsuga (Pinaceae) from the upper Miocene of eastern China: biogeographic and paleoclimatic implications. Rev. Palaeobot. Palynol., 285: 104358. DOI:10.1016/j.revpalbo.2020.104358 |
Du, B.-X., Yan, D.-F., Sun, B.-N., et al., 2012. Cunninghamia praelanceolata sp. nov. with associated epiphyllous fungi from the upper Miocene of eastern Zhejiang, SE China and their palaeoecological implications. Rev. Palaeobot. Palynol., 182: 32-43. DOI:10.1016/j.revpalbo.2012.06.002 |
EBVMC (Editor Board of Vegetation Map of China, Chinese Academy of Sciences), 2007. Vegetation Map of the People's Republic of China (1: 1000000). Geology Publishing House, Beijing.
|
Falcon-Lang, H.J., Mages, V., Collinson, M., 2016. The oldest Pinus and its preservation by fire. Geology, 44: 303-306. |
Fang, J., Wang, Z., Tang, Z., 2011. Atlas of Woody Plants in China: Distribution and Climate. Higher Education Press, Beijing; Springer, Heidelberg, Dordrecht, London, New York.
|
Farjon, A., 2018. Conifers of the world. Kew Bull., 73: 1-16. DOI:10.1007/s12225-017-9725-2 |
Farjon, A., 2021. Pines: Drawings and Descriptions of the Genus Pinus. Brill, Boston.
|
Farjon, A., Filer, D., 2013. An Atlas of the World's Conifers: an Analysis of Their Distribution, Biogeography, Diversity and Conservation Status. Brill, Boston.
|
Florin, R., 1963. The distribution of conifer and taxad genera in time and space. Acta Horti Bergiani, 20: 121-312. |
Frankis, M., 2002. Classification of the Genus Pinus. http://www.pinetum.org/Lovett/classification.htm.
|
Fu, L.G., Li, N., Mill, R.R., 1999. Pinaceae. In: Wu, Z.Y., Raven, P.H. (Eds.), Flora of China, 4. Science Press, Beijing and Missouri Botanical Garden Press, St. Louris, pp. 11-52.
|
Gernandt, D.S., López, G.G., García, S.O., et al., 2005. Phylogeny and classification of Pinus. Taxon, 54: 29-42. DOI:10.2307/25065300 |
Grote, P.J., Srisuk, P., 2021. Fossil Pinus from the Cenozoic of Thailand. Rev. Palaeobot. Palynol., 295: 104501. DOI:10.1016/j.revpalbo.2021.104501 |
He, W., Sun, B., Liu, Y.-S., 2012. Fokienia shengxianensis sp. nov. (Cupressaceae) from the late Miocene of eastern China and its paleoecological implications. Rev. Palaeobot. Palynol., 176–177: 24-34. |
He, Y., 2017. Geologic Time and Ecological Environment of the Shengxian Formation Flora from Eastern Zhejiang and Volcanic Activity Influence. PhD Thesis. Lanzhou University, Lanzhou.
|
Hernández-León, S.G., Gernandt, D.S., Pérez de la Rosa, J.A., et al., 2013. Phylogenetic relationships and species delimitation in Pinus section Trifoliae Inferrred from plastid DNA. PLoS One, 8: e70501. DOI:10.1371/journal.pone.0070501 |
Hilton, J., Riding, J.B., Rothwell, G.W., 2016. Age and identity of the oldest pine fossils: comment. Geology, 44: e400-e401. DOI:10.1130/G38050C.1 |
Ho, K.-S., Chen, J.-C., Lo, C.-H., et al., 2003. 40Ar–39Ar dating and geochemical characteristics of late Cenozoic basaltic rocks from the Zhejiang–Fujian region, SE China: eruption ages, magma evolution and petrogenesis. Chem. Geol., 197: 287-318. DOI:10.1016/S0009-2541(02)00399-6 |
Hou, X.-Y., 1983. Vegetation of China with reference to its geographical distribution. Ann. Mo. Bot. Gard., 70: 509-549. DOI:10.2307/2992085 |
Hu, Y., Liang, L., Xiao, L., et al., 2022. Fossils history of Pinus and its implications in biogeography. J. Earth Environ., 13: 243-256. |
Jia, H., Ferguson, D.K., Sun, B., et al., 2020. Phytogeographic implications of a fossil endocarp of Diploclisia (Menispermaceae) from the Miocene of eastern China. Geol. J., 56: 758-767. DOI:10.1109/aeeca49918.2020.9213451 |
Jia, H., Jin, P., Wu, J., et al., 2015. Quercus (subg. Cyclobalanopsis) leaf and cupule species in the late Miocene of eastern China and their paleoclimatic significance. Rev. Palaeobot. Palynol., 219: 132-146. DOI:10.1016/j.revpalbo.2015.01.011 |
Jin, W.-T., Gernandt, D.S., Wehenkel, C., et al., 2021. Phylogenomic and ecological analyses reveal the spatiotemporal evolution of global pines. Proc. Natl. Acad. Sci. U.S.A., 118: e2022302118. DOI:10.1073/pnas.2022302118 |
Klaus, W., 1980. Neue Beobachtungen zur Morphologie des Zapfens von Pinus und ihre Bedeutung für die Systematik, Fossil-bestimmung, Arealgestaltung und Evolution der Gattung. Plant Syst. Evol., 134: 137-171. DOI:10.1007/BF00986796 |
Klaus, W., 1989. Mediterranean pines and their history. Plant Syst. Evol., 162: 133-163. DOI:10.1007/BF00936915 |
Leslie, A.B., Beaulieu, J., Holman, G., et al., 2018. An overview of extant conifer evolution from the perspective of the fossil record. Am. J. Bot., 105: 1531-1544. DOI:10.1002/ajb2.1143 |
Li, H.M., Guo, S.X., 1982. Angiospermae, in: Nanjing Inst Geol Min Res. In: Paleontological Atlas of East China, Part 3, Volume of Mesozoic and Cenozoic. Geological Publishing House, Beijing, pp. 294-316.
|
Li, R., Sun, B., Wang, Q., et al., 2015. Two new Castanopsis (Fagaceae) species based on cupule and foliage from the upper Miocene of eastern Zhejiang, China. Plant Syst. Evol., 301: 25-39. DOI:10.1007/s00606-014-1051-7 |
Li, X.-C., Manchester, S.R., Xiao, L., et al., 2021. Ormosia (Fabaceae: Faboideae) from the Miocene of southeastern China support historical expansion of the tropical genus in East Asia. Hist. Biol., 33: 3561-3578. DOI:10.1080/08912963.2021.1877700 |
Li, X.-C., Sun, B.-N., Xiao, L., et al., 2014. Stratum characteristics of the Neogene Shengxian Formation in Zhejiang province and its related fossil studies. J. Lanzhou Univ., 50: 145-153. |
Li, X., 2010. The Late Cenozoic Floras from Eastern Zhejiang Province and Their Paleoclimatic Reconstruction. PhD Thesis. Lanzhou University, Lanzhou, pp. 1-138 (in Chinese with English abstract).
|
Li, X., Sun, B., Xiao, L., et al., 2010. Leaf macrofossils of Ilex protocornuta sp. nov. (Aquifoliaceae) from the late Miocene of East China: implications for palaeoecology. Rev. Palaeobot. Palynol., 161: 87-103. DOI:10.1016/j.revpalbo.2010.02.002 |
Li, Y., Yi, T.-M., Grote, P.J., et al., 2022. A new species of Pinus (Pinaceae) from the Miocene of Weichang, Hebei province, China and its evolutionary significance. Hist. Biol., 34: 885-896. DOI:10.1080/08912963.2021.1952197 |
Liu, Y.S., Zetter, R., Ferguson, D., et al., 2007. Discriminating fossil evergreen and deciduous Quercus pollen: a case study from the Miocene of eastern China. Rev. Palaeobot. Palynol., 145: 289-303. DOI:10.1016/j.revpalbo.2006.12.001 |
Liu, Y.S., Zetter, R., Ferguson, D.K., et al., 2008. Lagerstroemia (Lythraceae) pollen from the Miocene of eastern China. Grana, 47: 262-271. DOI:10.1080/00173130802457255 |
Mai, D., 1986. Über Typen und Originale tertiärer Arten von Pinus L. (Pinaceae) in mitteleuropäischen Sammlungen--ein Beitrag zur Geschichte der Gattung in Europa. Feddes Repert., 97: 571-605. DOI:10.1002/fedr.4910970904 |
Millar, C.I., 1998. Early evolution of pines. In: Richardson, D.M. (Ed.), Ecology and Biogeography of Pinus. Cambridge University Press, Cambridge, UK, pp. 69-91.
|
Miller Jr, C.N., 1976. Early evolution in the Pinaceae. Rev. Palaeobot. Palynol., 21: 101-117. DOI:10.1016/0034-6667(76)90024-5 |
Mirov, N.T., 1967. The genus Pinus. The Ronald Press Company, New York.
|
Molnar, P., England, P., 1990. Late Cenozoic uplift of mountain ranges and global climate change: chicken or egg?. Nature, 346: 29-34. DOI:10.1038/346029a0 |
Nagalingum, N., Marshall, C., Quental, T., et al., 2011. Recent synchronous radiation of a living fossil. Science, 334: 796-799. DOI:10.1126/science.1209926 |
Nichols, D.J., Johnson, K.R., 2008. Plants and the KT Boundary. Cambridge University Press, Cambridge, England.
|
Ohsawa, M., 1993. Latitudinal pattern of mountain vegetation zonation in southern and eastern Asia. J. Veg. Sci., 4: 13-18. DOI:10.2307/3235728 |
Price, R.A., Liston, A., Strauss, S.H., 1998. Phylogeny and Systematics of Pinus, in: Richardson, D.M., (Ed.), Ecology and Biogeography of Pinus. Cambridge University Press, Cambridge, UK, pp. 49-68.
|
Price, R.A., Olsen-Stojkovich, J., Lowenstein, J.M., 1987. Relationships among the genera of Pinaceae: an immunological comparison. Syst. Bot., 12: 91-97. DOI:10.2307/2419217 |
Raymo, M.E., Ruddiman, W.F., 1992. Tectonic forcing of late Cenozoic climate. Nature, 359: 117-122. DOI:10.1038/359117a0 |
Renner, S.S., 2011. Living fossil younger than thought. Science, 334: 766-767. DOI:10.1126/science.1214649 |
Richardson, D.M., Rundel, P.W., 1998. Ecology and Biogeography of Pinus: an introduction, in: Richardson, D.M. (Ed.), Ecology and Biogeography of Pinus. Cambridge University Press, Cambridge, pp. 3-46.
|
Ryberg, P.E., Rothwell, G.W., Stockey, R.A., et al., 2012. Reconsidering relationships among stem and crown group Pinaceae: oldest record of the genus Pinus from the Early Cretaceous of Yorkshire, United Kingdom. Int. J. Plant Sci., 173: 917-932. DOI:10.1086/667228 |
Steinthorsdottir, M., Coxall, H., De Boer, A., et al., 2021. The Miocene: the future of the past. Paleoceanogr. Paleoclimatol., 36: e2020PA004037. DOI:10.1029/2020PA004037 |
Stockey, R.A., 1983. Pinus driftwoodensis sp. n. from the early Tertiary of British Columbia. Bot. Gaz., 144: 148-156. DOI:10.1086/337355 |
Stults, D.Z., Axsmith, B.J., Liu, Y.-S.C., 2010. Evidence of white pine (Pinus subgenus Strobus) dominance from the Pliocene northeastern Gulf of Mexico coastal plain. Palaeogeogr. Palaeoclimatol. Palaeoecol., 287: 95-100. DOI:10.1016/j.palaeo.2010.01.021 |
Teodoridis, V., Sakala, J., 2008. Early Miocene conifer macrofossils from the most basin (Czech Republic). N. Jb. Geol. Paläont. Abh., 250: 287-312. DOI:10.1127/0077-7749/2008/0250-0287 |
Utescher, T., Bondarenko, O.V., Mosbrugger, V., 2015. The Cenozoic cooling–continental signals from the Atlantic and Pacific side of Eurasia. Earth Planet Sci. Lett., 415: 121-133. |
Wang, H., 1985. Atlas of the Paleogeography of China. Cartographic Publishing House, Beijing (in Chinese).
|
Wang, Q., Ma, F., Yang, Y., et al., 2014. Bamboo leaf and pollen fossils from the late Miocene of eastern Zhejiang, China and their Phytogeological significance. Acta Geol. Sin., 88: 1066-1083. DOI:10.1111/1755-6724.12274 |
Xiao, L., Sun, B., Li, X., et al., 2011. Anatomical variations of living and fossil Liquidambar leaves: a proxy for paleoenvironmental reconstruction. Sci. China Earth Sci., 54: 493-508. DOI:10.1007/s11430-010-4135-4 |
Xiao, L., Wu, Z., Guo, L., et al., 2022. Late Miocene leaves and endocarps of Choerospondias (Anacardiaceae) from Zhejiang, eastern China: implications for paleogeography and paleoclimate. Biology, 11: 1399. DOI:10.3390/biology11101399 |
Xing, Y., Liu, Y.-S.C., Su, T., et al., 2010. Pinus prekesiya sp. nov. from the upper Miocene of Yunnan, southwestern China and its biogeographical implications. Rev. Palaeobot. Palynol., 160: 1-9. |
Xu, Q., Zhou, W., Kodrul, T.M., et al., 2015a. Late Eocene white pines (Pinus subgenus Strobus) from southern China. Sci. Rep., 5: 1-12. DOI:10.1097/INF.0000000000000949 |
Xu, X.-H., Wang, Z.-X., Yang, G.-L., et al., 2015b. Two Pinus species from the upper Miocene in Zhejiang, China and their palaeobiogeographic significance. Rev. Palaeobot. Palynol., 215: 68-75. |
Yamada, T., Yamada, M., Tsukagoshi, M., 2014. Fossil records of subsection Pinus (genus Pinus, Pinaceae) from the Cenozoic in Japan. J. Plant Res., 127: 193-208. DOI:10.1007/s10265-013-0621-z |
Yamada, T., Yamada, M., Tsukagoshi, M., 2015. Taxonomic revision of Pinus fujiii (Yasui) Miki (Pinaceae) and its implications for the phytogeography of the section Trifoliae in East Asia. PLoS One, 10: e0143512. DOI:10.1371/journal.pone.0143512 |
Yang, Y., Jin, P., Dong, C., et al., 2015. Palynological assemblage from the late Miocene of Tiantai-Ninghai area, Zhejiang, China and its paleovegetation and paleoclimate. Quat. Sci., 35: 669-682. |
Yang, Y., Wang, W.-M., Shu, J.-W., et al., 2018. Miocene palynoflora from Shengxian Formation, Zhejiang Province, southeast China and its palaeovegetational and palaeoenvironmental implications. Rev. Palaeobot. Palynol., 259: 185-197. |
Yin, A., 2010. Cenozoic tectonic evolution of Asia: a preliminary synthesis. Tectonophysics, 488: 293-325. |
Yoshie, F., Sara, A., 1985. Types of Florin rings, distributional patterns of epicuticular wax, and their relationships in the genus Pinus. Can. J. Plant Sci., 63: 2150-2158. DOI:10.1139/b85-304 |
Zachos, J., Pagani, M., Sloan, L., et al., 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292: 686-693. |
Zeb, U., Dong, W.-L., Zhang, T.-T., et al., 2020. Comparative plastid genomics of Pinus species: insights into sequence variations and phylogenetic relationships. J. Syst. Evol., 58: 118-132. DOI:10.1111/jse.12492 |
Zeng, L., Wang, G., 2009. Modeling golden section in plants. Prog. Nat. Sci., 19: 255-260. |
Zhang, C., Zhang, S., 1986. Flora of Zhejiang Volume 1 Pteridophyta-Gymnospermae. Zhejiang Science and Technology Publishing House, Hangzhou (in Chinese with English Summary).
|
Zhang, J.-W., D'Rozario, A., Adams, J.M., et al., 2014. The occurrence of Pinus massoniana Lambert (Pinaceae) from the upper Miocene of Yunnan, SW China and its implications for paleogeography and paleoclimate. Rev. Palaeobot. Palynol., 215: 57-67. |
Zhang, J.W., Huang, J., D'Rozario, A., et al., 2015. Calocedrus shengxianensis, a late Miocene relative of C. macrolepis (Cupressaceae) from South China: implications for paleoclimate and evolution of the genus. Rev. Palaeobot. Palynol., 222: 1-15. |
Zidianakis, G., Iliopoulos, G., Zelilidis, A., et al., 2016. Pinus remains from the Pitsidia plant assemblage document coastal pine forests in southern Crete during the late Miocene. Rev. Palaeobot. Palynol., 235: 11-30. |



