b. Ural Branch of the Russian Academy of Sciences, Institute of Plant and Animal Ecology, 8 Marta Street, Ekaterinburg 620144, Russia
Plant taxonomy and phylogenetic systematics have long been based almost exclusively on characters of generative plant parts (Takhtadzhyan 1966; Cronquist, 1988). The defining power of the characters of vegetative organs for differentiation of taxonomic plant groups is obviously low. However, the structure of vegetative organs can also be taxonomically specific. For example, such features as arrangement of leaves on a stem, leaf shape, type of venation (Takhtadzhyan, 1966), characteristics of integumentary and conducting tissues (Esau, 1969; Gamaley, 2004), leaf thickness and density (Yudina et al., 2020). Phylogenetic groups of plants can differ in the characteristics of underground organ structures, including the type and architecture of the root system (Fitter, 1987; Bouda et al., 2018), as well as the diameter of absorbing roots (Chen et al., 2013). In addition, specific root symbioses are well known in plant families, for example, mycorrhizal and nitrogen-fixing. Mycorrhiza type and mycorrhizal colonization rates are considered taxonomically correlated characters (Kiers and Heijden, 2006; Markmann et al., 2008; Davison et al., 2011; Kiers et al., 2011).
The variety of root syndromes are mainly associated with two alternative strategies of plant nutrition – absorption (acquisition) of soil resources and nutrition with or without participation of mycorrhizal fungi (Bergmann et al., 2020). It has recently been shown that mycorrhizal hyphae can penetrate or colonize the roots of non-host plants without forming typical mycorrhizal structures, but these plants can indirectly participate in mycorrhizal nutrition by means of host plants (Wang et al., 2022).
It is well known that root systems and absorbing roots of mycorrhizal and non-mycorrhizal plants are constructed in different ways (John St, 1980; Brundert, 1991; Eissenstat, 1992; Eissenstat et al., 2000; Bergmann et al., 2020). Arbuscular mycorrhiza (AM), ectomycorrhizae and other fungi colonize parenchyma of root cortex of plants. High AM colonization rates generally accompany large root diameter (Veselkin and Betekhtina, 2013; Betekhtina and Veselkin, 2019), a small specific root length (SRL) (Kong et al., 2019) and root surface area relative to volume. Mycorrhizal plants have fewer root branching orders, but cortex is longer compared to non-mycorrhizal plants (Betekhtina and Veselkin, 2019). In non-mycorrhizal and facultatively mycorrhizal plants, roots are usually not only strongly branched, but often have a short or rapidly reducing cortex, which is accompanied by a small root diameter (Betekhtina and Veselkin, 2019) and a higher SRL (Ryser, 2006). In non-mycorrhizal plants, as a rule, root hairs are well developed and roots are adapted to direct absorption of substances from soil, since they have a large surface area in relation to their volume (Eissenstat et al., 2000). Under conditions of macronutrient deficiency, roots of non-mycorrhizal plants can form various modifications, for example, bulbous root hairs and dauciform roots in sedges (Lambers et al., 2008).
The syndromes of the structure of mycorrhizal and non-mycorrhizal roots are constant in a wide range of conditions and within different taxonomic groups. Root syndromes of mycorrhizal and non-mycorrhizal plants are only one of possible major root syndromes. Another subdivision of a similar scale can be, for example, root structure syndromes of monocots and dicots, due to absence/presence of a secondary structure, as well as taxa of the superorder level (Valverde-Barrantes et al., 2015). Along with major syndromes, root character syndromes have been described in a limited number of species of steppe plants (Zhou et al., 2021), species within the same genus (Ehmig and Linder, 2020) and subgenus (Konoplenko et al., 2017).
Monocots include about 85, 000 species from 11 to 12 orders. They dominate many biomes and are of crucial economic importance (Givnish et al., 2018). Monocot plants are convenient for studying various root traits for several reasons. Firstly, there is no secondary tissues in monocot roots. Secondly, secondary xylem and a cork layers are not formed in shoots and roots. This means that roots of different monocots have the same relatively simple morpho-anatomical structure. This creates a basis for a confident comparison of subtle or quantitative features of roots of different groups or taxa. Thirdly, monocots are characterized by a wide variety of symbiotic (arbuscular and orchid mycorrhiza, dark septate endophytes) and non-symbiotic (aerenchyma, dauciform roots, simple and bulbous root hairs) adaptations for soil resources exploitation. Characteristics of root hairs, such as high density and length, are closely related to non-mycorrhizal nutrition acquisition (John St, 1980). Monocots generally have well-developed root hairs that persist over long periods (Kauff et al., 2000). This trait makes it possible to compare the length of root hairs in taxa with different adaptations to soil nutrition in an effort to understand how soil nutrition drives diversity in root structure.
Angiosperm species that develop mycorrhizae tend to form relatively short root hairs, whereas those that do not form mycorrhizae develop relatively long root hairs (Baylis, 1975; John St, 1980). However, the relationship between the length of root hairs and the intensity of mycorrhizal colonization has rarely been studied. Monocots have a wide variety of relationships with mycorrhizal fungi. Thus, to understand strategies of nutrient absorption, we must examine root hair size in these plants.
Studies of root syndromes often use integral features such as specific root length (SRL), root tissue density (RTD), and root diameter (Bergmann et al., 2020). This approach is not fully applicable to describing structural transformations of roots in response to different types of soil nutrition. For example, in thicker roots, the cortex may have a relatively larger area (Kong et al., 2014) either due to a larger number of layers of cortical cells in mycorrhizal species, or due to a well-developed aerenchyma (Lynch, 2015) in non-mycorrhizal species, which in turn strongly affects the density of root tissue and its specific length. Because of this complication, it is not always possible to adequately describe the entire variety of root syndromes. Therefore, it seems to be more reasonable to find differences between anatomical features of roots. Absorbing roots of different taxa, which undoubtedly differ in many ways, are often attributed to the same type based on SRL and RTD. However, if we consider the syndromes of traits, we may encounter an unexpectedly great diversity of absorbing root structures within a class.
We studied structural features – syndromes – of root systems and absorbing roots in species of four multispecies boreal families of monocots: Poaceae, Cyperaceae, Orchidaceae, Iridaceae. We initially assumed that roots of Poaceae, Cyperaceae, Orchidaceae, and Iridaceae species should have different structures, primarily due to the peculiarities of their interaction with mycorrhizal fungi. Therefore, the aim of our work was to give an accurate and uniform description and quantitative characterization of root trait syndromes of Poaceae, Cyperaceae, Orchidaceae, and Iridaceae species.
2. Materials and methods 2.1. Study siteStudies were carried out in Ekaterinburg, Middle Urals, Russia (56°37′N, 61°04′E), in the southern part of the boreal zone of the Middle Urals. This region has a temperate continental climate. Intensive plant growth lasts from May to August. The temperature rises above 10 ℃ for 127 days a year on average. Average annual air temperature is +0.9℃, the highest average temperature, +17.6℃, occurs in July, and the lowest average temperature, −12.6℃, occurs in January. The average annual precipitation is 537 mm, with a maximum in July. Typical vegetation in the nearby territories is pine forest. The range of other habitats, however, is also quite wide: small-leaved forests, floodplains, meadows, petrophytic habitats, anthropogenic wastelands. Two main types of soils are common in this area – brown forest and sod-podzolic.
2.2. Field research and samplingOverall, 61 species were analyzed from the following families: Poaceae (16 species), Cyperaceae (24 species), Orchidaceae (14 species) and Iridaceae (7 species). Whole plants with intact roots were collected at the end of June–July 2008–2020 in their typical natural conditions, which cover a wide range of biotopes depending on moisture. Names and ciphers of habitats are given according to the EUNIS Habitat Classification (Chytrý et al., 2020): R1B continental dry grassland (true steppe) – 7 species; T35 temperate continental Pinus sylvestris forest – 17 species; R35 moist or wet mesotrophic to eutrophic hay meadow – 9 species; R36 moist or wet mesotrophic to eutrophic pasture – 1 species; R37 temperate and boreal moist or wet oligotrophic grassland – 1 species; Q22 poor fen – 4 species; Q42 extremely rich moss-sedge fen – 3 species; Q43 tall-sedge base-rich fen – 1 species; Q53 tall-sedge bed – 10 species; V15 bare tilled, fallow or recently abandoned arable land – 1 species. The species of the genus Iris became an exception, as they are rarely found in natural conditions. We sampled Iridaceae from the collection of Botanical Garden of Ural Federal University, which is based on wild specimens introduced from natural habitats.
2.3. Determination of roots structure characteristicsRoots were cleaned out from soil with water and fixed in 70% ethanol. For each individual, we determined the order of root branching in accordance with the centripetal ordering system, where orders are distributed from the distal end (Pregitzer et al., 2002). Roots at the distal end of the root system – roots of the first order – are formed on the second order roots, which are laid on the third order roots. Thus, in monocots, the highest order is the thickest adventitious roots extending from the rhizome or stem. We took five roots of the last branching order (i.e., the thinnest) from each individual. Then, root segments of the last order were cut transversely into sections 20–50 μm thick and examined using a Leica DM 5000 microscope (Leica, Germany) at 150× magnification. The following indicators were measured: root diameter; сortex thickness; diameter of central cylinder (stele); number of primary xylem vessels; length of root hairs; number of layers of cortical cells, including exodermis and parenchyma of cortex. Quantitative characteristics were measured using Simagis Mesoplant program (SI-AMS, Russia). Partial volume of cortex and stele was determined based on the obtained measurements by dividing the stele area by the root cross-sectional area and multiplying by 100%.
2.4. Definition of mycorrhiza developmentFor each species, one sample of roots from 5 individuals was randomly selected and fixed in 70% ethanol to analyze mycorrhiza development. Mycorrhizal colonization was determined for each sample on 15 fragments of thin roots of the last and penultimate orders, each 1 cm long. Fragments were randomly selected from the root system. Roots were subjected to maceration in a water bath in KOH for 30–60 min and colored by aniline blue (Selivanov, 1981). Hyphae, arbuscules, and vesicles of AM fungi were recorded on squashed preparations at 200× magnification. Species that had arbuscules were considered mycorrhizal. Species in which hyphae and vesicles were found, but arbuscules were absent, were not classified as mycorrhizal. This condition was important for the genus Carex, most of whose species are considered non-mycorrhizal according to previous studies (Miller et al., 1999). Presence of fungal structures (hyphae, vesicles, and arbuscules) was determined in 5 fields of the microscope on each centimeter-long fragment of the root. Thus, there were 75 visual fields for each individual and 375 visual fields for each species. The intensity of mycorrhization was determined by fungus occurrence in the roots: 100% - fungal structures were found in all studied visual fields in cortical cells; 50% - fungal structures were found in half of the studied visual fields in cortical cells, etc. In general, accounting for AM using this method (Selivanov, 1981) produces results close to those of a widely used method of accounting for AM development (McGonigle et al., 1990). This was previously demonstrated in Akhmetzhanova et al. (2012).
2.5. Data analysisIn the process of analyzing variability of individual variables, the following methods were used: for ranks – Kruskal–Wallis test; for dimensional and countable features – one-way analysis of variance (ANOVA). Principal components analysis (PCA) was used to determine the key features of the root structure and reduce the prediction interval. Continuum variables were transformed before ANOVA and PCA to achieve a greater compliance with normal distribution: values of dimensional and countable features were logarithmic using natural logarithm; values of features, expressed in shares, were subjected to arcsine transformation. To assess the degree of differences between species of different families according to several characters at a time, we used permutational analysis of variance – PERMANOVA (Anderson, 2001) with calculation of Euclidean distance and 9999 permutations. ANOVA and PCA were performed using STATISTICA 8.0 software (StatSoft Inc., 1984–2007). PERMANOVA calculations were performed using R program (R Core Team, 2022), 'vegan' package (Oksanen et al., 2020).
3. Results 3.1. Characteristics of root structureAll studied features of root structure differed among families of monocots (Fig. 1). Characteristics describing the overall transverse size of a root and how successful the AM formation is varied the most among families. Orchidaceae roots had the fewest branches, while Cyperaceae and Iridaceae roots had the most branches (Fig. 2). The largest average, root diameter, stele diameter and cortex thickness were found in Orchidaceae (Fig. 3); the smallest in Cyperaceae (Figs. 3 and 4). The difference between these families in terms of the average size was 18–25 times. The abundance of mycorrhiza differed between Cyperaceae and Iridaceae the most (30 times). The least variable between families – no more than 3 times – were the partial volume of stele, the number of layers of exodermis and core parenchyma.
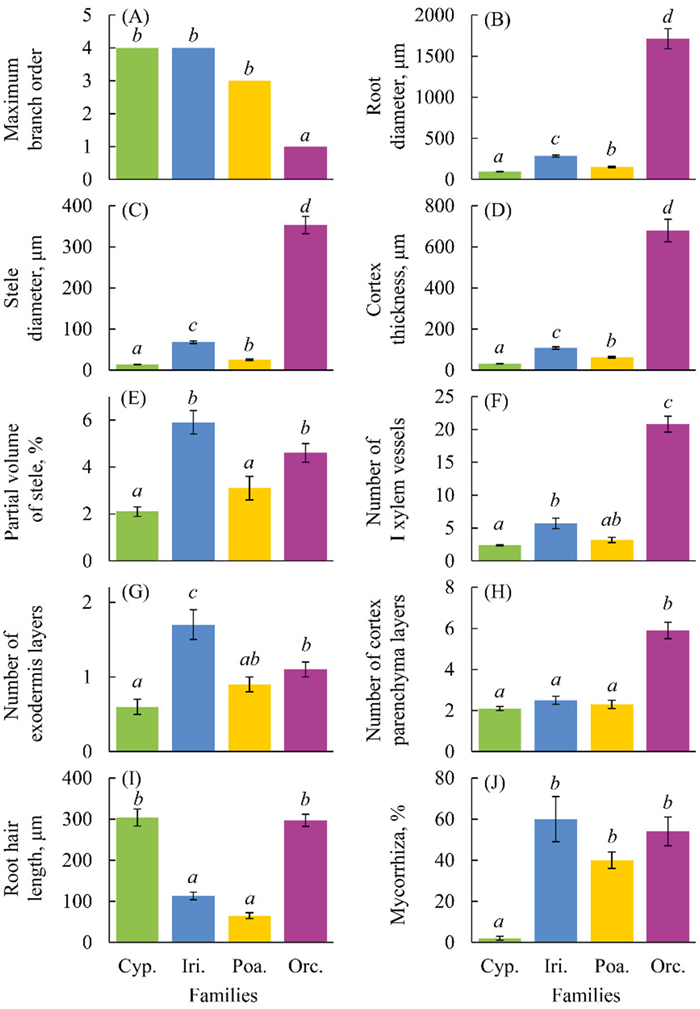
|
| Fig. 1 Traits of root structure and root system of 4 monocot families (Cyp. – Cyperaceae; Iri. – Iridaceae; Poa. – Poaceae; Orc. – Orchidaceae); alphabetic indexes show homogeneous values according to Mann–Whitney U test (maximum branch order) or Tukey's test (the other traits). |

|
| Fig. 2 Roots of Cypripedium guttatum (Orchidaceae) (A), Calamagrostis epigejos (Poaceae) (B), Iris aphylla (Iridaceae) (C), Сarex supina (Cyperaceae) (D). Roman numerals mark orders of root branching according to classification (Pregitzer et al., 2002). In Orchidaceae, the root system is represented by 1st order adventitious roots that do not branch. In Carex, 3rd order roots are formed from adventitious 4th order roots, then go 2nd order roots, and 1st order roots. The 1st order roots are the thinnest. In all species, we compared only 1st order roots. It can be seen how much 1st order roots differ in size in orchids and sedges. Bar for Сarex supina is 1 cm. |

|
| Fig. 3 Cross sections of the absorptive roots under visible light: Neottia nidus-avis (mycoheterotrophic orchid) (Orchidaceae) (A), Phragmites australis (Poaceae) (B), Iris pseudacorus (Iridaceae) (C), Carex pseudocyperus (Cyperaceae) (D). Legend: root hair (Rh); epidermis (Ep); exodermis (Ex); parenchyma of the cortex (C); endodermis (En); vessels of primary xylem (X). The bar is 100 microns. |

|
| Fig. 4 Cross sections of roots of Epipactis atrorubens (Orchidaceae) (A), Calamagrostis epigejos (Poaceae) (B), Iris pseudacorus (Iridaceae) (C), Carex pallescens (Cyperaceae) (D). Fluorescence microscopy under UV light 365 nm for detection of suberin (McKenzie and Peterson 1995) and lignin. Suberin and lignin have a more intense glow. Therefore, exodermis, endodermis and xylem vessels glow. Legend: root hair (Rh); parenchyma of the cortex (C); endodermis (En); vessels of primary xylem (X); fungal structures (F). In Iridaceae the epidermis (Ep) is strongly thickened and performs a supporting and barrier function. Therefore, when describing the syndrome of root traits, we referred to it as exodermis (Ex). Bar is 100 microns. |
Thus, species of each family had a well-defined list of root characteristics by which they could be distinguished (Table 1). Orchidaceae and Iridaceae had thick roots and a relatively large stele. The roots of Orchidaceae did not branch, but root hairs were long. In contrast, the roots of Iridaceae were highly branched but had short root hairs. Exodermis is strongly developed in Iridaceae and less developed in Orchidaceae. In addition, Orchidaceae and Iridaceae differed in mycorrhiza types: orchid (and possibly mycoheterotrophy) in Orchidaceae and typical AM in Iridaceae.
| Sign combinations | Root size | ||
| Thick roots with relatively thick stele | Thin roots with relatively small stele | ||
| Branching of root system | Unbranched and slightly branched root systems | Orchidaceae: long root hairs; a special type of mycorrhiza, including mycoheterotrophy |
Poaceae: short root hairs; typical AM |
| Branched root systems |
Iridaceae: short root hairs; typical AM |
Cyperaceae: long and specialized root hairs; random AM |
|
Poaceae and Cyperaceae had thin roots and a relatively thin stele. But in Poaceae, roots were less branched than in Cyperaceae. Families also differed in length of root hairs, with short root hairs in Poaceae and long hairs in Cyperaceae. Also, Poaceae species are usually mycorrhizal and, in our study, formed abundant typical AM. Cyperaceae, as a rule, only randomly form AM, and in our study, the abundance of AM structures in sedge roots was low.
3.2. Root structure syndromesThree of four families of monocots possessed syndromes, i.e., combinations of root traits that clearly differed from those of other families. In three families – Orchidaceae, Iridaceae and Cyperaceae – one or more characters that form the basis of a syndrome were at extremes, i.e., were either at their maximum or minimum compared to the values of species from other families. Orchidaceae had unbranched thick roots with a unique type of mycorrhizae. Iridaceae had relatively thick branched roots with developed exodermis, and AM. Cyperaceae had well-branched thin roots with long root hairs with sparse AM. Only Poaceae roots did not have any specific or extreme characteristics. By all indications, Poaceae occupy an intermediate position between other families.
Principal components analysis (Table 2) can either (ⅰ) identify the leading, main features from a larger set, or (ⅱ) collapse the original large set of measured variables into a limited number of new features – principal components. PCA has shown that some of the measured features of root structure are strongly interrelated and are combined into well-interpreted principal components. The first two principal components together explain more than 80% of the variability of the original 10 root structure characteristics, both when using the rotation of principal components and without rotation. Based on the structure of correlations obtained without their rotation, two informative, maximally unrelated, actually measured features can be distinguished: 1) diameter of stele, which characterizes a total transverse size of a root; 2) length of root hairs. The rest of the features are correlated to some extent with either the first or the second feature. Based on the structure of feature correlations obtained after principal components rotation, two easily interpreted principal components can be distinguished: 1) a component describing a transverse size of a root; 2) a component positively associated with the formation of exodermis and mycorrhiza and negatively associated with the length of root hairs.
| Root traits | Unrotated | Varimax raw | |||
| Principal component 1 – "Root size" | Principal component 2 – "Root hair length" | Principal component 1 – "Root size" | Principal component 2 – "Exodermis, root hair length and mycorrhiza" | ||
| Root order | 0.823 | −0.261 | −0.863 | 0.018 | |
| Root diameter | −0.981 | 0.109 | 0.972 | 0.172 | |
| Stele diameter | −0.990 | 0.017 | 0.954 | 0.263 | |
| Cortex thickness | −0.985 | 0.052 | 0.960 | 0.227 | |
| Partial volume of stele | −0.615 | −0.399 | 0.478 | 0.556 | |
| Number of I xylem vessels | −0.945 | 0.168 | 0.954 | 0.105 | |
| Number of exodermis layers | −0.487 | −0.626 | 0.291 | 0.738 | |
| Number of cortex parenchyma layers | −0.875 | 0.322 | 0.930 | −0.063 | |
| Root hair length | −0.136 | 0.832 | 0.365 | −0.760 | |
| Mycorrhiza | −0.716 | −0.549 | 0.532 | 0.729 | |
| Share of variance explained | 0.64 | 0.18 | 0.60 | 0.21 | |
Using PERMANOVA analysis, we found that the monocotyledonous families differ from each other in terms of root structure, regardless of the studied set of features. Statistically significant differences between the families were established by analyzing three sets of features: 1) according to the complex of all real root structure characteristics: F(3; 57) = 104.87; P < 0.001; belonging to a particular family determines 85% of the total variance of root features; 2) according to two key real features (Fig. 5 a): F(3; 57) = 207.53; P < 0.001; belonging to a particular family determines 92% of the total variance of root features; 3) in the space of two main principal components (Fig. 5b): F(3; 57) = 103.92; P < 0.001.

|
Fig. 5 Differentiation of species of four monocots families in Middle Urals (    |
In the process of comparing all four families simultaneously, PERMANOVA tests a hypothesis that there are significant differences in at least one out of six possible pairwise comparisons. In other words, based on the results of PERMANOVA, i.e., comparison of all four families, it is impossible to determine whether the root structure syndromes differ between the families that are closest to each other. Therefore, we conducted an additional study to see whether the differences between two families whose centroids are closest to each other (i.e., Iridaceae and Poaceae; see Fig. 4) are statistically significant. In the spaces specified by different combinations of features, the following significance of difference between these families was obtained: 1) according to the complex of all the real characteristics: F(1; 21) = 16.76; P < 0.001; 2) according to two key real characteristics (see Fig. 5): F(1; 21) = 15.39; P < 0.001; 3) in space of two main factors (see Fig. 5): F(1; 21) = 5.86; P = 0.009. Thus, root structure syndromes differ significantly even between two families with the closest character values. Therefore, the conclusion about the specificity of root structure syndromes in four monocotyledonous families has been proved and does not depend on specific methods of data analysis.
4. DiscussionAvailable information on the characteristics of root structure and their diverse combinations observed during adaptation to different soil and climatic conditions is incomplete. What is clear thus far is only the most general changes in root structure in response to major environmental factors (Freschet et al., 2021). This model (Freschet et al., 2021) does not consider the taxonomic specificity of a root structure.
To explain the variability of roots, the root economics approach has traditionally been used, which includes strategies for fast and slow acquisition of resources by roots (Reich, 2014; Weemstra et al., 2016). The diversity of root trait syndromes has recently been associated with two contrasting strategies of soil nutrition – the ability to absorb soil resources with the participation of mycorrhizal fungi or autonomously (Bergmann et al., 2020). Integral indicators have been used to isolate these syndromes: SRL, RTD, root diameter, cortical fraction (Bergmann et al., 2020). Apparently, syndromes are more difficult to identify when it comes to the analysis of anatomical features, where the roots of plants belonging to the same group (AM) may have diverse morphotypes that cannot be attributed to one group (Betekhtina and Veselkin, 2019).
Our work focuses on species from the four largest families of monocots (Givnish et al., 2018). These families belong to two orders: Poales (Cyperaceae and Poaceae) and Asparagales (Iridaceae and Orchidaceae) (Givnish et al., 2018). In total, we studied 61 species of monocots. This consists of 10% of cereal species, 23% of sedges and 44% of orchids from those growing in the Middle Urals. Our data convincingly confirm a high specificity of absorption root structural syndromes in four families of monocots.
In monocots, compared to dicots (Betekhtina and Veselkin, 2019), the overall variability of some root characteristics is high. For example, in herbaceous dicots, root system branching varies from 3 to 5 orders (Betekhtina and Veselkin, 2019), whereas in monocots within the present study – from 1 to 4 orders. In dicots, the thickness of first-order roots varied between families by no more than two times, and in monocots, up to 15–20 times. This is more than a 10-fold difference observed when comparing the thickness of absorptive roots (excluding roots with secondary thickening) of grasses on a global scale (Ma et al., 2018). High variability in root structure of monocots is explained by the difference between roots of Orchidaceae and the others. If we do not consider Orchidaceae, the difference between families in root system branching degree is leveled, and the characters describing transverse dimensions differ by only a factor of 2–3.
The central feature of Orchidaceae root syndrome is an interaction with basidiomycetes to form orchid mycorrhiza (Smith and Read, 2010). The root system of Orchidaceae consists of fleshy, non-branching roots that are laid down on stems or rhizomes. Weak root branching along with large transverse dimensions are also found in other plant groups (Comas and Eissenstat, 2009; Comas et al., 2014; Valverde-Barrantes et al., 2015). This structure is optimal for the habitat of mycorrhizal fungi, which can increase the absorptive surface of roots by a factor of 2–6 compared to a branching root system with fine roots (McCormack and Iversen, 2019). Fungal mycelium increases the absorptive surface area of roots (Dearnaley and Cameron, 2017). This may be why orchid roots usually form few root hairs (Hew and Yong, 2004). In our results, orchid root hairs are long. Thick parenchyma of the cortex in Orchidaceae, in addition to the symbiosis formation area, can perform storage functions. In some orchids, absorbing roots are perennial organs (Salazar et al., 2003; Hagsater et al., 2005). In orchids, along with the development of parenchyma, a strong development of the stele was recorded. Our results showed that cortical parenchyma is well developed in taxa with a well-developed stele, or, more precisely, in taxa with a well-developed xylem. The number and size of vessels in orchid roots is an order of magnitude higher than in roots of other families. In absorptive roots, stable allometric connections exist between cortex and stele (Kong et al., 2019). In relation to axial transport channels (phloem sieve tubes, xylem vessels), radial transport networks of parenchymal cells are the origins and ends of transport system, zones of its loading or unloading. The root parenchyma with developed mycorrhiza is accompanied by an increase in the ascending flow, compared with the parenchyma of non-mycorrhizal species (Gamaley, 2004). The specificity of orchid roots structure correlates with their other bioecological features: usually highly specialized entomophily; small seeds; ontogenesis with stage of protokorma and other unusual variants of ontogenesis; a special type of mycorrhizae not found in other plants (Waterman and Bidartondo, 2008; Fay and Chase 2009; Merckx, 2013).
The transverse dimensions of Cyperaceae, Iridaceae, and Poaceae roots were not the same, but quite similar. Significant differences among Cyperaceae, Iridaceae, and Poaceae were related to adaptations for absorbing soil resources. The structure of root features correlations with the main principal component "Exodermis, root hairs length and mycorrhiza" (Table 2) indicates that there is a negative correlation between the abundance of AM and the length of root hairs. Such a correlation is prominent if families with arbuscular mycorrhiza – Cyperaceae, Iridaceae, and Poaceae – are analyzed without Orchidaceae. This approach allows us to see possible ranges of AM abundance depending on the length of root hairs (Fig. 6). In Cyperaceae, the leading adaptation for absorption of soil resources is long root hairs, whereas in Iridaceae and Poaceae it is predominantly AM.
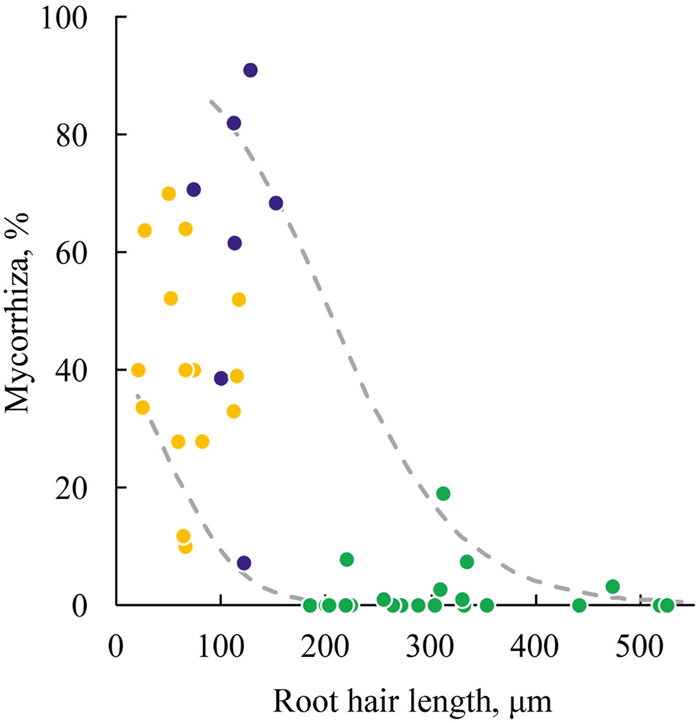
|
Fig. 6 Dependence between length of root hairs and abundance of arbuscular mycorrhiza in species of three monocots families in Middle Urals (   |
Our data clearly illustrate a well-known pattern: the fundamental ability to form one or another type of mycorrhiza is determined phylogenetically (Kiers and Heijden, 2006; Markmann et al., 2008; Davison et al., 2011; Kiers et al., 2011). However, the abundance of mycorrhiza depends not only on the taxonomic position of plants, but also on the environmental conditions (Hetrick, 1991). This is evidenced by a large range of variations in AM abundance in both Iridaceae and Poaceae.
The family Iridaceae is closely related to the orchid family (Givnish et al., 2018). In Iridaceae species, we found an unexpected combination of root characters: a high number of root branching orders, thick roots with thick cortex and stele, and short root hairs. Iridaceae were probably originally adapted to arid conditions (Rodionenko, 1961), and most modern iris species have pronounced xerophytic properties, while the presence of hygrophytes and hydrophytes among them is secondary. The root systems of irises, although branched, are rather short. This indirectly indicates adaptation to pulsating inflow of water with precipitation. The strong growth of cortex in Iridaceae may be associated not only with the creation of conditions for mycorrhizal fungi (Comas et al., 2012), but also with a water storage function. It is significant that some species of the Iridaceae family are adapted to dry conditions due to the formation of contractilis roots (Ruzin, 1979; Jernstedt, 1984), which is also accompanied by the strong growth of root parenchyma (North et al., 2008). Strong cortex growth requires strengthening mechanical tissue – exodermis. In turn, exodermis (ⅰ) maintains the shape of thick roots, (ⅱ) retains water in tissues, and (ⅲ) increases the sucking power of tissues due to the elastic deformation of the cell wall (Bakhari and Wendelbo, 1978).
In the Cyperaceae root syndrome, the leading characteristics are those that compensate for a weak connection with AM fungi. These are a highly branched root system, thin roots with poorly developed cortex and stele, sparse exodermis, and long root hairs. Such structural features are aimed at increasing the surface of contact with soil and allow sedges to explore large volumes of soil. In the absence of mycorrhiza, low-accessible elements, such as phosphorus, can be obtained from sedges using dauciform roots. Interestingly, Cyperaceae is characterized by the largest range of variability for principal component 2 in Fig. 5a. This may be Cyperaceae represented the largest number of species in this study. But it may also reflect Cyperaceae's great variety of original adaptations for absorbing soil resources. In addition to long root hairs and AM, a wide range of other root modifications are possible in Cyperaceae: dauciform roots (Shane et al., 2006); bulbous-based root hairs (Miller et al., 1999); ectomycorrhiza-like structures (Harrington and Mitchell, 2002); aerenchyma (Visser et al., 2000); and dark septate endophytes (Muthukumar et al., 2004; Weishampel and Bedford, 2006). These adaptations can be widely combined with each other both in one sedge species and in one individual (Veselkin et al., 2014; Konoplenko et al., 2017). Despite the fact that the species of Cyperaceae family are poorly related to mycorrhiza, their roots are characterized by a high percentage of cortex. Most of the Carex species grow in waterlogged habitats and cortex overgrowth may be associated with the formation of aerenchyma, which is necessary for storing oxygen inside the roots during flooding (Kutschera and Lichtenegger, 1982). At the same time, the death and lysis of cortical cells during the formation of aerenchyma can negatively affect the development of mycorrhiza. In addition, the lower Cyperaceae stele is characterized by the absence of moisture deficiency.
The Poaceae family is closely related to Cyperaceae (Givnish et al., 2018). The key features of the Poaceae root syndrome are intermediate between Iridaceae and Cyperaceae. These features are low-extended cortex and stele, and typical exodermis. The ability to form a typical AM is expectedly combined with short root hairs. However, the structural features of cereal roots leave them the opportunity to regulate the surface area of exchange with soil autonomously, i.e., without mycorrhiza, which can compensate for non-obligate relationships of grass roots with mycorrhizal fungi. At the same time, the nature of emerging compromises in roots of cereals is largely determined by adaptation to the specific habitat conditions (Fort et al., 2013; Wahl and Ryser, 2000).
5. ConclusionSyndromes of root features of four families of monocots – Cyperaceae, Iridaceae, Poaceae, and Orchidaceae – were predictably different. An unexpected and therefore new result of our work that the root syndromes of different families of monocots show large-scale and highly significant differences. In fact, root structure syndromes are unique in each family. It is expected that the main vector, with which all other structural features of roots are consistent, is set by the interaction with mycorrhizal fungi. This conclusion is fully consistent with similar comparisons only within dicots (Betekhtina and Veselkin, 2014) and with generalizations about plants in general (Bergmann et al., 2020). According to the peculiarities of the roots structure, the species of four families of monocots fall into three contrasting groups: Orchidaceae with a special type of mycorrhiza; Cyperaceae with rare or occasional arbuscular mycorrhiza; Iridaceae and Poaceae with typical arbuscular mycorrhiza. The features of roots of different families are so prominent that even Iridaceae and Poaceae differ significantly according to the majority of characters. The specificity of Cyperaceae, Iridaceae, Poaceae, and Orchidaceae root syndromes means that species of these families use different strategies for obtaining water and nutrients, including different symbiotic interactions in these strategies. But the traits that make up root syndromes are also correlated with other functions performed by roots, such as storing water and nutrients. The established patterns are important for understanding variability of root characters in species of different taxa growing within the same community.
We analyzed root syndromes of only four families of monocots. This, of course, does not give a complete picture of the diversity of root syndromes in all 59, 000 species of monocots and even in monocots of the boreal zone. It would be interesting to determine whether root syndromes are specific to other monocot families common in the boreal zone, such as Liliaceae and Alliaceae. Perhaps such a comparison would expand the diversity of described monocot root syndromes.
AcknowledgementsThe authors would like to express their deepest gratitude to A.N. Sozontov (Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences) for performing PERMANOVA analysis. The work of D.V. Veselkin is a part of the research project of the Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences (№122021000092–9).
Author contributions
AB and DV developed the ideas and designed methodology; AB and DT collected the data; DV analyzed the data; AB and DV wrote the manuscript. All the authors significantly contributed to the drafts and gave their final approval for publication.
Declaration of competing interest
No conflict of interest exits with the submission of this manuscript, and it is approved by all authors for publication.
Akhmetzhanova, A.A., Soudzilovskaia, N.A., Onipchenko, V.G., et al., 2012. A rediscovered treasure: mycorrhizal intensity database for 3000 vascular plant species across the former Soviet Union. Ecology, 93: 689-690. DOI:10.1890/11-1749.1 |
Anderson, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol., 26: 32-46. DOI:10.1111/j.1442-9993.2001.01070 |
Bakhari, M.H., Wendelbo, P., 1978. On anatomy, adaptations to xerophytism and taxanomy of Anabasis inclusive Esfandiaria (Chenopodiaceae). Bot. Not., 131: 279-292. |
Baylis, G.T.S., 1975. Magnolioid mycorrhiza and mycotrophy in root systems derived from it. In: Endomycorrhizas; Proceedings of a Symposium.
|
Bergmann, J., Weigelt, A., Van der Plas, F., et al., 2020. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv., 6: eaba3756. DOI:10.1126/sciadv.aba3756 |
Betekhtina, A.A., Veselkin, D.V., 2014. Relationship between root structure of herbaceous dicotyledonous plants and their mycorrhizal status. Dokl. Biol. Sci., 459: 348-350. DOI:10.1134/S0012496614060064 |
Betekhtina, A.A., Veselkin, D.V., 2019. Mycorrhizal and non-mycorrhizal dicotyledonous herbaceous plants differ in root anatomy: evidence from the Middle Urals, Russia. Symbiosis, 77: 133-140. DOI:10.1007/s13199-018-0571-2 |
Bouda, M., Brodersen, C., Saiers, J., 2018. Whole root system water conductance responds to both axial and radial traits and network topology over natural range of trait variation. J. Theor. Biol., 456: 49-61. DOI:10.1016/j.jtbi.2018.07.033 |
Brundert, М. , 1991. Mycorrhizas in natural ecosystems. Adv. Ecol. Res., 21: 171-313. DOI:10.1016/S0065-2504(08)60099-9 |
Chen, W., Zeng, H., Eissenstat, D.M., et al., 2013. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Global Ecol. Biogeogr., 22: 846-856. DOI:10.1111/geb.12048 |
Chytrý, M., Tichý, L., Hennekens, S.M., et al., 2020. EUNIS Habitat Classification: expert system, characteristic species com-binations and distribution maps of European habitats. Appl. Veg. Sci., 23: 648-675. DOI:10.1111/avsc.12519 |
Comas, L.H., Callahan, H.S., Midford, P.E., 2014. Patterns in root traits of woody species host-ing arbuscular and ectomycorrhizas: implications for the evolution of belowground strategies. Ecol. Evol., 4: 2979-2990. DOI:10.1002/ece3.1147 |
Comas, L.H., Eissenstat, D.M., 2009. Patterns in root trait variation among 25 co-existing North American forest species. New Phytol., 182: 919-928. DOI:10.1111/j.1469-8137.2009.02799.x |
Comas, L.H., Mueller, K.E., Taylor, L.L., et al., 2012. Evolutionary patterns and biogeochemical significance of angiosperm root traits. Int. J. Plant Sci., 173: 584-595. DOI:10.1086/665823 |
Cronquist, A., 1988. The Evolution and Classification of Flowering Plants. The New
York Botanical Garden, Bronx.
|
Davison, J., Öpik, M., Daniell, T.J., et al., 2011. Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microbiol. Ecol., 78: 103-115. DOI:10.1111/j.1574-6941.2011.01103.x |
Dearnaley, J.D., Cameron, D.D., 2017. Nitrogen transport in the orchid mycorrhizal symbiosis – further evidence for a mutualistic association. New Phytol., 213: 10-12. DOI:10.1111/nph.14357 |
Ehmig, M., Linder, H.P., 2020. Unexpected diversity and evolutionary lability in root architectural ecomorphs in the rushes of the hyperdiverse Cape flora. New Phytol., 227: 216-231. DOI:10.1111/nph.16522 |
Eissenstat, D.M., 1992. Costs and benefits of constructing roots of small diameter. J. Plant Nutr., 15: 763-782. DOI:10.1080/01904169209364361 |
Eissenstat, D.M., Wells, C.E., Yanai, R.D., et al., 2000. Building roots in a changing environment: implications for root longevity. New Phytol., 147: 33-42. DOI:10.1046/j.1469-8137.2000.00686.x |
Esau, K., 1969. Plant Anatomy. Publishing House. Mir, Moscow.
|
Fay, M.F., Chase, M.W., 2009. Orchid biology: from Linnaeus via Darwin to the 21st century. Ann. Bot., 104: 359-364. DOI:10.1093/aob/mcp190 |
Fitter, A.H., 1987. An architectural approach to the comparative ecology of plant root systems. New Phytol., 106: 61-77. DOI:10.1111/j.1469-8137.1987.tb04683.x |
Fort, F., Jouany, C., Cruz, P., 2013. Root and leaf functional trait relations in Poaceae species: implications of differing resource-acquisition strategies. J. Plant Ecol., 6: 211-219. DOI:10.1093/jpe/rts034 |
Freschet, G.T., Roumet, C., Comas, L.H., et al., 2021. Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol., 232: 1123-1158. DOI:10.1111/nph.17072 |
Gamaley, YuV., 2004. Transport System of Vascular Plants. St. Petersburg University Publishing House, St. Petersburg, p. 422.
|
Givnish, T.J., Zuluaga, A., Spalink, D., et al., 2018. Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots. Am. J. Bot., 105: 1888-1910. DOI:10.1002/ajb2.1178 |
Hágsater, E., Soto, M.A., Salazar, G.A., et al., 2005. The Orchids of México. Redacta, México City.
|
Harrington, T.J., Mitchell, D.T., 2002. Colonization of root systems of Carex flacca and C. pilu-lifera by Cortinarius (Dermocybe) cinnamomeus. Mycol. Res., 106: 452-459. DOI:10.1017/S0953756202005713 |
Hetrick, B.A.D., 1991. Mycorrhizas and root architecture. Experientia, 47: 355-362. DOI:10.1007/BF01972077 |
Hew, C.S., Yong, J.W.H., 2004. The Physiology of Tropical Orchids in Relation to the Industry, second ed. World Scientific Publishing Co., Pte. Ltd, p. 388.
|
Jernstedt, J.A., 1984. Root contraction in hyacinth. I. Effects of IAA on differential cell expansion. Am. J. Bot., 71: 1080-1089. DOI:10.1002/j.1537-2197.1984.tb11960.x |
John St, T.V., 1980. Root size, root hairs and mycorrhizal infection: a re-examination of Baylis's hypothesis with tropical trees. New Phytol., 84: 483-487. DOI:10.1111/j.1469-8137.1980.tb04555.x |
Kauff, F., Rudall, P.J., Conran, J.G., 2000. Systematic root anatomy of Asparagales and other monocotyledons. Plant Syst. Evol., 223: 139-154. DOI:10.1007/BF00985275 |
Kiers, E.T., Duhamel, M., Beesetty, Y., et al., 2011. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science, 333: 880-882. DOI:10.1126/science.1208473 |
Kiers, E.T., Heijden, M.G.A., 2006. Mutualistic stability in the arbuscular mycorrhizal symbiosis: exploring hypotheses of evolutionary cooperation. Ecology, 87: 1627-1636. DOI:10.1890/0012-9658(2006)87[1627:MSITAM]2.0.CO;2 |
Kong, D., Ma, C., Zhang, Q., et al., 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol., 203: 863-872. DOI:10.1111/nph.12842 |
Kong, D., Wang, J., Wu, H., et al., 2019. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun., 10: 1-9. DOI:10.1038/s41467-019-10245-6 |
Konoplenko, M.A., Güsewell, S., Veselkin, D.V., 2017. Taxonomic and ecological patterns in root traits of Carex (Cyperaceae). Plant Soil, 420: 37-48. DOI:10.1007/s11104-017-3292-z |
Kutschera, L., Lichtenegger, E., 1982. Wurzelatlas Mitteleuropaischer Grunlandpflanzen (Root Compendium of Central European Grassland Vegetation. Gustav
Fischer Verlag, Stuttgart, Germany, p. 516.
|
Lambers, H., Raven, J.A., Shaver, G.R., et al., 2008. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol., 23: 95-103. DOI:10.1016/j.tree.2007.10.008 |
Lynch, J.P., 2015. Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant Cell Environ., 38: 1775-1784. DOI:10.1111/pce.12451 |
Ma, Z., Guo, D., Xu, X., et al., 2018. Evolutionary history resolves global organization of root functional traits. Nature, 555: 94-97. DOI:10.1038/nature25783 |
Markmann, K., Giczey, G., Parniske, M., 2008. Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria. PLoS Biology, 6: e68. DOI:10.1371/journal.pbio.0060068 |
McCormack, M.L., Iversen, C.M., 2019. Physical and functional constraints on viable below-ground acquisition strategies. Front. Plant Sci., 1215. DOI:10.3389/fpls.2019.01215 |
McGonigle, T.P., Miller, M.H., Evans, D.G., et al., 1990. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol., 115: 495-501. DOI:10.1111/j.1469-8137.1990.tb00476.x |
McKenzie, B.E., Peterson, C.A., 1995. Root browning in Pinus banksiana Lamb. and Eucalyptus pilularis Sm. 2. Anatomy and permeability of the cork zone. Bot. Acta, 108: 138-143. DOI:10.1111/j.1438-8677.1995.tb00843.x |
Merckx, V.S., 2013. Mycoheterotrophy: the Biology of Plants Living on Fungi. Springer, New York.
|
Miller, R.M., Smith, C.I., Jastrow, J.D., et al., 1999. Mycorrhizal status of the genus Carex (Cyperaceae). Am. Jails, 86: 547-553. DOI:10.2307/2656816 |
Muthukumar, T., Udaiyan, K., Shanmughavel, P., 2004. Mycorrhiza in sedges – an overview. Mycorrhiza, 14: 65-77. DOI:10.1007/s00572-004-0296-3 |
North, G.B., Brinton, E.K., Garrett, T.Y., 2008. Contractile roots in succulent monocots: convergence, divergence and adaptation to limited rainfall. Plant Cell Environ., 31: 1179-1189. DOI:10.1111/j.1365-3040.2008.01832.x |
Oksanen, J., Blanchet, F.G., Friendly, M., et al., 2020. Vegan: Community Ecology Package v. 2.5-7. URL: cran.r-project.org/package=vegan.
|
Pregitzer, K.S., DeForest, J.L., Burton, A.J., et al., 2002. Fine root architecture of nine North American trees. Ecol. Monogr., 72: 293-309. DOI:10.1890/0012-9615(2002)072[0293:FRAONN]2.0.CO;2 |
R Core Team, 2022. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL:
|
Reich, P.B., 2014. The world-wide 'fast–slow'plant economics spectrum: a traits manifesto. J. Ecol., 102: 275-301. DOI:10.1111/1365-2745.12211 |
Rodionenko, G.I., 1961. Genus Iris-Iris L.: questions of morphology, biology, evolution and taxonomy. Izd. Akad. Science SSSR.: 215. |
Ruzin, S.E., 1979. Root contraction in Freesia (Iridaceae). Am. J. Bot., 66: 522-531. DOI:10.1002/j.1537-2197.1979.tb06254.x |
Ryser, P., 2006. The mysterious root length. Plant Soil, 286: 1-6. DOI:10.1007/s11104-006-9096-1 |
Salazar, G.A., Chase, M.W., Soto Arenas, M.A., et al., 2003. Phylogenetics of Cranichideae with emphasis on Spiranthinae (Orchidaceae, Orchidoideae): evidence from plastid and nuclear DNA sequences. Am. J. Bot., 90: 777-795. DOI:10.3732/ajb.90.5.777 |
Selivanov, I.A., 1981. Mycosymbiotrophism as a form of consortium relationships in plant cover of soviet union. Min. Sci., 232. |
Shane, M.W., Cawthray, G.R., Cramer, M.D., et al., 2006. Specialized «dauciform» roots of Cyperaceae are structurally distinct, but functionally analogous with «cluster» roots. Plant Cell Environ., 29: 1989-1999. DOI:10.1111/j.1365-3040.2006.01574.x |
Smith, S.E., Read, D.J., 2010. Mycorrhizal Symbiosis, Third ed. Academic Press, New
York.
|
Takhtadzhyan, A.L., 1966. System and Phylogeny of Flowering Plants. Nauka, Moscow/Leningrad, p. 611.
|
Valverde-Barrantes, O.J., Smemo, K.A., Blackwood, C.B., 2015. Fine root morphology is phylogenetically structured, but nitrogen is related to the plant economics spectrum in temperate trees. Funct. Ecol., 29: 796-807. DOI:10.1111/1365-2435.12384 |
Veselkin, D.V., Betekhtina, A.A., 2013. Testing hypotheses about difference in root sizes in connection with type of ecological strategy and mycotrophic status of plant species. Bull. MOIP. Dep. Biol., 118: 42-49. |
Veselkin, D.V., Konoplenko, M.A., Betekhtina, A.A., 2014. Means for soil nutrient uptake in sedges with different ecological strategies. Russ. J. Ecol., 45: 547-554. DOI:10.1134/S1067413614060149 |
Visser, E.J.W., Bögemann, G.M., Van de Steeg, H.M., et al., 2000. Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol., 148: 93-103. DOI:10.1046/j.1469-8137.2000.00742.x |
Wahl, S., Ryser, P., 2000. Root tissue structure is linked to ecological strategies of grasses. New Phytol., 148: 459-471. DOI:10.1046/j.1469-8137.2000.00775.x |
Wang, Y., He, X., Yu, F., 2022. Non-host plants: are they mycorrhizal networks players?. Plant Divers., 44: 127-134. DOI:10.1016/j.pld.2021.06.005 |
Waterman, R.J., Bidartondo, M.I., 2008. Deception above, deception below: linking pollination and mycorrhizal biology of orchids. J. Exp. Bot., 59: 1085-1096. DOI:10.1093/jxb/erm366 |
Weemstra, M., Mommer, L., Visser, E.J., et al., 2016. Towards a multidimensional root trait framework: a tree root review. New Phytol., 211: 1159-1169. DOI:10.1111/nph.14003 |
Weishampel, P.A., Bedford, B.L., 2006. Wetland dicots and monocots differ in colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza, 16: 495-502. DOI:10.1007/s00572-006-0064-7 |
Yudina, P.K., Ivanov, L.A., Ronzhina, D.A., et al., 2020. Effect of systematic position at family level on functional traits of steppe leaves plants. Sib. Ecol. J., 5: 647-661. DOI:10.15372/SEJ20200508 |
Zhou, M., Bai, W., Li, Q., et al., 2021. Root anatomical traits determined leaf-level physiology and responses to precipitation change of herbaceous species in a temperate steppe. New Phytol., 229: 1481-1491. DOI:10.1111/nph.16797 |



