b. School of Pharmacy and Life Science, Jiujiang University, Jiujiang 332005, China;
c. South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China
Mutong, the general name of the plants in the genus Akebia, are members of the Lardizabalaceae family that occur naturally in East Asia, specifically in China, Japan, and Korea. The Flora Reipublicae Popularis Sinicae (FRPS) states that the genus Akebia contains four species (and two associated subspecies) of climbing plants, namely, Akebia quinata (Houttuyn) Decaisne, Akebia longeracemosa Matsumura, Akebia pentaphylla (Makino) Makino, Akebia trifoliata (Thunberg) Koidzumi, A. trifoliata subsp. australis (Diels) T. Shimizu and A. trifoliata subsp. longisepala H.N. Qin (Editorial Commission of Chinese Flora of Chinese Academy of Sciences, 2001). The plants of this genus are perennial, deciduous woody vines that produce edible fruits commonly known as "Bayuezha" in China (Li et al., 2010a; Zou et al., 2019). The fruits of Akebia plants are usually set singly or in clusters of two to five fruit on the infructescence, and crack longitudinally along the ventral suture when they get ripe in August or September. Akebia fruits often contain many essential nutrients, such as sugars, minerals, and crude proteins, as well as metabolic components such as flavonoids, polyphenols, and vitamins (Zou et al., 2018, 2019). Experimental and clinical studies have revealed that Akebia species possess anti-microbial, anti-inflammatory, anti-oxidative, and anti-cancer properties (Lu et al., 2019; Wang et al., 2015, 2019). Akebia quinata, A. trifoliata, and A. trifoliata subsp. australis are the source plants of the traditional Chinese medicines "Akebiae Caulis" and "Akebiae Fructus", and are used to treat fever, activate blood circulation, and facilitate diuresis (Chinese Pharmacopoeia Commission, 2020). Therefore, Akebia fruit has been widely regarded as a new type of medicinal and edible fruit with high development potential in recent years (Huang et al., 2021a).
Many researchers and breeders have been attracted by the commercial value of Akebia. Akebia seeds contain up to 39% oil, with 77% unsaturated fatty acids, and are often used to produce edible oil in southern Chinese villages (Du et al., 2012). The fruit peel of Akebia is rich in pectin and has the potential to produce pectin for commercial food industry applications (Jiang et al., 2012). Akebia flowers are abundant with anthocyanins which are active compounds with various biological activities, and the flower anthocyanins can be successfully extracted using the radio frequency heating-assisted enzymatic extraction method (Jiang et al., 2020). However, in the past few decades, most studies on Akebia have mainly focused on its ethnopharmacology, phytochemistry, and pharmacology (An et al., 2016; Maciag et al., 2021; Peng et al., 2021), while few studies have focused on Akebia domestication, germplasm resources, and breeding methods, which are important for the genetic improvement of Akebia.
The plants of the genus Akebia are widely distributed in Shanxi, Henan, Sichuan, Chongqing, Hunan, Hubei, Jiangxi, Zhejiang and Fujian provinces of China (Li et al., 2010a). The wide geographical distribution of Akebia provides rich genetic resources and substantial genetic diversity for cultivar improvement and also provides the possibility to breed novel cultivars through inter- or intra-specific hybridization. Wild resources also play an important role in breeding Akebia as a medicinal plant, an oilseed crop, or an ornamental plant. Akebia species have been widely cultivated as medicinal and edible fruit crops in China (Huang et al., 2021a; Li et al., 2010a). Although the domestication and genetic improvement of Akebia for commercial cultivation remain in their infancy, the synthesis of knowledge on these species is needed to provide insights for the rational development and utilization of those germplasm resources and facilitate the development of new breeding lines or cultivars for commercial cultivation or production.
There is now intense research interest in the transcriptomics, genomics, growth and development, and plant physiology of Akebia (Jiang et al., 2022a; Niu et al., 2019, 2020, 2021; Zou et al., 2022). This review therefore considers the literature on the origin and distribution of Akebia, as well as its physiology and cross compatibility, and molecular biology on Akebia. Finally, Akebia selection and breeding strategies are summarized.
2. Origin and distribution of AkebiaChina has the earliest records of Akebia species. Around 100 BC, Akebia was recorded in Shen Nong's Herbal Classic, in which it was called "Tongcao". Akebia is called mutong or Akebiae Caulis in modern Chinese pharmacopoeia (Huang et al. 2013). According to the data published in the FRPS, the Lardizabalaceae family includes nine genera, Akebia, Lardizabala, Boquila, Stauntonia, Holboellia, Decaisnea, Sargentodoxa, Sinofranchetia and Archakebia representing around 50 plant species (Editorial Commission of Chinese Flora of Chinese Academy of Sciences, 2001). Akebia is the most populous genus of the Lardizabalaceae family, and China is the natural distribution center of Akebia, containing all Akebia species except for A. pentaphylla, which is endemic to Japan (Christenhusz, 2012; Li et al., 2010a). The germplasm resources of wild Akebia species are widespread in more than 20 provinces of China, especially in central and southwest China (Fig. 1A). Some of these provinces include Gansu, Ningxia, Shannxi, Shanxi, and Henan provinces in northern China; Yunnan, Guangdong, and Guangxi provinces in southern China; Sichuan, Chongqing, and Guizhou provinces in western China; and Shandong, Jiangsu, and Zhejiang provinces in eastern China.
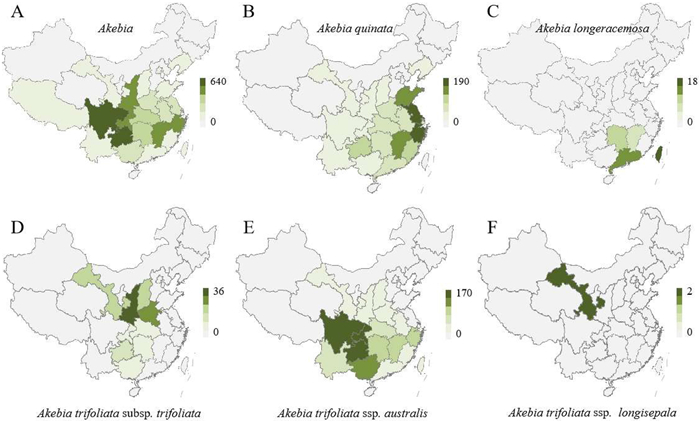
|
| Fig. 1 The geographic distribution of Akebia species. (A) Akebia; (B) Akebia quinata; (C) Akebia longeracemosa; (D) Akebia trifoliata subsp. trifoliata; (E) Akebia trifoliata subsp. australis; (F) Akebia trifoliata subsp. longisepala. The distribution heat map was made based on the specimen data of Akebia in Chinese virtual herbarium. |
The species A. quinata is known as chocolate vine or five-leaf akebia in Europe, America, and Japan, while it is usually called mutong or five-leaf akebia in China. Its flowers have a delicate, spicy chocolate fragrance that carries in the air. A. quinata plants are widely distributed in China, from an elevation of 300 m–1500 m, and are mainly distributed in southeast coastal provinces, such as Shandong, Jiangsu, and Zhejiang provinces (Fig. 1B). In contrast, the species A. longeracemosa has a narrow geographic distribution, from an elevation of 300 m–1600 m, and mainly occurs in Hunan, Jiangxi, Guangdong, and Taiwan provinces (Fig. 1C). A. trifoliata is not only the most widely distributed species in the genus Akebia, but is also the most promising species worth exploring as a new fruit crop, having larger fruits than A. quinata and A. longeracemosa. A. trifoliata consists of three subspecies, A. trifoliata subsp. trifoliata, A. trifoliata subsp. australis and A. trifoliata subsp. longisepala. A. trifoliata subsp. trifoliata has a wider geographic distribution in dimension, ranging from subtropical to temperate regions, from an elevation of 20 m–2800 m, while it has a narrow geographic distribution in longitude. This taxon is mainly distributed in Sichuan, Chongqing, Hunan, Hubei, Henan, Shaanxi, and southeastern Gansu province, and the Qinling mountain range is the core distribution area of A. trifoliata subsp. trifoliata (Fig. 1D). Akebia trifoliata subsp. australis and A. trifoliata subsp. longisepala are also subspecies of A. trifoliata that are endemic to China. The delimitation of these three subspecies mainly relies on the texture, shape, and margins of leaflets, or the shape, size, and length of inflorescences. The differences in botanical characteristics between Akebia species are discussed in the following sections. A. trifoliata subsp. australis plants are mainly found in southern China, ranging from the Yangtze River basin to the central part of Taiwan, and distributed northward to Henan, Shanxi, and Shaanxi provinces, from an elevation of 300 m–2100 m (Fig. 1E). The species A. trifoliata subsp. longisepala, which was recognized by Qin (1997), is found on the edges of the semi-deciduous forest by streams and hillsides in Wenxian county, Gansu province, from an elevation of 600 m–850 m (Fig. 1F).
3. Botanical characteristics of Akebia speciesAkebia species are deciduous or sub-evergreen twining vines that are usually found in patches in the wild due to clonal reproduction. When twined around tall trees, Akebia can reach a height of more than 10 m (Fig. S1). The branches that creep along the ground normally produce few flowers, while branches that climb trees or shrubs usually bloom the most profusely as they obtain more sunlight. Akebia plants are monoecious, with flowers that are functionally unisexual (Qin, 1997). Inflorescences are axillary, usually racemose, and sometimes subumbellate. These plants often have many staminate flowers in the terminal part of the raceme. The pistillate flowers are larger than staminate flowers, and are solitary or few at the bases of inflorescences, having three to 10 carpels, where each carpel secretes a large, viscous drop of fluid to receive pollen grains (Christenhusz, 2012; Li et al., 2010a; Qin, 1997). Flowers are protogynous, self-incompatible, and require cross-pollination. The pollination system of Akebia is dominated by wind pollination and supplemented by insect pollination. The main pollinators observed in A. quinata are small solitary bees and hoverflies (Kawagoe and Suzuki, 2002, 2003). The fruits of Akebia species are fleshy follicles that are produced singly or in clusters, and dehisce along ventral sutures when ripened on the vine (Zou et al., 2019, 2022). The geographic distribution and morphological features of Akebia species distributed in China are shown in Table 1.
| Akebia quinata | Akebia longeracemosa | Akebia trifoliata | |||
| ssp. trifoliata | ssp. australis | ssp. longisepala | |||
| Geographic distribution center | southeast coastal provinces | Jiangxi, Guangdong, Taiwan | Qinling Mountain Range | southern China | Wenxian county |
| Number of leaves | 5(-7) | 5 | 3 | 3 | 3 |
| Leaf texture | leathery to papery | subleathery | papery or thinly leathery | subleathery to leathery | leathery |
| Leaf margin | entire to shallowly lobed | entire | sinuate or lobed | entire | entire |
| Inflorescence length | 6–12 cm | 12–20 cm | 6–16 cm | 8–20 cm | 6–8 cm |
| Number of female flowers | 1–2 | 1–2 | 1–2 | 1–2 | 1–2 |
| Number of male flowers | 4–10 | 25–35 | 15–35 | 20–35 | 7–10 |
| Flower color | white, light green, pink, purple | pink to dark purple | pink to dark purple | light red to dark purple | purple black |
| Number of sepals | 3(-6) | 3 | 3 | 3–6 | 3(-4) |
| Female sepals length | 1–2 cm | 1–1.5 cm | 1–1.5 cm | 0.9–1.5 cm | 2.2–2.7 cm |
| Male sepals length | 0.6–0.8 cm | 0.4–0.5 cm | 0.3–0.5 cm | 0.2–0.5 cm | 0.9–1.2 cm |
| Flower scent | slightly sweet fragrant | unscented | unscented | unscented | unscented |
| Flowering time | March to April | March to April | March to May | March to April | March to April |
| Fruit ripening time | August to September | August to September | August to September | August to October | August to September |
A. quinata has palmate compound leaves consisting of five leaflets, occasionally three to four or six to seven, alternate or clustered on short branches (Fig. 2). Of all members of the genus Akebia, A. quinata is certainly the most ornamental plant, being very attractive with its divided leaves and unusual pale purple, pale green, or white flowers with a delicate, spicy chocolate scent, however, A. quinata flowers produce no nectaries (Fig. 3). The fruits of A. quinata are produced singly or in clusters and have great variation in size, shape, color and flavor. The fruit ripening period of A. quinata is from August to September depending on latitude. In the wild, the fruit weight of A. quinata is generally less than 150 g, which is usually smaller than A. trifoliata fruit. Fortunately, some wild germplasm resources of A. quinata have excellent taste, smaller seeds or thinner peel which provides excellent genetic resources for future genetic improvement.
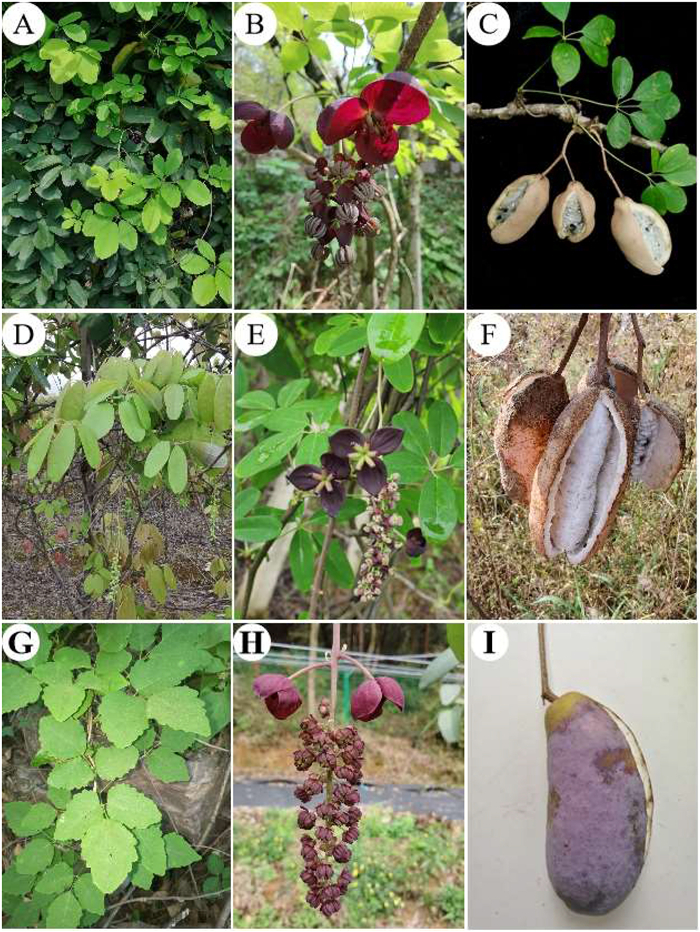
|
| Fig. 2 Akebia quinata, Akebia longeracemosa and Akebia trifoliata. Leaves, flowers, and fruits of A. quinata (A–C); leaves, flowers, and fruits of A. longeracemosa (D–F); leaves, flowers, and fruits of A. trifoliata (G–I). |

|
| Fig. 3 The phenotypic diversity of Akebia quinata flowers (A–I). |
A. quinata plants have a wide range of adaptability in different habitats, which contributes to their abundant phenotypic diversity. The species is very hardy and can be resistant to temperatures down to approximately −35 ℃ (Christenhusz and Rix, 2012). This wide range of geographical distribution and adaptability provides abundant phenotypic and genetic diversity and special genetic resources that can be explored for domestication and genetic improvement.
3.2. Akebia longeracemosaA. longeracemosa has palmate compound leaves consisting of five leaflets. Compared with A. quinata, the leaf phenotypic characteristics of these two species are similar. The major difference between these two species is that A. longeracemosa has longer inflorescences and more staminate flowers (Fig. 2D–F). In terms of geography, the distribution region of A. longeracemosa in the overlapping distribution regions of A. quinata and A. trifoliata subsp. australis. Moreover, the morphological characteristics of A. longeracemosa show hybrid characteristics; specifically, A. longeracemosa leaves resemble the leaves of A. quinata, while the long inflorescences resemble those of A. trifoliata subsp. australis. Interspecific artificial hybridization experiments conducted in our Akebia germplasm resources nursery demonstrated that there was no reproductive isolation between Akebia species, and fertile progeny could be produced. In addition, we have found specimens resembling intermediate plants (similar to A. longeracemosa, but with a shorter inflorescence length that was nevertheless longer than that of A. quinata) in the wild where both species occur. Li et al. (2010b) also found intermediate plants in Lushan mountain, Tianmu mountain, and Xixia. Thus, there is sufficient reason to speculate that A. longeracemosa may originate from the hybridization of A. quinata and A. trifoliata subsp. australis, forming a separate species after a long period of evolution. However, confirming whether A. longeracemosa is truly a hybrid requires more molecular and morphological evidence.
3.3. Akebia trifoliataA. trifoliata is the most widely distributed species in the genus Akebia, spanning more than 30 dimensions from east to west and 20 dimensions from north to south. This wide range of habitats leads to a variety of phenotypes and is particularly reflected in the size, shape, and margins of leaflets, as well as the floral composition. According to the data published in the FRPS, this species has three subspecies, and their varied traits are described in Table 1, including the shape of the leaflet margin and the shape and size of sepals in male and female flowers. The delimitation of these two subspecies (ssp. trifoliata and ssp. australis) mainly relies on the morphological characteristics of leaflets and their geographic distribution. In comparison with A. trifoliata subsp. trifoliata, A. trifoliata subsp. australis plants have narrower (leaflet width-to-length ratio below 0.8), thicker, and subcoriaceous leaflets with entire margins (occasionally shallowly lobed) (Table 1). In terms of geographical distribution, A. trifoliata subsp. trifoliata grows naturally in central China around the Yellow River Valley, especially along the Qinling mountain range, while A. trifoliata subsp. australis occurs mainly in southern China, ranging from the south of the Yangtze River to Taiwan island (Li et al., 2010a). A. trifoliata ssp. australis plants flower from late March to early May, and the morphological characteristics of flowers are not much different from those of ssp. trifoliata (Fig. 4A–C). The flower structure of Akebia is rather unusual, and flowers have only sepals but no petals. The sepals show petaloid characteristics and have functions in both attraction and protection. In Akebia, six sepals are initiated, but one to three sepals of the second whorl do not develop further in the latter development of the flower (Zhang and Ren, 2011). In A. trifoliata ssp. australis, stable mutant plants with two whorls of sepals in both female and male flowers have been observed, and flowers with five to eight conspicuous sepals that make them look larger and more ornamental (Fig. 4D–F). The flowers of Akebia are functionally unisexual because either the stamens or the carpels stop development in the latter development of the flower (Zhang and Ren, 2011). In A. trifoliata ssp. australis, it has also been observed that inflorescences have only (or many) female flowers which due to the carpels are normal development in the male flowers (Fig. 4G). Usually, in the male flowers, the carpels are reduced and sterile, although in some cases the carpels can develop normally. The molecular mechanism underlying this biological phenomenon remains to be studied and may play an important role in the molecular breeding of Akebia. In general, the inflorescence length of A. trifoliata ssp. australis ranges from 8 to 20 cm, while under artificial cultivation conditions, the length of inflorescences can reach as long as 40 cm (Fig. 4H). In comparison with A. quinata, A. trifoliata has relatively monotonous flower colors (usually light red to deep red) but exhibits great variability in leaf shape, leaf margin, sepal number, and inflorescence length.
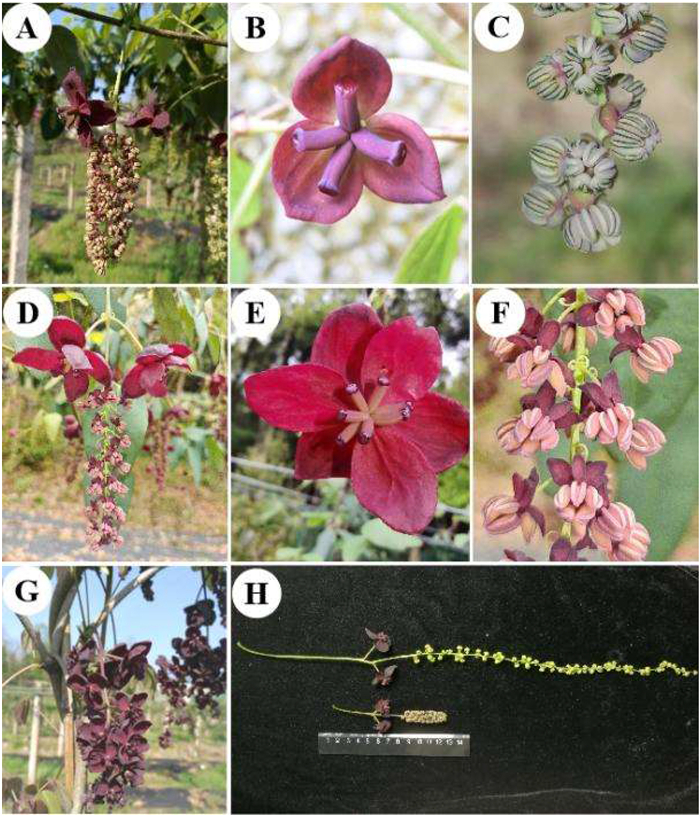
|
| Fig. 4 The flowers of Akebia trifoliata subsp. australis. Normal inflorescence (A), female flower (B), male flower (C); flowers with two whorls sepals (D–F); inflorescence with all female flowers (G); inflorescence length (H). |
It takes about 5 months for A. trifoliata ssp. australis fruits to mature from fruiting (April to May) to ripening (August to September) (Fig. 5A–C). The fruits of A. trifoliata dehisce along the ventral suture when ripening naturally, revealing soft, white, gelatinous, sweet pulp that tastes like a mixture of cream and litchi (Fig. 5C and D). A broad variety of fruit skin colors has been observed in A. trifoliata ssp. australis, from green to brown, light pink to light red, purplish to bluish, and purple to blackish-purple (Fig. 5E–J). The interspecific and intraspecific variations of fruit peel color have been widely observed in the genus Akebia. The mechanism of color change in A. trifoliata fruit was preliminarily elucidated by Jiang et al. (2022a), who identified 364 metabolites and 41 pathways that were potentially responsible for peel color change. Moreover, it was found that the decreased chlorophyll metabolism and carotenoid biosynthesis coupled with increased flavonoid and anthocyanin biosynthesis, as well as decreased levels of lipids, terpenoids, and steroid metabolites, jointly promoted the color transition from green to purple. Fruit peel (skin) color is an important characteristic that affects the commercial value of the fruit. Color is the first quality perceived by consumers, and affects consumer choice (Goulas and Manganaris, 2012; Karanjalker et al., 2018). In most plants, chlorophyll, betalains, anthocyanins, carotenoids, and flavonoids are the main factors affecting fruit color (Xin et al., 2021; Zhang et al., 2021a; Zhou et al., 2020), while several external factors, such as temperature, pH, nutrition, exposure to sunlight, and position of fruit on the tree canopy also affect fruit color (Kondo et al., 2002; Saure, 1990; Tyas et al., 1998; Wu et al., 2013). It has been observed that fruit bagging (with white sulfate paper) has a significant positive influence on the A. trifoliata ssp. australis fruit peel color and quality (unpublished data). Further studies on the genetic and molecular mechanism of Akebia fruit color changes provide fundamental insights for the in-depth exploitation of desirable fruit traits and breeding of new varieties with different peel colors.
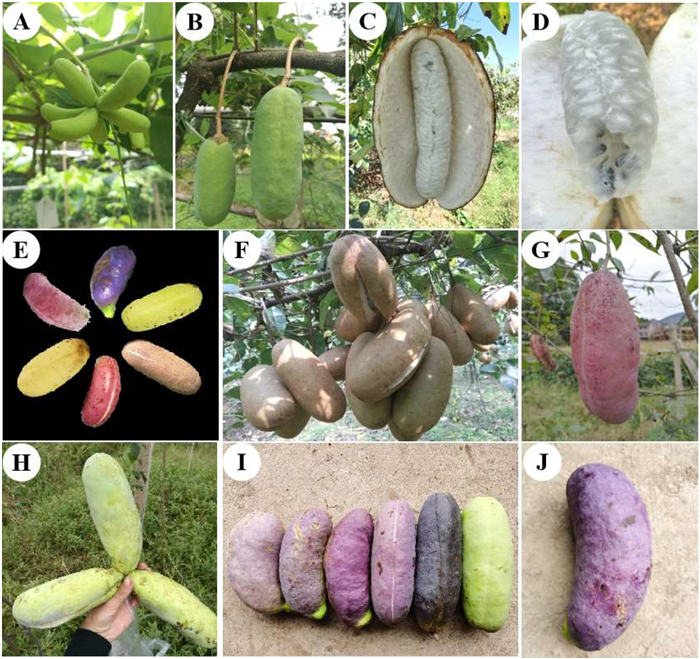
|
| Fig. 5 The fruits of Akebia trifoliata subsp. australis. Yong fruits (A–B), ripened and naturally cracked fruit (C), fruit pulp (D), fruits with different colors and shapes when ripened (E–J). |
A. trifoliata subsp. longisepala, another subspecies of A. trifoliata, was recognized by Qin in 1997 and is endemic to China (Qin, 1997). Compared with A. trifoliata subsp. australis, there is almost no difference in phenotypic characteristics between these two subspecies except for the flower characteristics. For A. trifoliata subsp. longisepala, the sepals of pistillate flowers are more than four times larger than the carpels, and the sepals of staminate flowers are more than two times longer than the anthers, while the sepal lengths of pistillate and staminate flowers of the other two subspecies of A. trifoliata are less than three times the lengths of the carpels and one time the length of the anthers, respectively. This subspecies has a very narrow geographical distribution range and is only found in Wenxian county, Gansu, China (Fig. 1F). In terms of geography, the geographic distribution of the three subspecies overlaps in the Qinling mountain range. Whether there is any gene flow between different subspecies of A. trifoliata needs further study.
4. Molecular biology of AkebiaThe wild germplasm resources provide abundant genetic materials for plant breeding, domestication, cultivation and improvement. Analysis and assessment of genetic diversity and patterns of wild resources may lay a good foundation for effective conservation and utilization of wild resources. Microsatellite markers have the properties of high reproducibility, codominant inheritance, abundance, and wide genome coverage, and have been found to be highly efficient molecular tools in the study of the genetic structure and diversity of germplasm resources (Qi et al., 2015). Hence, many molecular markers have been developed, such as genomic SSR, EST-SSR and ISSR (Li et al., 2009, 2018; Niu et al., 2019; Zhang et al., 2021b; Guan et al., 2022; Zhong et al., 2022). Those microsatellites also produced robust cross-species amplification in the species of Akebia, and those molecular markers provided a useful tool for the estimation of the levels of gene flow and hybridization introgression of Akebia species in their sympatric zone of natural distribution as well as the genetic diversity among different populations. Recently, a set of core germplasm collections were screened by using ISSR, SRAP or SSR markers (Zhang et al., 2020; Zhong et al., 2021). These core collections and molecular identity could reduce the management cost and improve the efficiency of conservation. In general, these abundant molecular markers are helpful for the study of genetic diversity, population structure and genome evolution as well as molecular marker-assisted breeding in the genus Akebia.
The ripened fruit of all Akebia species cracks longitudinally along the ventral, and this brings many knotty problems such as the pulp infected by pathogens, contaminated by impurities, or eaten by birds, and thus resulting in short shelf life, yield loss, and decreased acceptance by consumers (Zou et al., 2022). Thus, exploring the physiological and biochemical mechanisms underlying Akebia fruit cracking is essential for both breeding crack-resistant varieties and preventing fruit cracking during storage. Our previous studies confirmed the importance of maturity stages for A. trifoliata fruit quality and suggested that the A. trifoliata fruit should be harvested one week before fruit cracking, which was the ideal harvest maturity for long-distance transportation and greater consumer acceptability (Zou et al., 2022). However, few studies have been done for characterizing the underlying molecular mechanisms until recent years. Niu et al. (2020) identified 9301 differentially expressed genes (DEGs) and 223 differentially abundant proteins (DAPs) by integrating transcriptome and proteome profiles at different fruit ripening stages of A. trifoliata, and proposed that pectinesterase (PE), pectate lyase (PL), and beta-galactosidase (β-GAL2) may play important roles in the regulation of A. trifoliata fruit cracking. In this substantial research work, Niu et al. (2021) further disentangled the fruit softening mechanisms using tandem mass tag technology and identified 2839 proteins and 302 DAPs, including the proteins (PE, PL, and β-GAL) involved in cell wall degradation, as well as gibberellin-regulated protein, cysteine protease, thaumatin-like protein, and heat shock proteins that were involved in plant hormone, stress, and defense response. These proteins might also play important roles in A. trifoliata fruit ripening and softening. Jiang et al. (2022b) explored the mechanism of A. trifoliata fruit cracking based on cell-wall metabolism and found that the depolymerization of pectin and cellulose, disordered reactive oxygen species homeostasis, the degradation of starch, the movement of water, and decreased cell-wall strength jointly promoted A. trifoliata fruit cracking. Although some studies have evaluated fruit cracking in A. trifoliata, the key candidate genes that control the process of fruit cracking remain unclear, and this really needs more efforts. Fortunately, be benefit from the advance of sequencing technology, Huang et al. (2021b) published the first genome assembly of A. trifoliata. This genome assembly will provide valuable resources for further functional gene identification and accelerate the molecular breeding studies (Yu et al., 2021).
5. Selection and breeding for AkebiaA. trifoliata may be the most widely exploited species domesticated as a new fruit crop in the genus Akebia due to its high adaptability, greater fruit size, and high yield compared to other Akebia species. Although Akebia has been cultivated as a high-value medicinal and edible fruit crop in China, the cultivation and domestication of Akebia as a new fruit crop remain in their infancy. Only a small number of studies on Akebia breeding and cultivation have been conducted, and most efforts have focused on phytochemical and pharmacological analyses (Du et al., 2012; Gao and Wang, 2006; Iwanaga et al., 2012; Liu et al., 2018; Maciag et al., 2021; Ochmian et al., 2014; Xiao et al., 2012). The domestication of a wild fruit plant to allow for cultivation is a complex process involving a cyclical procedure over a long period. This process requires ongoing efforts in breeding programs. Thus, a clear breeding target and reasonable breeding methods are the key points that allow the wild fruit crop to be successfully domesticated and enter the market. In our previous studies, we have drafted six breeding goals for the domestication of Akebia as a new fruit, namely: fruit indehiscence, larger fruit, thinner fruit peel, higher edible ratio, few or no seeds, and shelf-stable fruit (Huang et al., 2021a; Li et al., 2010a; Zou et al., 2018, 2019). These six breeding objectives provide important breeding directions for the improvement of Akebia in the future. It has been found that 11 key fruit traits of A. trifoliata have high repeatability (> 0.90) and wide phenotypic correlation among traits. This finding provides essential genetic parameters for the further selection of elite genotypes and the formulation of appropriate selection strategies in Akebia breeding (Zou et al., 2018). In addition, Smith-Hazel-index-based multi-trait selection was successfully used to select superior lines in our breeding program. Akebia plants are self-incompatible and are pollinated mainly through wind, which makes a recurrent selection strategy suitable in the primary stage of Akebia domestication. We have demonstrated that recurrent selection is an effective method for the population improvement of fruit traits in A. trifoliata and maintaining their genetic variability (Huang et al., 2021a; Zou et al., 2019).
Thus, based on the practice of the domestication and breeding of seedling populations and the performance of cultivated characters of clonal lines, we have proposed the "1 + 3 + 2" model of Akebia breeding improvement in the primary stage of domestication (Huang et al., 2021a). In detail, "1" represented a core germplasm resource nursery of Akebia and was used to construct the basic populations of recurrent selection that contained excellent individuals according to the six breeding objectives outlined above; "3" referred to the basic population through about three generations of recurrent selection and produced an improved population; "2" referred to the selection of several superior individuals with excellent comprehensive characters from the improved population for 2 years fruit characters evaluation. Using this strategy, a series of elite clonal lines formed by superior individuals could be obtained through 3–5 years multi-point regional test. This breeding strategy includes traditional breeding approaches, such as wild population selection, intraspecific and interspecific hybridization, and clonal selection. Molecular marker-assisted selection can be used to accelerate the breeding process and improve breeding efficiency.
In general, we have put forward a clear goal for the domestication and improvement of Akebia and constructed a feasible breeding system for the domestication and improvement of Akebia that provides targets and methods for the domestication and improvement of Akebia in the future.
6. Conclusions and domestication strategies of new fruitsChina has abundant Akebia germplasm resources. The wide range of geographical distribution and adaptability of Akebia provides abundant phenotypic and genetic diversity and special genetic resources for domestication and genetic improvement. As an important multipurpose crop, Akebia is usually used as a traditional medicinal plant, an edible oil plant, an ornamental plant, or a fruit crop. In addition to those economic values, Akebia is a representative genus of the basal eudicot lineage and plays a crucial role in the study of the early evolution of eudicots (Liu et al., 2010). Thus, the genus Akebia has attracted increasing attention from both commercial farmers and evolutionary biologists. Therefore, it is important and necessary to systematically consolidate and evaluate the geographic distribution, biological characteristics, cross compatibility, and breeding methods of Akebia, which provide essential information for future related research. However, the domestication and commercial cultivation of Akebia as a fruit crop is still in its infancy stage, some prominent fruit traits need to be improved such as, seed numbers, crackingness, fruit peel thickness, and fruit storage, etc. Fruit commercial traits are vital for fruit crops which determining the selling price, market acceptability and development potential of the fruit. Consumers are reluctant to eat Akebia fruit with numerous seeds which has largely impeded the promotion of Akebia as a fruit, so seedless varieties must be created in further breeding programs. Polyploidy is widely distributed in the plant kingdom and plays an important role in evolution of new varieties and species in nature, and ploidy manipulation breeding is an effective means to create seedless fruit germplasm (Zhang et al., 2019). However, no polyploidy has been found in the wild and all known Akebia species are diploid. Thus, it is necessary to create tetraploids by ploidy manipulation breeding, carry out cross-ploidy crossing between diploids and tetraploids, and try to obtain triploid plants. Although ploidy manipulation breeding may not produce desired fruit traits, it is undoubtedly an attempt to obtain seedless fruit of Akebia.
No doubt the successful domestication of a new fruit tree from the wild has always been a long-term endeavor with tremendous difficulty and terrible uncertainty, and only few fruit trees have been successfully domesticated till now, e.g., kiwifruit (Actinidia L.), blueberry (Vaccinium L.), avocado (Persea Mill.) and macadamia nuts (Macadamia F. Muell.) (Huang, 2022). Though we have established a systematic breeding program of Akebia and achieved a remarked domestication progress, the domestication and improvement of Akebia still require much efforts. And it is possible to accelerate this breeding process and improve breeding efficiency with the help of modern biotechnology, e.g., genomic selection, marker-assisted selection, high-throughput phenotyping, genome editing and de novo domestication (Hickey et al., 2019). Undoubtedly, conventional breeding methods should be continued throughout the breeding and improvement process, especially superior genotypes selection, natural variation selection, intra- and interspecific hybridization which are still the main and effective technique in the initial domestication stage of new fruits.
Of course, the domestication of new fruit crops from the wild is seem an unending journey which driven by the human endless pursuit for new taste of fresh fruits. Looking to the future, what new fruit crops could be domesticated and how to domesticate effectively? With the rapid economical and societal development, people's health consciousness is constantly enhanced. Therefore, those wild fruit tree resources with excellent taste, high nutrition and therapeutic value will receive more concerns in the future. As for how to effectively and successfully domesticate a new fruit crop, we think there are five basic steps that should be implemented during domestication process. Firstly, one or more wild fruit trees with domestication potential should be screened from the wild which evaluated based on the edible security, taste, nutrition, health care, commercial traits, and etc. Secondly, clear goals of genetic improvement should be formulated for the traits that need to be improved in wild fruit crops. The target traits may including a set of fruit traits such as, fruit size, seed numbers, fruit palatability, fruit edible ratio, fruit storage, disease resistance, fruit yield, and etc. Nevertheless, the breeding goals must be realistic and achievable. Thirdly, core germplasm repositories of target species should be established. Apparently, a broad spectrum of wild germplasm resources plays an incredible role for the success of domesticating a new fruit crop, especially at the starting stage of domestication. In this step, the systematic investigation, evaluation and description of genetic diversity of germplasm collections is a prioritized tasks which could lay a solid foundation for their continuous genetic improvement. Fourthly, a well formulated breeding strategy should be drafted and implemented. Breeding programs should be designed according to the growth, genetic and reproductive characteristics of fruit trees. More importantly, the domestication strategy should has a long-term vision and can be constantly refined with the advancement of domestication process. Apart from the traditional breeding methods mentioned above, new breeding technologies and new ideas of fruit domestication are very important for the success of domesticating a new fruit plant. Finally, the market participation and the development of related downstream products are also crucial for the success of domesticating a new fruit plant. Particularly, market feedback is also a key factor in fruit domestication. The timely understanding of the market recognition of varieties can avoid losing the direction of fruit domestication.
AcknowledgmentsThis research was funded by Plant Germplasm Innovation Program, Biological Resources Programme, the Chinese Academy of Sciences (KFJ-BRP-007-001) and Youth Foundation of Lushan Botanical Garden, the Chinese Academy of Sciences (2021ZWZX07).
Author contributions
Shuai Zou: Conceptualization, Writing - original draft. Chen Feng: Review & editing. Puxin Gao: Investigation. Tongjian Li: Investigation. Tianjiao Jia: Investigation, Data curation. Hongwen Huang: Funding acquisition, Project administration, Review & editing. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.12.001.
An, J.P., Ha, T.K.Q., Kim, J., et al., 2016. Protein tyrosine phosphatase 1B inhibitors from the stems of Akebia quinata. Molecules, 21: 1091. DOI:10.3390/molecules21081091 |
Chinese Pharmacopoeia Commission, 2020. Pharmacopoeia of the People's Republic of China. China Medical Science Press, Beijing, China.
|
Christenhusz, M.J.M., 2012. An overview of Lardizabalaceae. Curtis's Bot. Mag., 29: 235-276. DOI:10.1111/j.1467-8748.2012.01790.x |
Christenhusz, M.J.M., Rix, M., 2012. Akebia quinata. Curtis's Bot. Mag., 29: 284-289. DOI:10.1111/j.1467-8748.2012.01792.x |
Du, Y., Jiang, Y., Zhu, X., et al., 2012. Physicochemical and functional properties of the protein isolate and major fractions prepared from Akebia trifoliata var. australis seed. Food Chem., 133: 923-929. DOI:10.1016/j.foodchem.2012.02.005 |
Editorial Commission of Chinese Flora of Chinese Academy of Sciences, 2001. Flora of China. Science Press, Beijing, China.
|
Gao, H., Wang, Z., 2006. Triterpenoid saponins and phenylethanoid glycosides from stem of Akebia trifoliata var. australis. Phytochemistry, 67: 2697-2705. DOI:10.1016/j.phytochem.2006.09.003 |
Goulas, V., Manganaris, G.A., 2012. Exploring the phytochemical content and the antioxidant potential of Citrus fruits grown in Cyprus. Food Chem., 131: 39-47. DOI:10.1016/j.foodchem.2011.08.007 |
Guan, J., Fu, P., Wang, X., et al., 2022. Assessment of the breeding potential of a set of genotypes selected from a natural population of Akebia trifoliata (Three–Leaf Akebia). Horticulturae, 8: 116. DOI:10.3390/horticulturae8020116 |
Hickey, L.T., Hafeez, A.N., Robinson, H., et al., 2019. Breeding crops to feed 10 billion. Nat. Biotechnol., 37: 744-754. DOI:10.1038/s41587-019-0152-9 |
Huang, H., 2022. Discovery and domestication of new fruit trees in the 21st century. Plants, 11: 2107. DOI:10.3390/plants11162107 |
Huang, H., Liang, J., Tan, Q., et al., 2021b. Insights into triterpene synthesis and unsaturated fatty-acid accumulation provided by chromosomal-level genome analysis of Akebia trifoliata subsp. australis. Hort. Res., 8: 33. DOI:10.1007/978-981-16-7570-6_4 |
Huang, H.P., Huang, L.Q., Wang, J., et al., 2013. Origin textual research, medicinal history and resources of Akebia. Chin. Traditional Patent Med., 35: 2488-2490. |
Huang, H.W., Zou, S.Y., Cheng, C.S., 2021a. Domestication and breeding strategy of wild fruit trees on track of plant introduction and domestication history. J. Plant Genet. Resour., 22: 1463-1473. |
Iwanaga, S., Warashina, T., Miyase, T., 2012. Triterpene saponins from the pericarps of Akebia trifoliata. Chem. Pharm. Bull., 60: 1264-1274. DOI:10.1248/cpb.c12-00448 |
Jiang, Y., Ding, Y., Wang, D., et al., 2020. Radio frequency-assisted enzymatic extraction of anthocyanins from Akebia trifoliata (Thunb.) Koidz. flowers: process optimization, structure, and bioactivity determination. Ind. Crop. Prod., 149: 112327. DOI:10.1016/j.indcrop.2020.112327 |
Jiang, Y., Du, Y., Zhu, X., et al., 2012. Physicochemical and comparative properties of pectins extracted from Akebia trifoliata var. australis peel. Carbohydr. Polym., 87: 1663-1669. DOI:10.1016/j.carbpol.2011.09.064 |
Jiang, Y., Wu, Y., Yin, H., et al., 2022a. Metabolic and bioactive comparative analyses reveal the mechanisms of color changes in Akebia trifoliata (Thunb.) Koidz fruit. Sci. Hortic., 295: 110819. DOI:10.1016/j.scienta.2021.110819 |
Jiang, Y., Yin, H., Wang, D., et al., 2022b. Exploring the mechanism of Akebia trifoliata fruit cracking based on cell-wall metabolism. Food Res. Int., 157: 111219. DOI:10.1016/j.foodres.2022.111219 |
Karanjalker, G.R., Ravishankar, K.V., Shivashankara, K.S., et al., 2018. Influence of bagging on color, anthocyanin and anthocyanin biosynthetic genes in peel of red colored mango cv. Lily'. Erwerbs-Obstbau, 60: 281-287. DOI:10.1007/s10341-018-0371-0 |
Kawagoe, T., Suzuki, N., 2002. Floral sexual dimorphism and flower choice by pollinators in a nectarless monoecious vine Akebia quinata (Lardizabalaceae). Ecol. Res., 17: 295-303. DOI:10.1046/j.1440-1703.2002.00489.x |
Kawagoe, T., Suzuki, N., 2003. Flower-size dimorphism avoids geitonogamous pollination in a nectarless monoecious plant Akebia quinata. Int. J. Plant Sci., 164: 893-897. DOI:10.1086/378659 |
Kondo, S., Maeda, M., Kobayashi, S., et al., 2002. Expression of anthocyanin biosynthetic genes in malus sylvestris L. 'Mutsu' nonred apples. J. Hortic. Sci. Biotechnol., 77: 718-723. DOI:10.1080/14620316.2002.11511562 |
Li, L., Chen, X.Z., Yao, X.H., et al., 2010b. Geographic distribution and resource status of three important Akebia species. J. Wuhan Bot. Res., 28: 497-506. |
Li, L., Yao, X.H., Chen, X.Z., et al., 2009. Development and characterization of microsatellite loci in Chinese medicinal plant Akebia trifoliata ssp. australis and cross-species amplification in closely related taxa. Conserv. Genet., 10: 959-962. DOI:10.1007/s10592-008-9666-2 |
Li, L., Yao, X.H., Zhong, C.H., et al., 2010a. Akebia: a potential new fruit crop in China. Hortscience, 45: 4-10. DOI:10.21273/hortsci.45.1.4 |
Li, T.J., Dong, J., Liao, L., et al., 2018. Isolation and characterization of microsatellite markers for Akebia trifoliata. Guihaia, 38: 1117-1124. DOI:10.1002/mawe.201700138 |
Liu, C., Zhang, J., Zhang, N., et al., 2010. Interactions among proteins of floral MADS-box genes in basal eudicots: implications for evolution of the regulatory network for flower development. Mol. Biol. Evol., 27: 1598-1611. DOI:10.1093/molbev/msq044 |
Liu, Y.C., Wang, H.M., Zeng, X.H., 2018. Research progress of active compounds and pharmacological effects in Akebia trifoliata (Thunb) Koidz stems. IOP Conf. Ser. Earth Environ. Sci., 185: 012034. DOI:10.1088/1755-1315/185/1/012034 |
Lu, W.L., Yang, T., Song, Q.J., et al., 2019. Akebia trifoliata (Thunb.) Koidz seed extract inhibits human hepatocellular carcinoma cell migration and invasion in vitro. J. Ethnopharmacol., 234: 204-215. DOI:10.1016/j.jep.2018.11.044 |
Maciag, D., Dobrowolska, E., Sharafan, M., et al., 2021. Akebia quinata and Akebia trifoliata - a review of phytochemical composition, ethnopharmacological approaches and biological studies. J. Ethnopharmacol., 280: 114486. DOI:10.1016/j.jep.2021.114486 |
Niu, J., Shi, Y., Huang, K., et al., 2020. Integrative transcriptome and proteome analyses provide new insights into different stages of Akebia trifoliata fruit cracking during ripening. Biotechnol. Biofuels, 13: 149. DOI:10.1186/s13068-020-01789-7 |
Niu, J., Sun, Z., Shi, Y., et al., 2021. Comparative analysis of Akebia trifoliata fruit softening at different flesh ripening stages using tandem mass tag technology. Front. Nutr., 8: 684271. DOI:10.3389/fnut.2021.684271 |
Niu, J., Wang, Y.J., Shi, Y.L., et al., 2019. Development of SSR markers via de novo transcriptome assembly in Akebia trifoliata (Thunb.). Koidz. Genome, 62: 817-831. DOI:10.1139/gen-2019-0068 |
Ochmian, I., Guan, T., Kubus, M., 2014. Description and assessment of chemical properties of fruits of the chocolate vine (five-leaf Akebia) Akebia quinata (Houtt.) Decne and dead man's fingers Decaisnea insignis (Griff.) Hokk.f. and Thomson, grown in Szczecin and in the Arboretum in Glinna (northwestern Poland). J. Elementol., 19: 1073-1084. |
Peng, P., Jia, D., Cao, L., et al., 2021. Akebia saponin E, as a novel PIKfyve inhibitor, induces lysosome-associated cytoplasmic vacuolation to inhibit proliferation of hepatocellular carcinoma cells. J. Ethnopharmacol., 266: 113446. DOI:10.1016/j.jep.2020.113446 |
Qi, W.H., Jiang, X.M., Du, L.M., et al., 2015. Genome-wide survey and analysis of microsatellite sequences in bovid species. PLoS One, 10: e0133667. DOI:10.1371/journal.pone.0133667 |
Qin, H.N., 1997. A taxonomic revision of the Lardizabalaceae. Cathaya, 8–9: 1-214. DOI:10.1111/j.1439-0396.1997.tb00850.x |
Saure, M.C., 1990. External control of anthocyanin formation in apple. Sci. Hortic., 42: 181-218. DOI:10.1016/0304-4238(90)90082-P |
Tyas, J.A., Hofman, P.J., Underhill, S.J., et al., 1998. Fruit canopy position and panicle bagging affects yield and quality of 'Tai So' lychee. Sci. Hortic., 72: 203-213. DOI:10.1016/S0304-4238(97)00125-8 |
Wang, J., Ren, H., Xu, Q.L., et al., 2015. Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata. Food Chem., 168: 623-629. DOI:10.1016/j.foodchem.2014.07.105 |
Wang, X., Yu, N., Peng, H., et al., 2019. The profiling of bioactives in Akebia trifoliata pericarp and metabolites, bioavailability and in vivo anti-inflammatory activities in DSS-induced colitis mice. Food Funct., 10: 3977-3991. DOI:10.1039/c9fo00393b |
Wu, H.X., Wang, S.B., Ma, X.W., et al., 2013. Effect of bagging on fruit quality in mango. Acta Hortic., 992: 587-592. DOI:10.17660/ActaHortic.2013.992.73 |
Xiao, T., Wang, L., Liu, T., et al., 2012. Analysis of volatile compounds in flowers of Akebia trifoliata by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry. Adv. Mater. Res., 602: 1313-1316. |
Xin, M., Li, C.B., He, X.M., et al., 2021. Integrated metabolomic and transcriptomic analyses of quality components and associated molecular regulation mechanisms during passion fruit ripening. Sci. Hortic., 42: 181-218. |
Yu, X., Zhong, S., Yang, H., et al., 2021. Identification and characterization of NBS resistance genes in Akebia trifoliata. Front. Plant Sci., 12: 758559. DOI:10.3389/fpls.2021.758559 |
Zhang, A., Zheng, J., Chen, X., et al., 2021a. Comprehensive analysis of transcriptome and metabolome reveals the flavonoid metabolic pathway is associated with fruit peel coloration of melon. Molecules, 26: 2830. DOI:10.3390/molecules26092830 |
Zhang, K., Wang, X., Cheng, F., 2019. Plant polyploidy: origin, evolution, and its influence on crop domestication. Hortic. Plant J., 5: 231-239. DOI:10.1016/j.hpj.2019.11.003 |
Zhang, X.H., Ren, Y., 2011. Comparative floral development in Lardizabalaceae (Ranunculales). Bot. J. Linn. Soc., 166: 171-184. DOI:10.1111/j.1095-8339.2011.01144.x |
Zhang, Z., Yang, Q., Niu, Y., et al., 2020. Diversity analysis and establishment of core collection among Akebia trifoliata (Thunb.) Koidz. in Qinba mountain area of China using ISSR and SRAP markers. Genet. Resour. Crop Evol., 68: 1085-1102. |
Zhang, Z., Zhang, J., Yang, Q., et al., 2021b. Genome survey sequencing and genetic diversity of cultivated Akebia trifoliata assessed via phenotypes and SSR markers. Mol. Biol. Rep., 48: 241-250. DOI:10.1007/s11033-020-06042-w |
Zhong, S., Chen, W., Yang, H., et al., 2022. Characterization of microsatellites in the Akebia trifoliata genome and their transferability and development of a whole set of effective, polymorphic, and physically mapped simple sequence repeat markers. Front. Plant Sci., 13: 860101. DOI:10.3389/fpls.2022.860101 |
Zhong, Y., Wang, Y., Sun, Z., et al., 2021. Genetic diversity of a natural population of Akebia trifoliata (Thunb.) Koidz and extraction of a core collection using simple sequence repeat markers. Front. Genet., 12: 716498. DOI:10.3389/fgene.2021.716498 |
Zhou, Z.X., Gao, H.M., Ming, J.H., et al., 2020. Combined Transcriptome and Metabolome analysis of Pitaya fruit unveiled the mechanisms underlying Peel and pulp color formation. BMC Genom., 21: 734. DOI:10.1186/s12864-020-07133-5 |
Zou, S., Gao, P., Jia, T., et al., 2022. Physicochemical characteristics and nutritional composition during fruit ripening of Akebia trifoliata (Lardizabalaceae). Horticulturae, 8: 326. DOI:10.3390/horticulturae8040326 |
Zou, S., Yao, X., Zhong, C., et al., 2018. Genetic analysis of fruit traits and selection of superior clonal lines in Akebia trifoliate (Lardizabalaceae). Euphytica, 214. |
Zou, S., Yao, X., Zhong, C., et al., 2019. Effectiveness of recurrent selection in Akebia trifoliata (Lardizabalaceae) breeding. Sci. Hortic., 246: 79-85. DOI:10.1016/j.scienta.2018.10.060 |



