b. School of Forest Resources and Conservation, University of Florida, Gainesville, FL, USA
Leaves are the main organ of plant carbon assimilation, water transportation, and a wealth of other important ecological processes (Wright et al., 2004; Sack and Scoffoni, 2013; He et al., 2020). Leaf functional traits are of great significance for predicting ecosystem function and community assembly under future global change scenarios (e.g., drought and nitrogen deposition) (Wright et al., 2005; Domínguez et al., 2012; Jager et al., 2015). Variation in leaf functional traits in response to light conditions have been shown to be closely related to plant ecological strategies and performance (Westoby et al., 2002; Blackman et al., 2016; Fajardo and Siefert, 2018). Thus, understanding how plant ecosystems function and communities are assembled requires investigation of trait variation in species with different shade tolerance. Recently, multi-dimensional traits (e.g., leaf economics or vein trait dimensions) have been shown to be closely related to plant adaptability (Laughlin and Wilson, 2014; Li et al., 2015; Liu et al., 2020a). Compared with covariant or simple dimensions, independent variation and diversified combinations of leaf traits play key roles in coping with complex environments. What is less clear, however, is whether leaf economics traits and leaf vein traits have independent responses to soil water content and soil total nitrogen in species with different shade tolerance strategies, e.g., shade-tolerant and shade-intolerant species.
Trade-offs in plant functional traits, especially traits associated with resource uptake and transport, are hypothesized to drive variation in life history strategies among plants (Westoby et al., 2002; Fortunel et al., 2012; Liu et al., 2019a). One approach to understanding the trade-offs that leaves make between resource conservation and acquisition is through a leaf economics spectrum, which describes species with 'fast' leaves (e.g., leaves with higher specific leaf area and higher nitrogen content per mass) or 'slow' leaves (e.g., leaves with lower specific leaf areas and nitrogen contents) (Wright et al., 2004; Poorter et al., 2009; Reich and Cornelissen, 2014). Leaf vein traits are often decoupled from economics traits due to differences in structure, evolution and ecology (Sack and Scoffoni, 2013; Sack et al., 2014; Li et al., 2015). Leaf vein traits often indicate the efficiency of leaf water transport and the cost of leaf construction (Sack et al., 2005, 2013) as they are closely related to stomata density (Sack and Scoffoni, 2013), leaf size (Murphy et al., 2012, 2014), and leaf photosynthetic capacity (Brodribb et al., 2007; Brodribb and Field, 2010). Previous studies have demonstrated that at a community scale leaf economics traits and leaf vein traits are decoupled (Sack et al., 2014; Li et al., 2015; Blackman et al., 2016). An additional indicator of a tree species' tolerance to low light environments is shade tolerance (Valladares and Niinemets, 2008). In a given environment, tree species with different shade tolerances can alter plant photosynthetic efficiency and water transport efficiency by adjusting their traits (Sack and Holbrook, 2006; Hallik et al., 2009). Therefore, analysis of plant adaptation strategies without considering shade tolerance of tree species may ignore important ecological processes. However, little is known about whether leaf economics traits and vein traits are similarly decoupled in shade-tolerant and shade-intolerant species.
The level of trait value is critical to species' leaf adaptation strategy and may depend on species' shade tolerance (Sack and Holbrook, 2006; Wright et al., 2010; Zhang et al., 2019). Shade-tolerant species generally adopt a conservative adaptation strategy. The specific leaf area (SLA) and chlorophyll content of these species is lower than in shade-intolerant species (Lusk and Warton, 2007; Wright et al., 2010). In contrast, shade-intolerant species use an acquisitive adaptation strategy. This strategy is characterized by having higher minor vein length per unit leaf area (VLAmin) (Sack and Frole, 2006), which may contribute to higher gas exchange (Li et al., 2015), mesophyll light capture (Brodribb and Field, 2010), and greater transport efficiency (Russin and Evert, 1984). However, the relationship between leaf traits and shade tolerance in empirical studies (see Valladares and Niinemets, 2008; Niinemets, 2010 for a review) is contradictory, likely due, in part, to the confounding effect of environmental factors (e.g., soil factors) that are not explicitly considered (Sack et al., 2013; Yin et al., 2018). Shade tolerance is an integrative term that encompasses many aspects of life history far beyond light use efficiency (such as the ability to resist damage caused by excessive UV radiation, Valladares and Niinemets, 2008) and this issue still requires further exploration to understand variation and trait associations at species level.
Global drought and nitrogen deposition can play a role in filtering individuals in a community through variation in water and nutrient supply. Soil water content and soil total nitrogen strongly relate to plant adaptation strategies as they can significantly affect plant photosynthesis efficiency, water transport efficiency, and resource uptake (Ordoñez et al., 2009, 2010; Maire et al., 2015; Lin et al., 2020). Soil water content varies greatly in different environments and significantly affects soil microbial vitality and root development, thereby causing changes in plant nutrient and water absorption and utilization efficiency. Generally, plants in drier environments are characterized by higher VLAmin (Yin et al., 2018) and the number of vein areoles per leaf area (VAA) (Sack and Scoffoni, 2013). Such a pattern of trait variation probably contributes to the area and path of water exchange between the xylem and the surrounding mesophyll cells (Nardini et al., 2010), and further affects plant water use efficiency and photosynthetic efficiency (Murphy et al., 2014). Nutrient availability is often considered to be closely related to plant relative aboveground biomass (Zheng and Ma, 2018), thus causing a switch from nutrient limited competition in low soil total nitrogen to light limited competition in high soil total nitrogen (Hautier, 2009; DeMalach et al., 2017). Plants adapted to high soil total nitrogen tend to have higher SLAs (Maire et al., 2015) and a higher VLAmin to underpin the greater light competitive capability (Sack and Scoffoni, 2013; Zheng and Ma, 2018). In recent years, studies have shown that differences in the shade tolerance of a plant species may affect the efficiency of its utilization of soil nutrients (Huang et al., 2012). Therefore, it is important to determine whether leaf economics traits and vein traits are correlated with abiotic factors such as soil water content and soil total nitrogen in shade-tolerant and shade-intolerant species.
In this study, we sought to determine whether the relationship between leaf economics traits and vein traits is constant between tree species. Because carbon assimilation and water transport differ between shade-tolerant and shade-intolerant species, we reasoned that in environments with low light conditions it may be more cost-effective for shade-tolerant species to couple leaf economics traits and vein traits. In contrast, shade-intolerant species may benefit more when economics traits and vein traits are decoupled, because this would provide a more diverse set of leaf strategies to adapt to complex environments. Thus, we first hypothesized that the shade tolerance of a species significantly alters the relationship between leaf economics traits and vein traits. Specifically, we predicted that leaf economics traits and vein traits would be coupled in shade-tolerant species and decoupled in shade-intolerant species. Second, we asked whether intraspecific leaf traits are correlated with local soil water and/or nutrient conditions. We hypothesized that intraspecific traits would have distinct responses to soil water content and soil total nitrogen. Specifically, traits related to leaf hydraulics (such as vein traits) would be more correlated with soil water content, and traits related to resource acquisition and utilization (such as leaf economics traits) would be more correlated with soil total nitrogen. To test these hypotheses, we measured eight leaf traits (four leaf economics traits and four vein traits), soil water content, and soil total nitrogen in one shade-tolerant and one shade-intolerant tree species. Betula platyphylla (Japanese white birch; hereafter birch) and Acer mono (painted maple; hereafter maple), which are common in broadleaved-Korean pine forests in Northeast China (Wang, 1994), show significant differences in their tolerance to shade (Niinemets and Valladares, 2006). The richness of these two species and the wide variety of soil factors in the study area provide an ideal setting for testing soil fertility–trait relationships at the intraspecific level.
2. Materials and methods 2.1. Sample designThe study area is situated in the broadleaved-Korean pine forests in Northeast China (Wang, 1994), which has a temperate continental monsoon climate (Table S1). We assessed variation in eight leaf traits in birch, Betula platyphylla, and maple, Acer mono, across five hierarchical ecological scales: (ⅰ) leaves in sun vs. leaves in shade; (ⅱ) sunned leaves and shaded leaves within different canopy strata (upper; middle; lower canopy); (ⅲ) leaves in different canopy strata within a tree; (ⅳ) leaves of different trees within a site; (ⅴ) leaves from trees at different sites (Table 1). For each trait measured from each species, we collected 720 leaves from trees situated at four sites (Table S1), three trees per site, three canopy strata per tree, two light exposures per canopy strata, 10 leaves per light exposure.
| Leaf traits | Abbreviation | Unit | Birch | Maple | |||||||
| Mean | Max | Min | SE | Mean | Max | Min | SE | ||||
| Economics traits | |||||||||||
| Specific leaf area | SLA | cm2 g−1 | 185.07b | 305.39 | 128.79 | 3.824 | 242.69a | 339.87 | 159.86 | 5.577 | |
| Leaf thickness | LT | mm | 0.14a | 0.20 | 0.08 | 0.003 | 0.12b | 0.18 | 0.08 | 0.003 | |
| Leaf nitrogen content per mass | Nmass | mg g−1 | 31.90b | 37.84 | 25.65 | 0.320 | 35.29a | 42.37 | 27.44 | 0.363 | |
| Leaf nitrogen content per area | Narea | mg cm−2 | 0.18a | 0.23 | 0.11 | 0.003 | 0.15b | 0.22 | 0.09 | 0.004 | |
| Vein traits | |||||||||||
| Major veins length per unit leaf area | VLAmaj | mm mm−2 | 0.19a | 0.26 | 0.12 | 0.003 | 0.20a | 0.25 | 0.15 | 0.003 | |
| Minor vein length per unit leaf area | VLAmin | mm mm−2 | 7.61a | 10.01 | 5.66 | 0.101 | 4.30b | 5.68 | 3.03 | 0.077 | |
| Interveinal vein distance | IVD | mm | 0.26b | 0.35 | 0.17 | 0.006 | 0.33a | 0.49 | 0.24 | 0.007 | |
| Number of vein areoles per leaf area | VAA | n mm−2 | 11.90a | 23.96 | 6.01 | 0.524 | 7.20b | 11.96 | 2.89 | 0.285 | |
| Different letters indicate significant differences in economics or vein traits between birch and maple at the 0.05 level. | |||||||||||
We sampled leaves from mid-July to August 2017 within each study site. The birch tree, B. platyphylla, is shade intolerant; the maple tree, A. mono, is shade tolerant (Niinemets and Valladares, 2006). For each species, three individuals were selected with similar tree height (measured by clinometer, meanheight = 21.5 ± 1.1 m for birch; meanheight = 15.7 ± 1.1 m for maple) and diameter at breast height (DBH, meanDBH = 38.1 ± 1.7 cm for birch; meanDBH = 35.6 ± 2.3 cm for maple) on the south facing slope with similar slope angles. We divided the canopy of each sample tree into upper, middle and lower layers. The canopy height is the distance from the first living branch to the top of the tree. Within each canopy layer, sun and shade directions were divided, then one large sample branch was cut via tree climbing for each direction, 6 branches per tree in total. Within each branch, we selected 5 healthy and fully expanded leaves to measure leaf economics traits, 5 leaves to measure vein traits, and 10–20 leaves to measure leaf nitrogen content. Leaves were preserved in a mixture 90:5:5 of 70% ethanol, formalin, and glacial acetic acid.
We collected three soil subsamples after removing the leaf litter within one meter around each sample tree (soil corer, soil depth was 0–10 cm, and the sample angle was ~120°, Yang et al., 2019; Liu et al., 2020b). We then homogenized the three subsamples in a plastic bag and immediately transported them to the laboratory for soil water content and soil total nitrogen measurements.
2.3. Leaf trait measurementsWe measured the leaf thickness of each leaf with a micrometer (avoiding the major veins, with an accuracy of 0.01 mm). Leaf fresh mass was measured with accuracy of 0.0001 g. A scanner (BenQ Corporation, China) was used to scan and measure leaf area (the accuracy is 0.01 cm2). We oven-dried the leaves until they achieved a constant mass (at 65 ℃ for at least 72 h) and then weighed (accuracy of 0.0001 g). SLA was calculated as leaf area divided by dry leaf mass (cm2 g−1).
After grinding and drying, 0.1 g leaf samples were subjected to a pre-digestion system for 40 min, followed by a double acid (H2O2 + H2SO4) digestion, then Nmass (mg g−1) was determined with a Hanon K9840 auto-Kjeldahl analyzer (Jinan Hanon Instruments Co., Ltd., Jinan, China), Narea was calculated by dividing Nmass by SLA.
VLAmaj (Fig. 1) was calculated manually by using ImageJ software (NIH Image) after scanning leaf images with a scanner (BenQ Corporation, China). For VLAmin, we first softened the leaves in a 5% sodium hydroxide solution, and then carefully removed the mesophyll to expose the minor veins. After bleaching to clear (5% sodium hypochlorite) and careful washing, the sample leaves were stained with 1% saffron (Sack et al., 2012). We then observed and photographed the samples under a light microscope (Olympus Electronics, Inc., Tsukuba, Japan) with electronic image analysis equipment. Interveinal vein distance (IVD), the number of vein areoles per leaf area (VAA) and minor veins were manually calculated by using ImageJ software (NIH Image). VLAmin was calculated as the total length of the minor veins per unit area (Blonder et al., 2011).
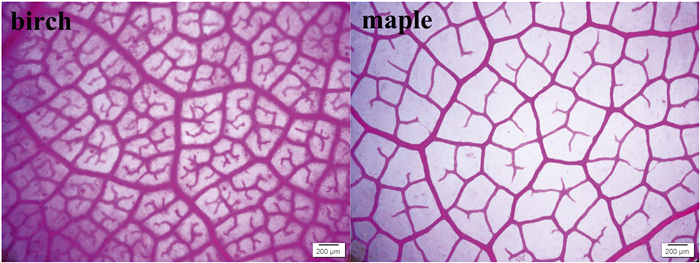
|
| Fig. 1 The venation networks for birch and maple after clearing and staining. |
Soil water content (g g−1) was determined using an oven drying method (Liu et al., 2020b). Soil total nitrogen (mg g−1) was measured by Hanon K9840 auto Kjeldahl analyzer (Jinan Hanon Instruments Co., Ltd., China).
2.5. Statistical analysisThe mean, maximum, minimum and standard error for eight leaf traits of birch and maple leaves were calculated. Least significant differences (LSD) were used to test whether there was a significant difference in the mean of traits between birch and maple. Correlations among leaf traits were analyzed using Pearson correlation analysis, prior to analyses, in order to satisfy the criteria of linear statistical methods, logarithmic transformation was performed on all traits. For each species, we first used principal component analysis (PCA) to obtain PC1 scores of economic and vein traits, and then used Pearson correlation analysis to analyze the correlation between these two sets of traits (Li et al., 2015). We used the total principal component scores of birch and maple to analyze the correlation between economic traits and leaf vein traits and soil water content and soil total nitrogen. Pearson correlation analysis was used in this step.
3. Results 3.1. Correlations of economic and vein traitsPCA was employed to evaluate the correlations between leaf economics and vein traits (Fig. 2). The first two principal components accounted for most leaf trait variation for birch and maple (59.90% and 73.2%, respectively, Fig. 2). The first axis represents vein trait variation and the second axis represents leaf economics trait variation (Fig. 2 and Table S2). These two sets of traits were decoupled in birch (p = 0.09) but significantly coupled in maple (p < 0.0001) (Figs. 2 and S1).
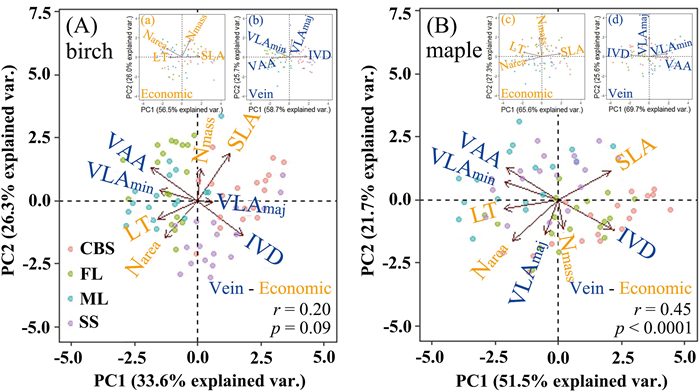
|
| Fig. 2 Principal component analysis of eight leaf traits for (A) birch and (B) maple. The inset graph shows Principal component analysis of economics traits and vein traits (a–d). The arrows represent the principal component loadings associated with the leaf traits. The correlation between leaf economics traits and vein traits of birch and maple were analyzed by the first principal component scores of economics traits and vein traits. Leaf trait abbreviations are provided in Table 1. CB: Changbai mountain; ML: Muling; FL: Fenglin; SS: Shengshan. |
Significant correlations between certain leaf traits were constant in birch and maple (Table 2). Among leaf economics traits, SLA was negatively correlated with leaf thickness and Narea, and leaf thickness was positively correlated with Narea. Among vein traits, IVD was negatively correlated with VAA and VLAmin, and VLAmin was positively correlated with VAA (Table 2). In both shade-intolerant and shade-tolerant species, part trait–trait relationships were changed, such as SLA versus Nmass and Nmass versus Narea. Leaf economics traits were not correlated with vein traits in birch except leaf thickness versus VLAmaj, IVD, VAA (Table 2); but in maple, significant correlations were observed except for leaf thickness versus VLAmaj and Nmass versus VLAmaj, VLAmin, IVD, VAA (Table 2).
| SLA | LT | Nmass | Narea | VLAmaj | VLAmin | IVD | VAA | |
| SLA | −0.79 | 0.05 | −0.91 | −0.38 | −0.54 | 0.41 | −0.38 | |
| LT | −0.49 | −0.01 | 0.73 | 0.04 | 0.58 | −0.64 | 0.61 | |
| Nmass | 0.46 | −0.20 | 0.36 | −0.07 | −0.08 | 0.17 | −0.15 | |
| Narea | −0.86 | 0.44 | 0.06 | 0.33 | 0.47 | −0.32 | 0.29 | |
| VLAmaj | 0.00 | −0.37 | −0.14 | −0.08 | 0.10 | 0.06 | −0.09 | |
| VLAmin | −0.22 | 0.18 | −0.15 | 0.16 | 0.04 | −0.86 | 0.86 | |
| IVD | −0.07 | −0.30 | −0.21 | −0.05 | 0.11 | −0.50 | −0.99 | |
| VAA | 0.01 | 0.32 | 0.17 | 0.09 | −0.13 | 0.56 | −0.98 |
For birch and maple, the principal component of economics traits indicates an axis from resource-conservative (lower SLA and Nmass) to resource-acquisitive (higher SLA and Nmass) strategies. The principal component of vein traits indicates an axis from high leaf construction cost (higher VLAmin, VAA) to low leaf construction cost (lower VLAmin, VAA) (Figs. 2 and 3).

|
| Fig. 3 Correlation between the first two principal component scores and soil water content and soil total nitrogen for birch (A, C) and maple (B, D). The first axis represents vein traits variation, from high leaf construction cost to low leaf construction cost; the second axis represents economics trait variation, from resource conservation to resource acquisition (See Fig. 2 for details). The solid line indicates that there is a significant correlation between the principal component scores and soil water content (SWC) or soil total nitrogen (STN), while the dashed line indicates that there is no significant correlation between the principal component value and SWC or STN. *, p < 0.05, **, p < 0.01, ***, p < 0.001. Abbreviations of the leaf traits are given in Table 1. SWC, soil water content; STN, soil total nitrogen. |
The correlation between the economics trait axis and soil water content was constant between tree species: the economics trait axis was positively correlated with soil water content (Fig. 3A and B). However, the relationship between the vein trait axis and soil water content changed from shade-intolerant to shade-tolerant species: the vein trait axis was positively correlated with soil water content in birch (Fig. 3A) but negatively correlated in maple (Fig. 3B).
The correlation between the leaf trait axis and soil total nitrogen was weaker. For birch, the economics trait axis was positively correlated with soil total nitrogen (Fig. 3C), but for maple, it was not correlated (Fig. 3D). The vein trait axis was negatively correlated with soil total nitrogen in maple (Fig. 3D), but not in birch (Fig. 3C).
4. Discussion 4.1. Adaptive mechanisms are distinct for shade-tolerant and shade-intolerant tree speciesOur findings that leaf economics and vein traits are coupled in birch but uncoupled in maple trees supports the hypothesis that shade tolerance alters adaptive strategies between species. Furthermore, our results emphasize the multi-dimensionality of leaf trait adaptation to environmental change.
In shade-intolerant birch trees, we identified two independent leaf trait axes. The first axis describes the relationship between SLA and Nmass. SLA reflects the efficiency of leaf dry-mass investment (Liu et al., 2019a, 2019b), while Nmass is integral to photosynthesis-related proteins. We discovered a negative relationship between these two traits, which is usually encountered in "fast-slow" species (Wright et al., 2004) and indicates a shift from resource-conservative (lower SLA and Nmass) to resource-acquisitive (higher SLA and Nmass) strategies. The second axis describes the variation in vein traits from high VLAmin, VAA and low IVD to low VLAmin, VAA and high IVD. This axis represents the trade-off between leaf construction cost and transportation distance (Sack and Scoffoni, 2013; Sack et al., 2013). Our finding that leaf economics and vein traits are decoupled (e.g., SLA and vein density) is consistent with previous studies (Sack and Frole, 2006; Dunbar-Co et al., 2009; Nardini et al., 2012; Blackman et al., 2016). One explanation for this decoupling of traits is that leaf economics and vein traits represent separate sub-systems (Li et al., 2015). For example, leaf economics traits are mainly located in palisade mesophyll tissues, whereas vein traits are mainly located in spongy mesophyll tissues. Consequently, these two sub-systems have likely undergone different evolutionary trajectories.
Our observation that leaf economics and vein traits are correlated in maple trees is similar to that of previous studies of woody species (Yin et al., 2018) and within eucalypt species (Blonder et al., 2013). The negative correlation between SLA and VAA, VAA, VLAmin and VLAmaj, and the positive correlation between SLA and IVD (Table 2) suggest that resource acquisitive species, such as maples, might improve resource use efficiency by reducing the cost of vein structure, while simultaneously improving leaf nutrient and water transport safety by increasing IVD (Sack et al., 2013). Furthermore, we found Nmass was not correlated with vein traits (Table 2), which may be explained by the correlation between vein traits and palisade tissue (Yin et al., 2018), as they both play roles in gas exchange and water transpiration. In palisade tissue, most nitrogen is related to photosynthesis, whereas mass-based N may have both structural and functional roles (Osnas et al., 2013). Taken together, our findings suggest that leaf economics and vein traits may be coupled to increase resource use efficiency and plant survival in shade (Flores-Moreno et al., 2019; Liu et al., 2020a). Thus, observed variation in how economics traits and vein traits are coupled in various shade-tolerant species may be due to differences in potential trade-offs between leaf light capture and water conduction.
4.2. Soil water content and nitrogen differentially correlate with multidimensional leaf traitsThe relationships of leaf economics and vein traits to soil water content and soil total nitrogen are distinct between birch and maple trees. We found that multidimensional leaf traits were significantly correlated with soil water content but weakly correlated with soil total nitrogen (Fig. 3). Along soil water content gradients, the economics axis of birch and maple trees shifted from resource-conservative strategies (lower SLA and Nmass) to resource-acquisitive strategies (higher SLA and Nmass) (Fig. 3A and B), which provides evidence that lower SLAs are generally distributed in relatively lower moisture areas (Niinemets, 2001; Wright et al., 2002; Stark et al., 2017; Luo et al., 2019). Lower SLAs in environments with low soil water content may be the result of thicker palisade tissue, which may contribute to maintaining high photosynthetic rate (He et al., 2017). Conversely, in environments with high soil water content, higher SLAs and larger surface areas may benefit plants with quick returns in leaves, e.g., investments in nutrients and dry mass (Wright et al., 2005).
Our results do not support the hypothesis that intraspecific traits have specific responses to soil water content and soil total nitrogen. The vein trait axis had species-specific responses to soil water content gradients (Fig. 3A and B). For maple, the vein trait axis shifted from less cost-effective (high VLAmin) to more cost-effective (low VLAmin) leaves (Fig. 3B). Higher VLAmin in environments with low soil water content (Table 2) enables higher leaf hydraulic conductance (Sack and Frole, 2006; Boyce et al., 2009), thus improving plant drought tolerance (Sack et al., 2013). VLAmin decreases with increasing soil water content, suggesting individuals with a conservative strategy actually require a lower leaf construction cost. The trade-off between leaf economics traits and vein traits along soil water content gradients is of great significance for improving the resource utilization efficiency of maple trees. For birch, the variation of the vein trait axis along soil water content gradients showed the opposite pattern: leaf economics traits with resource-conservative strategies correspond to more cost-effective vein traits in low soil water content conditions (Fig. 3A), which contrasts with the widespread observation of higher SLA being related to lower VLAmin. This is possible for birch because the weak correlation between economics traits and leaf vein traits may provide larger independent variation, however, this requires further investigation. Moreover, lower VLAmin reduces the cost of leaf construction and has been shown to make it possible for pioneer species to grow rapidly (Field et al., 2011).
The relationship between leaf economics and vein traits to soil total nitrogen was distinct between birch and maple trees (Fig. 3B and D). For birch, our study suggests that 'slow' leaf traits (low SLA) are favored in low soil total nitrogen environments. This is likely because a conservative resource strategy can minimize the sum of costs for acquiring and using nitrogen and water in photosynthesis (Shipley et al., 2006; Onoda et al., 2017). Forest aboveground biomass and canopy cover increase significantly in high nitrogen soils; in addition, plants in low nitrogen soils tend to compete for nutrients, whereas plants in high nitrogen soils tend to compete for light (Hautier et al., 2009; DeMalach et al., 2017). 'Fast' leaf traits (high SLA) are at an advantage in soils with high total nitrogen because they may enhance plant resource acquisition efficiency through larger leaf area and shorter water transport paths. However, some studies have failed to find a significant correlation between SLA and soil nitrogen gradients (Ordoñez et al., 2009). In maple, leaf vein traits decreased with increasing soil total nitrogen. For example, higher VLAmin was favored in environments with low soil total nitrogen. This may be because tough leaves can better resist animal and wind damage, while lower VLAmin can enhance survival by reducing leaf carbon consumption. Furthermore, no significant correlation was found between economics traits and soil total nitrogen in birch, nor was any found between vein traits and soil total nitrogen in maple (Fig. 3B and D). This finding may indicate that the adaption of plants to soil total nitrogen may differ depending on the shade tolerance of a species, which may promote coexistence between multiple species. Moreover, this suggests that adaptations to soil total nitrogen, e.g., changes in vein traits in birch or economic traits in maple, may not be as important as for other traits. In addition, these distinct correlations between species with different shade tolerances and soil total nitrogen may be related to the mycorrhizal type associated with these tree species. For example, arbuscular mycorrhizal (AM) fungi are commonly associated with shade-tolerant species, whereas ectomycorrhizal (ECM) fungi are often associated with shade-intolerant species. Previous studies have shown that AM-associated species usually grow in fertile areas with higher soil nitrogen content, whereas ECM-associated species grow in infertile areas with lower soil nitrogen content (Mao et al., 2019). Moreover, in plant nutrient economics strategies, ECM tree species tend to adopt resource-conservative strategies and AM tree species tend to adopt resource-acquisitive strategies (Valverde-Barrantes et al., 2018; Averill et al., 2019). These differences in habitat and nitrogen utilization strategies may be the reason why economics traits of birch and maple showed distinct correlations with soil total nitrogen.
5. ConclusionOur study shows that trait correlations between economics and vein traits in species of different shade tolerances are not consistent. These two groups of traits were decoupled in birch but coupled in maple. Our results provide direct evidence for the differences in ecological strategies of different shade-tolerant species. Moreover, local abiotic factors differentially drove changes in multidimensional leaf traits. Specifically, soil water content dominated the variation in the multi-dimensional trait variation of two different shade tolerant tree species; in contrast, soil nitrogen primarily dominated the variation in economic traits of birch and vein traits of maple. Our results suggest that the multidimensionality of leaf traits of tree species may be shaped by local abiotic factors, and suggest that the relative abundance of species of different shade tolerances should be considered when quantifying ecosystem function and community assembly variation along soil gradients.
AcknowledgementWe are grateful to Marco Visser and Zikun Mao for their helpful discussions and insightful comments in improving this manuscript. We also thank participants of Smithsonian CTFS-ForestGEO Workshop in Singapore for many meaningful suggestions about the first draft of the manuscript. This work was supported by the National Key R & D Program of China (2022YFD2201100), the National Natural Science Foundation of China (31971636) and the Fundamental Research Funds for the Central Universities (2572022DS13).
Author contributions
ZLL developed the idea; ZLL and MYJ carried out the experiment, MYJ analyzed the dataset; JDJ contributed to interpretation of data; and MYJ wrote the paper with substantial contributions from ZLL, JDJ, GZJ and QXG.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.03.001.
Averill, C., Bhatnagar, J.M., Dietze, M.C., et al., 2019. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc. Natl. Acad. Sci. U.S.A., 116: 23163-23168. DOI:10.1073/pnas.1906655116 |
Blackman, C.J., Aspinwall, M.J., Dios, V.R.D., et al., 2016. Leaf photosynthetic, economics and hydraulic traits are decoupled among genotypes of a widespread species of eucalypt grown under ambient and elevated CO2. Funct. Ecol., 30: 1491-1500. DOI:10.1111/1365-2435.12661 |
Blonder, B., Violle, C., Bentley, L.P., et al., 2011. Venation networks and the origin of the leaf economics spectrum. Ecol. Lett., 14: 91-100. DOI:10.1111/j.1461-0248.2010.01554.x |
Blonder, B., Violle, C., Enquist, B.J., et al., 2013. Assessing the causes and scales of the leaf economics spectrum using venation networks in Populus tremuloides. J. Ecol., 101: 981-989. DOI:10.1111/1365-2745.12102 |
Boyce, C.K., Brodribb, T.J., Field, T.S., et al., 2009. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. Roy. Soc. B-Biol. Sci., 276: 1771-1776. DOI:10.1098/rspb.2008.1919 |
Brodribb, T., Field, T., 2010. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett., 13: 175-183. DOI:10.1111/j.1461-0248.2009.01410.x |
Brodribb, T.J., Field, T.S., Jordan, G.J., 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol., 144: 1890-1898. DOI:10.1104/pp.107.101352 |
Carins Murphy, M.R., Jordan, G.J., Brodribb, T.J., 2012. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ., 35: 1407-1418. DOI:10.1111/j.1365-3040.2012.02498.x |
Carins Murphy, M.R., Jordan, G.J., Brodribb, T.J., 2014. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ., 37: 124-131. DOI:10.1111/pce.12136 |
DeMalach, N., Zaady, E., Kadmon, R., 2017. Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett., 20: 60-69. DOI:10.1111/ele.12706 |
Domínguez, M.T., Aponte, C., Pérez-Ramos, I.M., et al., 2012. Relationships between leaf morphological traits, nutrient concentrations and isotopic signatures for Mediterranean woody plant species and communities. Plant Soil, 357: 407-424. DOI:10.1007/s11104-012-1214-7 |
Dunbar-Co, S., Sporck, M.J., Sack, L., 2009. Leaf trait diversification and design in seven rare taxa of the Hawaiian plantago radiation. Int. J. Plant Prod., 170: 61-75. DOI:10.1086/593111 |
Fajardo, A., Siefert, A., 2018. Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology, 99: 1024-1030. DOI:10.1002/ecy.2194 |
Field, T.S., Brodribb, T.J., Iglesias, A., et al., 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl. Acad. Sci. U.S.A., 108: 8363-8366. DOI:10.1073/pnas.1014456108 |
Flores-Moreno, H., Fazayeli, F., Banerjee, A., et al., 2019. Robustness of trait connections across environmental gradients and growth forms. Global Ecol. Biogeogr., 28: 1806-1826. DOI:10.1111/geb.12996 |
Fortunel, C., Fine, P.V.A., Baraloto, C., Dalling, J., 2012. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Funct. Ecol., 26: 1153-1161. DOI:10.1111/j.1365-2435.2012.02020.x |
Hallik, L., Niinemets, Ü., Wright, I.J., 2009. Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora?. New Phytol., 184: 257-274. DOI:10.1111/j.1469-8137.2009.02918.x |
Hautier, Y., Pascal, A.N., Andy, H., 2009. Competition for light causes plant biodiversity loss after eutrophication. Science, 324: 636-638. DOI:10.1126/science.1169640 |
He, N.P., Liu, C.C., Tian, M., et al., 2017. Variation in leaf anatomical traits from tropical to cold-temperate forests and linkage to ecosystem functions. Funct. Ecol., 32: 10-19. |
He, N.P., Li, Y., Liu, C.C., et al., 2020. Plant trait networks: improved resolution of the dimensionality of adaptation. Trends Ecol. Evol., 10: 908-918. |
Huang, Y., Zhao, X., Zhou, D., et al., 2012. Phenotypic plasticity of early and late successional forbs in response to shifts in resources. PLoS One, 7: e50304. DOI:10.1371/journal.pone.0050304 |
Jager, M.M., Richardson, S.J., Bellingham, P.J., et al., 2015. Soil fertility induces coordinated responses of multiple independent functional traits. J. Ecol., 103: 374-385. DOI:10.1111/1365-2745.12366 |
Laughlin, D.C., Wilson, S., 2014. The intrinsic dimensionality of plant traits and its relevance to community assembly. J. Ecol., 102: 186-193. DOI:10.1111/1365-2745.12187 |
Li, L., McCormack, M.L., Ma, C., et al., 2015. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett., 18: 899-906. DOI:10.1111/ele.12466 |
Lin, G.G., Zeng, D.H., Mao, R., 2020. Traits and their plasticity determine responses of plant performance and community functional property to nitrogen enrichment in a boreal peatland. Plant Soil, 449: 151-167. DOI:10.1007/s11104-020-04478-4 |
Liu, C.C., Li, Y., Zhang, J., et al., 2020a. Optimal community assembly related to leaf economic-hydraulic-anatomical traits. Front. Plant Sci., 11: 341. DOI:10.3389/fpls.2020.00341 |
Liu, C.C., Li, Y., Xu, L., et al., 2019b. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep., 9: 5803. DOI:10.1038/s41598-019-42335-2 |
Liu, Z., L., Hikosaka, K., Li, F.R., et al., 2020b. Variations in leaf economics spectrum traits for an evergreen coniferous species: tree size dominates over environment factors. Funct. Ecol., 34: 1-10. DOI:10.1111/1365-2435.13362 |
Liu, Z.L., Jiang, F., Li, F.R., et al., 2019a. Coordination of intra and inter-species leaf traits according to leaf phenology and plant age for three temperate broadleaf species with different shade tolerances. For. Ecol. Manag., 434: 63-75. DOI:10.1016/j.foreco.2018.12.008 |
Luo, W.T., Zuo, X.A., Griffin-Nolan, R.J., et al., 2019. Long term experimental drought alters community plant trait variation, not trait means, across three semiarid grasslands. Plant Soil, 442: 343-353. DOI:10.1007/s11104-019-04176-w |
Lusk, C.H., Warton, D.I., 2007. Global meta-analysis shows that relationships of leaf mass per area with species shade tolerance depend on leaf habit and ontogeny. New Phytol., 176: 764-774. DOI:10.1111/j.1469-8137.2007.02264.x |
Maire, V., Wright, I.J., Prentice, I.C., et al., 2015. Global effects of soil and climate on leaf photosynthetic traits and rates. Global Ecol. Biogeogr., 24: 706-717. DOI:10.1111/geb.12296 |
Mao, Z.K., Corrales, A., Zhu, K., et al., 2019. Tree mycorrhizal associations mediate soil fertility effects on forest community structure in a temperate forest. New Phytol., 223: 475-486. DOI:10.1111/nph.15742 |
Nardini, A., Peda, G., La Rocca, N., 2012. Trade-offs between leaf hydraulic capacity and drought vulnerability: morpho-anatomical bases, carbon costs and ecological consequences. New Phytol., 196: 788-798. DOI:10.1111/j.1469-8137.2012.04294.x |
Nardini, A., Raimondo, F., Lo Gullo, M.A., et al., 2010. Leafminers help us understand leaf hydraulic design. Plant Cell Environ., 33: 1091-1100. |
Niinemets, Ü., 2001. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology, 82: 453-469. DOI:10.1890/0012-9658(2001)082[0453:GSCCOL]2.0.CO;2 |
Niinemets, Ü., 2010. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res., 25: 693-714. DOI:10.1007/s11284-010-0712-4 |
Niinemets, Ü., Valladares, F., 2006. Tolerance to shade, drought and waterlogging of temperate, Northern hemisphere trees and shrubs. Ecol. Monogr., 76: 521-547. DOI:10.1890/0012-9615(2006)076[0521:TTSDAW]2.0.CO;2 |
Onoda, Y., Wright, I.J., Evans, J.R., et al., 2017. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol., 214: 1447-1463. DOI:10.1111/nph.14496 |
Ordoñez, J.C., Bodegom, P.M., Witte, J.P.M., et al., 2009. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecol. Biogeogr., 18: 137-149. DOI:10.1111/j.1466-8238.2008.00441.x |
Ordoñez, J.C., Bodegom, P.M., Witte, J.P.M., et al., 2010. Plant strategies in relation to resource supply in mesic to wet environments: does theory mirror nature?. Am. Nat., 175: 225-239. DOI:10.1086/649582 |
Osnas, J.L., Lichstein, J.W., Reich, P.B., et al., 2013. Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science, 340: 741-744. DOI:10.1126/science.1231574 |
Poorter, H., Niinemets, Ü., Poorter, L., et al., 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol., 182: 565-588. DOI:10.1111/j.1469-8137.2009.02830.x |
Reich, P.B., Cornelissen, H., 2014. The world-wide 'fast-slow' plant economics spectrum: a traits manifesto. J. Ecol., 102: 275-301. DOI:10.1111/1365-2745.12211 |
Russin, W., Evert, R., 1984. Studies on the leaf of Populus deltoides (Salicaceae): morphology and anatomy. Am. J. Bot., 71: 1398-1415. DOI:10.1002/j.1537-2197.1984.tb11997.x |
Sack, L., Holbrook, N.M., 2006. Leaf hydraulics. Annu. Rev. Plant Biol., 57: 361-381. DOI:10.1146/annurev.arplant.56.032604.144141 |
Sack, L., Scoffoni, C., 2013. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol., 198: 983-1000. DOI:10.1111/nph.12253 |
Sack, L., Tyree, M.T., Holbrook, N.M., 2005. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol., 167: 403-413. DOI:10.1111/j.1469-8137.2005.01432.x |
Sack, L., Scoffoni, C., John, G.P., et al., 2013. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot., 64: 4053-4080. DOI:10.1093/jxb/ert316 |
Sack, L., Scoffoni, C., John, G.P., et al., 2014. Leaf mass per area is independent of vein length per area: avoiding pitfalls when modelling phenotypic integration (reply to Blonder et al. 2014). J. Exp. Bot., 65: 5115-5123. DOI:10.1093/jxb/eru305 |
Sack, L., Scoffoni, C., McKown, A.D., et al., 2012. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat. Commun., 3: 837. DOI:10.1038/ncomms1835 |
Sack, L., Frole, K., 2006. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology, 87: 483-491. DOI:10.1890/05-0710 |
Shipley, B., Lechowicz, M.J., Reich, W.P.B., 2006. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology, 3: 535-541. DOI:10.1890/05-1051 |
Stark, J., Lehman, R., Crawford, L., et al., 2017. Does environmental heterogeneity drive functional trait variation? A test in montane and alpine meadows. Oikos, 126: 1650-1659. DOI:10.1111/oik.04311 |
Valladares, F., Niinemets, Ü., 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst., 39: 237-257. DOI:10.1146/annurev.ecolsys.39.110707.173506 |
Valverde-Barrantes, O.J., Smemo, K.A., Feinstein, L.M., et al., 2018. Patterns in spatial distribution and root trait syndromes for ecto and arbuscular mycorrhizal temperate trees in a mixed broadleaf forest. Oecologia, 186: 731-741. DOI:10.1007/s00442-017-4044-8 |
Wang, Y.P., 1994. Korean Pine Forest. Northeast Forestry University Press, Harbin.
|
Westoby, M., Falster, D.S., Moles, A.T., et al., 2002. Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Evol. Syst., 33: 125-159. DOI:10.1146/annurev.ecolsys.33.010802.150452 |
Wright, I.J., Reich, P.B., Cornelissen, J.H.C., et al., 2005. Modulation of leaf economic traits and trait relationships by climate. Global Ecol. Biogeogr., 14: 411-421. DOI:10.1111/j.1466-822x.2005.00172.x |
Wright, I.J., Reich, P.B., Westoby, M., et al., 2004. The worldwide leaf economics spectrum. Nature, 428: 821-827. DOI:10.1038/nature02403 |
Wright, J.P., Sutton-Grier, A., Stevens, C., 2002. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytol., 155: 403-416. DOI:10.1046/j.1469-8137.2002.00479.x |
Wright, S.J., Kitajima, K., Kraft, N.J.B., et al., 2010. Functional traits and the growth-mortality trade-off in tropical trees. Ecology, 91: 3664-3674. DOI:10.1890/09-2335.1 |
Yang, D.X., Song, L., Jin, G.Z., 2019. The soil C: N: P stoichiometry is more sensitive than the leaf C: N: P stoichiometry to nitrogen addition: a four-year nitrogen addition experiment in a Pinus koraiensis plantation. Plant Soil, 442: 183-198. DOI:10.1007/s11104-019-04165-z |
Yin, Q., Wang, L., Lei, M., et al., 2018. The relationships between leaf economics and hydraulic traits of woody plants depend on water availability. Sci. Total Environ., 621: 245-252. DOI:10.1016/j.scitotenv.2017.11.171 |
Zhang, X.S., Jin, G.Z., Liu, Z.L., 2019. Contribution of leaf anatomical traits to leaf mass per area among canopy layers for five coexisting broadleaf species across shade tolerances at a regional scale. For. Ecol. Manag., 452: 117569. DOI:10.1016/j.foreco.2019.117569 |
Zheng, Z., Ma, P., 2018. Changes in above and belowground traits of a rhizome clonal plant explain its predominance under nitrogen addition. Plant Soil, 432: 415-424. DOI:10.1007/s11104-018-3815-2 |



